Abstract
Background
At ketamine and esketamine doses at which antidepressant doses are achieved, these agents are relatively selective, noncompetitive, N-methyl-D-aspartate receptor antagonists. However, at substantially higher doses, ketamine has shown mu-opioid receptor (MOR–gene symbol: OPRM1) agonist effects. Preliminary clinical studies showed conflicting results on whether naltrexone, a MOR antagonist, blocks the antidepressant action of ketamine. We examined drug-induced or endogenous MOR involvement in the antidepressant and dissociative responses to esketamine by assessing the effects of a functional single nucleotide polymorphism rs1799971 (A118G) of OPRM1, which is known to alter MOR agonist-mediated responses.
Methods
Participants with treatment-resistant depression from 2 phase III, double-blind, controlled trials of esketamine (or placebo) nasal spray plus an oral antidepressant were genotyped for rs1799971. Participants received the experimental agents twice weekly for 4 weeks. Antidepressant responses were rated using the change in Montgomery–Åsberg Depression Rating Scale (MADRS) score on days 2 and 28 post-dose initiation, and dissociative side effects were assessed using the Clinician-Administered Dissociative-States Scale at 40 minutes post-dose on days 1 and 25.
Results
In the esketamine + antidepressant arm, no significant genotype effect of single nucleotide polymorphism rs1799971 (A118G) on MADRS score reductions was detected on either day 2 or 28. By contrast, in the antidepressant + placebo arm, there was a significant genotype effect on MADRS score reductions on day 2 and a nonsignificant trend on day 28 towards an improvement in depression symptoms in G-allele carriers. No significant genotype effects on dissociative responses were detected.
Conclusions
Variation in rs1799971 (A118G) did not affect the antidepressant response to esketamine + antidepressant. Antidepressant response to antidepressant + placebo was increased in G-allele carriers, compatible with previous reports that release of endorphins/enkephalins may play a role in mediating placebo effect.
Trial Registration
Keywords: depression, esketamine, ketamine, mu-opioid receptor, polymorphism
Significance Statement
Ketamine and esketamine are N-methyl-D-aspartate receptor antagonists that produce robust and rapid antidepressant actions at subanesthetic doses. At substantially higher concentrations than needed for antidepressant effects, these agents stimulate the mu-opioid receptor (MOR–gene symbol: OPRM1). A small clinical study reported that MOR antagonism blocks the antidepressant action of ketamine, although this observation was inconsistent with evidence from other studies. Here, we examined whether the single nucleotide polymorphism rs1799971 (A118G) of OPRM1, which is known to alter MOR agonist-mediated responses, affected the antidepressant and dissociative responses to esketamine. Using data from 2 phase III studies of esketamine, we found no significant genotype effect on reductions in Montgomery–Åsberg Depression Rating Scale total score or on dissociative responses in patients with treatment-resistant depression treated with esketamine + oral antidepressant. These results did not support the hypothesis that the antidepressant effect of esketamine was mediated by MOR activation.
Introduction
Ketamine and esketamine are N-methyl-D-aspartate receptor (NMDAR) antagonists that in subanesthetic doses exert robust and rapid antidepressant effects in individuals with treatment-resistant depression (TRD) (Singh et al., 2016a, 2016b). However, ketamine has a known potential for abuse, and some individuals suffering from depression may be at risk (Kalsi et al., 2011; Schak et al., 2016). A recent report suggesting that the antidepressant effects of ketamine may be influenced by opiate receptor activation has added to this concern (Williams et al., 2018).
The inhibitory constant (Ki) values for ketamine and esketamine binding the NMDAR were approximately 1 μM and 0.5 μM, respectively (Zanos et al., 2018). Ketamine and esketamine showed weak potency for the mu-opioid receptor (MOR; gene symbol: OPRM1) with Ki values of 42 μM and 11 or 28.6 μM, respectively (Hustveit et al., 1995; Hirota et al., 1999). The half maximal effective concentration (EC50) of ketamine was 1000 μM for MOR in a functional assay (Hirota et al., 1999), while the estimated brain unbound ketamine level was approximately 1 μM when administered at antidepressant doses (Zanos et al., 2018) (see Discussion). These data suggest that ketamine and esketamine are relatively selective towards NMDAR at antidepressant doses. Thus, it appears highly unlikely that direct MOR agonist effects contribute to the antidepressant effects of ketamine as proposed by Williams et al. (Williams et al., 2018). It remains conceivable, however, that indirect MOR stimulation via release of endogenous opioid peptides may play a role in the antidepressant effects of ketamine and esketamine (Aalto et al., 2005). Williams et al. showed that naltrexone, a potent MOR antagonist that would putatively block both MOR-mediated and endogenous MOR stimulation, attenuated the antidepressant effects of ketamine (Williams et al., 2018). However, 2 follow-up reports did not observe the same blocking effects (Marton et al., 2019; Yoon et al., 2019).
Genetic variation in OPRM1 offers an opportunity to assess whether the antidepressant effects of ketamine and esketamine are influenced by MOR activation. The single nucleotide polymorphism (SNP) rs1799971 involves a nucleotide substitution at position 118 (A118G) in OPRM1, which results in a missense amino acid change (Asn44Asp) at an N-glycosylation site that affects the stability of the receptor protein (Bond et al., 1998; Huang et al., 2012). In clinical and preclinical models, these SNPs have been shown to alter the binding and activity of endogenous opioid peptides that mediate resilience to stress and the positive emotional expectancy to placebo administration (Bond et al., 1998). In genetically manipulated cells and brain tissue, the SNPs were associated with increased responses of endogenous opioid peptides (Bond et al., 1998), but decreased MOR mRNA expression, total MOR protein levels, and receptor binding (putatively as compensatory responses to the increased sensitivity to endogenous opioid peptides) (Mague et al., 2009; Huang et al., 2012). Models of the OPRM GG variant’s influence on function have displayed lower morphine-induced hyperlocomotion (Mague et al., 2009), reduced morphine-induced analgesia (Mague et al., 2009; Mahmoud et al., 2011; Henderson-Redmond et al., 2016), reduced buprenorphine-induced analgesia (Browne et al., 2017), blunted morphine-induced hypothermia (Henderson-Redmond et al., 2016), decreased MOR stimulation-induced excitatory responses in hippocampal neurons (Mague et al., 2015), and decreased morphine-induced suppression of electroencephalogram gamma band power (Mague et al., 2015). In studies assessing the effects of this variant on the response to drugs with potent MOR agonism, mice expressing the GG variant showed attenuation of antinociceptive and other responses to MOR agonists (Zubieta et al., 2005). Collectively, these preclinical data indicated that A118G was a functional variant of OPRM1.
Human G allele carriers of the rs1799971 polymorphism showed lower binding potential to [11C]carfentanil, a MOR-specific tracer sensitive to displacement by endogenous opioid peptides in whole brain (Weerts et al., 2013) and in brain regions involved in pain and affect (Peciña et al., 2015). For this radiotracer, a lower binding potential may thus reflect either increased endogenous ligand release or decreased receptor density or affinity. Crucially, a meta-analysis revealed that individuals homozygous for the OPRM1 (rs1799971) A-allele required less postsurgical opiate analgesic treatment compared with those homozygous for the G-allele (Choi et al., 2017), implying the net effect of the GG genotype was to reduce sensitivity to exogenous opioid receptor agonists. These clinical data provided further evidence that A118G was a functional variant.
To explore the role of MOR function on the antidepressant action of esketamine, we examined whether this functional OPRM1 variant altered the antidepressant effects of treatment with esketamine nasal spray. This analysis was performed as a post hoc assessment of data from pivotal trials that compared the antidepressant efficacy of esketamine nasal spray plus a newly initiated oral antidepressant (“esketamine” arm) compared with placebo nasal spray plus a newly initiated oral antidepressant (“placebo” arm) in patients with TRD. We also considered 2 additional MOR missense SNPs (rs1799972 and rs34427887). In our cohort, however, minor alleles for rs1799972 and rs34427887 occurred in 1.7% and 6.4% of the patients, respectively. As a result, statistical testing was not possible for rs1799972 and was underpowered for rs34427887, so the primary analysis was based on comparisons involving the rs1799971 polymorphism.
Methods and Materials
Study Samples and Evaluations
Whole blood for DNA samples was obtained from adult male and female patients with TRD in 2 separate randomized, double-blind, active-controlled, phase III clinical trials: TRANSFORM-1 (NCT02417064) (Fedgchin et al., 2019) and TRANSFORM-2 (NCT02418585) (Popova et al., 2019). The details of the methods and main outcomes of these trials were described previously (Fedgchin et al., 2019; Popova et al., 2019). For both studies, the protocols and their respective amendments were reviewed by an independent ethics committee or institutional review board at each site. The studies were conducted in accordance with the Declaration of Helsinki, Good Clinical Practices, and applicable regulatory requirements. Written informed consent was obtained from all patients before participating in the study.
Past treatment resistance was established by demonstrating nonresponse to at least 2 different antidepressant drugs within the current major depressive episode. The antidepressant effect was assessed by the difference in the change in Montgomery–Åsberg Depression Rating Scale (MADRS) total score on day 2 (24 hours after the initial esketamine dose) and day 28 (study endpoint) between depressed patients randomized to receive esketamine nasal spray plus a newly initiated oral antidepressant (esketamine + antidepressant [AD]) vs patients randomized to receive placebo nasal spray plus a newly initiated oral antidepressant (AD + placebo). The oral AD was selected as one to which the patient had not previously failed to tolerate (lifetime) or respond to (in the current depressive episode) from the following list: escitalopram, sertraline, duloxetine, or venlafaxine extended release. Measures of perceptual/dissociative effects were assessed by the Clinician-Administered Dissociative States Scale (CADSS) on study day 1 and day 25 at 40 minutes post treatment administration (corresponding to the time of maximum plasma concentration [Cmax] of esketamine, and approximately the highest CADSS total score, within each dosing session). Potential associations between the OPRM1 SNP and changes in MADRS total score at day 2 and day 28 were evaluated to test the a priori hypothesis. In an exploratory post hoc analysis, the effect of OPRM1 genotype and peak responses on the CADSS at day 1 and day 25 were also evaluated. Genomic DNA was extracted from whole blood using the FLEX STAR system (Autogen, Inc., Holliston, MA).
Genotyping
Patients were genotyped on the Illumina Omni2.5M v1.3 array and imputed to the 1000 Genomes reference panel (phase 3, v5) using MaCH/minimac (Howie et al., 2012). The SNP of interest in this analysis, rs1799971, was directly genotyped.
Statistical Analysis
Testing for associations was carried out with multiple linear regression models of outcome as a function of MOR SNP using a dominant model chosen due to the low frequency of G/G cases, as only 2 patients with the G/G genotype were randomized to the placebo group and 4 to the esketamine group. The primary outcome consisted of the MADRS total score change from baseline at day 2 and day 28. The peak CADSS response was rated on day 1 and day 25, each at 40 minutes post-dose; the change in peak CADSS response between day 1 and day 25 was also assessed (the magnitude of dissociation ratings typically attenuates across repeated dosing sessions, in contrast to the antidepressant effect, which is persistent) (Daly et al., 2018; Popova et al., 2019). All models were fit separately for patients receiving esketamine and patients receiving placebo, with covariates for age, sex, body mass index, and 4 multidimensional scaling components for ancestry. Models for the changes in MADRS and peak CADSS ratings also included baseline MADRS score and peak CADSS score as covariates, respectively. Tests were carried out on combined cohorts from the TRANSFORM-1 and TRANSFORM-2 studies with 2 treatment categories of esketamine and placebo. For the quantitative trait association conducted in this study and a minor allele frequency of 0.133 for rs1799971, a power calculation with n = 200 patients per arm demonstrated 80% power to detect an R2 with change in clinical outcome as small as 0.164 at (alpha) α = .05.
Results
The baseline demographics and clinical characteristics of patients from TRANSFORM-1 and TRANSFORM-2 studies are summarized in Supplementary Table 1. The demographic and baseline clinical characteristics of patients with different rs1799971 (A118G) variants and treatment are shown in Table 1. The treatment groups in both studies were similar with respect to demographic and baseline clinical characteristics (Table 1). The frequency of the minor allele G was 0.13 in our study, which was similar to the frequency in larger population studies of this exonic variant (Lek et al., 2016).
Table 1.
Baseline Demographics and Clinical Characteristics of Patients with OPRM1 SNP rs1799971 (A118G) Alleles
| Esketamine + AD | AD + placebo | |||
|---|---|---|---|---|
| AA (n = 176) | AG/GG (n = 57) | AA (n = 129) | AG/GG (n = 44) | |
| Age, year | ||||
| Mean (SD) | 45.6 (11.27) | 46.1 (12.76) | 48.1 (10.65) | 46.1 (11.27) |
| Range | 18–64 | 19–64 | 22–64 | 22–63 |
| Sex, n (%) | ||||
| Male | 58 (33.0) | 15 (26.3) | 47 (36.4) | 14 (31.8) |
| Female | 118 (67.0) | 42 (73.7) | 82 (63.6) | 30 (68.2) |
| Race, n (%) | ||||
| American Indian or Alaskan Native | 1 (0.6) | 0 | 0 | 0 |
| Asian | 2 (1.1) | 2 (3.5) | 2 (1.6) | 1 (2.3) |
| Black or African American | 15 (8.5) | 0 | 5 (3.9) | 1 (2.3) |
| White | 142 (80.7) | 52 (91.2) | 117 (90.7) | 36 (81.8) |
| Multiple, not reported, other | 16 (9.1) | 3 (5.3) | 5 (3.9) | 6 (13.6) |
| Age when diagnosed with MDD, year | ||||
| Mean (SD) | 31.3 (12.26) | 34.2 (13.97) | 35.9 (13.30) | 32.2 (12.20) |
| Range | 9–59 | 10–60 | 9–64 | 5–57 |
| Duration of current episode, weeks | ||||
| Mean (SD) | 158.2 (270.06) | 192.4 (210.08) | 126.5 (221.79) | 135.3 (172.51) |
| Range | 9–2288 | 15–1080 | 6–1720 | 14–832 |
| No. of previous antidepressant medicationsa, n (%) | ||||
| 1 or 2 | 118 (67.0) | 28 (49.1) | 83 (64.3) | 27 (61.4) |
| ≥3 | 58 (33.0) | 29 (50.9) | 46 (35.7) | 17 (38.6) |
| Class of oral antidepressant, n (%) | ||||
| SNRI | 108 (61.4) | 34 (59.6) | 83 (64.3) | 27 (61.4) |
| SSRI | 68 (38.6) | 23 (40.4) | 46 (35.7) | 17 (38.6) |
| Baseline CGI-S | ||||
| Mean (SD) | 5.1 (0.71) | 5.2 (0.68) | 5.1 (0.66) | 5.2 (0.76) |
| Range | 4–7 | 4–7 | 4–7 | 4–7 |
| Baseline PHQ-9 total score | ||||
| Mean (SD) | 20.5 (3.46) | 20.1 (4.46) | 20.4 (3.68) | 21.2 (3.67) |
| Range | 9–27 | 5–27 | 10–27 | 10–27 |
| Baseline MADRS total score | ||||
| Mean (SD) | 37.7 (5.45) | 37.8 (5.68) | 37.2 (6.14) | 38.2 (5.44) |
| Range | 22–49 | 26–48 | 18–52 | 29–53 |
Abbreviations: AD, antidepressant; CGI-S, Clinical Global Impression–Severity; MADRS, Montgomery–Åsberg Depression Rating Scale; MGH-ATRQ, Massachusetts General Hospital Antidepressant Treatment Response Questionnaire; PHQ-9, Patient Health Questionnaire 9-Item; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
aNumber of antidepressant medications with nonresponse (defined as ≤25% improvement) taken for at least 6 weeks during the current episode as obtained from MGH-ATRQ, in addition to one prospective antidepressant.
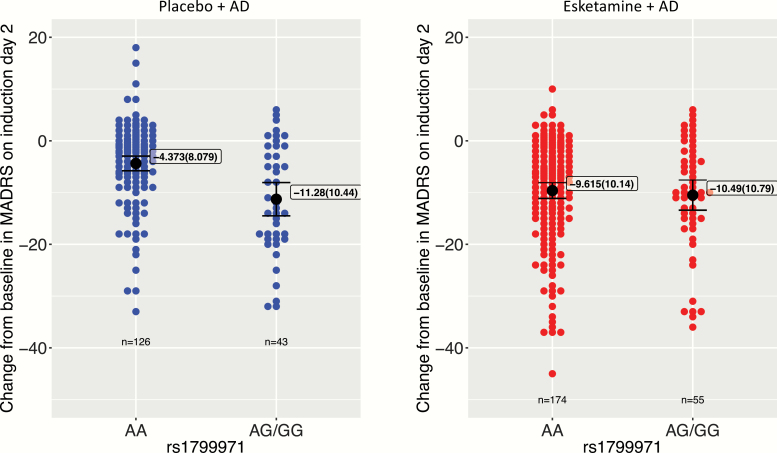
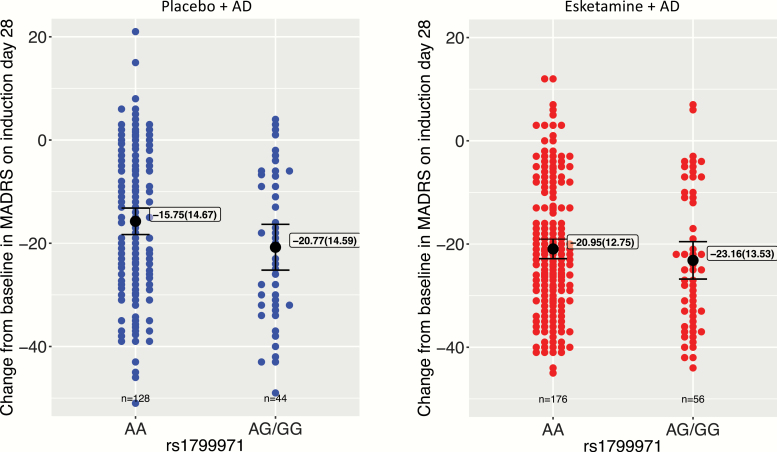
In the esketamine + AD arm, no significant genotype effects of SNP rs1799971 (A118G) on MADRS score reductions were detected on either day 2 (Table 2; Figure 1) or day 28 (Table 2; Figure 2). The mean (SD) reduction from baseline in MADRS total score was −9.62 (10.14) (AA genotype) and −10.49 (10.79) (AG/GG genotype) on day 2 and −20.95 (12.75) (AA genotype) and −23.16 (13.53) (AG/GG genotype) on day 28.
Table 2.
Effects of OPRM1 SNP rs1799971 (A118G) Variation on Improvements in Depression Severity, Assessed as Reductions from Baseline in the MADRS Score
| Esketamine + AD | AD + placebo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Minor allele frequency | n | Slope | P value | R2partial | Minor allele effect | n | Slope | P value | R2partial | Minor allele effect | |
| Day 2 | 0.13 | 229 | −0.63 | .69 | <0.5 % | None | 169 | −6.59 | <.001 | 10% | Greater response |
| Day 28 | 0.13 | 232 | −1.81 | .34 | <0.5 % | None | 172 | −4.30 | .07 | 2% | None |
Abbreviations: AD, antidepressant; MADRS, Montgomery–Åsberg Depression Rating Scale; P value, probability of rejecting H0: slope = 0 while H0 is true; R2partial, proportion of variance explained by the SNP regressor; Slope, regression coefficient for the SNP regressor; SNP, single nucleotide polymorphism.
Data are results of regression models for each outcome.
Figure 1.
Effects of OPRM1 single nucleotide polymorphism (SNP) rs1799971 (A118G) alleles on improvements in depression severity, assessed as reductions from baseline in the Montgomery–Åsberg Depression Rating Scale (MADRS) total score on day 2. Black dot, mean; boxed annotation: mean (SD); error bars, 95% mean confidence interval.
Figure 2.
Effects of OPRM1 single nucleotide polymorphism (SNP) rs1799971 (A118G) alleles on improvements in depression severity, assessed as reductions from baseline in the Montgomery–Åsberg Depression Rating Scale (MADRS) total score on day 28. Black dot, mean; boxed annotation, mean (SD); error bars, 95% mean confidence interval.
In the AD + placebo arm, a significant genotype effect of SNP rs1799971 (A118G) on the MADRS score reduction on day 2 was detected (Table 2; Figure 1) such that the patients with the AG and GG genotypes showed a greater reduction on MADRS total scores than those with the AA genotype. There was a nonsignificant trend towards a similar effect of SNP rs1799971 (A118G) on MADRS score reductions on day 28, with the reductions in patients with the AG/GG genotypes being numerically greater than those in patients with the AA genotype (Figure 2). Post hoc linear models including an interaction term between A118G SNP and treatment arm showed a significant interaction on day 2 (P < .05) but not on day 28 (P = .52). The mean (SD) reduction from baseline in MADRS total score was −4.37 (8.08) (AA genotype) and −11.28 (10.44) (AG/GG genotype) on day 2 and −15.75 (14.67) (AA genotype) and −20.77 (14.59) (AG/GG genotype) on day 28.
When broken down by trial, further post hoc testing showed that the placebo associations with rs1799971 (A118G) polymorphism were largely driven by 1 of the 2 cohorts. In TRANSFORM-2, at day 2 visit, patients treated with AD + placebo responded with an additional improvement of 10.53 points on MADRS total scores (P < .001) for the G-allele carriers compared with 1.39 (P = .53) for noncarriers. A similar interaction was not seen for the response on day 28, suggesting that the association seen in the AD + placebo arm may not be generalized across cohorts. No significant associations were seen for patients treated with esketamine + AD in either cohort.
In the post hoc exploration of genotype effects of SNP rs1799971 (A118G) on CADSS scores, no significant genotype effect was detected in either cohort on day 1 (first nasal spray treatment) or day 25 (last nasal spray treatment) (Table 3). In addition, no significant genotype effects of SNP rs1799971 (A118G) were detected on the changes in CADSS scores between day 1 and day 25 (i.e., reflecting the magnitude of the attenuation in this adverse event across repeated dosing) (Table 3).
Table 3.
Effects of OPRM1 SNP rs1799971 (A118G) Variation on Dissociative Symptoms Assessed Using the CADSSa
| Esketamine + AD | AD + placebo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Minor allele frequency | n | Slope | P value | R2partial | Minor allele effect | n | Slope | P value | R2partial | Minor allele effect | |
| Day 1 | 0.13 | 258 | 1.43 | .27 | <0.5 % | None | 182 | 0.27 | .64 | <0.5 % | None |
| Day 25 | 0.13 | 228 | 0.68 | .50 | <0.5 % | None | 171 | −0.15 | .64 | <0.5 % | None |
| Change | 0.13 | 225 | 0.31 | .74 | <0.5 % | None | 167 | −0.19 | .55 | <0.5 % | None |
Abbreviations: AD, antidepressant; CADSS, Clinician Administered Dissociative States Scale; Change, change of CADSS scores from day 1 to day 25; P value, probability of rejecting H0: slope = 0 while H0 is true; R2partial, proportion of variance explained by the SNP regressor; Slope, regression coefficient for the SNP regressor; SNP, single nucleotide polymorphism.
aThe data presented show results of the regression models applied to assess each outcome. The CADSS scores were obtained at 40 minutes post initiation of the nasal spray treatment on each of the study days indicated.
In a secondary analysis, no significant effects or consistent trends were detected for rs34427887 in any of the tests described above.
Discussion
In patients with TRD, this analysis did not reveal a significant genotype effect of OPRM1 rs1799971 (A118G) alleles on the antidepressant response to esketamine + AD on study days 2 or 28 (Table 2; Figures 1 and 2). In contrast, the analysis revealed significant genotype effects of these OPRM1 alleles on the antidepressant response to AD + placebo on day 2 (Table 2; Figure 1). The improvement in depressive symptoms was observed in the AD + placebo arm and was more prominent in the G allele carriers (AG/GG genotypes combined) than in the A homozygotes (Table 2; Figure 1). These findings were not explained by group differences in the baseline demographics or clinical characteristics of patients (Table 1). The partial R2 of 0.10 for the overall association was less than the minimum R2 of 0.16 that this analysis would be powered to detect at 80%. This association should be considered putative and would need to be confirmed in an independent cohort. On day 28, the rs1799971 (A118G) allele effect was no longer significant in the AD + placebo group, and there was no interaction between SNP and treatment arm (Table 2; Figure 2). Although not significant, the 2-point (esketamine + AD arm) and 5-point (AD + placebo arm) difference between the AA and AG/GG genotypes with regard to reduction in the MADRS total score on day 28 may be clinically relevant. Finally, no significant effect of genotype was found on the dissociation responses on study days 1 or 25 (Table 3).
Stimulating MOR has been proposed as a mode of action for some novel antidepressants (Lutz and Kieffer, 2013; Browne and Lucki, 2019). Preclinical studies showed MOR agonists produced antidepressant-like behavioral responses in animal models (Lutz and Kieffer, 2013). Recent studies showed that tianeptine was a selective full agonist at MOR and genetic deletion of MOR abolished the antidepressant-like action of tianeptine in mice given 10 or 30 mg/kg i.p. (Gassaway et al., 2014; Samuels et al., 2017), although the extent to which antidepressant doses of tianeptine activate the MOR in humans remains unclear. Moreover, efforts to demonstrate MOR-mediated clinical antidepressant effects have been proven unsuccessful; for example, the ETS6103 (tramadol, a potent controlled release MOR agonist) phase IIb trial failed to meet the primary endpoint of noninferiority compared with amitriptyline (ClinicalTrials.gov ID: NCT02014363). The mean reduction in MADRS scores was −6 for both doses tested of ETS6103 compared with −11 for amitriptyline.
As reviewed in the Introduction, Williams et al. reported that pretreatment with naltrexone, a MOR-preferring antagonist, attenuated the antidepressant effect of ketamine, suggesting that normal function of the opioid system was required to realize the full antidepressant effects of treatment (Williams et al., 2018). In contrast, Marton et al. (Marton et al., 2019) reported that concurrent use of buprenorphine (a MOR partial agonist), methadone (a potent MOR agonist), or naltrexone (a MOR antagonist) did not alter ketamine’s antidepressant activity in a small sample of patients with TRD (n = 7) compared with patients with TRD who did not receive agents with opioid agonist or antagonist effects (n = 27). Similarly, a recent paper by Yoon et al. reported 5 patients with TRD who had been chronically treated with naltrexone for a history of alcoholism showed adequate antidepressant responses to ketamine administration (Yoon et al., 2019). In preclinical studies of mice subjected to the chronic social defeat stress or lipopolysaccharide-treated models of depression, naltrexone did not block the antidepressant effects of ketamine, also suggesting that opioid receptors did not play a role in the antidepressant-like behavioral effects of ketamine (Zhang and Hashimoto, 2019). Thus, while all 3 of the above-mentioned human pharmacological studies remain difficult to interpret due to their small samples sizes, it is noteworthy that the results of 2 of the 3 converge with those of the current genetic study in providing no support for the hypothesis that MOR stimulation plays a major role in mediating the antidepressant actions of ketamine and esketamine (Williams et al., 2018; Marton et al., 2019; Yoon et al., 2019).
Williams et al. additionally showed that pretreatment with naltrexone did not affect dissociation responses as assessed by the CADSS in depressed patients treated with i.v. ketamine (Williams et al., 2018). These data are consistent with the results of the current study, which showed a lack of significant effects of the OPRM1 functional variants on dissociative side effects encountered in depressed patients treated with esketamine (Table 3). These clinical pharmacology and genetic data thus agree in providing no evidence for the involvement of MOR stimulation in the dissociation responses to ketamine and esketamine.
Moreover, the extant receptor pharmacology data provide no evidence that direct MOR stimulation contributes to the antidepressant effects of ketamine and esketamine. The dose-antidepressant response relationship for ketamine reveals that increasing the dose beyond 0.5 mg/kg i.v. does not provide incremental improvement in the antidepressant response (Fava et al., 2018). This information is critical because the reported plasma Cmax of i.v. ketamine at this dose was just below 1 μM (Zanos et al., 2018). Using the ketamine brain to plasma ratio of 4.2 and the unbound fraction of ketamine in brain of 0.25 (Shaffer et al., 2014), the estimated unbound concentration in brain of ketamine at the antidepressant dose level is also approximately 1 μM. This concentration is far lower than the EC50 of ketamine for MOR, which is reportedly 1000 μM in a functional assay (Hirota et al., 1999), indicating that the brain concentrations achieved at antidepressant doses of ketamine would be too low to produce direct MOR receptor activation. In addition, the reported Ki values of ketamine and esketamine for MOR are 42.1 μM for ketamine and 11 or 28.6 μM for esketamine (Zanos et al., 2018). Thus, the difference is 42-fold between the estimated ketamine brain Cmax level at antidepressant dose and ketamine’s Ki value for MOR. Similarly, using the reported plasma Cmax level of esketamine nasal spray (Janssen Research & Development, 2019) and the method for simulating brain unbound ketamine levels mentioned above, the estimated brain unbound Cmax value of 84 mg esketamine nasal spray is 0.4 μM. Thus, the difference between the estimated brain unbound Cmax and Ki values of esketamine for MOR is 28- or 72-fold. These differences between the brain concentrations achieved at antidepressant doses of ketamine and esketamine and their respective potencies for the MOR do not support the hypothesis that direct activation of MOR contributes to the antidepressant effects of these agents.
In addition, MOR stimulation is known to cause euphoria and respiratory depression. Neither ketamine nor esketamine induces respiratory depression when administered in the antidepressant dose range. For example, in the esketamine registration trials, no case of respiratory depression was observed across 78 244 esketamine dosing sessions, as assessed by respiratory rate and oxygen saturation; in addition, euphoria was generally mild, occurring in about 4% of patients (Janssen Research & Development, 2019). Moreover, the presence of euphoria in this small minority of patients is nonspecific and may be attributable directly or indirectly to esketamine’s NMDAR antagonist effects. For example, esketamine and ketamine administered in the antidepressant range have been shown to increase striatal dopamine release in human PET studies (Vollenweider et al., 2000; Kokkinou et al., 2018), which is thought to reflect indirect effects of NMDAR antagonism.
While the pharmacological evidence reviewed above makes it highly unlikely that direct MOR agonist effects contribute to the antidepressant effects of ketamine and esketamine, it remains conceivable that indirect MOR stimulation via release of endogenous opioid peptides may play a role in the antidepressant effects of ketamine and esketamine (Aalto et al., 2005). Among the human pharmacological challenge data reviewed above, the finding from the Williams et al. study that oral naltrexone (50 mg) pretreatment reduced ketamine-induced antidepressant effects in 7 patients with depression (Williams et al., 2018) appears compatible with such a hypothesis.
Furthermore, naltrexone may conceivably detract from the restoration of positive mood and affect by ketamine via direct effects on emotional processing. Human imaging studies showed that a single oral dose of naltrexone (50 mg) produced an 80% MOR blockade for at least 3 days (Lee et al., 1988; Rabiner et al., 2011). Human behavioral studies showed that naltrexone treatment attenuates positive emotional and hedonic responses to food (Yeomans and Gray, 1996, 1997; Murray et al., 2014; Eikemo et al., 2016), exercise (Daniel et al., 1992; Järvekülg and Viru, 2002), social interaction (Inagaki et al., 2016), music (Mallik et al., 2017), and sexual encounter (Murphy et al., 1990; Chelnokova et al., 2014). Moreover, Williams et al. terminated their study of naltrexone’s effect on antidepressant responses to i.v. ketamine early because the side effects of naltrexone were “noxious,” including severe nausea and vomiting, which may have confounded the assessment of improvement in depression ratings and/or resulted in functional unblinding of patients and raters (Williams et al., 2018).
Finally, pharmacological alteration of the endogenous opioid peptide signaling has been associated with high placebo response rates commonly reported with pain, mood, and anxiety disorders (Pecina and Zubieta, 2015; Sanacora, 2019). Similarly, the results from the Williams et al. study may indicate that the endogenous opioid mechanism is required for complete attainment of antidepressant effect size of ketamine and do not implicate the involvement of exogenous opioid response of ketamine for the mediation of antidepressant effects (Williams et al., 2018; Sanacora, 2019). Therefore, future studies aiming at addressing the impact of MOR blockade on antidepressant responses to ketamine (or esketamine) must consider including control arms to evaluate (1) the effects of naltrexone alone on depression severity and recovery in general, (2) whether naltrexone also attenuates attainment of the full clinical effects of other antidepressants, and (3) whether the attenuation of the antidepressant effect extends to placebo as well as to ketamine.
The importance of addressing the specificity of such effects among antidepressants was highlighted by a study exploring associations between MOR variants and the antidepressant response to citalopram in depressed participants from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study (Garriock et al., 2010). The SNP (rs540825), a distinct variant from that studied herein, was associated with an altered response to citalopram treatment, suggesting variation in the MOR potentially influences antidepressant response from a selective serotonin reuptake inhibitor antidepressant (although the lack of a placebo arm in the STAR*D trial also leaves open the possibility of an association with the placebo effect of introducing a new treatment). In contrast, the OPRM1 variant rs1799971 analyzed in the current study was not associated with antidepressant effects to any antidepressant treatment used in the STAR*D study (Garriock et al., 2010).
The finding of the current study that the OPRM1 variant rs179997 influences the placebo effect is noteworthy in view of preclinical and clinical evidence suggesting that endogenous opioid peptide release mediates the positive emotional expectancy in response to placebo administration (Zubieta et al., 2005). A PET imaging study using [11C]carfentanil showed that a greater response to antidepressant treatment was associated with higher baseline µ-opioid receptor binding in the nucleus accumbens (Pecina et al., 2015). In addition, a greater response to placebo was associated with increased placebo-induced endogenous µ-opioid transmission in the subgenual anterior cingulate cortex, nucleus accumbens, midline thalamus, and amygdala (Pecina et al., 2015). Our analysis revealed a significant effect on day 2 and a nonsignificant trend on day 28 towards an influence of the OPRM1 variant rs1799971 on antidepressant responses in AD + placebo treated patients with TRD (Table 2; Figures 1 and 2). Limitations to the interpretation of this finding, however, are that patients in the active control arm who showed this association were receiving both placebo nasal spray and an oral AD. The effect was not consistent when examined in each trial cohort separately, and the analysis was underpowered to detect the observed effect size of R2partial = 0.2. Further research is therefore needed to interpret more specifically the nature of the observed control arm effects in large cohorts of placebo-controlled, antidepressant clinical trials. SNPs in other genes such as catechol-O-methyltransferase (COMT rs4680) and fatty acid amide hydrolase (FAAH rs324420) also have been reported in the literature to alter the placebo response. However, these SNPs were not examined in the current study (Aslaksen et al., 2018; Colloca et al., 2019).
In summary, the data from the current analyses do not support a direct or robust MOR stimulation at antidepressant doses of esketamine. In contrast, these data add to previous evidence that MOR receptor signaling participates in mediating the placebo effect.
Supplementary Material
Acknowledgments
Stacey E. Shehin, PhD (PRA Health Sciences), and Priya Ganpathy, MPharm CMPP (SIRO Clinpharm Pvt. Ltd), provided writing assistance, and Harry Ma, PhD CMPP (Janssen Global Services), provided additional editorial support. Portions of this study were previously presented at the American College of Neuropsychopharmacology (ACNP) 57th Annual Meeting, December 9–13, 2018, Hollywood, FL, USA. The authors thank the study participants, without whom this study would not have been accomplished, and the investigators for their participation in this study.
This study was funded by Janssen Research & Development, LLC.
Statement of Interest
Z.S., M.F., V.P., M.L.F., H.K., W.C.D., and G.C. are employees of Janssen Research & Development, LLC and may hold company equity. J.B.S. and D.H. were employees of Janssen Research & Development at the time the study was conducted.
References
- Aalto S, Ihalainen J, Hirvonen J, Kajander J, Scheinin H, Tanila H, Någren K, Vilkman H, Gustafsson LL, Syvälahti E, Hietala J (2005) Cortical glutamate-dopamine interaction and ketamine-induced psychotic symptoms in man. Psychopharmacology (Berl) 182:375–383. [DOI] [PubMed] [Google Scholar]
- Aslaksen PM, Forsberg JT, Gjerstad J (2018) The opioid receptor mu 1 (OPRM1) rs1799971 and catechol-O-methyltransferase (COMT) rs4680 as genetic markers for placebo analgesia. Pain 159:2585–2592. [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L (1998) Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A 95:9608–9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CA, Erickson RL, Blendy JA, Lucki I (2017) Genetic variation in the behavioral effects of buprenorphine in female mice derived from a murine model of the OPRM1 A118G polymorphism. Neuropharmacology 117:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CA, Lucki I (2019) Targeting opioid dysregulation in depression for the development of novel therapeutics. Pharmacol Ther 201:51–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelnokova O, Laeng B, Eikemo M, Riegels J, Løseth G, Maurud H, Willoch F, Leknes S (2014) Rewards of beauty: the opioid system mediates social motivation in humans. Mol Psychiatry 19:746–747. [DOI] [PubMed] [Google Scholar]
- Choi SW, Lam DMH, Wong SSC, Shiu HHC, Wang AXM, Cheung CW (2017) Effects of single nucleotide polymorphisms on surgical and postsurgical opioid requirements: a systematic review and meta-analysis. Clin J Pain 33:1117–1130. [DOI] [PubMed] [Google Scholar]
- Colloca L, Wang Y, Martinez PE, Chang YC, Ryan KA, Hodgkinson C, Goldman D, Dorsey SG (2019) OPRM1 rs1799971, COMT rs4680, and FAAH rs324420 genes interact with placebo procedures to induce hypoalgesia. Pain 160:1824–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, Thase ME, Winokur A, Van Nueten L, Manji H, Drevets WC (2018) Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 75:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M, Martin AD, Carter J (1992) Opiate receptor blockade by naltrexone and mood state after acute physical activity. Br J Sports Med 26:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikemo M, Løseth GE, Johnstone T, Gjerstad J, Willoch F, Leknes S (2016) Sweet taste pleasantness is modulated by morphine and naltrexone. Psychopharmacology (Berl) 233:3711–3723. [DOI] [PubMed] [Google Scholar]
- Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, Ionescu DF, Mathew SJ, Chang LC, Iosifescu DV, Murrough J, Debattista C, Schatzberg AF, Trivedi MH, Jha MK, Sanacora G, Wilkinson ST, Papakostas GI (2018) Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry Published online 2018 Oct 3. doi: 10.1038/s41380-018-0256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedgchin M, Trivedi M, Daly EJ, Melkote R, Lane R, Lim P, Vitagliano D, Blier P, Fava M, Liebowitz M, Ravindran A, Gaillard R, Ameele HVD, Preskorn S, Manji H, Hough D, Drevets WC, Singh JB (2019) Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol 22:616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriock HA, Tanowitz M, Kraft JB, Dang VC, Peters EJ, Jenkins GD, Reinalda MS, McGrath PJ, von Zastrow M, Slager SL, Hamilton SP (2010) Association of mu-opioid receptor variants and response to citalopram treatment in major depressive disorder. Am J Psychiatry 167:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassaway MM, Rives ML, Kruegel AC, Javitch JA, Sames D (2014) The atypical antidepressant and neurorestorative agent tianeptine is a μ-opioid receptor agonist. Transl Psychiatry 4:e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson-Redmond AN, Yuill MB, Lowe TE, Kline AM, Zee ML, Guindon J, Morgan DJ (2016) Morphine-induced antinociception and reward in “humanized” mice expressing the mu opioid receptor A118G polymorphism. Brain Res Bull 123:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Sikand KS, Lambert DG (1999) Interaction of ketamine with mu2 opioid receptors in SH-SY5Y human neuroblastoma cells. J Anesth 13:107–109. [DOI] [PubMed] [Google Scholar]
- Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR (2012) Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet 44:955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Chen C, Mague SD, Blendy JA, Liu-Chen LY (2012) A common single nucleotide polymorphism A118G of the μ opioid receptor alters its N-glycosylation and protein stability. Biochem J 441:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hustveit O, Maurset A, Oye I (1995) Interaction of the chiral forms of ketamine with opioid, phencyclidine, sigma and muscarinic receptors. Pharmacol Toxicol 77:355–359. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Ray LA, Irwin MR, Way BM, Eisenberger NI (2016) Opioids and social bonding: naltrexone reduces feelings of social connection. Soc Cogn Affect Neurosci 11:728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen Research & Development LLC (2019) Esketamine nasal spray for patients with treatment-resistant depression. Advisory Committee Briefing Document. https://www.fda.gov/media/121377/download. Accessed 22 July 2019.
- Järvekülg A, Viru A (2002) Opioid receptor blockade eliminates mood effects of aerobic gymnastics. Int J Sports Med 23:155–157. [DOI] [PubMed] [Google Scholar]
- Kalsi SS, Wood DM, Dargan PI (2011) The epidemiology and patterns of acute and chronic toxicity associated with recreational ketamine use. Emerg Health Threats J 4:7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinou M, Ashok AH, Howes OD (2018) The effects of ketamine on dopaminergic function: meta-analysis and review of the implications for neuropsychiatric disorders. Mol Psychiatry 23:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Wagner HN Jr, Tanada S, Frost JJ, Bice AN, Dannals RF (1988) Duration of occupancy of opiate receptors by naltrexone. J Nucl Med 29:1207–1211. [PubMed] [Google Scholar]
- Lek M, et al. ; Exome Aggregation Consortium (2016) Analysis of protein-coding genetic variation in 60,706 humans. Nature 536:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL (2013) Opioid receptors: distinct roles in mood disorders. Trends Neurosci 36:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Isiegas C, Huang P, Liu-Chen LY, Lerman C, Blendy JA (2009) Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior. Proc Natl Acad Sci U S A 106:10847–10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Port RG, McMullen ME, Carlson GC, Turner JR (2015) Mouse model of OPRM1 (A118G) polymorphism has altered hippocampal function. Neuropharmacology 97:426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud S, Thorsell A, Sommer WH, Heilig M, Holgate JK, Bartlett SE, Ruiz-Velasco V (2011) Pharmacological consequence of the A118G μ opioid receptor polymorphism on morphine- and fentanyl-mediated modulation of Ca²⁺ channels in humanized mouse sensory neurons. Anesthesiology 115:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallik A, Chanda ML, Levitin DJ (2017) Anhedonia to music and mu-opioids: evidence from the administration of naltrexone. Sci Rep 7:41952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton T, Barnes DE, Wallace A, Woolley JD (2019) Concurrent use of buprenorphine, methadone, or naltrexone does not inhibit ketamine’s antidepressant activity. Biol Psychiatry 85:e75–e76. [DOI] [PubMed] [Google Scholar]
- Murphy MR, Checkley SA, Seckl JR, Lightman SL (1990) Naloxone inhibits oxytocin release at orgasm in man. J Clin Endocrinol Metab 71:1056–1058. [DOI] [PubMed] [Google Scholar]
- Murray E, Brouwer S, McCutcheon R, Harmer CJ, Cowen PJ, McCabe C (2014) Opposing neural effects of naltrexone on food reward and aversion: implications for the treatment of obesity. Psychopharmacology (Berl) 231:4323–4335. [DOI] [PubMed] [Google Scholar]
- Peciña M, Bohnert AS, Sikora M, Avery ET, Langenecker SA, Mickey BJ, Zubieta JK (2015) Association between placebo-activated neural systems and antidepressant responses: neurochemistry of placebo effects in major depression. JAMA Psychiatry 72:1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña M, Love T, Stohler CS, Goldman D, Zubieta JK (2015) Effects of the Mu opioid receptor polymorphism (OPRM1 A118G) on pain regulation, placebo effects and associated personality trait measures. Neuropsychopharmacology 40:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña M, Zubieta JK (2015) Molecular mechanisms of placebo responses in humans. Mol Psychiatry 20:416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, Mazzucco C, Hough D, Thase ME, Shelton RC, Molero P, Vieta E, Bajbouj M, Manji H, Drevets WC, Singh JB (2019) Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry 176:428–438. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Beaver J, Makwana A, Searle G, Long C, Nathan PJ, Newbould RD, Howard J, Miller SR, Bush MA, Hill S, Reiley R, Passchier J, Gunn RN, Matthews PM, Bullmore ET (2011) Pharmacological differentiation of opioid receptor antagonists by molecular and functional imaging of target occupancy and food reward-related brain activation in humans. Mol Psychiatry 16:826–835, 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BA, Nautiyal KM, Kruegel AC, Levinstein MR, Magalong VM, Gassaway MM, Grinnell SG, Han J, Ansonoff MA, Pintar JE, Javitch JA, Sames D, Hen R (2017) The behavioral effects of the antidepressant tianeptine require the mu-opioid receptor. Neuropsychopharmacology 42:2052–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G. (2019) Caution against overinterpreting opiate receptor stimulation as mediating antidepressant effects of ketamine. Am J Psychiatry 176:249. [DOI] [PubMed] [Google Scholar]
- Schak KM, Vande Voort JL, Johnson EK, Kung S, Leung JG, Rasmussen KG, Palmer BA, Frye MA (2016) Potential risks of poorly monitored ketamine use in depression treatment. Am J Psychiatry 173:215–218. [DOI] [PubMed] [Google Scholar]
- Shaffer CL, Osgood SM, Smith DL, Liu J, Trapa PE (2014) Enhancing ketamine translational pharmacology via receptor occupancy normalization. Neuropharmacology 86:174–180. [DOI] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, Tadic A, Sienaert P, Wiegand F, Manji H, Drevets WC, Van Nueten L (2016a) Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry 80:424–431. [DOI] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, Pinter C, Murrough JW, Sanacora G, Shelton RC, Kurian B, Winokur A, Fava M, Manji H, Drevets WC, Van Nueten L (2016b) A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry 173:816–826. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vontobel P, Oye I, Hell D, Leenders KL (2000) Effects of (S)-ketamine on striatal dopamine: a [11C]raclopride PET study of a model psychosis in humans. J Psychiatr Res 34:35–43. [DOI] [PubMed] [Google Scholar]
- Weerts EM, McCaul ME, Kuwabara H, Yang X, Xu X, Dannals RF, Frost JJ, Wong DF, Wand GS (2013) Influence of OPRM1 Asn40Asp variant (A118G) on [11C]carfentanil binding potential: preliminary findings in human subjects. Int J Neuropsychopharmacol 16:47–53. [DOI] [PubMed] [Google Scholar]
- Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, Hawkins J, Birnbaum J, Lyons DM, Rodriguez CI, Schatzberg AF (2018) Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry 175:1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans MR, Gray RW (1996) Selective effects of naltrexone on food pleasantness and intake. Physiol Behav 60:439–446. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Gray RW (1997) Effects of naltrexone on food intake and changes in subjective appetite during eating: evidence for opioid involvement in the appetizer effect. Physiol Behav 62:15–21. [DOI] [PubMed] [Google Scholar]
- Yoon G, Petrakis IL, Krystal JH (2019) Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiatry 76:337–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr, Gould TD (2018) Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev 70:621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Hashimoto K (2019) Lack of opioid system in the antidepressant actions of ketamine. Biol Psychiatry 85:e25–e27. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS (2005) Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci 25:7754–7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.