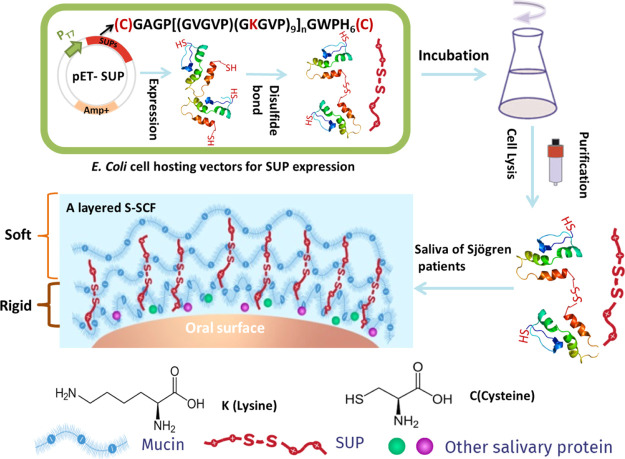
Abstract

Insufficient retention of water in adsorbed salivary conditioning films (SCFs) because of altered saliva secretion can lead to oral dryness (xerostomia). Patients with xerostomia sometimes are given artificial saliva, which often lacks efficacy because of the presence of exogenous molecules with limited lubrication properties. Recombinant supercharged polypeptides (SUPs) improve salivary lubrication by enhancing the functionality of endogenously available salivary proteins, which is in stark contrast to administration of exogenous lubrication enhancers. This novel approach is based on establishing a layered architecture enabled by electrostatic bond formation to stabilize and produce robust SCFs in vitro. Here, we first determined the optimal molecular weight of SUPs to achieve the best lubrication performance employing biophysical and in vitro friction measurements. Next, in an ex vivo tongue-enamel friction system, stimulated whole saliva from patients with Sjögren syndrome was tested to transfer this strategy to a preclinical situation. Out of a library of genetically engineered cationic polypeptides, the variant SUP K108cys that contains 108 positive charges and two cysteine residues at each terminus was identified as the best SUP to restore oral lubrication. Employing this SUP, the duration of lubrication (Relief Period) for SCFs from healthy and patient saliva was significantly extended. For patient saliva, the lubrication duration was increased from 3.8 to 21 min with SUP K108cys treatment. Investigation of the tribochemical mechanism revealed that lubrication enhancement is because of the electrostatic stabilization of SCFs and mucin recruitment, which is accompanied by strong water fixation and reduced water evaporation.
Keywords: biolubrication, recombinant supercharged polypeptides, ex vivo oral lubrication system, salivary substitutes, saliva, mucins, dry mouth, Sjögren’s syndrome, protein adsorption
1. Introduction
Biomacromolecules play a vital role in maintaining physiological functions in living systems especially at sliding interfaces, where conditioning films consisting of adsorbed macromolecules like proteins, glycoproteins, and polysaccharides support a wide range of normal and shear stresses.1 Salivary conditioning films (SCFs) in the human oral cavity are just one of the biofilms capable of withstanding contact pressures of ∼86 MPa during mastication2 with very low friction, which is unmatched by any man-made macromolecular coating. SCFs provide lubrication through glycoproteins, that is, mucins with molecular weights up to 20 MDa,3 that retain water molecules to generate repulsive hydration forces at the sliding interface even when the two surfaces are brought in close contact.4
Oral lubrication by the adsorbed SCFs is essential to facilitate mastication and speech, SCFs also protect against wear causing rashes and pain. An insufficient amount of water molecules retained in the adsorbed SCFs because of reduced (hyposalivation) or altered saliva secretion because of impaired salivary glands can be accompanied by xerostomia, that is, a subjective dry mouth feel.5 Radiation therapy in the maxillofacial region, Sjögren’s syndrome, polypharmacy (<5 medications), and high age can cause xerostomia.6 Although not being fatal, xerostomia can be chronic and drastically reduce quality of life of patients.7 Generally, these patients can be treated with artificial saliva, which contains lubricants and thickeners extracted from animal or plant sources like procine gastric mucins (PGM), hydroxyethyl cellulose, aloe vera, etc. Unfortunately, these formulations provide only a temporary relief because of their limited ability to retain sufficient water and a specific environment is required like for PGM, which is only effective under specific conditions of acidic pH and low ionic strength.8,9 Most of the current artificial saliva developments focus on optimizing the viscosity although it has been shown that there is only little correlation between viscosity and ability to lubricate the oral cavity.10 Ongoing research devoted to saliva substitutes aims to mimic natural saliva to achieve reduction in friction (termed as “Relief11” later in this study) and a long-lasting lubrication (termed as the “Relief Period11” later in this study) but unfortunately with little effect.12−15 These approaches do not take advantage of the patient’s own altered endogenous saliva secretion but focus on exogenous components, leading to temporary effects. The exogenous components of many saliva substitutes are often easily removed from the SCF by swallowing or drinking leading to limited duration of moistening and lubrication.16 The aim of this study is to demonstrate that the functionality of naturally remaining lubricating moieties can be boosted without replacing and masking them with exogenous components. Cationic supercharged polypeptides (SUPs) with the repetitive motif (GVGKP)n show excellent biocompatibility and act as biolubrication enhancers by interacting with the negatively charged salivary mucins.17 In previous publications, two variants with the number of repeat units (n) of 72 (K72) and 36 (K36) were applied revealing better lubrication for K72 than for K36 because of recruitment of mucins.17,18
Although the aforementioned study introduced a proof of concept to ameliorate biolubrication by a combination of exogenous and native entities, several important features for successful translation remained to be explored. Important questions still to be answered are: (i) does an additional increase of molecular weight of the SUP lead to improved biolubrication? (ii) Can the increased biolubrication observed on the nanoscale be generalized and transferred to the macroscale with relevant oral tissue? (iii) Do SUPs improve lubrication with saliva from patients suffering with xerostomia? All these questions were addressed in the current study by expressing pristine SUPs and SUPs containing two cysteine units at both ends of the peptide chain allowing dimerization of SUPs upon disulfide formation and doubling the molecular weight (Scheme 1). After identification of the best SUP yielding the highest lubrication performance assessed by quartz crystal microbalance and atomic force microscopy experiments,17 a recent tongue-enamel friction system11 was used for further characterization. Therefore, saliva from healthy volunteers and Sjögren’s patients was collected and their lubrication properties were measured on a tongue-enamel friction system with intermediate exposure to SUPs. Finally, a germanium-silicon rubber tribopair with simultaneous infrared spectroscopy was used to understand the tribochemical mechanism of the enhanced lubrication.
Scheme 1. Schematic Representation of SUP Fabrication via Recombinant Protein Expression and Interaction with Naturally Occurring Saliva from Healthy Volunteers and Patients Suffering from Sjögren’s Syndrome.
2. Experimental Section
2.1. Polypeptide Expression and Purification
Escherichia coli BLR (DE3) cells (Novagen) were transformed with the pET25b expression vectors containing the respective SUP genes (for details, see Supporting Information). For SUP production, Terrific Broth medium (12 g/L tryptone and 24 g/L yeast extract) enriched with phosphate buffer (2.31 g/L potassium phosphate monobasic and 12.54 g/L potassium phosphate dibasic) and supplemented with 100 μg mL–1 ampicillin was inoculated with an overnight starter culture to an initial density at 600 nm (OD600) of 0.1 and incubated at 37 °C with orbital agitation at 250 rpm until OD600 reached 0.6. Polypeptide production was induced by a temperature shift to 30 °C for an additional 16 h. Subsequently, cells were harvested by centrifugation (5000 rpm, 30 min, 4 °C, JLA-16.250 rotor, USA), then resuspended in lysis buffer (50 mM sodium phosphate buffer, pH 8.0, 300 mM NaCl, 20 mM imidazole) to an OD600 of 100, and subsequently disrupted with a constant cell disrupter (Constant Systems Ltd., Daventry Northants, UK). Cell debris was removed by centrifugation (15,000g, 30 min, and 4 °C). Polypeptides were purified from the supernatant under native conditions by Ni-sepharose chromatography. Product-containing fractions were dialyzed extensively against ultrapure water. The product purity was determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% polyacrylamide gel. Afterward, gels were stained with Coomassie staining solution (40% methanol, 10% glacial acetic acid, and 1 g/L Brilliant Blue R250). Photographs of the gels after staining were taken with a LAS-3000 Image Reader (Fuji Photo Film GmbH, Dusseldorf, Germany). Mass spectrometric analysis was performed using a 4800 matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF)/TOF Analyzer in the linear positive mode. The polypeptide samples were mixed with theα-cyano-4-hydroxycinnamic acid matrix (SIGMA) (100 mg mL–1 in 70% ACN and 0.1% trifluoroacetyl) (1:1 v/v). Mass spectra were analyzed with the Data Explorer V4.9. The concentrations of the purified polypeptides were determined by measuring absorbance at 280 nm by using a spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA).
2.2. Saliva Collection from Healthy Volunteers and Sjögren’s Syndrome Patients
A standard protocol11 was adopted to collect and prepare stimulated (SWS) and reconstituted (RWS) whole saliva as described in detail below. SWS was collected from 4 healthy volunteers (age 28.2 ± 2.8 years, 1 male, and 3 females) with flow rates of 1.6, 1.76, 1.45, and 1.15 mL/min. Healthy volunteers did not use any type of medication, did not smoke, and were free of history with radiotherapy or autoimmune diseases. Collecting of whole saliva was done on the same day of the week and at 10:00 a.m. The healthy adult donors were recruited from the department of Biomedical Engineering of the University Medical Centre Groningen, the Netherlands. All collections were performed in accordance with the relevant guidelines and regulations under the approval of the Medical Ethics Review Board of the University Medical Center Groningen (approval no. M17.217043, M09.069162 and UMCG IRB #2008109). Pathological samples were collected from 4 patients (age 56.2 ± 16.6, 1 male, and 3 females) suffering from Sjögren’s syndrome treated at the Maxillofacial Surgery Department of the University Medical Center Groningen (UMCG). Sjögren’s patients had been subjected to a diagnostic Sjögren’s work-up from the Department of Rheumatology and Clinical Immunology of the University Medical Center Groningen, the Netherlands. The Sjögren’s patients fulfilled the 2016 ACR-EULAR classification criteria for Sjögren’s syndrome.19 Patients and healthy volunteers gave written informed consent. The patients had reduced stimulated salivary flow rates of 0.48, 0.72, 0.45, and 0.98 mL/min. Accordingly, patients completed the validated xerostomia inventory, a questionnaire containing eleven questions on subjective dry mouth20,21 and scored 22, 31, 32, and 17, respectively, on the 11–55 scale. Participants were not allowed to eat or drink for 1 h prior to saliva collection. Before collecting any saliva, their mouth was rinsed well with tap water. Salivary flow was mechanically stimulated (by chewing on parafilm) for 5 min, every 5 min after collecting the saliva. Cells and food particles were removed by centrifugation (10,000 rpm, 10 °C, 5 min, JLA-16.250 rotor, USA) and a protease inhibitor phenylmethylsulfonyl fluoride (1 mM) was added to stabilize the saliva that is to prevent the breakdown of salivary proteins and glycoproteins. Saliva from individual patient and healthy volunteer was used for ex vivo friction measurements on the tongue-enamel model and the Tribochemist. For all the in vitro measurements, reconstituted saliva was used, which was prepared by the same protocol as described above but the saliva collected from 30 healthy volunteers recruited from the Department of Biomedical Engineering of the University Medical Centre Groningen, the Netherlands, was pooled, stabilized, and freeze-dried for storage.11 The lyophilized stock was dissolved in buffer (2 mM potassium phosphate, 50 mM KCl, 1 mM CaCl2, and pH 6.8) at 1.5 mg mL–1 for all in vitro experiments.
2.3. In Vitro S-SCF Formation Monitored Using a Quartz Crystal Microbalance with Dissipation and Zeta Potential Measurements
QCM-D device model Q-sense E4 (Q-sense, Gothenburg, Sweden) was used to study the structural softness and formation kinetics of SCFs in real time with Au-coated quartz crystals (5 MHz) as the substrata. Before each experiment, crystals were cleaned by 10 min UV/ozone treatment, followed by immersion into a 3:1:1 mixture of ultrapure-water, NH3 and H2O2 at 75 °C for 10 min, drying with N2, and another UV/ozone treatment. The chamber was perfused with buffer using a peristaltic pump until stable base lines were achieved both in frequency and dissipation, then RWS was flowed through the chamber for 2 h at 25 °C with a flow rate of 50 μL/min, equivalently a shear rate of about 3 s–1. Next, the chambers were perfused with buffer or 0.05% w/v of SUP for 2 min, after that another 2 h of RWS flow through to form a S-SCF. In between steps, buffer was flowed through the chamber for 15 min to remove the free salivary protein. The low salivary flow rate (50 μL/min) in the QCM-D was chosen to mimic a low oral salivary flow rate of dry mouth patients. After experiments, crystals were taken out of the QCM-D device and immediately used for further experiments. Zeta potentials of the SCFs in the absence and presence of the adsorbed SUPs were measured using a zetasizer nano series (model number ZEN3600, Malvern Ltd, UK). Silica spheres (diameter 1.7 μm) were coated with the SCF by suspending in saliva for 2 h. Subsequently, the spheres were suspended in buffer or K72, K108, K144, K108cys, and K144cys solutions (0.05% w/v) for 2 min. After each coating step, the spheres were rinsed with buffer for 10 min. The zeta potential of the different spheres was measured in buffer (2 mM potassium phosphate, 1 mM CaCl2, 50 mM KCl, and pH 6.8).
2.4. Elemental Composition of the S-SCF with SUP Modification and the Lubrication Property on the Nanocale
The elemental composition of the S-SCF surface was acquired from the X-ray photoelectron spectroscopy (XPS, S-Probe, surface science instruments, mountain view, CA, USA). Both low resolution for broad scans and high resolution for C1s and O1s peaks were made, where the O1s peak can be split into two components, the fraction of the O1s peak at 532.7 eV (% O532.7) from carboxyl groups was used to calculate the amount of oxygen in glycoproteins, which include mucins (% Oglyco).17
| 1 |
where % Ototal is the total percentage of oxygen.
Friction force and surface morphology were determined by AFM (Nanoscope IV Dimension 3100) with a Dimension Hybrid XYZ SPM scanner head on the differently S-SCFs in buffer. Rectangular, tipless cantilevers (length 300±5 μm, width 35±3 μm) were calibrated for their torsional and normal stiffness by AFM Tune IT v2.5 software.17,22 The normal stiffness (Kn) was between 0.01 and 0.07 N/m and the torsional stiffness (Kt) was between 1 and 5 × 10–9 N m/rad. Then, a silica-particle of 21.83 μm diameter (d) (Bangs Laboratories, Fishers, IN, USA) was glued to the cantilever with an epoxy glue. The deflection sensitivity (α) of the colloidal probe was recorded at a constant compliance with bare crystal in buffer to calculate the normal force (Fn) applied using
| 2 |
where ΔVn is the output voltage from the AFM photodiode because of the normal deflection of the colloidal probe. The torsional stiffness and geometrical parameters of the probe were used to calculate the friction force (Ff)17,22 according to
| 3 |
where t represents the thickness of the cantilever, δ represents the torsional detector sensitivity of the AFM, and ΔVL is the voltage output from the AFM photodiode because of the lateral deflection of the probe. Then, lateral deflection was observed at a scanning angle of 90° over a scan area of 25 × 25 μm2 with a scanning frequency of about 1 Hz. The colloidal probe was incrementally loaded and unloaded up to a normal force of 40 nN. For each normal force, friction loops were recorded to generate the friction force and to calculate the coefficient of friction (COF).
2.5. Tongue-Enamel Friction System
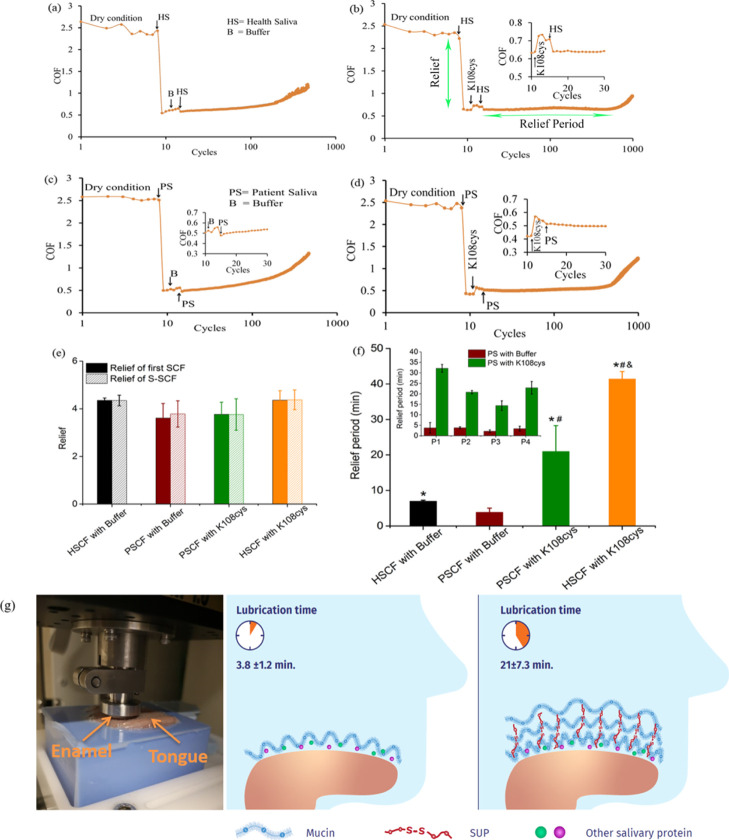
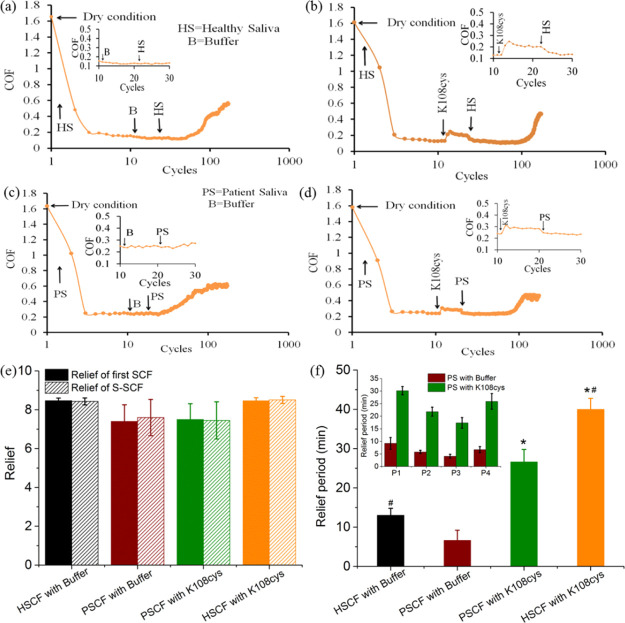
Fresh porcine tongues (Kroon Vlees BV, Groningen, The Netherlands) were carefully rinsed and dried followed the protocol described in detail by Vinke et al.11 Care was taken not to remove the protein and glycoprotein layer on the tongue surface. The tongues were placed upside down inside a handmade box and the rest of the space was filled with Wirosil duplicating silicone (Bego, Bremen, Germany), which looked like the one shown in Figure 3g after setting. The bovine enamel was also prepared according to the protocol of Vinke et al.,11 briefly the rounded and polished piece of enamel with a radius of curvature of 55 mm fixated in a stainless-steel holder. The final surface finish was obtained by sliding the enamel against a wetted polishing cloth with 0.05 μm alumina micro-polish thus the dental film was removed during the rubbing. It was used as the pin sliding against the tongues with the help of the universal mechanical tester (UMT-3, CETR Inc., USA). The applied normal force (Fn) was experimentally determined at 0.25 N as the minimal force could sense on a weighing spoon using their tongues.11 The sliding speed was 4 mm/s with a 10 mm sliding distance. UMT-3 recorded the friction force (Ff) every 0.01 s during all cycles. The COF was calculated using eq 4. To mimic dry mouth surfaces, each experiment was performed with following steps. First, the enamel was slid against tongue for 10 cycles under dry conditions.11 The stabilized COF in this step was called COFdry. Then, the sliding was stopped and a drop of 20 μL of healthy stimulated saliva or patient stimulated saliva was placed at the tongue-enamel interface rubbing 4 cycles followed by the step 3 where 20 μL of buffer or K108cys added. To reflect best the in vivo situation of immediate reflow of saliva in the oral cavity in step 4, another 20 μL of healthy or patient stimulated saliva was added to the surface again under continued rubbing. During the 4 steps of rubbing, a quick drop in the COF was observed (COFsaliva). The drop in the COF was termed “Relief” and calculated using eq 5. The duration of a low COF was designated to as the “Relief Period”. The end of the relief period was taken as the point, where a rapid change in the slope was observed.
| 4 |
| 5 |
Figure 3.
Ex vivo, macroscale lubrication properties of the SUP-modified S-SCFs involving healthy and patient saliva. Relief and relief period of the S-SCF measured with healthy saliva (HSCF) and saliva from patient individuals (HSCF) in an ex vivo tongue-enamel friction system.11 (a) Healthy S-SCF with intermediate buffer treatment. (b) Healthy S-SCF with intermediate K108cys treatment. (c) Patient S-SCF with intermediate buffer treatment. (d) Patient S-SCF with intermediate K108cys treatment. (e) Relief of the SCF and S-SCF involving healthy saliva and saliva from patient individuals. (f) Relief period for patient saliva and healthy saliva. Error bars represent the SD over three independent measurements. Stimulated human whole saliva (SWS) and Sjögren patient saliva was used for the HSCF and the PSCF, respectively. (g) Schematic representation of the SUP restoring the oral lubrication.*Statistically significant (P < 0.05) differences in the relief period of the S-SCF with intermediate K108cys treatment with respect to the S-SCF with intermediate buffer treatment both for healthy and patient saliva. #Statistically significant (P < 0.05) differences between healthy and patient S-SCF, respectively, either for intermediate buffer treatment or K108cys treatment.
2.6. Mechanism Investigated using Tribochemist
The Tribochemist (Ducom Instruments Pvt. Ltd, Bangalore, India) is an instrument that provides information on the chemical dynamics of the adsorbed layers during sliding. It is an apparatus, combining infrared spectroscopy with macroscopic-tribology to provide real-time information on the adsorbed layer composition during sliding. This helps to follow molecular changes during sliding in relation to friction and the understanding of the lubrication mechanisms.23 It consists of a tribometer (Ducom Instruments Pvt. Ltd, Bangalore, India) and an attenuated total reflection (ATR)–Fourier transform infrared (FTIR) spectrometer (Cary 600 series FTIR Spectrometer; Agilent Technologies, Santa Clara, CA, USA). The FTIR spectrometer was used for acquiring the IR spectra of the adsorbed layer on the germanium prism (Ge, Pike Technologies, USA) while the tribometer monitored the COF. The motion drive is linear using a stepper motor to reciprocate sliding with a polydimethylsiloxane (PDMS) pin (hemispherical, radius of 3 mm) against the germanium prism. For the current experiments, stroke length was 10 mm, velocity was 1 mm/s, load force was 450 mN, set with the Winducom 2010 (Ducom Instruments Pvt. Ltd) software developed using the LabVIEW platform. The protocol used is similar to that used for a tongue-enamel friction system that is dry friction; introduction of 20 μL pooled saliva by pipetting from healthy subjects or Sjögren’s patients to form SCF and sliding 10 cycles, introduction of 20 μL SUP k108cys and sliding for another 10 cycles, and then introduction of 20 μL of saliva to form S-SCFs under continuous sliding. The friction force generated by the software and the COF can be calculated by using eq 4. After the S-SCFs were formed, the FTIR spectra were collected within the wavenumber range of 400–4500 cm–1 at a resolution of 4 cm–1, with one spectrum being averaged from 12 interferograms. After the S-SCFs were formed on the germanium prism the ATR-FTIR spectra was recorded every 10 minutes under continuous sliding. Integration of each absorption bands in IR spectra were done by the ORIGIN PRO v. 9.0 program (Origin Lab Corporation, Northampton, MA, USA).
2.7. Statistical Analysis
All the data are expressed as means ± standard deviation (SD), calculated from three independent experiments. Statistical analysis was performed with GraphPad Prism version 5.0 for windows (GraphPad Software, La Jolla, California, USA). Significant differences between two groups were compared by using two-tailed Student’s t analysis. Correlation analyses were evaluated by Pearson r2, *p < 0.05.
3. Results and Discussion
3.1. Recombinant Expression and Characterization of SUPs
Cationic SUPs consist of repetitive pentapeptide units with the sequence (GVGKP)n including glycine (G), valine (V), proline (P), and lysine (K). Five different variants were employed in this study that can be divided into two groups. One group consists of K72, K108, and K144. The number indicates the total amounts of charges in each SUP molecule. Specific details can be found in Table S1. The other group, K108cys and K144cys, consists of SUPs modified with cysteines at both N and C termini, which are able to form either intramolecular or intermolecular disulfide bonds. A description of the related genes and amino acid composition of SUPs with the general sequences (GAGP[(GVGVP)(GKGVP)9]nGWPH6, CGAGP[(GVGVP)(GKGVP)9]nGWPH6C) is given in Table S1 and Figure S1, respectively. The expression yields of SUPs are 40 mg of purified protein per liter of the culture medium. The proteins were purified from the supernatant under native conditions by Ni-sepharose affinity chromatography mediated through a terminal hexahistidine tag appended to the polypeptide chains. The purity was characterized by SDS-PAGE as shown in Figure S2 where the clear bands show the purity of SUPs obtained. The dimerization yields of K108cys and K144cys were quantified to be around 30 and 50%, respectively. Additional structure verification was obtained by MALDI-TOF mass spectrometry (Figure S3). Each SUP variant yielded a sharp peak and the observed molecular weights were in good agreement with the expected masses of the proteins (Table S1). Molecular cloning and the recombinant expression of perfectly defined, genetically engineered, unfolded polyelectrolytes enabled the increase of the molecular weight of the SUPs from K72 (Mw: 36313 g/mol) over K108 (Mw: 53870 g/mol) to K144 (Mw: 71294 g/mol). Again by genetic engineering, two Cys moieties were terminally introduced into the polypeptide chains for further molecular weight increase to obtain dimers of K108cys and K144cys. The SUPs containing the Cys residues dimerized partially, which leads to doubling of their molecular weight.
3.2. Kinetics of SCF Formation and SUP-Induced Viscoelastic and Topographic Modification
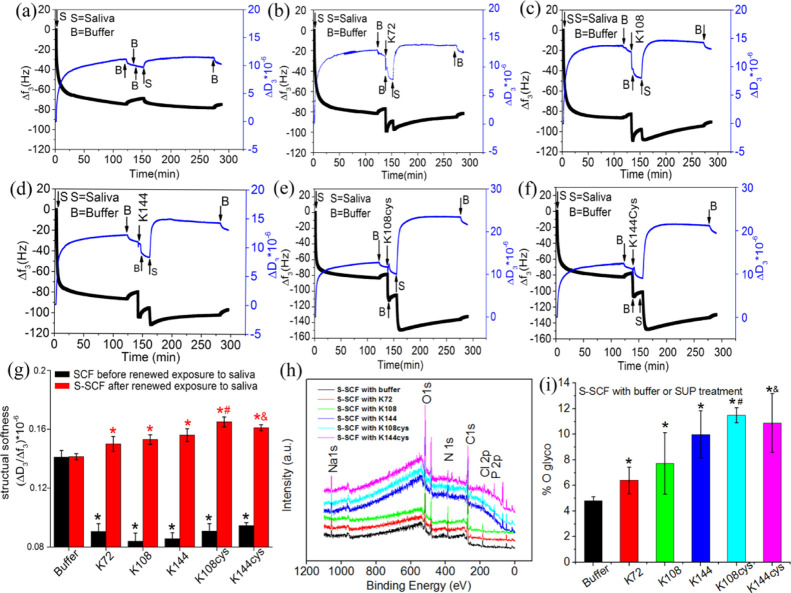
A quartz crystal microbalance with dissipation (QCM-D) was used to monitor the formation of an initial SCF on a gold (Au)-coated QCM-D sensor surface followed by the investigation of exposure to different recombinant SUPs or buffer, and finally renewed adsorption of salivary proteins in real-time to form secondary SCF (S-SCF) (Figure 1a–f). SCF formation on a bare sensor surface for 2 h caused a frequency shift (Δf3) of about −80 Hz and a dissipation (ΔD3) change greater than 10, indicating a large amount of salivary protein adsorption on the top of the sensor. The ratio of dissipation and frequency shift (ΔD3/Δf3) larger than 10–6 indicated the formation of a highly viscoelastic SCF. The higher value of ΔD3/Δf3 indicates higher layer softness because of water-filled nature of the adsorbed layer.24,25 Exposure of the SCF to buffer (Figure 1a) yielded a small change in Δf3 and ΔD3, while exposure to SUP solutions (0.5 mg/mL) yielded a significant change (Figure 1b–f) with the ΔD3/Δf3 drastically decreasing (black bars in Figure 1g). A decrease in ΔD3/Δf3 indicates electrostatic stabilization, that is increased compaction or decreased structural softness of the existing SCF because of the exposure to SUPs establishing strong electrostatic bonds between positively charged SUPs and negatively charged salivary glycoproteins. Reflow of saliva caused renewed adsorption of salivary proteins and the formation of S-SCF (Figure 1a–f). With increasing molecular weight of the SUP, the final frequency shift Δf3 for the S-SCF was higher in the order: K72 (−95 ± 10.2 Hz), K108 (−110 ± 8.8 Hz), K144 (−120 ± 6.7 Hz), K108cys (−140 ± 5.5 Hz), and K144cys (−140 ± 6.3 Hz). The structural softness of the S-SCF with intermediate exposure to buffer did not change much but for SUPs exposed a much higher structural softness compared to the initial SCF was detected (red bars in Figure 1g). Both the above observations support the mechanism of mucin recruitment on the surface17 by electrostatic forces and increasing frequency shifts indicate that SUPs with higher molecular weights recruit larger amounts of salivary glycoproteins. Mucin recruitment is also evident from the increased glycosylation of the S-SCFs with an intermediate treatment of SUPs (Figures 1h,i, S4, Table S2) measured using XPS. Full peak description in Figure 1h and Table S2 shows that the relative content of C, O, and N changes upon exposure to SUPs indicating the different protein adsorbed on the surface. The O1s spectra could be deconvoluted into two components: O=C–N and C–O–H considered as the O from protein and the glycol group, respectively. The relative contents of glycoprotein17 could be calculated by the integral of O1s at 532.7ev (Figure 1i and Table S2). A higher amount of O1s at 532.7 eV represents glycoprotein about 11.94 ± 0.6 and 10.88 ± 2.3 was achieved in the S-SCF with K108cys and K144cys modification, respectively, compared to the SCF with buffer or SUPs without termination of cysteine. The dimerization of SUPs upon disulfide formation and doubling the molecular weight and the chain length increase the mucin recruitment yield a softer overlayer. Thus, the exposure of the SCF to SUPs and addition of further saliva give rise to a composite structure that is composed of a relatively rigid initial SCF and a surface layer of the extremely soft S-SCF.
Figure 1.
Kinetics of SCF formation and SUP induced viscoelastic modification. The quartz crystal microbalance with a dissipation (QCM-D) response to adsorption of salivary proteins forming a SCF, and the effect of intermediate SUP adsorption and renewed exposure to saliva to form the secondary SCF (S-SCF). (a–f) The control with intermediate buffer/no SUP adsorption, with SUP K72, K108, K144, K108cys, and K144cys, respectively. (g) Structural softness of the SCF after intermediate exposure to buffer or SUP (black columns) and after renewed exposure to saliva (S-SCF, red columns). (h) The full spectrum XPS scans, showing the chemical element of each surface. (i) The amount of glyco group on each surface. The error bars represent the SD over three independent measurements. *Statistically significant (p < 0.05) differences in structural softness with respect to control. #Significant differences (P < 0.05) in structural softness and glycosylation of S-SCF treated with K108cys with respect to K72, K108, and K144. &Significant difference in structural softness and glycosylation of S-SCF treated with K144cys with respect to K72.
Because of dimerization, both cysteine-modified SUPs (K108cys and K144cys) recruited more salivary glycoproteins leading to a higher structural softness. The roughness of the assembled layers was investigated using an AFM as shown in Figure S5. Bare Au-coated crystals exhibited a smooth surface with heights of around 3 nm (Supporting Information Figure S5a) while after adsorption of salivary protein (Figure S5b), the height increased to over 15 nm. Similar structures were observed when SUPs were involved but the heights were around 30 nm (Figure S5c–h). The globular structure and rougher topography could be attributed to the adsorption of mucins, which in lubricating films with a loop and chain architecture can bear high loads during movement to give rise to low friction.26,27 The higher roughness of the S-SCF with intermediate exposure to the SUP can be explained by the additional salivary glycoprotein recruitment on the top layer. The more efficient glycoprotein recruitment on the SCF with intermediate exposure to K108cys and K144cys can be attributed to the higher positive zeta potential as shown in Figure S6. The zeta-potential of the SCF-coated silica spheres was −12.2 ± 4.9 mV because of the negatively charged salivary proteins including mucins, which is consistent with our previous findings.17 After exposure to K72, the zeta potential increased to −0.99 ± 3.07 mV, with further increase to 12.8 ± 0.76 and 12.9 ± 1.4 mV after exposure to K108cys and K144cys, respectively. The highly positively charged surface of the K108cys and K144cys exposed SCF triggered heavy adsorption to yield higher negative frequency shifts (Figure 1) upon re-exposure to saliva to give rise to a very soft S-SCF (Figure 1g).
3.3. In Vitro, Nanoscale Lubrication Properties of the SUP-Modified SCFs
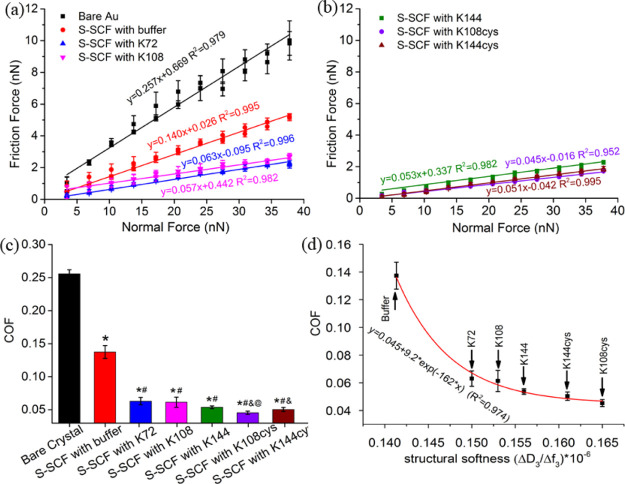
The S-SCFs both with and without intermediate exposure to the SUP were investigated under a colloidal probe AFM and the COF was measured against a spherical 22 μm silica particle. The friction force (Ff) was measured by applying a normal load (Fn) in the range of 3–38 nN and the slope of a linear fit was taken as the COF (Figure 2). On the bare gold (Au), the Ff increased linearly (R2 = 0.98) with Fn, corresponding to a COF of 0.26 (Figure 2a). The COF was reduced to 0.14 after the SCF was exposed to buffer (Figure 2a). S-SCFs with an intermediate recombinant SUP layer exhibited an even further decreased COF (Figure 2b,c) giving rise to better lubricity. The highest structural softness of S-SCFs with intermediate exposure to K108cys and K144cys led to extremely low COFs with values of 0.045 and 0.051, respectively (Figure 2d). The structural softness-induced COF variation was further evaluated with the first-order kinetic model. The correlation could be formulated as
| 6 |
where “y” is the COF of the S-SCF, “x” is the structural softness (ΔD3/Δf3) of the S-SCF,24,25 and “a” and “y0” are the constants. “k” is the kinetic rate constant, and negative values of “k” indicate an inverse correlation between “x” (structural softness) and “y” (COF). The kinetic parameters of eq 6 were estimated statistically using a data-fitting procedure based on a nonlinear least-square regression method. As shown in Figure 2d, the higher structural softness was rebuilt through salivary mucin recruitment by the polypeptide, which led to a lower COF. With an increase of the molecular weight or the length of the SUPs, the electrostatic stabilization, that is, rigidity of the SCF and mucin recruitment and softness of the S-SCF increase (Figure 1g). Furthermore, higher molecular weights of the SUPs generate a larger amount of excess charges on the surface to recruit higher amounts of mucins to further increase the softness of the S-SCF. In vitro, K108cys provided the best recruitment resulting in the softest S-SCF (Figure 1g) and largest enhancement in salivary lubrication (Figure 2d). As also observed earlier, in our study, the structural softness of the surface layer (SCF) correlates with increasing water content.28 The softer S-SCF enabled by mucin recruitment yielded a different mesh size in the S-SCF that affected the water content,29 which gives rise to aqueous lubrication.4,8,30 Although the roughness increased after mucin recruitment, the increased softness and hydration overwhelmed the effect of roughness increase and gave rise to low friction. A similar phenomenon was found in the synovial fluid film in knee joints.31 The structural softness increase of the S-SCF induced by recombinant SUPs determined the lubrication behavior of the S-SCFs (Figure 2d), and the same principle may be applied to other articulating surfaces where water lubrication is mediated by an adsorbed conditioning film, for example eye and cartilage.
Figure 2.
In vitro, nanoscale lubrication properties of the SUP-modified S-SCFs for different SUP molecular weights. The friction force vs normal force measured using a colloid probe atomic force microscope, plots (a,b) used to calculate the COF as a slope of the linear fits presented in (c). (d) The correlation between structural softness of the S-SCF after interaction with SUPs and the resulting COF. Reconstituted human whole saliva (RWS) was used for these measurements. *Statistically significant (p < 0.05) differences in the COF of S-SCFs with respect to bare crystals. #Significant differences (p < 0.05) in the COF of all S-SCF’s treated with SUPs with respect to the S-SCF treated with buffer. &Significant difference in the COF of K108cys- and K144cys-treated S-SCFs with respect to the S-SCFs fabricated with K72 or K108. @Significant difference in the COF between films generated by K144 and K108cys.
3.4. Ex Vivo Demonstration of the Efficacy of K108cys to Enhance Lubrication Using Sjögren’s Patient Saliva
In vitro measurement of lubrication between a silica ball and a gold surface using a laboratory source of saliva RWS (human reconstituted whole saliva) on the nanoscale helped identifying K108cys as the SUP, which gives rise to highest S-SCF structural softness and lowest COF. In order to translate this strategy closer to the clinic, the lubrication needs to be measured in terms of relevant parameters and between realistic sliding surfaces. Thus, the ex vivo evaluation of K108cys with regard to salivary lubrication with samples from 4 healthy volunteers and 4 dry mouth patients suffering from Sjögren’s syndrome were performed with a customized tongue-enamel friction system,11 which mimics dry mouth and allows measurement of “Relief” (COF reduction) and “Relief Period” (lubrication duration). Here, we differentiate between the healthy SCF (HSCF) formed of saliva from healthy volunteers and the patient SCF (PSCF) originating from patient saliva.
The lubrication measurements were performed in 3 steps (Figure 3). Enamel was slid against the tongue for 2.5 s (10 cycles) under dry conditions and from these data, the COF was calculated using eq 4. Then, 20 μL saliva from healthy individuals or Sjögren’s patients was introduced to create the initial SCF by enamel-tongue sliding for 4 cycles. The sharp drop in the COF was called “Relief” and calculated using eq 5 (clearly marked in Figure 3b). Afterward, 20 μL of K108cys or buffer solution was introduced for 4 sliding cycles followed by reflow of 20 μL of saliva from healthy individuals or Sjögren’s patients to create the S-SCF under continuous sliding. The COF was monitored till it started increasing and this time duration was called the relief period.
After producing the initial SCF, pooled SWS provided a relief of 4.5 ± 0.8 fold whereas the patient saliva provided a relief in the range of 3.7 ± 0.6 fold. Introduction of the SUPs caused a slight increase in the COF (Figure 3b,d inset), which is probably because of an increase in layer (SCF) rigidity induced by electrostatic stabilization as shown by the QCM-D data (black bars in Figure 1g). Reflow of saliva and formation of S-SCF restored the COF immediately, as shown in Figure 3b,d. The relief between the initial SCF and S-SCF both for buffer and SUPs was similar. A slightly higher relief was observed for pooled healthy saliva compared to the average value of relief from the 4 patient saliva samples, probably because Sjögren’s patient saliva might contain either modified32 or less amounts of lubricating molecules compared to healthy saliva.33 Intermediate exposure to SUPs does not affect the relief.
The duration for which the COF remained low (Figure 3a–d) was designated as the “Relief Period” and quantified using the conversion factor of 12 cycles/minute. The end of the relief period was taken as the point, where a rapid change in the slope was observed (clearly marked in Figure 3b). The relief period for the S-SCF with intermediate buffer was only about 6 and 3 min in the healthy S-SCF and the patient S-SCF, respectively, while for the S-SCF with intermediate K108cys exposure, the relief period increased significantly both in the patient saliva and healthy saliva. For pooled healthy SWS, the relief period increased up to 41 ± 3 min. For saliva from 4 patients, the relief period increased from 15 ± 2.5 (lowest) to 30 ± 3.6 (highest) min (inset Figure 3f). In this contribution, our in vitro strategy to achieve low friction was successfully translated to the ex vivo stage with the help of a tongue-enamel friction system, by using xerostomia patient saliva, and by focusing on the relief period, which is inaccessible to be determined in vitro with surface friction studies. For xerostomia patients, decreasing oral friction and making the relief similar to healthy humans is necessary, but maintaining low friction for a long duration, that is a longer relief period, is possibly more important to avoid frequent reapplication of saliva substitutes. Figure 3f clearly shows that an intermediate exposure to K108cys helps to enhance the relief period for both saliva samples.
Although the layered composite structure of the SCFs (S-SCFs) entails strong electrostatic complexation between the natural components and the cationic lysine residues of the SUP, the relief remains as good as without SUP treatment (Figure 3e). In vitro, the intermediate treatment with SUPs, as compared to buffer, show a clear decrease in friction (Figure 2c,d), but this decrease is not reflected in relief ex vivo (Figure 3e). The reason could be that the friction pairs in vitro and ex vivo are different. Furthermore, it is well known that the frictional properties often differ between the nanoscale and the macroscale.34,35 Besides scale, the surface properties (topography, roughness etc.) of tongue and enamel would be very different as compared to the smooth silica ball and QCM crystals.
3.5. Tribochemical Mechanism of the Role Played by SUPs in S-SCF Lubrication
Tribochemist enables real-time in situ ATR–FTIR spectroscopy during continuous sliding while both SCF and S-SCFs were established with or without SUPs. The protocol was similar to that used in tongue-enamel friction in Figure 4 but each sliding step consisted of 10 cycles. The COF of the SCF increased after K108cys treatment (inset Figure 4b,d), which is consistent with the increase measured on the tongue-enamel friction system. Reflow of saliva and formation of S-SCF restored the COF and, as can be seen from Figure 4e, the relief was not different between the initial SCF and S-SCF both for buffer and SUPs. Moreover, no significant difference was detected between the healthy saliva-conditioning film (HSCF) and patient saliva-conditioning film (PSCF) after treatment with buffer or K108cys. Relief was higher as compared to the tongue-enamel friction system, which could be because of the difference in the tribo-pair, the PDMS-germanium on the Tribochemist versus tongue-enamel on the UMT. The relief period (Figure 4f) increased both for the HSCF, from 13 ± 1.8 to 40 ± 2.8 min, and the PSCF, from 6.6 ± 2.6 to 26.6 ± 3.2 min, after treatment with K108cys.
Figure 4.
In vitro, macroscale lubrication properties of the SUP-modified SCFs from healthy and patient saliva. Relief and the relief period of the S-SCF with patient saliva (PSCF) and healthy saliva (HSCF) at the silicon rubber-germanium sliding interface. (a) Healthy S-SCF with intermediate buffer treatment. (b) Healthy S-SCF with intermediate K108cys treatment. (c) Patient S-SCF with intermediate buffer treatment and (d) patient S-SCF with intermediate K108cys treatment. (e) Relief of the SCF and the S-SCF in patient saliva and healthy saliva. (f) Relief period for patient saliva and healthy saliva. Error bars represent the SD over three independent measurements. SWS and Sjögren patient saliva was used for HSCF and PSCF, respectively. *Statistically significant (P < 0.05) differences in the relief period of the S-SCF with intermediate K108cys treatment with respect to the S-SCF with intermediate buffer treatment both for healthy and patient saliva. #Statistically significant (P < 0.05) differences between the healthy and the patient S-SCF, respectively, either for intermediate buffer treatment or K108cys treatment.
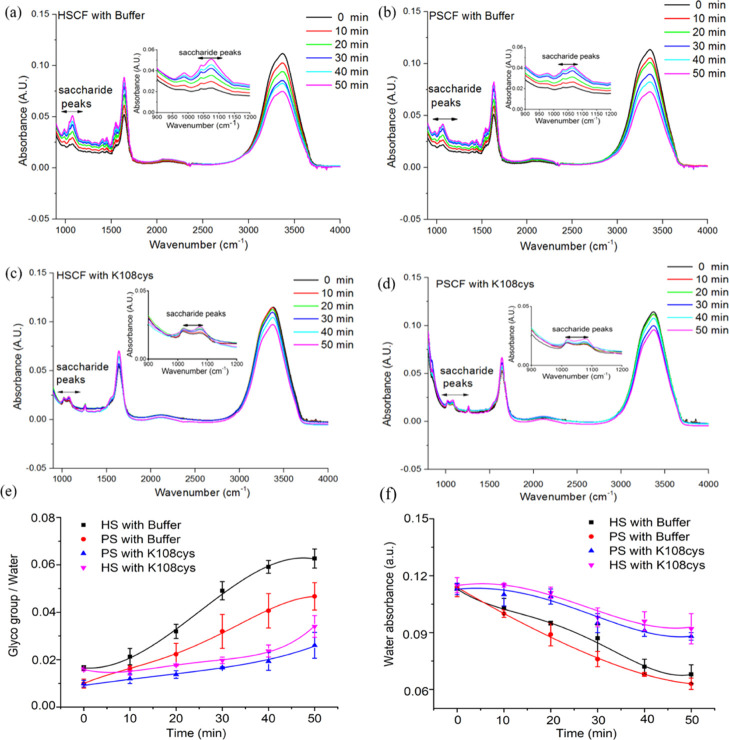
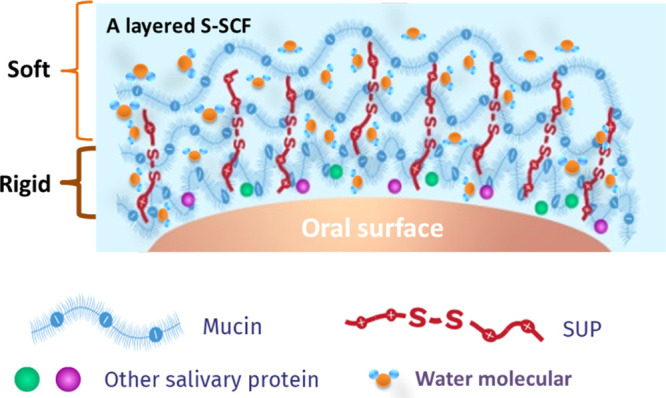
During sliding of S-SCFs, the ATR–FTIR spectra were recorded every 10 min. Three different regions can be distinguished in the FTIR spectra shown in Figure 5a–d, that is saccharide peaks in the 960–1200 cm–1 region representing skeletal vibrations, peaks between 1600 and 1700 cm–1 corresponding to amide I vibrations36 from the salivary protein, and peaks between 2500 and 3800 cm–1 belonging to water.23 Polysaccharide and water peak areas were quantified and the ratio of the polysaccharide and water peak area as a function of time is presented in Figure 5e. Polysaccharide adsorption peaks are observed both for the healthy (HSCF) and patient (PSCF) S-SCF, but the polysaccharide to water ratio, that is glycoprotein concentration for HSCF (0.017 ± 0.002) was significantly higher than for PSCF (0.01 ± 0.003) (Figure 5e), indicating lower amounts of glycosylated proteins in patient saliva.32,33,37 For the buffer-treated SCF (Figure 5a,b), the polysaccharide peaks increased while water peaks decreased with time causing an increase in the polysaccharide/water peak ratio (Figure 5e). This can be caused by the loss of water-like evaporation leading to an increase of the glycoprotein concentration upon sliding in a short time. Both for HSCF and PSCF treated with SUP K108cys, the polysaccharide/water ratio remained constant at 0.0236 ± 0.0025 for 40 min and 0.0166 ± 0.0012 for 30 min of sliding, respectively (Figure 5e). The constant polysaccharide/water ratios indicate that water was retained on the surface to maintain a low COF and a long relief period as shown in Figures 3f and 4f. The strong water fixation also confirmed by the lower rate of water loss in Figure 5f when SCF was treated with K108cys. The buffer-treated HSCF and PSCF show a relief period of 6 to over 10 min (Figures 3f and 4f), which could be because of a fast increase of the polysaccharide/water ratio upon sliding (Figure 5e). The S-SCF treated with K108cys resulted in a soft layer on the top of a relatively rigid charge-stabilized layer, which assists to retain water on the surface and provides high lubricity for a longer period of time (Scheme 2). The results clearly prove that an intermediate layer of K108cys causes electrostatic stabilization of SCFs, which is accompanied by strong water fixation and delayed water evaporation giving rise to a longer relief period not only for healthy but also patient saliva.
Figure 5.
Tribochemistry of the SUP-modified S-SCFs from healthy and patient saliva. Typical FTIR adsorption bands for the S-SCF with patient saliva (PSCF) and healthy saliva (HSCF) treated with K108cys or buffer on a Ge crystal surface during sliding with PDMS pin (1 mm/s; loading force 450 mN) as a function of time. Clearly visible are the polysaccharide peaks (950–1200 cm–1), the amide I peaks indicative of proteins (1600 and 1700 cm–1), and the peaks between 2500 and 4000 cm–1 are indicative of water. (a) HSCF treated with buffer. (b) PSCF treated with buffer. (c) HSCF treated with K108cys. (d) PSCF treated with K108cys. (e) The ratio between the saccharide and water peak area for the HSCF and PSCF treated with K108cys and buffer, respectively. (f) The absorbance of water on the HSCF and PSCF with K108cys or buffer treatment in function of time. Each data point and error bar on HSCF is an average and SD from triplicate measurements performed with healthy saliva and saliva from Sjögren’s syndrome patient.
Scheme 2. Schematic Illustration Showing the Strong Water Immobilization of the Layered S-SCF by Introduction of SUP.
There is an urgent need to develop biomimetic lubricants to restore oral lubrication for xerostomia patients. In the present study, a novel approach of electrostatic stabilization and mucin recruitment with recombinant SUPs17 was pursued to create a layered composite SCF (S-SCF) from patient’s endogenous saliva to enhance oral biolubrication. In contrast to other salivary lubrication research, where PDMS ball on disk sliding yields a 100-fold drop in the COF to values of 0.025,38,39 the tongue-enamel friction system used here shows a realistic drop in the COF to values of 0.5. Furthermore, the relief highly correlates with in vivo dry mouth feel (r = 0.97).11 K108cys treatment of patient saliva showed an average relief period of 21 ± 7 min, which was better than 0.5 min for the use of Dentaid Xeros, a typical artificial saliva substitute.11
Conditioning films present on articulating interfaces, such as the SCF in the oral cavity,17 tear-conditioning film on ocular surfaces,40 and lamina splendens on cartilage surfaces41 are essential for effortless sliding, while conditioning film impairment because of auto immune diseases, age, and medication42−44 leads to a variety of symptoms like dry eye, dry mouth, or excessive cartilage wear in articular joints.17 Our approach is to utilize the existing salivary glycoproteins, stabilize them electrostatically with the help of K108cys, and enhance lubrication. A proof of principle is obtained for oral lubrication, the most challenging environment for biolubrication, but similar recruitment mechanisms may be applied in other parts of body where lubrication is required. In this study, we have based our conclusions on in vitro and ex vivo salivary lubrication measurements. We did not perform any in vivo experiments because of the lack of a suitable animal model, which can mimic xerostomia. For application in patients, the expression yields of SUPs need to be increased and the production of proteins needs to be scaled up besides developing in vivo models. Altogether, this research provides new important insights into restoring the functionality of the conditioning films at articulating tissues in a living system.
4. Conclusions
We successfully increased the molecular weight of SUPs by using genetic engineering to obtain K72, K108, and K144. By introducing two cysteines at the N- and C-termini of the SUPs, we produced K108cys and K144cys, allowing partial dimerization by disulfide formation, doubling the molecular weights of the SUPs. QCM-D and AFM measurements show that an increase in the length of the SUP backbone enhances lubrication with K108cys having the optimal length for salivary lubrication enhancement. K108cys did not adversely affect the relief and was able to significantly enhance the relief period for saliva from patients suffering from xerostomia because of Sjögren’s syndrome. In situ infrared spectroscopy during the lubrication process revealed the ability of K108cys to function synergistically with the SCF to bind water molecules and thereby delay evaporation. A proof of principle was obtained for oral lubrication and suggested to be an alternative solution by exploiting residual saliva components in even the diseased state. Here, we demonstrate that the functionality of naturally remaining lubricating moieties can be strongly improved through a layered architecture with the help of genetically engineered polypeptide materials instead of replacing and masking original lubricants with exogenous components. This strategy may also be beneficial for other parts of the body where aqueous lubrication is essential at articulating interfaces.
Acknowledgments
We are thankful to all Sjögren’s patients and the healthy volunteers who donated stimulated saliva at the Department of Biomedical Engineering, UMCG. The UMT-3 tribometer (Bruker) setup was purchased thanks to the grant no. ZonMW91112026 from the Netherlands Organization for Health Research and Development. The Tribochemist device (Ducom) was purchased thanks to the Netherlands Organization for Scientific Research grant ZonMW91113014. We also would like to thank the China Scholarship Council for a 4 year scholarship to H.W. and C.M. to pursue their PhD studies in The Netherlands. A.H. gratefully acknowledges funding by the European Research Council (ERC Advanced Grant SUPRABIOTICS, grant no. 694610).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.0c06159.
Cloning/gene oligomerization and expression vector construction; SUP expression and purification; mass spectrum and SDS-page of the purified SUPs; XPS of each S-SCF with or without exposure to the SUP; surface topography of each S-SCF (PDF)
Author Contributions
H.W. and C.M. have equally contributed to the article and thus share the first authorship. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. The authors declare no conflict of interest.
The authors declare no competing financial interest.
Supplementary Material
References
- Seror J.; Zhu L.; Goldberg R.; Day A. J.; Klein J. Supramolecular Synergy in the Boundary Lubrication of Synovial Joints. Nat. Commun. 2015, 6, 6497. 10.1038/ncomms7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejak B.; Młotkowski A.; Romanowicz M. Finite Element Analysis of Stresses in Molars during Clenching and Mastication. J. Prosthet. Dent. 2003, 90, 591–597. 10.1016/j.prosdent.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Coles J. M.; Chang D. P.; Zauscher S. Molecular Mechanisms of Aqueous Boundary Lubrication by Mucinous Glycoproteins. Curr. Opin. Colloid Interface Sci. 2010, 15, 406–416. 10.1016/j.cocis.2010.07.002. [DOI] [Google Scholar]
- Lee S.; Spencer N. D. Sweet, Hairy, Soft, and Slippery. Science 2008, 319, 575–576. 10.1126/science.1153273. [DOI] [PubMed] [Google Scholar]
- Herrmann G.; Müller K.; Behr M.; Hahnel S. Xerostomie Und Ihr Einfluss Auf Die Mundgesundheitsbezogene Lebensqualität. Z. Gerontol. Geriatr. 2017, 50, 145–150. 10.1007/s00391-015-0968-y. [DOI] [PubMed] [Google Scholar]
- Cooper B. G.; Catalina Bordeianu C.; Nazarian A.; Snyder B. D.; Grinstaff M. W. Active Agents, Biomaterials, and Technologies to Improve Biolubrication and Strengthen Soft Tissues. Biomaterials 2018, 181, 210–226. 10.1016/j.biomaterials.2018.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellema A. P.; Slotman B. J.; Doornaert P.; Leemans C. R.; Langendijk J. A. Impact of Radiation-Induced Xerostomia on Quality of Life after Primary Radiotherapy among Patients with Head and Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 751–760. 10.1016/j.ijrobp.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Yakubov G. E.; McColl J.; Bongaerts J. H. H.; Ramsden J. J. Viscous Boundary Lubrication of Hydrophobic Surfaces by Mucin. Langmuir 2009, 25, 2313–2321. 10.1021/la8018666. [DOI] [PubMed] [Google Scholar]
- Lee S.; Müller M.; Rezwan K.; Spencer N. D. Porcine Gastric Mucin (PGM) at the Water/Poly(Dimethylsiloxane) (PDMS) Interface: Influence of PH and Ionic Strength on Its Conformation, Adsorption, and Aqueous Lubrication Properties. Langmuir 2005, 21, 8344–8353. 10.1021/la050779w. [DOI] [PubMed] [Google Scholar]
- Hahnel S.; Behr M.; Handel G.; Bürgers R. Saliva Substitutes for the Treatment of Radiation-Induced Xerostomia—a Review. Support. Care Canc. 2009, 17, 1331–1343. 10.1007/s00520-009-0671-x. [DOI] [PubMed] [Google Scholar]
- Vinke J.; Kaper H. J.; Vissink A.; Sharma P. K. An Ex Vivo Salivary Lubrication System to Mimic Xerostomic Conditions and to Predict the Lubricating Properties of Xerostomia Relieving Agents. Sci. Rep. 2018, 8, 9087. 10.1038/s41598-018-27380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouth J.Oral Moisturizers; The American Dental Association, July, 2007; Vol. 138. [PubMed] [Google Scholar]
- Hatton M. N.; Levine M. J.; Margarone J. E.; Aguirre A. Lubrication and Viscosity Features of Human Saliva and Commercially Available Saliva Substitutes. J. Oral Maxillofac. Surg. 1987, 45, 496–499. 10.1016/s0278-2391(87)80009-5. [DOI] [PubMed] [Google Scholar]
- Chałas R.; Rykwa D.; Łabno P. Artificial Tears and Saliva Substitutes. Curr. Issues Pharm. Med. Sci. 2012, 25, 52–54. 10.12923/j.2084-980x/25.1/a.11. [DOI] [Google Scholar]
- Preetha A.; Banerjee R. Comparison of Artificial Saliva Substitutes. Trends Biomater. Artif. Organs 2005, 18, 178–186. [Google Scholar]
- Vinke J.; Kaper H. J.; Vissink A.; Sharma P. K. Dry Mouth: Saliva Substitutes Which Adsorb and Modify Existing Salivary Condition Films Improve Oral Lubrication. Clin. Oral Invest. 2020, 10.1007/s00784-020-03272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeregowda D. H.; Kolbe A.; van der Mei H. C.; Busscher H. J.; Herrmann A.; Sharma P. K. Recombinant Supercharged Polypeptides Restore and Improve Biolubrication. Adv. Mater. 2013, 25, 3426–3431. 10.1002/adma.201300188. [DOI] [PubMed] [Google Scholar]
- Sharma P. K.; Herrmann A.; Kolbe A.; Veeregowda D. H.; van der Mei H. C.; Busscher H. J.. Biolubricant Polypeptides and Therapeutic Uses Thereof. U.S. Patent 9,334,312 B2, 2016.
- Shiboski C. H.; Shiboski S. C.; Seror R.; Criswell L. A.; Labetoulle M.; Lietman T. M.; Rasmussen A.; Scofield H.; Vitali C.; Bowman S. J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. 10.1002/art.39859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson W. M.; Chalmers J. M.; Spencer A. J.; Williams S. M.; Spencer A. J.; Williams S. M. The Xerostomia Inventory: A Multi-Item Approach to Measuring Dry Mouth The Xerostomia Inventorv: A Multi-Item Approach to Measuring Dry Mouth. Community Dent. Health 1999, 16, 12–17. [PubMed] [Google Scholar]
- van der Putten G.-J.; Brand H. S.; Schols J. M. G. A.; de Baat C. The Diagnostic Suitability of a Xerostomia Questionnaire and the Association between Xerostomia, Hyposalivation and Medication Use in a Group of Nursing Home Residents. Clin. Oral Invest. 2011, 15, 185–192. 10.1007/s00784-010-0382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducker W. A.; Senden T. J.; Pashley R. M. Direct Measurement of Colloidal Forces Using an Atomic Force Microscope. Nature 1991, 353, 239–241. 10.1038/353239a0. [DOI] [Google Scholar]
- Hou J.; Veeregowda D. H.; van de Belt-Gritter B.; Busscher H. J.; van der Mei H. C. Extracellular Polymeric Matrix Production and Relaxation under Fluid Shear and Mechanical Pressure in Staphylococcus Aureus Biofilms. Appl. Environ. Microbiol. 2018, 84, e01516 10.1128/aem.01516-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontturi K. S.; Tammelin T.; Johansson L.-S.; Stenius P. Adsorption of Cationic Starch on Cellulose Studied by QCM-D. Langmuir 2008, 24, 4743–4749. 10.1021/la703604j. [DOI] [PubMed] [Google Scholar]
- Dolatshahi-Pirouz A.; Rechendorff K.; Hovgaard M. B.; Foss M.; Chevallier J.; Besenbacher F. Bovine Serum Albumin Adsorption on Nano-Rough Platinum Surfaces Studied by QCM-D. Colloids Surf., B 2008, 66, 53–59. 10.1016/j.colsurfb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Macakova L.; Yakubov G. E.; Plunkett M. A.; Stokes J. R. Colloids and Surfaces B: Biointerfaces Influence of Ionic Strength Changes on the Structure of Pre-Adsorbed Salivary Films . A Response of a Natural Multi-Component Layer. Colloids Surf., B 2010, 77, 31–39. 10.1016/j.colsurfb.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Macakova L.; Yakubov G. E.; Plunkett M. a.; Stokes J. R. Influence of Ionic Strength on the Tribological Properties of Pre-Adsorbed Salivary Films. Tribol. Int. 2011, 44, 956–962. 10.1016/j.triboint.2010.12.006. [DOI] [Google Scholar]
- Salour F.Moisture influence on structural behaviour of pavements: Field and laboratory investigations. Ph.D. Dissertation, KTH Royal Institute of Technology, 2015. [Google Scholar]
- Urueña J. M.; Pitenis A. A.; Nixon R. M.; Schulze K. D.; Angelini T. E.; Gregory Sawyer W. Mesh Size Control of Polymer Fluctuation Lubrication in Gemini Hydrogels. Biotribology 2015, 1–2, 24–29. 10.1016/j.biotri.2015.03.001. [DOI] [Google Scholar]
- Dedinaite A.; Thormann E.; Olanya G.; Claesson P. M.; Nyström B.; Kjøniksen A.-L.; Zhu K. Friction in Aqueous Media Tuned by Temperature-Responsive Polymer Layers. Soft Matter 2010, 6, 2489–2498. 10.1039/c003320k. [DOI] [Google Scholar]
- Wan H.; Ren K.; Kaper H. J.; Sharma P. K. A bioinspired mucoadhesive restores lubrication of degraded cartilage through reestablishment of lamina splendens. Colloids Surf., B 2020, 193, 110977. 10.1016/j.colsurfb.2020.110977. [DOI] [PubMed] [Google Scholar]
- Chaudhury N. M. A.; Shirlaw P.; Pramanik R.; Carpenter G. H.; Proctor G. B. Changes in Saliva Rheological Properties and Mucin Glycosylation in Dry Mouth. J. Dent. Res. 2015, 94, 1660–1667. 10.1177/0022034515609070. [DOI] [PubMed] [Google Scholar]
- Agha-Hosseini F.; Imanpour M.; Mirzaii-Dizgah I.; Moosavi M.-S. Mucin 5B in Saliva and Serum of Patients with Oral Lichen Planus. Sci. Rep. 2017, 7, 12060. 10.1038/s41598-017-12157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W.; Wang Y.; Wang L.; Bai M.; Xue Q. Influence of Heat Treatment on the Micro/Nano-Tribological Properties of Ultra-Thin Ionic Liquid Films on Silicon. Colloids Surf., A 2010, 361, 118–125. 10.1016/j.colsurfa.2010.03.018. [DOI] [Google Scholar]
- Pu J.; Jiang D.; Mo Y.; Wang L.; Xue Q. Micro/Nano-Tribological Behaviors of Crown-Type Phosphate Ionic Liquid Ultrathin Films on Self-Assembled Monolayer Modified Silicon. Surf. Coatings Technol. 2011, 205, 4855–4863. 10.1016/j.surfcoat.2011.04.089. [DOI] [Google Scholar]
- Song L.; Hou J.; Van Der Mei H. C.; Veeregowda D. H.; Busscher H. J.; Sjollema J. Antimicrobials Influence Bond Stiffness and Detachment of Oral Bacteria. J. Dent. Res. 2016, 95, 793–799. 10.1177/0022034516634631. [DOI] [PubMed] [Google Scholar]
- Adamczak M. I.; Martinsen Ø. G.; Smistad G.; Hiorth M. Polymer Coated Mucoadhesive Liposomes Intended for the Management of Xerostomia. Int. J. Pharm. 2017, 527, 72–78. 10.1016/j.ijpharm.2017.05.032. [DOI] [PubMed] [Google Scholar]
- Wang X.; Du M.; Han H.; Song Y.; Zheng Q. Boundary Lubrication by Associative Mucin. Langmuir 2015, 31, 4733–4740. 10.1021/acs.langmuir.5b00604. [DOI] [PubMed] [Google Scholar]
- Bongaerts J. H. H.; Rossetti D.; Stokes J. R. The Lubricating Properties of Human Whole Saliva. Tribol. Lett. 2007, 27, 277–287. 10.1007/s11249-007-9232-y. [DOI] [Google Scholar]
- Peng C.-C.; Cerretani C.; Braun R. J.; Radke C. J. Evaporation-Driven Instability of the Precorneal Tear Film. Adv. Colloid Interface Sci. 2014, 206, 250–264. 10.1016/j.cis.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Jahn S.; Seror J.; Klein J. Lubrication of Articular Cartilage. Annu. Rev. Biomed. Eng. 2016, 18, 235–258. 10.1146/annurev-bioeng-081514-123305. [DOI] [PubMed] [Google Scholar]
- Al-Hashimi I. Xerostomia Secondary to Sjögren’s Syndrome in the Elderly. Drugs Aging 2005, 22, 887–899. 10.2165/00002512-200522110-00001. [DOI] [PubMed] [Google Scholar]
- Gipson I. K.; Hori Y.; Argüeso P. Character of Ocular Surface Mucins and Their Alteration in Dry Eye Disease. Ocul. Surf. 2004, 2, 131–148. 10.1016/s1542-0124(12)70149-0. [DOI] [PubMed] [Google Scholar]
- Neu C. P.; Reddi A. H.; Komvopoulos K.; Schmid T. M.; Di Cesare P. E. Increased Friction Coefficient and Superficial Zone Protein Expression in Patients with Advanced Osteoarthritis. Arthritis Rheum. 2010, 62, 2680–2687. 10.1002/art.27577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.