Although hydroxychloroquine and azithromycin, alone or in combination, have been proposed for treatment of patients with coronavirus disease 2019 (COVID-19),1 their potential cardiovascular toxicities had limited consideration in this new clinical environment. We aimed to determine whether these drugs were associated with an increased reporting of cardiovascular (CV) adverse drug reactions (ADR) in a real-world population, before start of prescription for COVID-19 in Europe and America.
In this observational, retrospective study, we used VigiBase,2,3 the World Health Organization pharmacovigilance database encompassing more than 21 million reports from more than 130 countries, to compare CV-ADR reporting in patients who received hydroxychloroquine, azithromycin, or their combination with CV-ADRs reported with all other drugs in the full database (clinicaltrials.gov identifier NCT04314817). Association between hydroxychloroquine, azithromycin, and their combination with CV-ADR was assessed using reporting odds ratio (ROR) and information component (IC), an indicator value for disproportionate Bayesian reporting that compares observed and expected values to find associations between drugs and ADR.2 The lower end of the IC’s 95% credibility interval is IC025. It is considered significant when above 0.2 Reporting odds ratios were computed to compare ADR frequencies between different exposures, using χ2 tests.3 These methods are similar to those used to study CV-ADR related to anticancer and hormonal drugs.2,3 For each report, age, sex, time to onset (TTO), fatalities, concurrent ADRs, and medications (drugs associated with a known or possible risk of QT prolongation as reported at crediblemeds.org)4 were collected. TTO were compared using nonparametric tests. The French National Commission for Data Protection and Liberties and institutional review board approved the use of confidential electronically processed patient data. All CV-ADRs were included, classified by group queries,2 according to the Medical Dictionary for Regulatory Activities, between November 14, 1967, and March 1, 2020.
We extracted 76 822 ADR cases associated with hydroxychloroquine alone, 89 692 with azithromycin alone, and 607 with the combination of both drugs. The cases were retrieved from 21 275 867 total ADR reports in VigiBase. Hydroxychloroquine was a suspected (versus concomitantly used) drug in 21 808/76 822 (28.4%) cases and azithromycin in 54 533/89 692 (60.8%) cases.
There was significant greater reporting of prolonged-QT (LQT) and/or ventricular tachycardia including Torsades-de-Pointes (TdP/VT) for each drug individually in suspected cases (n=480 [223 LQT; 257 TdP/VT], IC025=1.67 for azithromycin and n=136 [53 LQT; 83 TdP/VT], IC025=1.04 for hydroxychloroquine; Figure). Hydroxychloroquine was also associated with conduction disorders (atrioventricular and bundle-branch blocks; n=75, IC025=1.04) and heart failure (n=203, IC025=0.06). No other CV-ADR (including cardiac ischemia and myocarditis)2 was significantly associated with these drugs. The IC values over time and intersecting cases for significant CV-ADRs associated with hydroxychloroquine or azithromycin are presented in the Figure.
Figure.
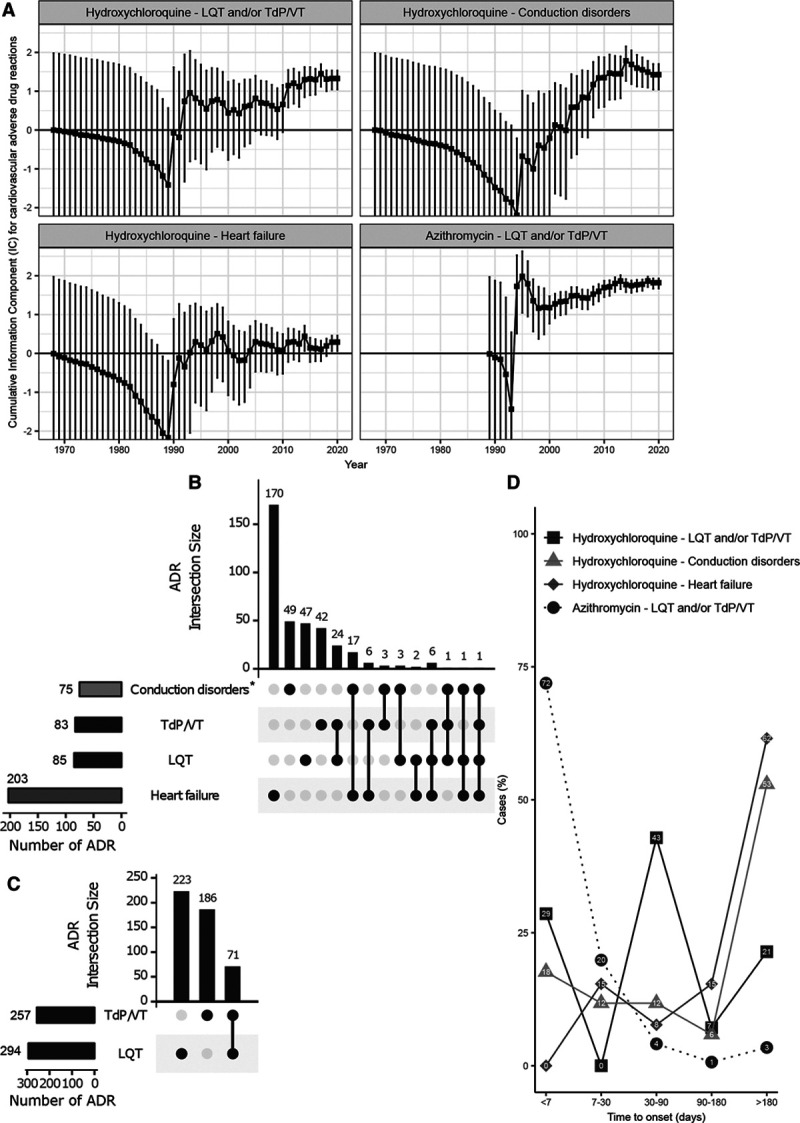
Temporal evolution (last accessed March 1, 2020) of the information component (IC) for cardiovascular (CV) adverse drug reactions (ADR) significantly associated with hydroxychloroquine or azithromycin (see statistics below). Whiskers are IC025, IC975. IC025 and IC975 are the lower and upper end of the IC 95% credibility interval, respectively. Significance is IC025>0 (black line). LQT, long QT syndrome; TdP/VT, ventricular tachyarrhythmias (VT) including Torsades-de-Pointes (TdP). Years of first ADR report within VigiBase for hydroxychloroquine and azithromycin were respectively 1967 and 1989 (A). Intersection for selected CV-ADR (IC025>0) in case reports from VigiBase, for hydroxychloroquine (B) and azithromycin (C). Time to onset (in days) between first treatment intake and the CV-ADR associated with hydroxychloroquine or azithromycin (D). *Of the conduction disorders (n=75), 50/75 (67%) were atrioventricular blocks, 24/75 (32%) were bundle-branch blocks, and 1/75 (1%) was a sinus block. Time to onset data are available for hydroxychloroquine and LQT and/or TdP/VT (n=90), for hydroxychloroquine and conduction disorders (n=77), for hydroxychloroquine and heart failure (n=94), and for azithromycin and LQT and/or TdP/VT (n=70). Statistics:2 IC = log2[(Nobserved + 0.5)/(Nexpected + 0.5)], where Nexpected = (Ndrug × Nreaction)/Ntotal, with Nexpected being the number of individual case safety reports (ICSRs) expected for the drug-ADR combination; Nobserved being the actual number of ICSRs observed for the drug-ADR combination; Ndrug being the number of ICSRs for the drug, regardless of ADR; Nreaction being the number of ICSRs for the ADR, regardless of the drug; and Ntotal being the total number of ICSRs in the database.
Azithromycin monotherapy was associated with a greater reporting of LQT and/or TdP/VT than hydroxychloroquine monotherapy (736/89 085 [0.8%] versus 263/76 215 [0.3%], respectively; reporting odds ratio, 2.36 [95% CI, 2.05–2.71). The combination of azithromycin and hydroxychloroquine was associated with a greater reporting of LQT and/or TdP/VT reporting than either drug in monotherapy (999/165 300 [0.6%] versus 9/607 [1.5%], reporting odds ratio, 2.48 [95% CI, 1.28–4.79]).
Most CV-ADRs reports were in women (516/772, 66.8%). Reporting regions were mostly Americas (549/851, 64.5%) and Europe (185/851, 21.7%). Reporters were mostly healthcare professionals (674/706, 91.1%). In most cases, ADR was attributed to a single drug (492/844, 58.3%). Concurrent reporting of drugs with a known risk of TdP in TdP/VT cases was 31.5% (81/257) with azithromycin and 16.9% (14/83) with hydroxychloroquine.4
TTO of LQT and/or TdP/VT with azithromycin was shorter compared with hydroxychloroquine (3 [interquartile range=1;7] versus 51 [IQR=11;113] days, P<0.01). With hydroxychloroquine, TTO of LQT and/or TdP/VT was shorter than heart failure (51 [IQR=11;113] versus 348 [IQR=91;2016] days, P=0.027). This longer TTO observed in hydroxychloroquine may reflect chronic use in lupus or rheumatoid arthritis.
The proportion that resulted in death for TdP/VT cases was 8.4% (7/83) with hydroxychloroquine and 20.2% (52/257) with azithromycin versus 0% (0/53) and 5.4% (12/223) for LQT without TdP/VT with hydroxychloroquine and azithromycin, respectively (P<0.001 for both). The corresponding death rate was 20.7% (42/203) for heart failure associated with hydroxychloroquine. The dose of hydroxychloroquine was higher in heart failure compared with LQT and/or TdP/VT cases (200 [IQR=200;400] versus 200 [IQR=200;200] mg/d, P=0.033).
The main limitation is that without data on numbers exposed in VigiBase, this work cannot assess the incidence or risk for QT prolongation with these drugs. However, our results are consistent with the facts that these CV-ADRs are found in the US Food and Drug Administration’s labels of hydroxychloroquine and azithromycin, and that both drugs are referenced as having a known risk of TdP on the CredibleMeds website.4 These CV-ADRs are important to bear in mind in the setting of COVID-19 with patients presenting additional risk factors of LQT/TdP caused by inflammation with elevated interleukin-6, hypokalemia, numerous interacting drugs, bradycardia, and higher hydroxychloroquine dosages.5
In conclusion, reports of potentially lethal acute cardiac proarrhythmogenic effects leading to ventricular arrhythmias have been described mainly with azithromycin but also with hydroxychloroquine. Their combination yielded an even stronger signal. Hydroxychloroquine was also associated with potentially lethal heart failure when exposure was prolonged over several months.
Acknowledgments
The authors thank Banook group/Cardiabase, Nancy–France http://www.banookgroup.com/ (Yasmin Khan) for providing the Holter and ECG systems to evaluate prospectively the impact of hydroxychloroquine and azithromycin on ventricular arrhythmia in a current cohort of treated patients with COVID-19, as a follow-up study of this work. They also thank the staff of the AP.HP.Sorbonne clinical investigation center (Maria Martin, Francois Gueguin, Dr Paul Gougis, Dr Bruno Pinna) for their involvement in the latter study and their current effort to better study adverse drug reactions associated with COVID-19 drugs.
Disclosures
The supplied data from VigiBase come from various sources. The likelihood of a causal relationship is not the same in all reports. The information does not represent the opinion of the World Health Organization. The authors report no conflicts.
Footnotes
Data sharing: The data, analytic methods, and study materials are available to other researchers for purposes of reproducing the results or replicating the procedure at http://www.vigiaccess.org/.
References
- 1.Jakhar D, Kaur I. Potential of chloroquine and hydroxychloroquine to treat COVID-19 causes fears of shortages among people with systemic lupus erythematosus. Nat Med. 2020;26:632. doi: 10.1038/s41591-020-0853-0. doi: 10.1038/s41591-020-0853-0. [DOI] [PubMed] [Google Scholar]
- 2.Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, Gobert A, Spano JP, Balko JM, Bonaca MP, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 2018191579–1589doi: 10.1016/S1470-2045(18)30608-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salem JE, Yang T, Moslehi JJ, Waintraub X, Gandjbakhch E, Bachelot A, Hidden-Lucet F, Hulot JS, Knollmann BC, Lebrun-Vignes B, et al. Androgenic effects on ventricular repolarization: a translational study from the International Pharmacovigilance Database to iPSC-Cardiomyocytes. Circulation 20191401070–1080doi: 10.1161/CIRCULATIONAHA.119.040162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woosley RL, Heise CW, Gallo T, Tate J, Woosley D, Romero KA. QTdrugs List. www.CredibleMeds.org. Accessed June 29, 2020.
- 5.Funck-Brentano C, Salem JE, Nguyen LS, Drici MD, Roden DM. Response to the editorial “COVID-19 in patients with cardiovascular diseases”: Covid-19 treatment with hydroxychloroquine or chloroquine and azithromycin: a potential risk of Torsades de Pointes. Arch Cardiovasc Dis 2020113367–368doi: 10.1016/j.acvd.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]


