Abstract
BACKGROUND
In humans, inadequate trophoblast invasion into the decidua is associated with the ‘great obstetrical syndromes’ which include pre-eclampsia, foetal growth restriction (FGR) and stillbirth. The mechanisms regulating invasion remain poorly understood, although interactions with the uterine environment are clearly of central importance. Extravillous trophoblast (EVT) cells invade the uterus and transform the spiral arteries. Progress in understanding how they invade has been limited due to the lack of good in vitro models. Firstly, there are no non-malignant cell lines that have an EVT phenotype. Secondly, the invasion assays used are of limited use for the small numbers of primary EVT available from first-trimester placentas. We discuss recent progress in this field with the generation of new EVT lines and invasion assays using microfluidic technology.
OBJECTIVE AND RATIONALE
Our aim is to describe the established models used to study human trophoblast invasion in vivo and in vitro. The difficulties of obtaining primary cells and cell lines that recapitulate the phenotype of EVT are discussed together with the advantages and pitfalls of the different invasion assays. We compare these traditional end point assays to microfluidic assays where the dynamics of migration can be measured.
SEARCH METHODS
Relevant studies were identified by PubMed search, last updated on February 2020. A search was conducted to determine the number of journal articles published using the cell lines JEG-3, BeWo, JAR, HTR-8/Svneo, Swan-71 and primary human extravillous trophoblast in the last 5 years.
OUTCOMES
Deep trophoblast invasion into the maternal decidua is a particular feature of human pregnancy. This invasion needs to be finely regulated to allocate resources between mother and baby. A reliable source of EVT is needed to study in vitro how the uterine environment regulates this process. First, we critically discuss the issues with the trophoblast cell lines currently used; for example, most of them lack expression of the defining marker of EVT, HLA-G. Recently, advances in human stem cell and organoid technology have been applied to extraembryonic tissues to develop trophoblast cell lines that can grow in two (2D) and three dimensions (3D) and differentiate to EVT. This means that the ‘trophoblast’ cell lines currently in use should rapidly become obsolete. Second, we critically discuss the problems with assays to study trophoblast invasion. These lack physiological relevance and have simplified migration dynamics. Microfluidic assays are a powerful tool to study cell invasion because they require only a few cells, which are embedded in 3D in an extracellular matrix. Their major advantage is real-time monitoring of cell movement, enabling detailed analysis of the dynamics of trophoblast migration.
WIDER IMPLICATIONS
Trophoblast invasion in the first trimester of pregnancy remains poorly understood despite the importance of this process in the pathogenesis of pre-eclampsia, FGR, stillbirth and recurrent miscarriage. The new technologies described here will allow investigation into this critical process.
Keywords: trophoblast , invasion assays , organoids , microfluidics , human
Introduction
A unique feature of placentation in the great apes compared to other primates is the deep invasion of placental trophoblast cells into the decidua and myometrium. This invasion is necessary to transform the uterine spiral arteries, ensuring an adequate maternal blood supply into the intervillous space for normal foetal growth and development. Extravillous trophoblast (EVT) cells remodel the maternal spiral arteries resulting in highly dilated terminal portions as they enter the intervillous space (Moffett and Loke, 2006; Pijnenborg et al., 2006). This adaptation reduces maternal blood flow velocity and pressure, whilst accommodating the increased uteroplacental perfusion necessary to meet the requirements of the developing foetus (Burton et al., 2009). When invasion is inadequate, the arteries remain narrow at their openings, and blood enters the intervillous space at a higher velocity, in a turbulent, jet-like flow pattern (Collins et al., 2012). This leads to rupturing of the anchoring villi, resulting in a globular-shaped placenta, damage to the villous architecture and impaired placental function (Kingdom et al., 2018). Inadequate invasion is associated with the great obstetrical syndromes, which include pre-eclampsia, FGR and still birth (Fig. 1) (Moffett and Loke, 2006). Trophoblast invasion is a complex process involving interactions with stromal cells, glands, arteries, macrophages and decidual natural killer (dNK) cells. Numerous publications have suggested roles for a range of factors including cytokines, metalloproteinases (MMPs), hormones, products of endometrial glands, oxygen tension and extracellular matrix (ECM) proteins (Fig. 2) (Burton et al., 2002; Burton et al., 2010; Harris et al., 2010; Whitley and Cartwright, 2010; Zhu et al., 2012; Xiong et al., 2013; Pollheimer et al., 2018).
Figure 1.
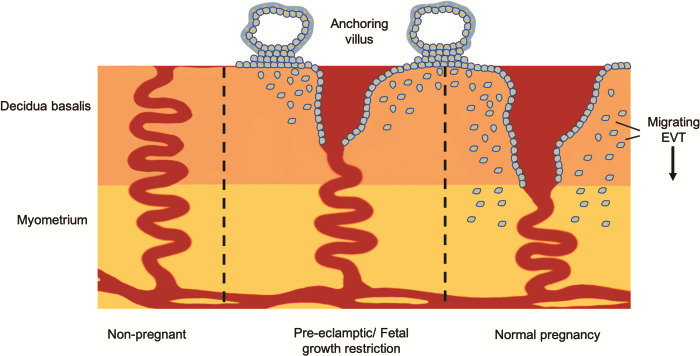
Human trophoblast invasion. Extravillous trophoblast (EVT) cells migrate from the anchoring villus and invade into the decidua basalis. Their primary function is to remodel the uterine spiral arteries, which results in a four to six times times increase in the diameter of the spiral artery: from high-resistance low-flow vessels into large dilated vessels. In the great obstetrical syndromes of pre-eclampsia, foetal growth restriction (FGR) and still birth, invasion is inadequate and the arteries remain narrow at the opening into the intervillous space.
Figure 2.
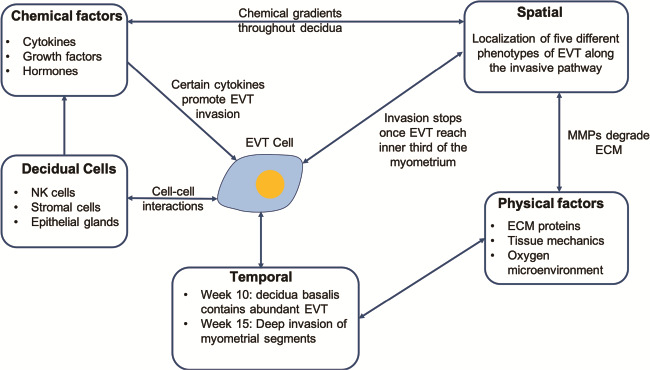
Regulators of trophoblast invasion. (EVT) cells invade the decidua coming into contact with decidual cells, where certain cell–cell interactions are known to release cytokines that promote invasion. Before the onset of maternal circulation, EVT cells are supported by secretions from the nutrient-rich endometrial glands. There are physical factors that are thought to regulate invasion: the oxygen environment, extracellular matrix (ECM) proteins the EVT have to cleave during migration, and tissue stiffness. Trophoblast invasion is spatially and temporally controlled with varying cell populations and physical factors as EVT migrate into the decidua. Decidua basalis contains abundant EVT by week 10 and at week 15 deep invasion of the myometrial segments is observed.
Overall, the findings are conflicting, reflecting the difficulty of studying trophoblast invasion in vitro and in vivo. Placentation in mammals is highly variable, especially in the extent of invasion into the uterus by trophoblast cells. This ranges from no invasion (epitheliochorial placentation) to trophoblast cells penetrating through uterine blood vessels to come into direct contact with maternal blood (haemochorial placentation) (Moffett and Loke, 2006). Because of the lack of good animal models to study human trophoblast invasion, in vitro assays have been developed using human cell lines and explant tissues; the use of primary human trophoblast cells has been limited. These assays monitor cell migration in response to chemoattractants and/or inhibiting compounds and vary in their use of ECM proteins, experimental throughput and cost. This review is an assessment of the current technologies used to study human trophoblast cell behaviour and their ability to represent physiological conditions in vivo.
Trophoblast Invasion
Trophoblast cells develop from the trophectoderm that forms the wall of the blastocyst and subsequently differentiates into two main lineages, villous (VCT) and EVT. The villous trophoblast that differentiates into overlying syncytiotrophoblast (SCT) covers the placental villi and EVT invade from the anchoring villi into the decidua. The physiological function of EVT is to attach the placenta to the uterine wall and remodel the spiral arteries. This process whereby interstitial trophoblasts encircle spiral arteries and then destroy the arterial media, which is replaced by ‘fibrinoid’ material, is unique to the great apes and humans (Pijnenborg et al., 2011). The endovascular trophoblast then moves down the arterial lumen from the cytotrophoblast shell to form loose plugs during the first trimester and transiently line the arteries. Trophoblast invasion is spatially and temporally finely controlled during the first half of pregnancy. The decidua basalis contains abundant EVT by Week 10, which extends into the myometrium by Week 15 (Pijnenborg et al., 2006). In normal pregnancies, as EVT move deeper they fuse into multi-nucleated giant cells and stop at the inner third of the myometrium (Pijnenborg 1980; Al Lamki & Burton 1999). The extent of trophoblast invasion is reduced in pathological pregnancies, resulting in inadequate transformation of the spiral arteries and disordered blood flow into the intervillous space (Burton et al., 2009; Pijnenborg et al., 2011).
Excessive invasion deep into the myometrium can also occur when decidua is absent or thin. (Goldman-Wohl and Yagel, 2002; Jauniaux and Jurkovic, 2012). This is known as placenta percreta, a condition that vividly demonstrates that the decidua plays a critical role in the regulation of trophoblast invasion but the exact contribution of the various immune cells, stromal cells and other factors is still uncertain (Jauniaux et al., 2017).
Are Animal Models Appropriate for Studying Human Trophoblast Invasion?
The placenta is evolutionary highly diverse, and therefore, it is important to ask whether there is any suitable animal model for studying human trophoblast invasion. The mouse is most frequently used in placental research as, together with simian primates and humans, it possesses a haemochorial placenta where there is direct contact between the trophoblast and maternal blood with no separation by the endothelium and/or epithelium (Moffett and Loke, 2006; Schmidt et al., 2015). However, there is variation in the depth of trophoblast invasion into decidua in species with haemochorial placentas with minimal invasion in the mouse. In contrast to humans, the blood vessels of the decidua are lined by the endothelium rather than trophoblast cells, and it is the dNK cells that move into the media of the arteries directly contributing to the arterial remodelling (Adamson et al., 2002; Moffett and Loke, 2006; Carter, 2007). The ‘soil’ that the trophoblast invades into in humans, the decidualised endometrium, only starts forming at implantation in mice and is restricted to the decidua basalis (Moffett and Loke, 2006). The extent of decidualisation correlates with the degree of invasion into the uterus, and this is most pronounced in humans where ‘pre-decidual’ change is first seen around the arteries in the secretory phase of the menstrual cycle (Brosens et al., 2002; Johnson et al., 2003). Other rodents, such as the rat and guinea pig, have extensive trophoblast invasion, but in these species, it is primarily the endovascular trophoblast that are involved in arterial remodelling (Pijnenborg et al., 1981; Verkeste et al., 1998).
Placentation in our nearest evolutionary relatives, the great apes, closely resembles human placentation in the depth and route of trophoblast invasion (Moffett and Loke, 2006; Carter and Pijnenborg, 2011). Despite the limited sampling of the placental bed that is possible in apes, it does seem that deep invasion and arterial transformation in the myometrium is restricted to humans, gorillas and chimpanzees (Carter et al., 2015). This deep invasion, which presumably results in the increased blood flow needed to accommodate the longer intrauterine period for development of the larger brains, seems to have come at a price with the high frequency of reproductive disorders such as obstructive labour and pre-eclampsia in humans (Moffett and Loke, 2006; Hofman, 2014; Burton et al., 2015). There is little reliable evidence that great apes experience these disorders, which limits their use as models for trophoblast invasion. Ethical and financial considerations also mean that there is limited demand for primates in human pregnancy research.
Despite the differences in trophoblast invasion between species, it does not mean that animal models should be discounted altogether. They have advantages that are important in the study of placental dysfunction. For example, mice can be genetically manipulated in order to generate strains with gene knock-outs that result in changes analogous to those seen in complications of human pregnancy (Perez-Garcia et al., 2018; Woods et al., 2018). The short gestational time of the mouse (19–21 days) allows for routine analysis of downstream health effects on foetal physiology such as that of FGR (Grigsby, 2016). Nonetheless, studies that use animal models to study trophoblast invasion should in parallel use human trophoblast cells in vitro to validate the findings.
From Cell Lines to Organoids
Controversial use of trophoblast cell lines
One of the problems in studying trophoblast migration is the reliance on trophoblast cell lines, driven by the difficulty in obtaining pure, primary, first-trimester, human trophoblast. We conducted a PubMed search for journal articles published over the past 5 years, and of the 1044 papers, only 76 studies used primary human EVT. This is compared to 968 for the cell lines JEG-3, BeWo, JAR, HTR-8/Svneo and Swan-71 (Fig. 3A). The choriocarcinoma cell lines, BeWo, JEG-3 and JAR, are highly malignant, contain abnormal numbers of chromosomes, have been passaged through the hamster cheek pouch for several years and have a substantially different transcriptomic profile from EVT (Fig. 3B) (Hertz, 1959; Kohler and Bridson, 1971; Apps et al., 2011; King, 2000). Recently, HTR-8/SVneo was shown to contain a heterogeneous population of both trophoblast and mesenchymal cells (Abou-Kheir et al., 2017). Whether these are truly representative of normal trophoblast, either villous or extravillous, is controversial. In an attempt to define characteristics typical of normal first-trimester trophoblast, we proposed four criteria: (i) expression of markers (GATA3, KRT7 and TFAPC2), (ii) HLA class I profile, (iii) hypomethylation of the ELF5 promoter and (iv) expression of C19MC (Lee et al., 2016). The challenge with ‘marker’ genes is that none are unique to trophoblast and all these criteria used together are needed for identification. For the HLA class I profile, SCT and VCT are HLA class I null whereas EVT express HLA-C, HLA-E and HLA-G. The HLA-G molecule is uniquely expressed by EVT cells, compared to any other normal cell type, and is the standard marker used for identification in immunohistochemistry and analysis by flow cytometry (Apps et al., 2011). The only cell line to express endogenous HLA-G is JEG-3, making it useful as a positive control for this extravillous marker (Apps et al., 2009, 2011; Manaster et al., 2012). However, the large number of transcripts that are differentially expressed compared to primary EVT and the malignant nature of JEG-3 cells make their use largely obsolete for use in invasion assays. Primary cells should be used where possible, because there is limited evidence a cell line exists that is a good model for the extravillous phenotype, with its unique invasive function and ability to form HLA-G+ multinucleated ‘placental bed giant’ cells.
Figure 3.

The range of cells used to study human trophoblast invasion. (A) A PubMed search was conducted for journal articles published over the past 5 years, last updated February 2020 for studies that use JEG-3, BeWo, JAR, HTR-8/Svneo, Swan-71 and primary human EVT. (B) Timeline of the establishment of these cell lines as well as hTSC and organoids.
The trophoblast community could learn from those working in the cancer field where there is a healthy critical attitude to the problems of cell lines. Selection for cells surviving passaging, grown in 2D rather than 3D and at high oxygen levels are some of the features that poorly reproduce the in vivo situation (Burdall et al., 2003; Hughes et al., 2007; Gillet et al., 2013).
Trophoblast organoid and stem cells
In recent years, the development of 3D organoid culture systems has emerged as a promising platform to study the functions of human organs (Sato et al., 2011; Lancaster et al., 2013; Clevers, 2016; Nantasanti et al., 2016). The organoid cultures are in 3D and can be derived from human embryonic stem cells (hESCs) or adult progenitor cells. In a defined culture medium, they self-organise and recapitulate the differentiation and function of the organ of origin (Clevers, 2016). A major advancement in the trophoblast field has been the establishment of trophoblast organoids that can differentiate from VCT into the two main trophoblast cell lineages: SCT and EVT (Haider et al., 2018; Turco et al., 2018). These organoids can be kept in culture for at least 6 months with continuous passaging, can be frozen to build up Bio Banks and produce human chorionic gonadotropin (hCG), the pregnancy hormone produced by SCT in the first trimester of pregnancy (Turco et al., 2018). These will clearly be an important tool for studying trophoblast invasion as they also differentiate to HLAG+ EVT with appropriate media that vigorously invade in 3D into the Matrigel® drop. Importantly and unlike primary cultures, cell number is not a limiting factor with organoids. These models could be combined with organoids of the endometrium to study maternal regulation of trophoblast differentiation and invasion (Turco et al., 2017).
In addition to organoids, human trophoblast stem cells (hTSCs) have also been derived from blastocysts and first-trimester placentas. Unlike organoids, these cultures are in 2D but can also be differentiated to produce EVT (Okae et al., 2018). The development of both 2D and 3D self-renewing hTSC is a paradigm shift in the tools used to study trophoblast invasion. The trophoblast cell lines currently in use should rapidly become obsolete.
Traditional In Vitro Assays
Immunohistological observations of the placental bed throughout gestation in normal and abnormal pregnancies and in vitro migration or invasion assays have provided much information about the complex events occurring during placentation. Seminal observations made by Pijnenborg on pregnant hysterectomy samples have described the different invasion routes, endovascular versus interstitial (Pijnenborg et al., 1980), the contribution of interstitial trophoblast in vascular remodelling in the myometrium (Pijnenborg et al., 1983) and the extent of trophoblast invasion between normal and pre-eclamptic pregnancies (Meekins et al., 1994). However, these provide only a snapshot and cannot provide insights into cellular and molecular mechanisms regulating invasion.
Over the years, a number of in vitro assays have been developed to understand the factors that regulate trophoblast invasion. These assays vary in their simplicity, readouts, cost and use of cell lines, primary explant tissue or primary cells (Table I).
Table I.
In vitro assays to study trophoblast invasion.
| Assay | Measurement | 2D or 3D | Chemotaxis | HTS | Type of analysis | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| Scratch assay | Velocity | 2D | No | Yes | Endpoint/kinetic | Simple imaging analysis Direction of cell movement is defined Inexpensive Easy to use |
Large number of cells needed Collective cell migration is observed Not suitable for chemical gradients Not suitable for non-adherent cells |
| Transwell (Boyden chamber) | Cell count Cell labelling |
3D | Yes | Yes | End point | ECM components can be added Selective cytokines can be added Inexpensive Compatible with adherent and non-adherent cells |
High volume to surface ratio No control of cytokine gradient Inaccurate and tedious analysis Large number of cells needed |
| Explant culture/ Artery assay |
Cell count Cell labelling Migration route |
3D | No | No | End point |
In vivo-like microenvironment More complex than single cell or cell culture study |
Maintaining long-term cell viability challenging Large variability with samples Time-consuming Qualitative assay Artery assays only available at term |
| Microfluidics | Velocity Directionality Motility |
3D | Yes | Yes | Kinetic | Reduced consumption of cells Reduced consumption of reagents Flexibility of device design Real-time data acquisition using live microscopy |
Permeability of device material to water vapour can cause drying Experimental set-up can be time-consuming Syringe pumps can be expensive |
Note: HTS denotes high-throughput screening.
Wound healing assay
The wound healing assay, known also as the scratch assay, is a popular and simple two-dimensional (2D) migration assay (Liang et al., 2007). A confluent monolayer of cells is grown on a plastic or glass substrate coated with ECM proteins and then wounded using a pipette tip (Fig. 4A). With time lapse microscopy, closure of the wound is monitored over hours or days. Changes in cell polarity and measurement of speed and migration distance can be quantified using image acquisition software such as ImageJ (Liang et al., 2007; Jonkman et al., 2014). This assay is particularly suitable to study the effects of cell–matrix interactions and chemical factors on cell migration. The limitation of wound healing assays, however, is that they are 2D and thus of limited physiological relevance to trophoblast invasion in the decidual tissue (Friedl and Bröcker, 2000; Cukierman et al., 2001). Evaluating single-cell migration and the number of migrating cells is challenging as cells mostly migrate without losing cell–cell contacts to neighbouring cells. Moreover, there are questions over the relevance of an assay that only detects the rate that cells move to fill a gap, which is substantially different to EVT invasion in 3D.
Figure 4.

Conventional in vitro and ex vivo assays used to study trophoblast invasion. (A) The wound healing or scratch assay is simple, economical and 2D. A scratch is made, and the cells migrate to close the gap. The rate of migration is quantified using time-lapse microscopy. (B) The Transwell or Boyden chamber is 3D and cells are seeded in an insert and placed in a well with a cytokine. Cells migrate through a semi-permeable membrane with migration quantified by counting the number of cells that have crossed the membrane. (C) Villous explants from first trimester pregnancies are cultured on an extracellular matrix (ECM) or decidual tissue and extravillous trophoblast (EVT) outgrowth is quantified. (D) Uterine spiral arteries isolated from term pregnancies are embedded in a fibrin gel and cultured with EVT to study invasion under various regulating factors.
A major stumbling block in the wound healing assay is the large numbers of cells required and this limits the use of primary HLA-G+ trophoblast cells. Trophoblast cell lines are an alternative, but then the question arises as to whether wound closure is caused by cell proliferation rather than migration. For example, when interleukin (IL) 12 was added in increasing concentrations to a monolayer of JEG-3 cells to assess its influence on trophoblast migration (Karmakar et al., 2004), cell motility was reduced as IL-12 concentration increased, but this coincided with a reduction in cell number within the wound. Thus, has IL-12 caused a reduction in motility or inhibition in cell proliferation? This experiment demonstrates the weakness of the wound healing assay as well as the problem of using highly malignant cell lines.
Transwell assay
The transwell assay (Boyden chamber) has been traditionally used to study cell invasion. An insert containing a cell-permeable membrane is placed in a multi-well tissue culture plate, with cells seeded on one side and chemoattractant added to the medium on the other (Fig. 4B). To create an invasion assay, the cell permeable membrane can be coated with a layer of ECM protein to mimic cell invasion into the basement membrane or stroma. To quantify the number of cells that have migrated, a cell-permanent dye is added to the cells, which then convert the dye into a fluorescent signal (Albini and Benelli, 2007; Justus et al., 2014). When homogenous cell lines are used, the assays can be robust and reproducible, but they are more difficult with primary trophoblast cells, both because of the cell numbers needed and the problem that contaminating non-trophoblast cells are always present in the isolates. For studies with primary trophoblast, cells that remain on the membrane are removed using a cotton swab whilst those that have moved to the lower surface of the filter are stained for HLA-G and counted. This approach has shown the promoting effect of IL-8 on the migration of HLA-G+ EVT (Hanna et al., 2006) due to upregulation of MMP-2 and MMP-9 and integrin expression (Jovanović et al., 2010). Using the transwell assay, many other cytokines and hormones such as chemokine (C–X–C motif) ligand (CXCL) 16, IL-1β, CXCL6, human placental growth hormone (hPGH) and granulocyte-macrophage colony-stimulating factor (GM-CSF) have been used to study the migration of primary trophoblast cells isolated from first trimester placenta (Lacroix et al., 2005; Huang et al., 2006; Prutsch et al., 2012; Xiong et al., 2013).
One of the major disadvantages of the transwell assay is the simplification of the migration events. Whereas the number of cells that have crossed the cell permeable membrane can be quantified, it is not possible to obtain cell speed and direction. Therefore, it is difficult to distinguish between cells crossing the membrane at high speed due to random migration or directed migration towards the chemoattractant. The stochastic nature of cell migration means that comparing the effect of multiple cytokines on a cell population is challenging. To conclude that one cytokine has a greater effect on cell migration than another requires kinetic readouts not possible with the transwell assay. There are, however, adaptations to the traditional transwell assay that are now available commercially. xCELLigence® uses microelectrodes underneath the microporous membrane that generate an electric current, which is impeded when cells migrate. This provides a label-free method to monitor cell migration kinetically, but this adaptation is expensive.
Explant cultures
Explant culture is a technique in which organs or small pieces of tissue are removed and cultured in vitro. In trophoblast research, primary villous tissue of first-trimester pregnancies containing cytotrophoblast cell columns are dissected and cultured on ECM (Fig. 4C) (Genbacev et al., 1992; Caniggia et al., 2000; Bauer et al., 2004; James et al., 2006). Trophoblast proliferation and invasion can be studied using the Transwell assay where the cells remain in their appropriate cellular and extracellular environment. Factors that are thought to influence trophoblast invasion can be tested individually or to assess the impact of factors secreted by decidua using conditioned medium (Wright et al., 2006). To quantify cytotrophoblast proliferation, the area of explant outgrowth from the anchoring villi is quantified and cells are stained for markers of proliferation. The number of cells that cross the cell-permeable membrane is counted to assess the effect of chemoattractant on trophoblast invasion and immunohistochemistry is used to determine if the invading cells are extravillous by staining for HLA-G (Librach et al., 1991; Wright et al., 2006).
To study maternal–foetal cell interactions and spiral artery remodelling in vitro, villous explants can be cultured with decidual explants on ECM proteins (Vićovac et al., 1995; Dunk et al., 2003; Moser et al., 2010). The main advantage of this system is that the spatial relationship between migrating trophoblast and stroma is preserved. Explant cultures, however, have several major disadvantages. There is extensive degeneration of SCT within 4 h of culture and cell death of VCT and the villous mesenchyme after 48 h of culture, which could affect invading EVT (James et al., 2005). The number of cells that invade or proliferate depends on the size of the villi sampled. Achieving a consistent sample size between test and control experiments is difficult; therefore, a note of caution is needed when interpreting data from explant cultures. Tissue from early pregnancies is needed as the outgrowth decreases with increasing gestational age (James et al., 2006). Studying trophoblast migration in response to cytokines is challenging because of the difficulty in creating a chemotactic gradient with explant tissue and the inability to track cell movement live.
Spiral artery assay
Studying remodelling of uterine spiral arteries by EVT has been a challenge in the field, despite this being a critical step in establishing the maternal circulation to the placenta. To observe direct interaction between EVT and cells of the spiral arteries, normal arteries isolated from the non-placental bed myometrium of term caesarean sections have been cultured with trophoblast cells (Fig. 4D). To study interstitial invasion, the arteries are embedded in a fibrin hydrogel and fluorescently labelled trophoblast cells are seeded on top and left for up to 5 days before sectioning the construct (Cartwright et al., 2002). For endovascular invasion, the arteries are mounted on a pressure myograph and perfused with trophoblast cells. Using this set-up, trophoblast cells caused apoptosis and loss of the endothelial cell layer within 96 h of co-culture compared to perfusion with media (Ashton et al., 2005). This assay has also been used to show a role for oxygen saturation and inflammatory molecules such as TNFα in mediating arterial transformation (Ashton et al., 2005).
This experimental model is an attempt to study the mechanisms that control trophoblast arterial remodelling in vitro, given the limitations of in vivo and animal models. However, there are questions about the viability of the arterial cells and extent of degradation of its ECM with the long culture periods used. The source of the normal uterine arteries is from myometria of non-placental bed areas at term and not from mucosal spiral arteries early in pregnancy where there is evidence that dNK have a role in modulating the media independently of EVT (Moffett-King, 2002; Apps et al., 2009; Abbas et al., 2017). There are questions as to whether the arteries sampled are actual spiral arteries given the caesarean sections incision is through the lower segment, just above attachment of the urinary bladder to the uterus, which does not contain spiral arteries. Given these drawbacks, it is important to ask whether the information gained goes beyond that of detailed examination by immunohistochemistry on sections of the decidua and myometrium from first trimester pregnancies? Since chemical gradients cannot be generated and quantification of migration dynamics is simplified, we feel that this arterial assay has limited use.
Moving Forward: Microfluidic-Based Invasion Assays
Microfluidics is a technology characterised by the manipulation of fluids in micrometre-sized structures (Sackmann et al., 2014). It has become increasingly popular in biological research as it allows observation of cell behaviour in highly controlled microenvironments, at the length scale of biological systems (e.g. macromolecules, cells, small organisms). Chemical and physical factors such as cytokine and pressure gradients can be controlled in a 3D microscale environment. Rapid and low-cost microfabrication techniques have led to implementation in many areas, such as organ-on-a-chip (Huh et al., 2010), PCR-on-a-chip (Jha et al., 2012) and single-cell sequencing using droplet microfluidics (Zilionis et al., 2016).
The cancer field has driven the use of microfluidics in migration and invasion studies (Polacheck et al., 2011; Jeon et al., 2015; Ma et al., 2018). Tumour cell invasion and metastasis lead to the formation of secondary tumours, which occurs when cancer cells penetrate surrounding basement membranes, move through stroma and penetrate into the lymphatic or vascular systems (Wong and Hynes, 2006; van Zijl et al., 2011). Microfluidic assays allow tracking of cancer cells as they invade into basement membranes in 3D in response to chemical or physical cues. Cells can be tracked in real time, and dynamic information such as velocity and the direction of cell movement can be gained. This is particularly beneficial for chemotactic studies where it is not always clear if cell migration is random or directed (Fig. 5).
Figure 5.

Microfluidics assay to study EVT invasion. EVTs are isolated from first-trimester placentas, then stained with a cell tracker dye and embedded in Matrigel® in the central hydrogel channel. A constant flow of medium in the two side channels, either with or without a cytokine, is applied to create a chemical gradient across the hydrogel channel. Individual cell tracks are generated from time-lapse confocal microscopy.
Microfluidics to study trophoblast invasion
Whilst primary human samples are preferred, the low number of EVT cells that can be isolated from first-trimester samples, typically 50–100K, limits their use. However, an advantage of the microfluidic assays is that the small channel volumes mean only a few thousand cells are needed. Commercial chips (AIM Biotech, IBIDI) with multiple devices on one microscope slide can be used to test cytokines and growth factors in a high-throughput manner. The good optical clarity of microfluidic devices allows for imaging in 3D and in real time. Microscopes can be programmed to move between chips to enable parallel experiments.
Microfluidics has been adopted to study trophoblast invasion in response to GM-CSF, a cytokine produced by dNK cells when one of the KIR genes (KIR2DS1) is activated (Abbas et al., 2017). dNK, which make up 70% of the maternal leukocytes present in the decidua, signal using a range of receptor–ligand interactions (Moffett-King, 2002). Genetic case–control studies show that in pregnancies where mothers possess the activating KIR2DS1 gene and the foetus has an HLA-C allele belonging to the C2 group, the mothers are less likely to have pre-eclampsia. This may be because with this maternal/foetal combination there is increased secretion of cytokines that promote trophoblast invasion (Xiong et al., 2013; Abbas et al., 2017). Primary EVT were found to move with more directionality towards increasing concentrations of both human recombinant (hr) GM-CSF and GM-CSF produced by activated dNK cells. This confirms that EVT movement is directed and not random, which is not always clear from end point assays. For each experiment, approximately 2000 cells were embedded in Matrigel® and cell migration was monitored for 12 h. The presence of the extravillous phenotype was confirmed by fixing and staining the cells for HLA-G.
The use of microfluidics to study EVT migration is a major step forward compared to the conventional assays discussed in this review. Cells can be tracked in real-time enabling detailed analysis of the dynamics of trophoblast migration. The cells are embedded in a physiological relevant matrix in 3D, and the low cell number required for this assay allows the use of primary EVT. Furthermore, the introduction of commercial microfluidic chips removes a barrier to biological labs, which often do not have access to facilities to fabricate chips.
Microfluidics in the wider reproduction field
Beyond the study of cell invasion, microfluidics has been utilised by the wider reproduction field. In an elegant study, the human reproductive tract and 28-day menstrual cycle was modelled in a single microfluidic unit to integrate cytokine and endocrine signals between tissues of the ovary, fallopian tube, uterus, cervix and liver (Xiao et al., 2017). The novelty of this study is to show how hormones produced by follicles during each ovarian cycle can regulate downstream tissue functions, such as endometrial proliferation and menstruation. This organ-on-a-chip device (and others like it that integrate multiple tissues) are emerging as prime candidates for toxicology and drug discovery because of the high analytical throughput and lower reagent cost from miniaturised devices (Neužil et al., 2012; Cui and Wang, 2019).
Microfluidic devices that have previously been used to study cancer and trophoblast invasion have been recently adapted to study early human embryonic development (Zervantonakis et al., 2012; Abbas et al., 2017). A controllable and reproducible model of human epiblast and amnion development (Zheng et al., 2019) used a three-channel device containing a central gel channel with two side channels used for either cell loading and flow of basal medium or addition of chemical supplements. Seeding of human embryonic stem cells (ES) results in attachment onto the gel between evenly spaced supporting posts that partition the channels. Epiblast like cysts are generated by 36 h, and chemicals can be added in a controlled fashion to induce other hallmarks of embryonic development.
Limitations of Proposed Tools to Study Trophoblast Invasion
There are a number of limitations in the new technologies to study trophoblast invasion. For microfluidics, the barrier of entry has been the expertise, cost and time required to design and make chips using soft lithography. However, this is becoming less of an issue as commercial products are now available, with more sophisticated designs that can be bought off the shelf. Inevitably, there is a learning curve with new technology set-up and analysing real-time migration data compared to the end point systems. Open-source imaging analysis systems such as Fiji and packages using the programming language R, are available and designed to make this analysis accessible (Schindelin et al., 2012; Wortel et al., 2019).
For organoids, ethical restrictions or access to tissue from first-trimester terminations are a barrier to entry for some labs. Disease modelling is not possible for both organoids and trophoblast stem cells, as the outcome of pregnancy is unknown in the first trimester when the tissue is obtained. There are also questions about where these stem cells originate from in the tissue, with renewed efforts to establish the trophoblast stem cell niche. Finally, the culture of organoids and hTSC has a higher cost compared to cell lines, as growth factors and basement membrane extract are expensive.
Moving Forward: Incorporating the Decidual Microenvironment
The in vitro assays that we have discussed are models of invasion of acellular ECM and do not involve interaction with maternal decidual or immune cells. This shortcoming should be addressed in an effort to design invasion models that better recapitulate the complex decidual microenvironment. There have been advances in the organoid field to develop co-culture systems between epithelia and lymphocytes, with the creation of an air–liquid interface model (Neal et al., 2018). The recently established trophoblast organoids offer an opportunity to study immune cell interaction with invasive EVT (Turco et al., 2018). Further to this, the regulation of EVT invasion by decidual glands could be studied by combining the trophoblast and endometrial gland organoid systems together (Turco et al., 2017; Abbas et al., 2020).
The mechanical microenvironment has been shown to influence cell differentiation and migration but has been largely ignored in the trophoblast field. Substrate stiffness can induce actin cytoskeletal reorganisation and contractility resulting in a change in the number of focal adhesion points and thus migration speed (Plotnikov et al., 2012; Bangasser et al., 2017). Therefore, migration studies involving EVT in vitro should aim to replicate the mechanical microenvironment that the cells feel in vivo. We have previously characterised the mechanical stiffness of human non-pregnant endometrium, decidua and placenta. The stiffness of decidua basalis, the site of EVT invasion was found to be 1250 Pa (Abbas et al., 2019). Matrigel®, the artificial basement membrane extract and the substrate most commonly used to study EVT migration in 3D, was an order of magnitude softer, at 331 Pa, than decidua basalis and does not represent a physiological environment for investigating EVT migration into decidual basalis. Chemically defined hydrogels can be tuned to better reflect both mechanics and ECM protein content of the in vivo microenvironment (Gjorevski et al., 2016; Gjorevski and Lutolf, 2017).
Concluding Remarks
A characteristic feature of placentation in humans is the deep invasion of EVT into the decidua and myometrium. The mechanisms that govern trophoblast invasion and that lead to defective placentation are not yet fully understood. In part, this is because of the lack of good models to study EVT both in vivo and in vitro. Placentation in laboratory animals differ from that of humans and ethical restrictions prevent experiments in primates. We need to acknowledge that choriocarcinoma cell lines are poor models of the uniquely invasive EVT cell, which have substantially different transcriptomic profiles, are highly malignant and contain abnormal numbers of chromosomes. Recent advances in organoid culture systems offer a platform to study multiple trophoblast cell types, which are patient-derived and can be biobanked and cultured long-term. In addition, advancements in microfluidics offer a new method of studying EVT cell invasion. These assays require only a few thousand cells and are embedded in 3D in a relevant ECM. The major advantage of this assay is real-time monitoring of cell movement, enabling detailed analysis of the dynamics of trophoblast migration.
Authors’ roles
Y.A. prepared the manuscript. A.M., G.J.B. and M.Y.T. helped write the paper, which all of the authors approve.
Funding
Centre for Trophoblast Research and the Wellcome Trust (090108/Z/09/Z, 085992/Z/08/Z); Y.A. was supported by an Isaac Newton Grant: G100391, grant awarded to M.Y.T. M.Y.T. is supported by a Royal Society Dorothy Hodgkin Fellowship Grant: DH160216. M.Y.T. is supported by a Royal Society Dorothy Hodgkin Fellowship and has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (Grant agreement No. [853546])
Conflict of interest
There is no conflict of interest.
References
- Abbas Y, Brunel LG, Hollinshead MS, Fernando RC, Gardner L, Duncan I, Moffett A, Best S, Turco MY, Burton GJ et al. Generation of a three-dimensional collagen scaffold-based model of the human endometrium. Interface Focus 2020;10:20190079.The Royal Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas Y, Carnicer-Lombarte A, Gardner L, Thomas J, Brosens JJ, Moffett A, Sharkey AM, Franze K, Burton GJ, Oyen ML. Tissue stiffness at the human maternal-fetal interface. Hum Reprod 2019;34:1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas Y, Oefner CM, Polacheck WJ, Gardner L, Farrell L, Sharkey A, Kamm R, Moffett A, Oyen ML. A microfluidics assay to study invasion of human placental trophoblast cells. J R Soc Interface 2017;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Kheir W, Barrak J, Hadadeh O. Daoud G. HTR-8/SVneo cell line contains a mixed population of cells. Placenta 2017;50:1–7. [DOI] [PubMed] [Google Scholar]
- Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol 2002;250:358–373. [DOI] [PubMed] [Google Scholar]
- Albini A, Benelli R. The chemoinvasion assay: a method to assess tumor and endothelial cell invasion and its modulation. Nat Protoc 2007;2:504–511Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- Al-Lamki RS, Skepper JN, Burton GJ. Are human placental bed giant cells merely aggregates of small mononuclear trophoblast cells? An ultrastructural and immunocytochemical study. Hum Reprod 1999;14:496–504. [DOI] [PubMed] [Google Scholar]
- Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 2009;127:26–39Wiley-Blackwell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Sharkey A, Gardner L, Male V, Trotter M, Miller N, North R, Founds S, Moffett A. Genome-wide expression profile of first trimester villous and extravillous human trophoblast cells. Placenta 2011;32:33–43Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton SV, Whitley GSJ, Dash PR, Wareing M, Crocker IP, Baker PN, Cartwright JE. Uterine spiral artery remodeling involves endothelial apoptosis induced by extravillous trophoblasts through Fas/FasL interactions. Arterioscler Thromb Vasc Biol 2005;25:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser BL, Shamsan GA, Chan CE, Opoku KN, Tüzel E, Schlichtmann BW, Kasim JA, Fuller BJ, McCullough BR, Rosenfeld SS et al. Shifting the optimal stiffness for cell migration. Nat Commun 2017;8:15313.Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knöfler M. Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J Clin Endocrinol Metab 2004;89:812–822. [DOI] [PubMed] [Google Scholar]
- Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies. Am J Obstet Gynecol 2002;187:1416–1423Mosby Inc. [DOI] [PubMed] [Google Scholar]
- Burdall SE, Hanby AM, Lansdown MR, Speirs V. Breast cancer cell lines: friend or foe? Breast Cancer Res 2003;5:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E, Charnock-Jones DS. The influence of the intrauterine environment on human placental development. Int J Dev Biol 2010;54:303–312. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Moffett A, Keverne B. Human evolution: brain, birthweight and the immune system. Philos Trans R Soc Lond B Biol Sci 2015;370:20140061.The Royal Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab 2002;87:2954–2959. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Woods AW, Jauniaux E, JCP K. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009;30:473–482Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta 2000;21:S25–S30W.B. Saunders. [DOI] [PubMed] [Google Scholar]
- Carter AM. Animal models of human placentation--a review. Placenta 2007;28:S41–S47. [DOI] [PubMed] [Google Scholar]
- Carter AM, Enders AC, Pijnenborg R. The role of invasive trophoblast in implantation and placentation of primates. Philos Trans R Soc Lond B Biol Sci 2015;370:20140070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AM, Pijnenborg R. Evolution of invasive placentation with special reference to non-human primates. Best Pract Res Clin Obstet Gynaecol 2011;25:249–257. [DOI] [PubMed] [Google Scholar]
- Cartwright JE, Kenny LC, Dash PR, Crocker IP, Aplin JD, Baker PN, Whitley GSJ. Trophoblast invasion of spiral arteries: a novel in vitro model. Placenta 2002;23:232–235. [DOI] [PubMed] [Google Scholar]
- Clevers H. Modeling development and disease with organoids. Cell 2016;165:1586–1597. [DOI] [PubMed] [Google Scholar]
- Collins SL, Birks JS, Stevenson GN, Papageorghiou AT, Noble JA, Impey L. Measurement of spiral artery jets: general principles and differences observed in small-for-gestational-age pregnancies. Ultrasound Obstet Gynecol 2012;40:171–178John Wiley & Sons, Ltd. [DOI] [PubMed] [Google Scholar]
- Cui P, Wang S. Application of microfluidic chip technology in pharmaceutical analysis: a review. J Pharm Anal 2019;9:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science 2001;294:1708–1712. [DOI] [PubMed] [Google Scholar]
- Dunk C, Petkovic L, Baczyk D, Rossant J, Winterhager E, Lye S. A novel in vitro model of trophoblast-mediated decidual blood vessel remodeling. Lab Invest 2003;83:1821–1828. [DOI] [PubMed] [Google Scholar]
- Friedl P, Bröcker EB. The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci 2000;57:41–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Schubach SA, Miller RK. Villous culture of first trimester human placenta--model to study extravillous trophoblast (EVT) differentiation. Placenta 1992;13:439–461. [DOI] [PubMed] [Google Scholar]
- Gillet J-P, Varma S, Gottesman MM. The clinical relevance of cancer cell lines, J Natl Cancer Inst. 2013;105:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorevski N, Lutolf MP. Synthesis and characterization of well-defined hydrogel matrices and their application to intestinal stem cell and organoid culture. Nat Protoc 2017;12:2263–2274Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez-Morán P, Clevers H, Lutolf MP. Designer matrices for intestinal stem cell and organoid culture. Nature 2016;539:560–564. [DOI] [PubMed] [Google Scholar]
- Goldman-Wohl D, Yagel S. Regulation of trophoblast invasion: from normal implantation to pre-eclampsia. Mol Cell Endocrinol 2002;187:233–238. [DOI] [PubMed] [Google Scholar]
- Grigsby PL. Animal models to study placental development and function throughout normal and dysfunctional human pregnancy. Semin Reprod Med 2016;34:11–16Thieme Medical Publishers, Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider S, Meinhardt G, Saleh L, Kunihs V, Gamperl M, Kaindl U, Ellinger A, Burkard TR, Fiala C, Pollheimer J et al. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Reports 2018;11:537–551Cell Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006;12:1065–1074. [DOI] [PubMed] [Google Scholar]
- Harris LK, Smith SD, Keogh RJ, Jones RL, Baker PN, Knöfler M, Cartwright JE, Whitley GSJ, Aplin JD. Trophoblast- and vascular smooth muscle cell-derived MMP-12 mediates elastolysis during uterine spiral artery remodeling. Am J Pathol 2010;177:2103–2115Elsevier Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz R. Choriocarcinoma of women maintained in serial passage in hamster and rat. Proc Soc Exp Biol Med 1959;102:77–81. [DOI] [PubMed] [Google Scholar]
- Hofman MA. Evolution of the human brain: when bigger is better. Front Neuroanat 2014;8:15.Frontiers Media SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zhu X-Y, Du M-R, Wu X, Wang M-Y, Li D-J. Chemokine CXCL16, a scavenger receptor, induces proliferation and invasion of first-trimester human trophoblast cells in an autocrine manner. Hum Reprod 2006;21:1083–1091. [DOI] [PubMed] [Google Scholar]
- Hughes P, Marshall D, Reid Y, Parkes H, Gelber C. The costs of using unauthenticated, over-passaged cell lines: how much more data do we need? Biotechniques 2007;43:577–578581-2 passim. [DOI] [PubMed] [Google Scholar]
- Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science 2010;328:1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JL, Stone PR, Chamley LW. Cytotrophoblast differentiation in the first trimester of pregnancy: evidence for separate progenitors of extravillous trophoblasts and syncytiotrophoblast. Reproduction 2005;130:95–103. [DOI] [PubMed] [Google Scholar]
- James JL, Stone PR, Chamley LW. The effects of oxygen concentration and gestational age on extravillous trophoblast outgrowth in a human first trimester villous explant model. Hum Reprod 2006;21:2699–2705. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol 2017;218:75–87. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta 2012;33:244–251. [DOI] [PubMed] [Google Scholar]
- Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc Natl Acad Sci USA 2015;112:214–219National Academy of Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha SK, Chand R, Han D, Jang Y-C, Ra G-S, Kim JS, Nahm B-H, Kim Y-S. An integrated PCR microfluidic chip incorporating aseptic electrochemical cell lysis and capillary electrophoresis amperometric DNA detection for rapid and quantitative genetic analysis. Lab Chip 2012;12:4455–4464The Royal Society of Chemistry. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Burghardt RC, Joyce MM, Spencer TE, Bazer FW, Pfarrer C, Gray CA. Osteopontin expression in uterine stroma indicates a decidualization-like differentiation during ovine pregnancy. Biol Reprod 2003;68:1951–1958. [DOI] [PubMed] [Google Scholar]
- Jonkman JEN, Cathcart JA, Xu F, Bartolini ME, Amon JE, Stevens KM, Colarusso P. An introduction to the wound healing assay using live-cell microscopy. Cell Adh Migr 2014;8:440–451Taylor & Francis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanović M, Stefanoska I, Radojcić L, Vićovac L. Interleukin-8 (CXCL8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (MMP)2 and MMP9 and integrins alpha5 and beta1. Reproduction 2010;139:789–798. [DOI] [PubMed] [Google Scholar]
- Justus CR, Leffler N, Ruiz-Echevarria M, Yang LV. In vitro cell migration and invasion assays. J Vis Exp 2014;88 MyJoVE Corporation. doi: 10.3791/51046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar S, Dhar R, Das C. Inhibition of cytotrophoblastic (JEG-3) cell invasion by interleukin 12 involves an interferon gamma-mediated pathway. J Biol Chem 2004;279:55297–55307American Society for Biochemistry and Molecular Biology. [DOI] [PubMed] [Google Scholar]
- King A, Thomas L, Bischof P. Cell culture models of trophoblast II: Trophoblast cell lines - A workshop report. Placenta 2000;21:p. S113–S119. [DOI] [PubMed] [Google Scholar]
- Kingdom JC, Audette MC, Hobson SR, Windrim RC, Morgen E. A placenta clinic approach to the diagnosis and management of fetal growth restriction. Am J Obstet Gynecol 2018;218:S803–S817. [DOI] [PubMed] [Google Scholar]
- Kohler PO, Bridson WE. Isolation of hormone-producing clonal lines of human choriocarcinoma. J Clin Endocrinol Metab 1971;32:683–687. [DOI] [PubMed] [Google Scholar]
- Lacroix M-C, Guibourdenche J, Fournier T, Laurendeau I, Igout A, Goffin V, Pantel J, Tsatsaris V, Evain-Brion D. Stimulation of human trophoblast invasion by placental growth hormone. Endocrinology 2005;146:2434–2444. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature 2013;501:373–379Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CQE, Gardner L, Turco M, Zhao N, Murray MJ, Coleman N, Rossant J, Hemberger M, Moffett A. What is trophoblast? A combination of criteria define human first-trimester trophoblast. Stem cell reports 2016;6:257–272Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C-C, Park AY, Guan J-L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2007;2:329–333. [DOI] [PubMed] [Google Scholar]
- Librach CL, Werb Z, Fitzgerald ML, Chiu K, Corwin NM, Esteves RA, Grobelny D, Galardy R, Damsky CH, Fisher SJ. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol 1991;113:437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y-HV, Middleton K, You L, Sun Y. A review of microfluidic approaches for investigating cancer extravasation during metastasis. Microsystems Nanoeng 2018;4:17104.Nature Publishing Group. [Google Scholar]
- Manaster I, Goldman-Wohl D, Greenfield C, Nachmani D, Tsukerman P, Hamani Y, Yagel S, Mandelboim O. MiRNA-mediated control of HLA-G expression and function. In Bobé P, editor. PLoS One 2012;7:e33395 Public Library of Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, Van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol 1994;101:669–674. [DOI] [PubMed] [Google Scholar]
- Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol 2006;6:584–594Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol 2002;2:656–663. [DOI] [PubMed] [Google Scholar]
- Moser G, Gauster M, Orendi K, Glasner A, Theuerkauf R, Huppertz B. Endoglandular trophoblast, an alternative route of trophoblast invasion? Analysis with novel confrontation co-culture models. Hum Reprod 2010;25:1127–1136. [DOI] [PubMed] [Google Scholar]
- Nantasanti S, Bruin A, De Rothuizen J, Penning LC, Schotanus BA. Concise review: organoids are a powerful tool for the study of liver disease and personalized treatment design in humans and animals. Stem Cells Transl Med 2016;5:325–330Wiley-Blackwell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, Liu IH, Chiou S-H, Salahudeen AA, Smith AR et al. Organoid modeling of the tumor immune microenvironment. Cell 2018;175:1972–1988e16. Cell Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neužil P, Giselbrecht S, Länge K, Huang TJ, Manz A. Revisiting lab-on-a-chip technology for drug discovery. Nat Rev Drug Discov 2012;11:620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okae H, Toh H, Sato T, Hiura H, Takahashi S, Shirane K, Kabayama Y, Suyama M, Sasaki H, Arima T. Derivation of human trophoblast stem cells. Cell Stem Cell 2018;22:50–63e6. Elsevier. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia V, Fineberg E, Wilson R, Murray A, Mazzeo CI, Tudor C, Sienerth A, White JK, Tuck E, Ryder EJ et al. Placentation defects are highly prevalent in embryonic lethal mouse mutants. Nature 2018;555:463–468Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 1983;4:397–413. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980;1:3–19. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Robertson WB, Brosens I, Dixon G. Review article: trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta 1981;2:71–91. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Carter AM. Deep trophoblast invasion and spiral artery remodelling in the placental bed of the lowland gorilla. Placenta 2011;32:586–591. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 2006;27:939–958. [DOI] [PubMed] [Google Scholar]
- Plotnikov SV, Pasapera AM, Sabass B, Waterman CM. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. 2012;Cell, 151:1513–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacheck WJ, Charest JL, Kamm RD. Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc Natl Acad Sci U S A 2011;108:11115–11120National Academy of Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollheimer J, Vondra S, Baltayeva J, Beristain AG, Knöfler M. Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front Immunol 2018;9:2597.Frontiers Media SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prutsch N, Fock V, Haslinger P, Haider S, Fiala C, Pollheimer J, Knöfler M. The role of interleukin-1β in human trophoblast motility. Placenta 2012;33:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature 2014;507:181–189. [DOI] [PubMed] [Google Scholar]
- Sato T, Stange DE, Ferrante M, Vries RGJ, Es JH, Brink S, Houdt WJ, Pronk A, Gorp J, Siersema PD et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011;141:1762–1772. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012;9:676–682Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Morales-Prieto DM, Pastuschek J, Fröhlich K, Markert UR. Only humans have human placentas: molecular differences between mice and humans. J Reprod Immunol 2015;108:65–71. [DOI] [PubMed] [Google Scholar]
- Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, Hollinshead M, Marsh SGE, Brosens JJ, Critchley HO et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol 2017;19:568–577Nature Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco MY, Gardner L, Kay RG, Hamilton RS, Prater M, Hollinshead MS, McWhinnie A, Esposito L, Fernando R, Skelton H et al. Trophoblast organoids as a model for maternal–fetal interactions during human placentation. Nature 2018;564:263–267Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkeste CM, Slangen BF, Daemen M, Straaten H, Kohnen G, Kaufmann P, Peeters LL. The extent of trophoblast invasion in the preplacental vasculature of the guinea-pig. Placenta 1998;19:49–54. [DOI] [PubMed] [Google Scholar]
- Vićovac L, Jones CJ, Aplin JD. Trophoblast differentiation during formation of anchoring villi in a model of the early human placenta in vitro. Placenta 1995;16:41–56. [DOI] [PubMed] [Google Scholar]
- Whitley GSJ, Cartwright JE. Cellular and molecular regulation of spiral artery remodelling: lessons from the cardiovascular field. Placenta 2010;31:465–474Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SY, Hynes RO. Lymphatic or hematogenous dissemination: how does a metastatic tumor cell decide? Cell Cycle 2006;5:812–817NIH Public Access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods L, Perez-Garcia V, Hemberger M. Regulation of placental development and its impact on fetal growth—new insights from mouse models. Front Endocrinol (Lausanne) 2018;9: Frontiers Media SA. doi: 10.3389/fendo.2018.00570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortel IMN, Dannenberg K, Berry JC, Miller MJ, Textor J. CelltrackR: an R package for fast and flexible analysis of immune cell migration data. bioRxiv 2019;670505.Cold Spring Harbor Laboratory. doi: 10.1101/670505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JK, Dunk CE, Perkins JE, Winterhager E, Kingdom JCP, Lye SJ. EGF modulates trophoblast migration through regulation of Connexin 40. Placenta 2006;27:S114–S121. [DOI] [PubMed] [Google Scholar]
- Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, McKinnon KE, Dokic D, Rashedi AS, Haisenleder DJ et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun 2017;8:14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong S, Sharkey AM, Kennedy PR, Gardner L, Farrell LE, Chazara O, Bauer J, Hiby SE, Colucci F, Moffett A. Maternal uterine NKcell-activating receptor KIR2DS1 enhances placentation. J Clin Invest 2013;123:4264–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci USA 2012;109:13515–13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Xue X, Shao Y, Wang S, Esfahani SN, Li Z, Muncie JM, Lakins JN, Weaver VM, Gumucio DL et al. Controlled modelling of human epiblast and amnion development using stem cells. Nature 2019;573:421–425Springer Science and Business Media LLC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J-Y, Pang Z-J, Yu Y-H. Regulation of trophoblast invasion: the role of matrix metalloproteinases. Rev Obstet Gynecol 2012;5:e137–e143MedReviews, LLC. [PMC free article] [PubMed] [Google Scholar]
- Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res Mutat Res 2011;728:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilionis R, Nainys J, Veres A, Savova V, Zemmour D, Klein AM, Mazutis L. Single-cell barcoding and sequencing using droplet microfluidics. Nat Protoc 2016;12:44–73. [DOI] [PubMed] [Google Scholar]


