Abstract
Study Objectives:
Patients with chronic kidney disease (CKD) often report poor sleep quality, but they commonly exhibit OSA. The aim of this study was to evaluate the influence of OSA severity and of estimated glomerular filtration rate impairment on objective sleep quality in nondialyzed patients with CKD, defined as an estimated glomerular filtration rate <60 mL/min/1.73m2.
Methods:
Polysomnographic sleep characteristics were compared between patients with (n = 430) and without CKD (n = 6,639) in the European Sleep Apnea Database cohort. Comparisons were repeated in 375 patients with CKD and 375 control patients without CKD matched for sleep center, age, sex, and AHI, and in 310 matched CKD and non-CKD patients without psychiatric disturbances.
Results:
Among all patients with and without CKD, total sleep time was similar but sleep stage N1 (median 8.7% [IQR 4.8–18.0] vs 6.7% [3.6–12.7], respectively) and sleep stage R (12.6% [6.8–17.7] vs 14.2% [8.8–19.8], respectively) significantly differed (P < .0001). No difference in sleep characteristics was observed between matched patients either with or without psychiatric disturbances. After subdividing the matched patients according to AHI tertile (<25, ≥25 to <49, and ≥49 events/h) and estimated glomerular filtration rate (≥60, 45 to <60, <45 mL/min/1.73m2), we found a significant effect of AHI on sleep stages N2, N3, and R (P < .001), but there was no effect of CKD.
Conclusions:
In nondialyzed patients with CKD, objective sleep quality is influenced similarly by AHI as in patients without CKD but is not affected by CKD severity. Previously reported poor sleep quality in CKD may partly result from the high prevalence of OSA in CKD.
Citation:
Marrone O, Cibella F, Roisman G, et al. Effects of sleep apnea and kidney dysfunction on objective sleep quality in nondialyzed patients with chronic kidney disease: an ESADA study. J Clin Sleep Med. 2020;16(9):1475–1481.
Keywords: chronic kidney disease, estimated glomerular filtration rate, sleep apnea, sleep quality
BRIEF SUMMARY
Current Knowledge/Study Rationale: Patients with chronic kidney disease often report poor sleep quality but commonly exhibit OSA. In this study, the impact of coexisting OSA and worsening estimated glomerular filtration rate on polysomnographic sleep characteristics was evaluated in non-dialyzed renal patients.
Study Impact: The severity of OSA, but not of the estimated glomerular filtration rate impairment, influenced objective sleep quality. In chronic kidney disease, addressing OSA could be crucial to improve sleep quality.
INTRODUCTION
Sleep characteristics are altered in a broad range of diseases. The relationship between sleep and disease is often bidirectional. Worsening of sleep quality can reflect the detrimental effects of an ongoing disease on the macro- and microarchitecture of sleep. However, poor sleep quality and abnormal sleep duration can negatively influence the progression of a disease.
Chronic kidney disease (CKD) is one of the conditions in which abnormal sleep characteristics have attracted the attention of many researchers in the last decade. The interest in the alteration of sleep characteristics in CKD is related not only to their impact on quality of life but also to patients’ prognosis, because poor sleep quality1,2 and short sleep duration1,3–6 can predict a worse evolution of the disease.
Among patients with CKD, the poorest sleep characteristics have usually been observed in patients with end-stage renal disease, but abnormal sleep features have also been described in less-advanced stages of CKD. Questionnaire-based studies have often showed altered self-reported sleep quality in nondialyzed patients with CKD,7–11 although a relationship between renal function and the degree of impairment of sleep quality has not always been found in cross-sectional7,9,10,12 or longitudinal analyses.10,12 Accordingly, data collected using actigraphic monitoring has shown a relationship between various sleep parameters, including short sleep duration and sleep fragmentation, and CKD severity12,13 or progression.1,6 However, in CKD, alterations in sleep quality may also be related to disorders frequently coexisting with renal dysfunction, like periodic leg movements (PLM) and sleep-disordered breathing (SDB), which have not been considered in previous studies.14,15
Sleep quality assessed by questionnaires or actigraphic recordings is imperfectly correlated with objective electroencephalogram sleep characteristics. Only few studies have evaluated sleep quality and duration on electroencephalogram recordings in nondialyzed patients with CKD using polysomnographic studies. Among them, one small study showed a short sleep time and low sleep efficiency in stage 4–5 CKD but reported a mean apnea-hypopnea index (AHI) of just 1.9 events/h in the sample of patients with CKD.16 In the general population HypnoLaus cohort, which included participants with stage 1–3 CKD and participants with mostly mild to moderate OSA, sleep efficiency was the only objective sleep quality parameter independently correlated to the CKD stage and estimated glomerular filtration rate (eGFR); SDB severity and PLM index were other variables showing independent correlations with total sleep time, slow-wave sleep, and REM percentages.11 However, most patients with OSA in the cohort had mild SDB, so the conclusions of that study were mainly based on interactions between mild CKD and mild OSA.
Neuropsychiatric disorders, including depression and anxiety, which are highly prevalent in CKD,17 represent another potential confounder in the analysis of the relationship between CKD and sleep quality. Their possible role in disturbing the sleep of patients with CKD has not been evaluated.
The aim of this study was to assess in nondialyzed patients with a low eGFR the independent contribution of the severity of SDB and eGFR impairment on sleep duration and quality, evaluated using an electroencephalogram. For this purpose, we conducted a case-control analysis in a population with a high rate of severe SDB and considered the coexistence of psychiatric disturbances.
METHODS
Data from patients included in the European Sleep Apnea Database (ESADA) were analyzed. The ESADA study prospectively collects data from unselected patients aged 18–80 years with suspected OSA referred to European sleep centers. Data recorded in the ESADA include anthropometrics, information on comorbidities, blood tests, degree of daytime somnolence assessed by the Epworth Sleepiness Scale, and baseline polysomnography or cardiorespiratory polygraphy data. Additional information that may define the health conditions of each patient more precisely is recorded in a separate column. Exclusion criteria included a previous OSA diagnosis, limited estimated life expectancy, and current alcohol or drug abuse.18
Enrollment in the ESADA started in March 2007. Written informed consent to anonymous use of data was obtained from all patients. Each sleep center obtained approval from the ethical committee of its own institution.
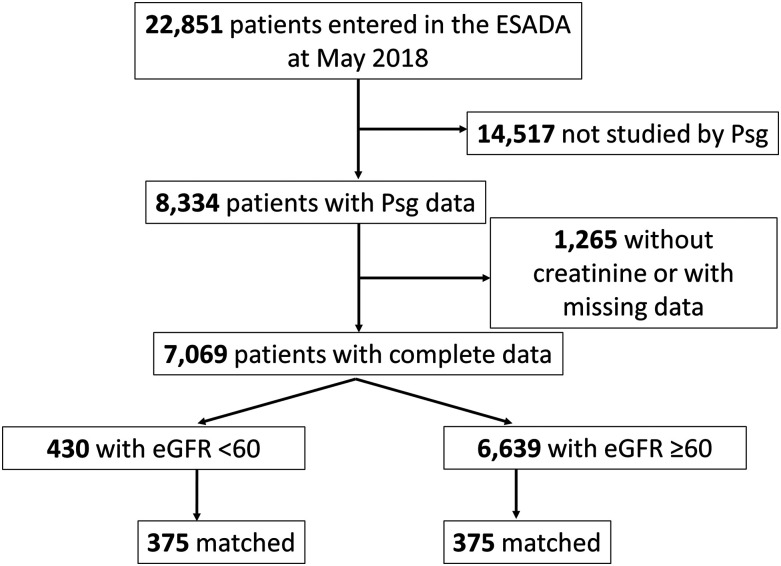
Data recorded between March 2007 and December 2017 for 22,851 participants were analyzed. The methods used for polysomnography scoring were previously reported.19 After we excluded patients studied by polygraphy (n = 14,517) and those without data on creatinine (n = 381) or other variables of interest (n = 884), data collected for 7,069 participants were available for the analysis. The Chronic Kidney Disease-Epidemiology Collaboration equation was used for the calculation of eGFR in these patients.20 Four hundred thirty patients were found with an eGFR <60 mL/min/m2; in the database, dialytic therapy was not reported for any of these patients. For the purposes of this study, we considered these participants as CKD patients. Next, each of these patients was matched to one patient with an eGFR >60 mL/min/m2. Matching criteria included sleep center, sex, age ±4 years, and AHI ±4 events/h if less than the median (34 events/h) or AHI ±8 events/h if higher than the median. Finally, 375 patients and 375 control patients could be matched. The flowchart for patients’ inclusion is shown in Figure 1.
Figure 1. Schematic representation of patients’ inclusion criteria.
Data on sleep duration and distribution of sleep stages obtained from polysomnography were compared between patients with CKD and control patients. Psychiatric disorders are often associated with abnormal polysomnographic sleep characteristics.21 The analysis was then repeated after excluding patients with psychiatric disorders, defined as a psychiatric diagnosis and/or assumption of drugs classified as psycholeptic or psychoanaleptic (codes N05 or N06 of the Anatomical Therapeutic Chemical (ATC) classification). For this analysis, 310 patients with an eGFR <60 and 310 control patients were compared.
Statistical analysis
Results are presented as mean ± standard deviation for continuous variables if normally distributed and as median (IQR) if not. Continuous variables relevant to patient and sleep characteristics were compared between CKD patients and control patients by one-way analysis of variance or Mann-Whitney U-test, as appropriate. The normality of variable distribution was evaluated by Kolmogorov-Smirnov test. Categorical variables were compared by chi-square test. The same analyses were repeated after excluding patients with psychiatric disorders. Then, all the selected patients were subdivided into 3 groups according to AHI tertile. In turn, each group was subdivided into 3 subgroups according to eGFR: ≥60, 45 to <60 (CKD stage 3a), and <45 mL/min/m2 (CKD stages 3b, 4, and 5). A two-way analysis of variance was adopted to explore the influence of AHI and eGFR and of their interaction on sleep characteristics. A two-tailed P value of <.05 was considered significant.
RESULTS
Patients with CKD as compared with patients without CKD were older, were more often female, were slightly more obese, and had a higher number of comorbidities and worse SDB parameters (Table 1). Their sleep was characterized by a similar duration and a lower efficiency, a higher representation of sleep stage N1, and a lower representation of sleep stage R (Table 2).
Table 1.
Characteristics of patients in ESADA cohort with and without CKD.
| CKD (n = 430) | Non-CKD (n = 6,639) | P Value | |
|---|---|---|---|
| eGFR, mL/min/1.73 m2, mean ± SD | 48.1 ± 11.5 | 92.0 ± 15.4 | <.0001* |
| Age, y, mean ± SD | 63.5 ± 10.4 | 51.0 ± 11.9 | <.0001* |
| Sex, female, % | 39.5 | 26.9 | <.0001† |
| BMI, kg/m2, mean ± SD | 33.6 ± 7.3 | 31.3 ± 6.3 | <.0001* |
| ESS score, mean ± SD | 9.6 ± 5.2 | 10.0 ± 5.3 | .102* |
| Arterial hypertension, % patients | 72.1 | 41.4 | <.0001† |
| Diabetes, % patients | 27.6 | 12.5 | <.0001† |
| CHF, % patients | 11.2 | 2.4 | <.0001† |
| AHI, events/h, median (IQR) | 34 (20–57) | 26 (12–49) | <.0001‡ |
| Mean SpO2, %, median (IQR) | 92 (90–94) | 94 (92–95) | <.0001‡ |
| Lowest SpO2, %, median (IQR) | 79 (71–84) | 83 (76–88) | <.0001‡ |
One-way analysis of variance. †χ2 test. ‡Mann-Whitney U test. BMI = body mass index, CHF = chronic heart failure, CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, ESADA, European Sleep Apnea Database, ESS = Epworth Sleepiness Scale, IQR = interquartile range, SD = standard deviation, SpO2 = oxyhemoglobin saturation.
Table 2.
Sleep characteristics of patients in ESADA cohort with and without CKD.
| CKD (n = 430) | Non-CKD (n = 6,639) | P Value | |
|---|---|---|---|
| TST, min, mean ± SD | 370.6 ± 74.7 | 376.8 ± 66.1 | .063* |
| Sleep efficiency, %, median (IQR) | 83.0 (72.4–91.5) | 85.1 (75.8–92.7) | .0004† |
| Stage N1, %, median (IQR) | 8.7 (4.8–18.0) | 6.7 (3.6–12.7) | <.0001† |
| Stage N2, %, mean ± SD | 55.3 ± 15.9 | 55.8 ± 15.1 | .528* |
| Stage N3, %, median (IQR) | 16.4 (7.8–26.1) | 17.6 (9.0–27.8) | .053† |
| Stage R, %, median (IQR) | 12.6 (6.8–17.7) | 14.2 (8.8–19.8) | <.0001† |
One-way analysis of variance. †Mann-Whitney U test. CKD = chronic kidney disease, ESADA = European Sleep Apnea Database, IQR = interquartile range, SD = standard deviation, TST = total sleep time.
The characteristics of the patients selected after matching are shown in Table 3. In this sample, patients with CKD and the control patients did not differ in age, sex, and AHI, by design, or in perceived daytime sleepiness assessed by the Epworth Sleepiness Scale, but the patients with CKD still had more comorbidities, were slightly more obese, and had slightly worse nocturnal hypoxemia. There was no difference in sleep characteristics between the patients with CKD and the matched control patients (Table 4).
Table 3.
Characteristics of patients selected for matching.
| CKD Patients (n = 375) | Control Patients (n = 375) | P Value | |
|---|---|---|---|
| eGFR, mL/min/1.73m2, mean ± SD | 48.3 ± 11.5 | 84.3 ± 13.7 | <.0001* |
| Age, y, mean ± SD | 62.2 ± 9.6 | 62.0 ± 9.4 | .765* |
| Sex, female, % | 50.0 | 50.0 | — |
| BMI, kg/m2, mean ± SD | 33.7 ± 7.3 | 31.8 ± 6.5 | .0002* |
| ESS score, mean ± SD | 9.7 ± 5.3 | 9.3 ± 5.2 | .294* |
| Arterial hypertension, % patients | 70.7 | 61.6 | .009† |
| Diabetes, % patients | 27.4 | 16.7 | .004† |
| CHF, % patients | 11.0 | 4.6 | .001† |
| AHI, events/h, median (IQR) | 34 (19–56) | 35 (19–56) | .815‡ |
| Mean SpO2, %, median (IQR) | 92 (90–94) | 93 (91–95) | .027‡ |
| Lowest SpO2, %, median (IQR) | 80 (71–85) | 80 (72–85) | .228‡ |
One-way analysis of variance. †χ2 test. ‡Mann-Whitney U test. BMI = body mass index, CHF = chronic heart failure, CKD = chronic kidney disease, ESS = Epworth Sleepiness Scale, IQR = interquartile range, SD = standard deviation, SpO2 = oxyhemoglobin saturation.
Table 4.
Sleep characteristics of matched patients.
| CKD Patients (n = 375) | Control Patients (n = 375) | P Value | |
|---|---|---|---|
| TST, min, mean ± SD | 373.0 ± 70.8 | 373.6 ± 70.9 | .913* |
| Sleep efficiency, %, median (IQR) | 83.8 (72.9–91.6) | 83.0 (72.4–92.8) | .816† |
| Stage N1, %, median (IQR) | 8.3 (4.6–17.5) | 8.1 (4.3–14.1) | .452† |
| Stage N2, %, mean ± SD | 55.3 ± 15.3 | 55.4 ± 16.1 | .955* |
| Stage N3, %, median (IQR) | 16.6 (8.4–26.6) | 15.4 (8.0–28.8) | .684† |
| Stage R, %, median (IQR) | 12.5 (6.8–17.9) | 12.7 (7.1–19.0) | .436† |
One-way analysis of variance. †Mann-Whitney U test. CKD = chronic kidney disease, IQR = interquartile range, SD = standard deviation, TST = total sleep time.
Altogether, 14.2% of patients with CKD and 11.5% of control patients had a psychiatric disorder (P = .263) resulting from a reported previous psychiatric diagnosis (3patients with CKD vs control patients, 9.3% vs 7.4%; P = .344) and/or from the assumption of N05-psycholeptic (5.9% vs 1.9%; P = .005) or N06-psychoanaleptic drugs (7.9% vs 6.7%; P = .336). Differences in characteristics between the matched patients with and without CKD unaffected by psychiatric disorders are shown in Table S1 in the supplemental material. Again, no differences in sleep characteristics were found between patients with CKD and control patients (Table S2).
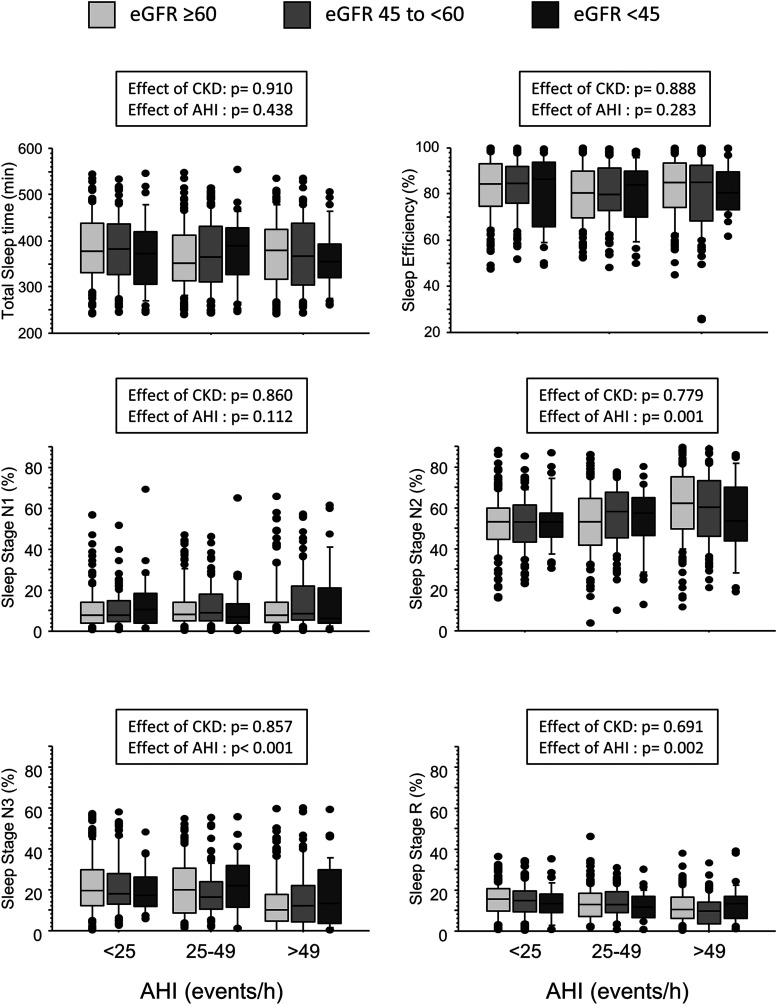
AHI tertiles in the matched patients with and without CKD (including the patients with psychiatric disorders) were <25, ≥25 to <49, and ≥49 events/h. Among the patients with CKD, 278 (74%) were in stage 3a, 71 (19%) were in stage 3b, 14 (4%) were in stage 4, and 12 (3%) were in stage 5 CKD. When the matched patients were subdivided into subgroups according to AHI tertile and to eGFR category, no effect of either AHI or CKD severity on total sleep time or sleep efficiency was observed. Whereas the percentage of sleep stage N2 increased, stages N3 and R decreased with increasing AHI (P for all changes <.0001) but no effect of CKD or AHI-CKD interaction was found (Figure 2).
Figure 2. Data distribution of polysomnographic variables, separately for patients with different eGFR and AHI classes.
Horizontal bars within each box represent median values. Bars closing inferiorly and superiorly each box represent, respectively, 25th and 75th percentiles. Values below 10th and above 90th percentiles are plotted as circles. P values were evaluated by two-way analysis of variance. CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate.
DISCUSSION
In our cohort of patients studied for suspected OSA, objective sleep characteristics were worse in nondialyzed patients with an eGFR <60 mL/min/m2 than in those with a higher eGFR in terms of sleep efficiency and sleep stage distribution. However, when comparisons were made between patients with low eGFR and control patients matched for AHI and other possible confounders, all differences in sleep parameters disappeared despite slightly worse nocturnal hypoxemia and a higher prevalence of cardiovascular comorbidities and diabetes mellitus in the patients with CKD. Further analysis showed that sleep quality worsened in a parallel fashion in patients with and control patients as AHI increased, pointing out the role of OSA as a possible cause of poor sleep quality in CKD. By contrast, increasing eGFR impairment did not affect sleep characteristics.
Altered sleep quality is considered a part of the symptom burden of patients with CKD, not only in end-stage renal disease but also in the early stages of the disease.22 Today, the prominent role of frequently coexistent OSA and PLM as factors disturbing sleep in CKD is clearly established. However, an association between poor sleep quality or short sleep duration and CKD could also be expected independently of these comorbidities. On the one hand, habitually short sleep duration and altered sleep quality have been reported as precursors of deteriorated kidney function, possibly as an effect of increased sympathetic activity and its cardiovascular and metabolic consequences.1,3,5,6,23 On the other hand, CKD is associated with neurological impairment that could easily result in both self-reported and objective sleep disturbances.24 In addition, fluid retention and uremia that characterize CKD, especially in its most advanced stages, have been suggested as possible causes of an objectively altered sleep quality, even independent of SDB and PLM.25
Studies based on self-reports have confirmed that sleep quality is poor in nondialyzed patients with CKD, but they did not consider the possible role of coexistent OSA.9,26 In addition, self-reported and objective sleep quality in each individual may differ.27 The only large study on objective sleep quality in mild CKD (stages 1–3), in patients in the HypnoLaus cohort, found that only sleep efficiency and possibly total sleep time were independently correlated to CKD stage and eGFR, whereas other sleep parameters were correlated to the SDB and PLM indexes.11 As expected, we found significant relationships between OSA severity and deteriorated sleep stage distribution. However, we were not able to show any association between CKD and sleep parameters that was independent of SDB. We cannot explain the difference between our findings in the ESADA cohort and those described in the HypnoLaus cohort. However, a strength of our study was the larger sample of patients with CKD and the very accurate matching between the patients with CKD and the control patients, which considered the most important potential confounding factors.
Psychiatric disturbances are more common in patients with CKD than in the general population.17 In our sample, the prevalence of psychiatric diagnoses did not differ between the patients with CKD and the control patients. Underreporting of psychiatric problems could have masked their higher prevalence among the patients with CKD. However, in that case we would expect a worse sleep quality in the sample of patients with CKD, which was not observed. Nevertheless, the very small difference in sleep characteristics between the patients with CKD and the control patients matched before and after the exclusion of those with a recognized psychiatric comorbidity suggests that psychiatric problems in CKD, when present, had little influence on sleep quality, possibly because they were mild or well controlled by pharmacological therapy.
A weakness of the study includes the availability of information on PLM only in about 50% of the patients. Although in this subsample the PLM index did not differ between patients with CKD and control patients (median 5.6 [IQR 0.0–22.2] vs 4.8 [0.4–14.9 events/h], respectively; P = .45), we cannot draw any conclusion on this issue. However, the confounding role of missing records regarding PLM could be called into question if differences between patients with CKD and control patients had been observed. Our data suggest that regardless of PLM, objective sleep quality is not affected in nondialyzed patients with CKD independent of SDB.
Furthermore, the analysis of sleep characteristics was limited to total sleep time, sleep efficiency, and sleep-stage distribution and thus could not be sensitive enough to identify subtle sleep characteristics that could distinguish patients with CKD. As for PLM, the arousal index was available only for approximately one-half of the patients. We cannot rule out that a more sophisticated electroencephalogram analysis, with arousal index, spectral analysis, odds ratio product, or cycling alternating pattern, would reveal differences that were not apparent in the variables we examined.
Another limitation was that we relied on single creatinine measurements for the identification of CKD, which could lead to CKD misdiagnosis.28 However, in the vast majority of patients, evaluations were performed in a stable clinical situation. In addition, other large epidemiological studies have been based on single measurements and were nonetheless able to show a relationship between sleep quality and kidney function.26
Finally, the patients with CKD were not selected from the general population but from a cohort of patients studied for suspected OSA. Therefore, our results may not apply to the entire spectrum of patients with CKD. Nevertheless, the characteristics of our population allowed us to explore the interaction between CKD and OSA in depth, including many patients with severe OSA, whereas studies performed in the general population have included mostly patients with mild forms of OSA.
In conclusion, SDB influences sleep-stage distribution in a similar fashion in nondialyzed patients with CKD and in patients without CKD, but no differences in objective sleep duration and quality are evident between patients with different degrees of eGFR impairment and similar OSA severity. This result suggests that CKD does not per se significantly affect objective sleep characteristics. Previously self-reported poor sleep quality and short sleep duration in CKD can partly be explained by poor correlation between self-reported and objective sleep assessment and by the high prevalence of OSA in CKD. Further studies on the effects of CPAP treatment on sleep quality in patients with CKD with OSA may lend further support to this hypothesis.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. By submitting the manuscript to the journal, the authors affirm that it is an original manuscript, is unpublished work if not as an abstract, and is not under consideration elsewhere. Work for this study was performed at institutions listed in the acknowledgments. This study was funded by support for the European Sleep Apnea Database network from the European Union COST Action B26 and the European Respiratory Society–funded Clinical Research Collaboration (funding 2015–2020). Unrestricted seed grants from the ResMed Foundation and the Philips Respironics Foundation for the establishment of the database in 2007 and 2011 are gratefully acknowledged. The European Sleep Apnea Database has a scientific collaboration with Bayer AG. O.M., F.C, G.R., P.S., P.J., O.K.B., I.B., S.S., A.P., R.S., and J.V. do not have any financial interests or connections, direct or indirect, or other situations that might raise the question of bias in the work reported or the conclusions, implications, or opinions stated. J.H. reports grants from ResMed, Philips Respironics, Bayer, and the European Respiratory Society related to maintenance of the database on behalf of the European Sleep Apnea Database group. L.G. reports a collaboration between the European Sleep Apnea Database network and Bayer. M.R.B. reports research financial support by Vivisol and Medicair Italy and honoraria from ResMed and Bioproject.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
ESADA Collaborators: Ulla Anttalainen and Tarja Saaresranta (Turku University Hospital, Division of Medicine, Department of Pulmonary Diseases and Sleep Research Center and Department of Pulmonary Diseases and Clinical Allergology, University of Turku, Turku, Finland); Sebastien Bailly, Jean-Louis Pépin, and Renaud Tamisier (Université Joseph Fourier and Université Grenoble Alpes, Grenoble, France); Ozen K. Basoglu and Sezai Tasbakan (Department of Chest Diseases, Ege University School of Medicine, Izmir, Turkey); Maria R. Bonsignore, Fabio Cibella, and Oreste Marrone (Institute for Biomedical Research and Innovation, National Research Council, Palermo, and PROMISE Department, University of Palermo, Palermo, Italy); Izolde Bouloukaki and Sophia Schiza (Sleep Disorders Center, University of Crete, Heraklion, Greece); Zoran Dogas (Sleep Medicine Center, Department of Neuroscience, University of Split School of Medicine, Split, Croatia); Marta Drummond and Mafalda von Zeller (Pulmonology Department Hospital São João, Medicine Faculty of Porto University, Porto, Portugal); Pierre Escourrou and Gabriel Roisman (Service d’Explorations Fonctionnelles Multidisciplinaires and Unité de Médecine du Sommeil, Hôpital Antoine Béclère, Clamart, France); Ingo Fietze and Thomas Penzel (Centre of Sleep Medicine, Charité Universitätsmedizin Berlin, Berlin, Germany); Ludger Grote, Jan Hedner, and Ding Zou (Center of Sleep and Wake Disorders, Sahlgrenska Academy, Gothenburg University and Sleep Medicine, Sahlgrenska University Hospital and Sahlgrenska Academy, Gothenburg, Sweden); Haralampos Gouveris (ENT Department at Mainz University Hospital, Mainz, Germany); Pavol Joppa and Ruzena Tkacova (Department of Respiratory Medicine and Tuberculosis, Faculty of Medicine, P.J. Safarik University and L. Pasteur University Hospital, Kosice, Slovakia); Holger Hein (Sleep Disorders Center, St. Adolf Stift, Reinbeck, Germany); Brian D. Kent, Walter T. McNicholas, and Silke Ryan (School of Medicine and Medical Science, University College Dublin and Department of Respiratory and Sleep Medicine, St. Vincent’s Hospital Group, Dublin, Ireland); John A. Kvamme (ENT Department, Førde Central Hospital, Førde, Norway); Carolina Lombardi and Gianfranco Parati (Sleep Disorders Center, Department of Cardiovascular Neural and Metabolic Sciences, IRCCS Istituto Auxologico Italiano, Milano-Bicocca University, Milan, Italy); Ondrej Ludka (Department of Cardiology, University Hospital Brno and International Clinical Research Center, St. Ann’s University Hospital, Brno, Czech Republic); Stefan Mihaicuta (Sleep Disorders Center, University Hospital, Timisoara, Rumania); Athanasia Pataka (Respiratory Failure Unit, G. Papanikolau Hospital, Exohi, Thessaloniki, Greece); Robert Plywaczewski and Pawel Sliwinski (Institute of Tuberculosis and Lung Diseases, Warsaw, Poland); Martin Pretl (Sleep Disorders Centre, Department of Neurology, Charles University, Prague, Czech Republic); Renata Riha (Department of Sleep Medicine, Edinburgh Royal Infirmary, Edinburgh, UK); Richard Staats (Department of Pneumology, University Hospital de Santa Maria, Lisbon, Portugal); Paschalis Steiropoulos (Sleep Unit, Department of Pneumonology, Democritus University of Thrace, Alexandroupolis, Greece); Georgia Trakada (Pulmonary Medicine, National and Kapodistrian University of Athens, Athens, Greece); and Johan Verbraecken (Multidisciplinary Sleep Disorders Centre, Antwerp University Hospital and University of Antwerp, Edegem-Antwerp, Belgium).
ABBREVIATIONS
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- ESADA
European Sleep Apnea Database
- PLM
periodic leg movements
- SDB
sleep-disordered breathing
Contributor Information
Collaborators: Ulla Anttalainen, Tarja Saaresranta, Sebastien Bailly, Jean-Louis Pépin, Renaud Tamisier, Ozen K. Basoglu, Sezai Tasbakan, Maria R. Bonsignore, Fabio Cibella, Oreste Marrone, Izolde Bouloukaki, Sophia Schiza, Zoran Dogas, Marta Drummond, Mafalda von Zeller, Pierre Escourrou, Gabriel Roisman, Ingo Fietze, Thomas Penzel, Ludger Grote, Jan Hedner, Ding Zou, Haralampos Gouveris, Pavol Joppa, Ruzena Tkacova, Holger Hein, Brian D. Kent, Walter T. McNicholas, Silke Ryan, John A. Kvamme, Carolina Lombardi, Gianfranco Parati, Ondrej Ludka, Stefan Mihaicuta, Athanasia Pataka, Robert Plywaczewski, Pawel Sliwinski, Martin Pretl, Renata Riha, Richard Staats, Paschalis Steiropoulos, Georgia Trakada, and Johan Verbraecken
REFERENCES
- 1.Petrov ME, Kim Y, Lauderdale DS, et al. Objective sleep, a novel risk factor for alterations in kidney function: the CARDIA study. Sleep Med. 2014;15(9):1140–1146. 10.1016/j.sleep.2014.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamamoto R, Shinzawa M, Isaka Y, et al. Sleep quality and sleep duration with CKD are associated with progression to ESKD. Clin J Am Soc Nephrol. 2018;13(12):1825–1832. 10.2215/CJN.01340118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto R, Nagasawa Y, Iwatani H, et al. Self-reported sleep duration and prediction of proteinuria: a retrospective cohort study. Am J Kidney Dis. 2012;59(3):343–355. 10.1053/j.ajkd.2011.08.032 [DOI] [PubMed] [Google Scholar]
- 4.Sasaki S, Yoshioka E, Saijo Y, Kita T, Tamakoshi A, Kishi R. Short sleep duration increases the risk of chronic kidney disease in shift workers. J Occup Environ Med. 2014;56(12):1243–1248. 10.1097/JOM.0000000000000322 [DOI] [PubMed] [Google Scholar]
- 5.McMullan CJ, Curhan GC, Forman JP. Association of short sleep duration and rapid decline in renal function. Kidney Int. 2016;89(6):1324–1330. 10.1016/j.kint.2015.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricardo AC, Knutson K, Chen J, et al. The association of sleep duration and quality with CKD progression. J Am Soc Nephrol. 2017;28(12):3708–3715. 10.1681/ASN.2016121288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iliescu EA, Yeates KE, Holland DC. Quality of sleep in patients with chronic kidney disease. Nephrol Dial Transplant. 2004;19(1):95–99. 10.1093/ndt/gfg423 [DOI] [PubMed] [Google Scholar]
- 8.Kurella M, Luan J, Lash JP, Chertow GM. Self-assessed sleep quality in chronic kidney disease. Int Urol Nephrol. 2005;37(1):159–165. 10.1007/s11255-004-4654-z [DOI] [PubMed] [Google Scholar]
- 9.Kumar B, Tilea A, Gillespie BW, et al. Significance of self-reported sleep quality (SQ) in chronic kidney disease (CKD): the Renal Research Institute (RRI)-CKD study. Clin Nephrol. 2010;73(2):104–114. 10.5414/CNP73104 [DOI] [PubMed] [Google Scholar]
- 10.Sabbatini M, Pisani A, Crispo A, et al. Sleep quality in patients with chronic renal failure: a 3-year longitudinal study. Sleep Med. 2008;9(3):240–246. 10.1016/j.sleep.2007.04.005 [DOI] [PubMed] [Google Scholar]
- 11.Ogna A, Forni Ogna V, Haba Rubio J, et al. Sleep characteristics in early stages of chronic kidney disease in the HypnoLaus cohort. Sleep. 2016;39(4):945–953. 10.5665/sleep.5660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal R, Light RP. Sleep and activity in chronic kidney disease: a longitudinal study. Clin J Am Soc Nephrol. 2011;6(6):1258–1265. 10.2215/CJN.10581110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knutson KL, Lash J, Ricardo AC, et al. Habitual sleep and kidney function in chronic kidney disease: the Chronic Renal Insufficiency Cohort study. J Sleep Res. 2018;27(2):283–291. 10.1111/jsr.12573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voulgaris A, Marrone O, Bonsignore MR, Steiropoulos P. Chronic kidney disease in patients with obstructive sleep apnea. A narrative review. Sleep Med Rev. October 2019;47:74–89. 10.1016/j.smrv.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 15.Lin Z, Zhao C, Luo Q, Xia X, Yu X, Huang F. Prevalence of restless legs syndrome in chronic kidney disease: a systematic review and meta-analysis of observational studies. Ren Fail. 2016;38(9):1335–1346. 10.1080/0886022X.2016.1227564 [DOI] [PubMed] [Google Scholar]
- 16.Parker KP, Bliwise DL, Bailey JL, Rye DB. Polysomnographic measures of nocturnal sleep in patients on chronic, intermittent daytime haemodialysis vs those with chronic kidney disease. Nephrol Dial Transplant. 2005;20(7):1422–1428. 10.1093/ndt/gfh816 [DOI] [PubMed] [Google Scholar]
- 17.Simões E Silva AC, Miranda AS, Rocha NP, Teixeira AL. Neuropsychiatric disorders in chronic kidney disease. Front Pharmacol. August 2019;10:932. 10.3389/fphar.2019.00932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hedner J, Grote L, Bonsignore M, et al. The European Sleep Apnoea Database (ESADA): report from 22 European sleep laboratories. Eur Respir J. 2011;38(3):635–642. 10.1183/09031936.00046710 [DOI] [PubMed] [Google Scholar]
- 19.Escourrou P, Grote L, Penzel T, et al. The diagnostic method has a strong influence on classification of obstructive sleep apnea. J Sleep Res. 2015;24(6):730–738. 10.1111/jsr.12318 [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baglioni C, Nanovska S, Regen W, et al. Sleep and mental disorders: A meta-analysis of polysomnographic research. Psychol Bull. 2016;142(9):969–990. 10.1037/bul0000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown SA, Tyrer FC, Clarke AL, et al. Symptom burden in patients with chronic kidney disease not requiring renal replacement therapy. Clin Kidney J. 2017;10(6):788–796. 10.1093/ckj/sfx057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bo Y, Yeoh EK, Guo C, et al. Sleep and the risk of chronic kidney disease: a cohort study. J Clin Sleep Med. 2019;15(03):393–400. 10.5664/jcsm.7660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jabbari B, Vaziri ND. The nature, consequences, and management of neurological disorders in chronic kidney disease. Hemodial Int. 2018;22(2):150–160. 10.1111/hdi.12587 [DOI] [PubMed] [Google Scholar]
- 25.Elias RM, Chan CT, Bradley TD. Altered sleep structure in patients with end-stage renal disease. Sleep Med. 2016;20:67–71. 10.1016/j.sleep.2015.10.022 [DOI] [PubMed] [Google Scholar]
- 26.Kim CW, Chang Y, Sung E, et al. Sleep duration and quality in relation to chronic kidney disease and glomerular hyperfiltration in healthy men and women. PLoS One. 2017;12(4):e0175298. 10.1371/journal.pone.0175298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan KA, Hardas PP, Redline S, Zeitzer JM; Sleep Heart Health Study Research Group . Correlates of sleep quality in midlife and beyond: a machine learning analysis. Sleep Med. 2017;34:162–167. 10.1016/j.sleep.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delanaye P, Glassock RJ, De Broe ME. Epidemiology of chronic kidney disease: think (at least) twice! Clin Kidney J. 2017;10(3):370–374. 10.1093/ckj/sfw154 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.