Abstract
PURPOSE
NCI-MATCH is a nationwide, histology-agnostic, signal-finding, molecular profile–driven trial for patients with refractory cancers, lymphomas, or myelomas. Patients with tumors harboring actionable aberration(s) in fibroblast growth factor receptor (FGFR) 1-3 were treated with AZD4547, an oral FGFR1-3 inhibitor.
METHODS
Patients’ tumors were screened by next-generation sequencing for predefined FGFR amplification, activating mutations, or fusions. Patients were treated with AZD4547, 80 mg orally twice daily until progression of disease or drug intolerance. A response rate of 16% was considered promising.
RESULTS
Between July 2016 and June 2017, 70 patients were assigned and 48 received protocol therapy and are eligible for analysis. Patients’ tumors harbored FGFR1 or FGFR2 amplification (n = 20), FGFR2 or FGFR3 single-nucleotide variants (n = 19), or FGFR1 or FGFR3 fusions (n = 9). The most common primary tumors were breast (33.3%), urothelial (12.5%), and cervical cancer (10.4%).Grade 3 adverse events were consistent with those described in previous clinical trials. Confirmed partial responses were seen in 8% (90% CI, 3% to 18%) and were observed only in patients whose tumors harbored FGFR1-3 point mutations or fusions. Stable disease was observed in 37.5% (90% CI, 25.8% to 50.4%). The median progression-free survival (PFS) was 3.4 months, and the 6-month PFS rate was 15% (90% CI, 8% to 31%). For patients with tumors harboring FGFR fusions, the response rate was 22% (90% CI, 4.1% to 55%), and 6-month PFS rate was 56% (90% CI, 31% to 100%).
CONCLUSION
Preliminary signals of activity appeared to be limited to cancers harboring FGFR activating mutations and fusions, although AZD4547 did not meet the primary end point. Different FGFR somatic alterations may confer different levels of signaling potency and/or oncogene dependence.
INTRODUCTION
The fibroblast growth factor/fibroblast growth factor receptor (FGF/FGFR) is a receptor tyrosine kinase signaling pathway that regulates several basic biologic processes, including tissue development, angiogenesis, and tissue regeneration.1-3 Genomic aberrations along this pathway in the form of FGFR gene amplification, activating point mutations, and gene fusions have been identified in tumors arising from diverse primary sites,1,4,5 most notably glioblastoma,6 and non–small-cell lung (predominantly squamous),7-9 bladder,10 endometrial,11,12 breast,13 and gastric carcinoma.14 Aberrant signaling through this pathway has been shown to drive tumor growth and proliferation, promote angiogenesis, and mediate resistance to anticancer therapies.2 The oncogenic role of aberrant FGFR signaling and its sensitivity to pharmacologic inhibition in preclinical models8,15,16 have provided a strong rationale to discover and develop FGFR-targeted therapies.
The National Cancer Institute–Molecular Analysis for Therapy Choice (NCI-MATCH) trial is a histology-agnostic, signal-finding, molecular profile–driven trial in patients with refractory cancer. Its primary aim is to establish whether patients with advanced cancer would derive clinical benefit from treatments selected based on their molecular profile regardless of the tissue of origin.
Tyrosine kinase inhibitors (TKIs) are small molecules that directly inhibit receptor kinase activity, most commonly by interfering with the binding of ATP at the tyrosine kinase domain.1,2 Because of the structural similarities of the kinase domains of FGFR, VEGFR, and PDGFR families,2,4 several nonselective TKIs originally developed to inhibit VEGFRs also inhibited FGFR.4 Although simultaneous inhibition of multiple receptor tyrosine kinases may increase treatment efficacy by concomitant inhibition of redundant or bypassing pathways, toxicity constraints and lack of durable and specific bioactivity against FGFRs limited the efficacy of these agents in tumors with aberrant FGFR signaling.2 Newer FGFR tyrosine kinase inhibitors that are more potent and highly selective against FGFR have already shown greater antitumor activity in malignancies with FGFR alterations.17
AZD4547 is a small molecule, orally bioavailable, FGFR1,2,3 inhibitor. Unlike nonselective FGFR tyrosine kinase inhibitors that also target other receptor tyrosine kinases and usually have modest bioactivity against the FGFR family, AZD4547 has high potency against FGFR1-3 with half maximal inhibitory concentrations (IC50s) for the isolated recombinant receptor in the nanomolar range (IC50 = 0.2 nmol/L for FGFR1, 2.5 nmol/L for FGFR2, and 1.8 nmol/L for FGFR3).18 AZD4547 has a much lower potency against FGFR4 (IC50 = 165 nmol/L),18 whose kinase domain is structurally distinct.4,19 The phase II dose of AZD4547 was determined to be 80 mg orally twice a day.20,21 Pharmacodynamic studies have shown that the drug exposures achieved with this dose were consistent with the exposures that induced tumor responses in preclinical models.22 AZD4547 has reached phase II clinical investigations in malignancies most likely to be driven by FGFR genomic aberrations. These malignancies include advanced squamous cell lung cancer,20 gastric cancer,23 bladder cancer, and hormone receptor–positive breast cancer that has progressed through endocrine therapies (ClinicalTrials.gov identifier: NCT01202591).24 Responses have been seen with AZD4547 in patients selected based on their tumors’ genomic profile and tissue of origin.20,22,23 Here we report the results of one of the subprotocols of the NCI-MATCH trial (Subprotocol W).
METHODS
Study Design and Eligibility
The NCI-MATCH trial is an ongoing, nationwide clinical trial with integrated multiple independent single subprotocols, each addressing an actionable molecular alteration. Each subprotocol consists of a single agent or combination for which at least a recommended phase II dose has been determined.
Patients with histologically documented solid tumors, lymphomas, or myelomas whose disease had progressed after at least one line of standard systemic therapy or for whom no standard therapy exists were registered on the screening step of the NCI-MATCH protocol to undergo molecular profiling analysis on fresh tumor biopsy samples or archived tumor tissue. The latter was performed in specific Clinical Laboratory Improvement Amendments–accredited laboratories and consisted of next-generation sequencing (NGS) using an investigational targeted Ampliseq panel.25 The assay was validated to detect ≥ 7× FGFR1-3 amplification, as well as several FGFR 1-3 single-nucleotide variants (SNVs) and targeted fusions. Patients whose tumors were found to harbor actionable FGFR 1-3 aberrations (Appendix Table A1, online only) were offered participation in Subprotocol W (Data Supplement). The actionable FGFR1-3 aberrations were selected by the subprotocol translational principal investigators (H.H.C. and P.H.). Mutations within the valine gatekeeper position of the FGFRs (FGFR1 V561, FGFR2 V564, FGFR3 V550, and FGFR3 V555), which are predicted to confer resistance to reversible FGFR kinase inhibitors, were excluded. The study was initiated after approval from the institutional review board of the participating sites and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from patients.
Patients were excluded from treatment if they had history of disorders of calcium and/or phosphate homeostasis and evidence of extensive tissue calcification. Medications that could elevate the calcium and/or phosphate levels were withheld. All patients underwent a full ophthalmologic evaluation before treatment, and those with current evidence of corneal or retinal disorders were excluded.
Patients were treated with 80 mg orally twice a day continuously. A cycle was defined as 28 days. Dose reductions to 60 mg orally twice a day were allowed for adverse events (AEs; any grade 4 or unmanageable grade 3 toxicity, grade 3 neutropenia for > 7 days or in the presence of mucositis, grade 3 thrombocytopenia in the presence of grade 2 or greater bleeding, hyperphosphatemia > 7.5 mg/dL). Treatment could be withheld up to twice, for up to 3 weeks for each occurrence. Treatment was permanently discontinued if AZD4547 was held for toxicity for the third time or for > 3 weeks.
Because of new availability of clinical trial data in FGFR1-3 amplified squamous cell lung carcinoma,26 gastric cancer, and cancer of the gastroesophageal junction,23 the protocol was amended to exclude these molecular alterations in these histologies, because AZD4547 did not show promising activity in these cohorts.23,27
After the initial accrual of 35 patients, the protocol was expanded to accrue up to 70 patients, until either the response data were available on the first 31 eligible patients or for 6 additional months (whichever came first) and up to a total of 70 patients. In this expansion, we also excluded histologies in which 10 or more patients had already been accrued (eg, breast cancer harboring FGFR1 amplifications).
Measurement of Effect
Response was assessed using revised RECIST criteria version 1.1.28 AEs were reported according to the NCI Common Terminology Criteria for Adverse Events version 4.0.
Statistical Considerations
The original accrual goal was 35 patients, to obtain 31 eligible patients. Because of brisk enrollment, expansion criteria were triggered. In the expansion phase (beyond 35 patients), the number of patients with each tumor type who could be enrolled was capped at 10, and histologies with at least 10 patients already enrolled on the subprotocol in the initial accrual phase were excluded. The primary objective of this NCI-MATCH subprotocol was to evaluate the proportion of patients who had objective response to targeted study agent. The overall response rate was compared against a null benchmark value of 5%. If the observed objective response rate were ≥ 5 of 31 (16%), it would then be concluded that the agent is promising and worthy of further investigations. Allowing for a 10% ineligibility rate, 35 patients were to be accrued to each subprotocol, to obtain 31 eligible and treated patients per subprotocol. With this design, the power is 91.8% to conclude an agent is promising if its true response rate is 0.25; type 1 error rate (one-sided) is 1.8%.
Secondary objectives included the proportion of patients who were progression free at 6 months (PFS6), progression-free survival (PFS), toxicity assessment, and evaluation of potential predictive biomarkers beyond the genomic alteration by which treatment is assigned.
RESULTS
Patient Characteristics and Demographics
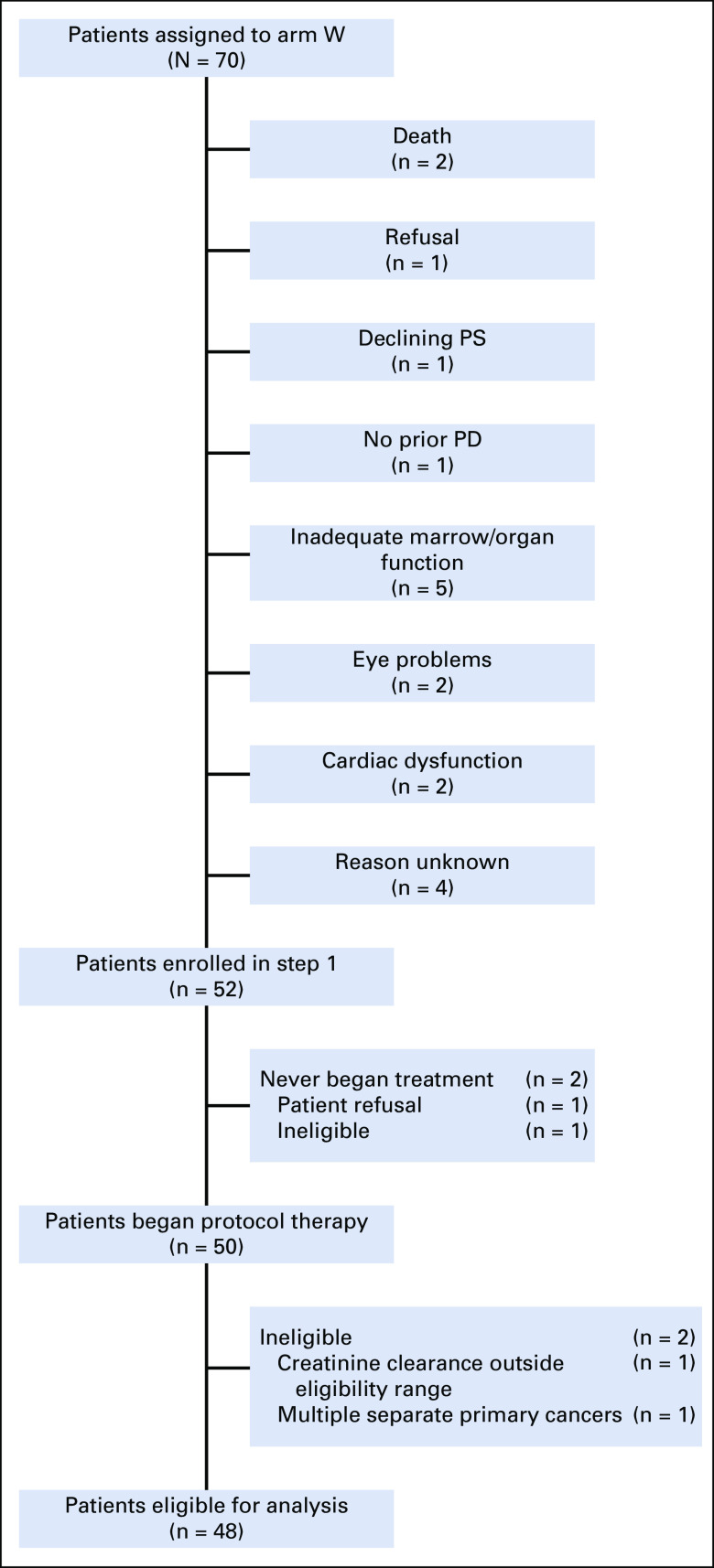
Seventy patients were assigned to Subprotocol W between July 2016 and June 2017 (Fig 1). Fifty-two patients from 47 different sites enrolled in the study, of whom 50 started protocol therapy; two patients who received protocol therapy were ineligible for analysis (pretreatment serum creatinine and creatinine clearance outside of eligibility range, n = 1; multiple separate primary cancers at study entry, n = 1; Fig 1). FGFR amplification (Amp) occurred in 20 patients, SNV in 19 patients (Appendix Fig A1, online only), and fusion in 9 patients.
FIG 1.
Patient flow diagram of NCI-MATCH Subprotocol W. Reasons that patients did not start protocol therapy were patient refusal (n = 1) and screening laboratories and imaging outside pretreatment window (also ineligible on the basis of pretreatment laboratories, n = 1). Reasons that patients were ineligible for analysis were pretreatment serum creatinine and creatinine clearance outside of eligibility range (n = 1) and multiple separate primary cancers at study entry (n = 1). PD, progressive disease; PS, performance status.
Demographics and patient characteristics are shown in Table 1. Most patients were female and white. Patients had received multiple lines of prior therapy (range, 0-14), with half of them having received at least 3 (Appendix Figs A2 and A3, online only).
TABLE 1.
Demographics and Patient Characteristics
The distribution among the histologic types is shown in Figure 2A (Appendix Table A2, online only). The predominant histologic subtype was breast cancer. Other rare malignancies (salivary gland carcinoma, anaplastic thyroid cancer, and prostate cancer with neuroendocrine differentiation) were also represented. Collectively, tumors from 16 distinct primary sites were accrued.
FIG 2.
(A) Pie chart of the primary histologic types (type of malignancy) of the 48 eligible patients. (B) Donut chart of the genomic aberrations of the eligible patients. The inner circle shows the type of genomic aberration (FGFR amplification, single-nucleotide variant, and fusion), and the outer circle shows the specific aberration (genomic aberration). (*) Two fusions present.
Landscape of FGFR1-3 Genomic Aberrations
The type of the genomic aberrations and their respective distribution among the 48 patients eligible for analysis is shown in Figure 2B. The most frequent genomic aberration was FGFR1 amplification, which can be attributed to the enrichment of the patient population in breast cancer, where FGFR1 amplifications frequently underpin therapeutic resistance to antihormonal therapy.13,29,30 The range of abnormal FGFR1 copy number was 7.1-33.9 (median, 9.89). Urothelial carcinomas (n = 6) harbored FGFR3 aberrations exclusively (point mutations and fusions), and endometrioid endometrial carcinomas (n = 3) exclusively harbored FGFR2 mutations.11,12
The landscape of co-occurring mutations is shown in Figure 3A. The most frequent co-occurring mutations were TP53 (48%) and PIK3CA (32%); these mutations did not correlate with histologic type. ESR1 (estrogen receptor α) mutations were seen exclusively in breast cancer, consistent with fact that gain-of-function ESR1 mutations generally occur on progression on antiestrogen and anti-HER2 therapy.30-33
FIG 3.

(A) Co-occurring mutations (left column) by histologic tumor type (lowest panel) and best response (lower panel) and their cumulative frequency (left side of the graph). Germline variants not subtracted. Note the high frequency of TP53 and PIK3CA mutations, which did not show a particular histologic predilection. Urothelial carcinomas harbored exclusively FGFR3 aberrations, whereas endometrial carcinomas most frequently had FGFR2 point mutations. (B) Focus on the co-occurrence of PIK3CA mutations and potential association with response. There was a significant association between progressive disease (PD) as best response to AZD4547 and co-occurrence of PIK3CA mutations (P = .03 by Fisher’s exact test). IHC, immunohistochemistry; NSCLC, non–small-cell lung cancer; PR, partial response; SCL, small-cell lung cancer; SD, stable disease; UE, unevaluable.
AEs
Of 49 patients who received protocol therapy and were evaluable for AEs (no toxicity data, n = 1), 39 experienced any AEs (80%), with 19 (39%) and 1 (2%) having grade 3 or 4 AEs, respectively. AEs with a frequency of at least 10% and/or of grade ≥ 3 severity and potentially associated with the investigational therapy are listed in Table 2. Common grade 1 or 2 AEs were constitutional (fatigue, 39%; anorexia, 27%; weight loss, 14%), GI (xerostomia, 43%; oral mucositis, 24%; nausea, 24%; constipation, 22%; vomiting, 11%; diarrhea, 10%; and altered taste, 10%), cutaneous (alopecia; xeroderma; nail ridging, discoloration or loss; rash; and palmar-plantar erythrodysesthesia), and ophthalmic (xerophthalmia, 22%; blurred vision, 12%). Elevated liver function tests, anemia, leukopenia, and hypercalcemia constituted the most common laboratory abnormalities. Grade 3-4 AEs included oral mucositis (14%), elevated liver function tests (8%), and palmar-plantar erythrodysesthesia (6%). Remarkably, hypophosphatemia (grade 1/2, n = 3 [6%]; grade 3, n = 1 [2%]) instead of hyperphosphatemia, which has been attributed to FGF23 signaling blockade,23,34,35 was seen. Consistent with the selectivity of this drug to FGFRs, toxicities attributable to the blockade of other receptors (most notably VEGFR), such as hypertension, proteinuria, and cardiovascular events,36 were not seen.
TABLE 2.
Adverse Events Potentially Associated With the Investigational Therapy, With a Frequency of ≥ 10% and/or Grade 3 Severity

Eight (16.3%) patients required a dose modification (reduction, delay, or discontinuation) during cycle 1. Twelve (24.5%), patients discontinued treatment because of AEs after a median of 3.5 cycles. The most frequent grade ≥ 3 events at least possibly associated with the investigational therapy among these patients were mucositis (n = 3), palmar-plantar erythrodysesthesia (n = 2), diarrhea (n = 1), dizziness (n = 1), peripheral sensory neuropathy (n = 1), syncope (n = 1), elevated transaminases (n = 2), and elevated alkaline phosphatase (n = 2). Of note, ocular toxicity not meeting criteria for a serious AE was reported in 2 patients who discontinued therapy (undefined, n = 1; retinopathy grade 2, n = 1).
Exploratory Evaluation of Activity and Efficacy
By data cutoff on December 7, 2018 (median follow-up for PFS of 9.2 months), 41 of 48 eligible patients were evaluable for response by RECIST (Table 3). Reasons for nonevaluable response were death before follow-up (n = 4), symptomatic deterioration (n = 1), and inadequate assessment (n = 2).
TABLE 3.
Best Confirmed Response According to RECIST 1.1, Classified by Genomic Aberration and in Total
Confirmed partial responses (PRs) were seen in 4 of 48 (8%; 90% CI, 3% to 18%) patients. Patients who achieved PR harbored the following aberrations: FGFR2 Y376C (n = 1), FGFR3 A393E (n = 1), and FGFR3-TACC3 fusions (n = 2). Eighteen patients (37.5%; 90% CI, 25.8% to 50.4%) achieved stable disease (SD), and 19 patients (39.6%; 90% CI, 27.7% to 52.5%) had progressive disease (PD; Fig 4A; Appendix Fig A4A, online only). In patients who achieved a PR, this was observed by completion of cycle 2, first radiographic evaluation of response, and was sustained for at least 2 cycles (Fig 4B; and Appendix Fig A4B). The median duration of response was 7.9 months. Two patients achieved a > 30% reduction of their target lesions but were coded as PD and SD. The first patient achieved a 36% reduction of her target lesion(s), but a new lesion was reported at the next scan. The second patient achieved a 42% reduction, but a new lesion appeared after cycle 3. The estimated median PFS was 3.4 months. Five patients had PR or SD for > 6 months, resulting in a 6-month PFS rate of 15% (90% CI, 8% to 31%; Fig 4C). All objective responses and PR or SD for > 6 months were seen in patients with FGFR mutations or fusions (Appendix Table A3, online only; Appendix Fig A4C).
FIG 4.

(A) Waterfall plot (color coded by type of genomic aberration) of best confirmed response of target lesion(s) according to RECIST1.1 (n = 36). Seven patients were unevaluable and 5 had a new lesion (classified as progressive disease [PD]) without a measurement of their target lesion(s). Also note that 2 patients achieved a > 30% reduction of their target lesions but were coded as PD and stable disease (SD): the first case achieved a 36% reduction of her target lesion(s), but a new lesion was reported at the next scan, and the second case achieved a 42% reduction, but a new lesion appeared after cycle 3. (B) Swimmer’s plot (color coded by type of genomic aberration) of the 22 patients who achieved SD or better as their best response. The time when partial response (PR) was achieved and when disease progressed are indicated with green and yellow triangles, respectively. (C) Overall progression-free survival (PFS). Kaplan-Meier curve shows PFS for all patients eligible for analysis. (D) Kaplan-Meier curve shows PFS by genomic aberration (median 1.8, 3.6, and 10.0 months for amplification, SNV, and fusion, respectively). (*) New lesions. SNV, single-nucleotide variant.
The 6-month PFS was 0% for patients harboring FGFR amplifications, 6% (90% CI, 1% to 30%) for SNVs, and 56% (90% CI, 31% to 100%) for FGFR fusions (Fig 4D). Two of the 4 patients who achieved a PR had a PFS > 9.5 months; their primary tumors were intrahepatic cholangiocarcinoma and urothelial carcinoma of the renal pelvis. Three of the 9 patients with FGFR fusions achieved a PFS > 10 months. Their primary tumors were urothelial carcinoma of the renal pelvis, diffuse astrocytoma WHO grade 2, and epithelial-myoepithelial carcinoma of the salivary gland. The median overall survival was 7.2 months (Appendix Fig A4D).
To identify potential mechanisms that may have contributed to the low overall response rate (ORR), we performed secondary pathway analysis. After TP53 mutations, which were frequent but untargetable, the most frequent co-occurring mutations were found in PIK3CA. There was a significant association between PD as best response to AZD4547 and co-occurrence of PIK3CA mutations (P = .03, Fisher’s exact test; Fig 3B).
DISCUSSION
In this study, AZD4547 did not meet the 16% ORR threshold across all FGFR 1-3 alterations, which constituted the primary end point of the study, but showed modest activity in patients with FGFR mutations and fusions. Dose reductions and discontinuations due to AEs cannot account for this result. It is becoming increasingly recognized that the oncogenic driver role of FGFR aberrations is complex.38 Careful patient selection with well-defined FGFR aberrations underpins the most successful results with FGFR inhibitors, even in trials in urothelial carcinoma and cholangiocarcinoma (ie, histologies where FGFR inhibitors have shown the greatest efficacy).17,37-41
In our study, the predominant genomic aberration was FGFR1 amplification, where responses with selective FGFR inhibitors have been infrequent.20,22,26,38-40,42 This absence of responses may rely on the fact that only a very high FGFR1 amplification is associated with FGFR1 dependence.43 The patients with amplification had a median of 9.89 copies. This level of amplification may have been too low to select for these patients. In addition, the predominant histologic subtype with FGFR1 amplification was breast cancer, where the FGFR1 amplicon is usually broad and encompasses other genes that can potentially contribute to tumor progression, most notably ZNF703.44-46 Neither erdafitinib38 nor BGJ39839,40 induced responses in FGFR-amplified tumors, with the possible exception of BGJ398 in FGFR1-amplified squamous cell carcinoma.40 Given the poor correlation between FGFR1 amplification and expression,20 BGJ398’s efficacy in squamous cell lung carcinoma remains to be confirmed in further studies.
Higher response rates have been reported in tumors harboring FGFR2 amplifications; however, in a phase II clinical trial in FGFR2-amplified gastric adenocarcinoma, AZD4547 was not superior to paclitaxel.23 This negative result has been attributed to the heterogeneity of FGFR2 amplification, which contrasts with the homogeneous amplification of the preclinical models.23 Akin to trastuzumab, which is effective mainly or only in combination with chemotherapy, the possibility exists that higher response rates may be achieved in combinatorial trials of AZD4547 and chemotherapy. AZD4547 at pharmacokinetically meaningful doses was well tolerated in combination with cisplatin plus gemcitabine47 and cisplatin plus capecitabine.48
The second most frequent genomic aberrations in this study were activating mutations of the FGFR2 and FGFR3. Most FGFR1-3 mutations occur in the extracellular, linker, and transmembrane domains and lead to enhanced ligand affinity or constitutive receptor dimerization and ligand-independent signaling.4 Mutations in the kinase domain that constitutively activate the receptor and transform cell lines are rare. The 10.5% response rate with AZD4547 in patients with point mutations suggests that, despite the evidence for the transformative capacity of the FGFR mutations in cell lines, other factors may be involved in rendering these tumors addicted to FGFR signaling and consequently sensitive to FGFR inhibition. Also, broad inclusion of less well-defined FGFR abnormalities may in part explain the low response rate.37
Tumors with FGFR fusions seem to have a high response rate to FGFR inhibition.40 Our data support this observation: 2 of the 4 patients who achieved PR and 3 of the 5 patients with PFS > 6 months harbored FGFR fusions. Although exploratory, these patients achieved the highest 6-month PFS rate as well, compared with their counterparts harboring amplifications and SNVs. However, patients with FGFR fusions constituted a minority in our study. This result can provide a potential lead for patient selection in future clinical trials.
The high prevalence of co-occurring mutations along the PI3K/AKT/mTOR pathway, a signal transduction pathway downstream of FGFR, among nonresponders is a biologically relevant finding. FGFR1 signal transduction relies on MAPK, and blockade of FGFR1 signaling results in inhibition of MAPK but not PI3K.34 Thus, FGFR blockade would not interrupt oncogenic signaling driven by somatic alterations in the PI3K/AKT/mTOR pathway. The presence of such comutations may typify a patient population primarily resistant to FGFR inhibition and, if confirmed, inform patient selection in future trials. A high degree of synergism between PI3K and FGFR inhibitors has been reported in preclinical models.49 However, the translation of this finding into the clinic may be challenging because of overlapping toxicities of the available agents. As the results of other subprotocols of the NCI-MATCH as well as other basket trials become available, it will be interesting to investigate whether mutations in the PI3K/AKT/mTOR and/or other intracellular signaling pathways underpin short-lived or the absence of responses to therapies targeting upstream tyrosine kinase receptors.
ACKNOWLEDGMENT
Investigational drug and limited funding for safety assessments was kindly provided by AstraZeneca. This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O’Dwyer, MD, and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health.
Appendix
FIG A1.

Lollipop plots. (A, B, D) Lollipop plots of the eligible mutations in (A) FGFR1, (B) FGFR2, and (D) FGFR3. Height on the y-axis is proportionate to the number of eligible base substitutions. (C, E) Lollipop plots of the mutations in (C) FGFR2 and (E) FGFR3 in the study population. Height on the y-axis is proportionate to number of patients harboring the respective mutation. Activating mutations of the FGFR1 are rare in cancer and were not seen in our patient population.
FIG A2.

Type and duration of medical therapies according to tumor type received before enrollment to NCI-MATCH Subprotocol W, as reported by the participating sites. Not shown are one patient with breast cancer who received 14 prior lines of therapy for whom the type and duration of treatments were not recorded, and one patient with salivary gland carcinoma who underwent prior surgery and radiation therapy but had not received any prior medical therapy. Day 0 is the day that the first medical therapy was started. The figure does not include and prior surgery and radiation therapy.
FIG A3.

Lines of therapy, including radiation therapy and surgery, as reported by the participating sites. One patient with cervical cancer harboring an FGFR3-TACC3.F17T8 gene fusion underwent prior surgery and radiation therapy and received 2 months of cisplatin approximately 8.5 years before enrollment.
FIG A4.

(A) Waterfall plot (color coded by tissue of origin) of best confirmed response of target lesion(s) according to RECIST (n = 36). Seven patients were unevaluable and 5 had a new lesion (classified as progressive disease [PD]) without a measurement of their target lesion(s). Also note that 2 patients achieved a > 30% reduction of their target lesions but coded as PD and stable disease (SD): the first case achieved a 36% reduction of her target lesion(s), but a new lesion was reported at the next scan; the second case achieved a 42% reduction, but a new lesion appeared after cycle 3. (B) Swimmer’s plot (color coded by tissue of origin) of the 22 patients who achieved SD or better as their best response. The time when partial response (PR) was achieved and when disease progressed are indicated with green and yellow triangles, respectively. (C) Bar plot of best confirmed response according to RECIST by specific genomic aberration. x-axis, number of patients. (D) Kaplan-Meier curve for the overall survival of the 48 patients eligible for analysis. (*) New lesions. NSCLC, non–small-cell lung cancer; SCL, small-cell lung cancer.
TABLE A1.
List of Actionable FGFR1-3 Aberrations for Subprotocol W (FGFR genomic aberrations) of the MATCH Trial
TABLE A2.
List of Primary Sites, Overall and by Genomic Aberration (n = 48)
TABLE A3.
Clinical Characteristics and Genomic Aberrations of the Patients Who Achieved PR (n = 4) and SD for > 6 months (n = 3)
PRIOR PRESENTATION
Presented at the ASCO Annual Meeting, Chicago, IL, June 1-5, 2018
SUPPORT
CLINICAL TRIAL INFORMATION
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
AUTHOR CONTRIBUTIONS
Conception and design: Young K. Chae, Peter Hammerman, Edith P. Mitchell, James A. Zwiebel, Robert J. Gray, Shuli Li, Lisa M. McShane, Larry V. Rubinstein, David Patton, P. Mickey Williams, Stanley R. Hamilton, Barbara A. Conley, Carlos L. Arteaga, Peter J. O’Dwyer, Alice P. Chen, Keith T. Flaherty
Financial support: Stanley R. Hamilton, Lyndsay N. Harris
Administrative support: S. Percy Ivy, P. Mickey Williams, Stanley R. Hamilton, Barbara A. Conley, Lyndsay N. Harris, Peter J. O’Dwyer, Alice P. Chen
Provision of study material or patients: Edith P. Mitchell, S. Percy Ivy, Stanley R. Hamilton, Alice P. Chen
Collection and assembly of data: Young K. Chae, Fangxin Hong, Christos Vaklavas, Heather H. Cheng, Edith P. Mitchell, S. Percy Ivy, Robert J. Gray, David Patton, P. Mickey Williams, Stanley R. Hamilton, Aaron Mansfield
Data analysis and interpretation: Young K. Chae, Fangxin Hong, Christos Vaklavas, Heather H. Cheng, Edith P. Mitchell, James A. Zwiebel, Robert J. Gray, Lisa M. McShane, Larry V. Rubinstein, David Patton, P. Mickey Williams, Stanley R. Hamilton, Aaron Mansfield, Lyndsay N. Harris, Alice P. Chen, Keith T. Flaherty
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Study of AZD4547 in Patients With Tumors Harboring Aberrations in the FGFR Pathway: Results from the NCI-MATCH Trial (EAY131) Subprotocol W
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Young K. Chae
Consulting or Advisory Role: Foundation Medicine, Boehringer Ingelheim, Biodesix, Counsyl, AstraZeneca, Guardant Health, Takeda, Roche/Genentech, ImmuneOncia, Hanmi, Lilly, Tempus
Speakers' Bureau: Genentech/Roche, Merck, AstraZeneca, Lilly
Research Funding: AbbVie, Bristol-Myers Squibb, Lexent Bio, Freenome, Biodesix
Travel, Accommodations, Expenses: Hanmi
Fangxin Hong
Consulting or Advisory Role: Merck Sharp & Dohme
Christos Vaklavas
Consulting or Advisory Role: Daiichi Sankyo
Research Funding: Genentech (Inst), Roche (Inst), Pfizer (Inst), Incyte (Inst), Pharmacyclics (Inst), Novartis (Inst), TRACON Pharmaceuticals (Inst), Innocrin Pharmaceuticals (Inst), Zymeworks (Inst), H3 Biomedicine (Inst)
Other Relationship: Puma Biotechnology, Takeda, Daiichi Sankyo
Uncompensated Relationships: Genentech
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1306968
Heather H. Cheng
Research Funding: Inovio Pharmaceuticals (Inst), Sanofi (Inst), Astellas Medivation (Inst), Janssen (Inst), Clovis Oncology (Inst), Color Foundation (Inst)
Peter Hammerman
Employment: Novartis, Pfizer (I)
Stock and Other Ownership Interests: Novartis, Pfizer (I)
Edith P. Mitchell
Honoraria: Sanofi
Consulting or Advisory Role: Genentech, Novartis, Merck, Bristol Myers Squib
Speakers' Bureau: Ipsen
Research Funding: Genentech (Inst), Sanofi (Inst)
James A. Zwiebel
Consulting or Advisory Role: Boston Pharmaceuticals, Scandion Oncology
Robert J. Gray
Research Funding: Agios, Amgen, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Genentech/Roche, Genomic Health, Genzyme, GlaxoSmithKline, Janssen-Ortho, Onyx, Pfizer, Sequenta, Syndax, Novartis, Takeda, AbbVie, Sanofi, Merck Sharp & Dohme
P. Mickey Williams
Research Funding: Illumina (Inst)
Patents, Royalties, Other Intellectual Property: I was a co-inventor of the DLBCL cell of origin patent recently filed by the NIH
Stanley R. Hamilton
Stock and Other Ownership Interests: The Johns Hopkins University School of Medicine
Consulting or Advisory Role: HalioDx, Thermo Fisher Scientific, Bristol-Myers Squibb, Loxo, Merck, Guardant Health, Cell Medica Limited
Aaron Mansfield
Consulting or Advisory Role: Genentech (Inst), Bristol-Myers Squibb (Inst), AbbVie (Inst), Astra Zeneca (Inst)
Research Funding: Novartis (Inst), BMS (Inst)
Travel, Accommodations, Expenses: AbbVie, Roche
Carlos L. Arteaga
Leadership: American Association for Cancer Research
Stock and Other Ownership Interests: Provista Diagnostics, Y-Trap
Consulting or Advisory Role: Novartis, Lilly, Sanofi, Radius Health, Taiho Pharmaceutical, Puma Biotechnology, Merck, H3 Biomedicine, Symphogen, Origimed, Petra Pharma, Third Rock Ventures, Immunomedics, Daiichi Sankyo, Athenex, G1 Therapeutics, Clovis Oncology
Research Funding: Puma Biotechnology, Pfizer, Lilly, Radius Health, Takeda, Bayer
Other Relationship: Susan G. Komen for the Cure
Lyndsay N. Harris
Patents, Royalties, Other Intellectual Property: Philips Healthcare
Peter J. O’Dwyer
Consulting or Advisory Role: Genentech, Array
Research Funding: Bristol-Myers Squibb, Pfizer, Novartis, Genentech, Mirati Therapeutics, Celgene, GlaxoSmithKline, BBI Healthcare, Merck, Pharmacyclics, Bayer, Five Prime Therapeutics, Forty Seven, Amgen, H3 Biomedicine (Inst), Taiho (Inst), Array BioPharma (Inst), Lilly/ImClone (Inst)
Expert Testimony: Bayer, Lilly
Keith T. Flaherty
Stock and Other Ownership Interests: Clovis Oncology, Loxo, X4 Pharma, Strata Oncology, PIC Therapeutics, Fount Therapeutics, Shattuck Labs, Apricity Health, Oncoceutics, Fog Pharma, Tvardi Therapeutics, Checkmate Pharmaceuticals, Kinnate
Consulting or Advisory Role: Novartis, Genentech, Merck, Lilly, Amgen, Sanofi, Oncoceutics, Bristol-Myers Squibb, Adaptimmune, Aeglea Biotherapeutics, Loxo, Roche, Asana Biosciences, Incyte, Shattuck Labs, Tolero Pharmaceuticals, Array BioPharma, FOG Pharma, Neon Therapeutics, Tvardi, Takeda, Verastem, Boston Biomedical, Pierre Fabre, Cell Medica, Debiopharm Group
Research Funding: Novartis, Sanofi
Travel, Accommodations, Expenses: Pierre Fabre, Debiopharm Group
No other potential conflicts of interest were reported.
REFERENCES
- 1.Chae YK, Ranganath K, Hammerman PS, et al. Inhibition of the fibroblast growth factor receptor (FGFR) pathway: The current landscape and barriers to clinical application. Oncotarget. 2017;8:16052–16074. doi: 10.18632/oncotarget.14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Touat M, Ileana E, Postel-Vinay S, et al. Targeting FGFR signaling in cancer. Clin Cancer Res. 2015;21:2684–2694. doi: 10.1158/1078-0432.CCR-14-2329. [DOI] [PubMed] [Google Scholar]
- 3.Itoh N, Ornitz DM. Fibroblast growth factors: From molecular evolution to roles in development, metabolism and disease. J Biochem. 2011;149:121–130. doi: 10.1093/jb/mvq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer. 2017;17:318–332. doi: 10.1038/nrc.2017.8. [DOI] [PubMed] [Google Scholar]
- 5.Wu YM, Su F, Kalyana-Sundaram S, et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 2013;3:636–647. doi: 10.1158/2159-8290.CD-13-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh D, Chan JM, Zoppoli P, et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science. 2012;337:1231–1235. doi: 10.1126/science.1220834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R, Wang L, Li Y, et al. FGFR1/3 tyrosine kinase fusions define a unique molecular subtype of non-small cell lung cancer. Clin Cancer Res. 2014;20:4107–4114. doi: 10.1158/1078-0432.CCR-14-0284. [DOI] [PubMed] [Google Scholar]
- 8.Weiss J, Sos ML, Seidel D, et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med. 2010;2:62ra93. doi: 10.1126/scitranslmed.3001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monaco SE, Rodriguez EF, Mahaffey AL, et al. FGFR1 amplification in squamous cell carcinoma of the lung with correlation of primary and metastatic tumor status. Am J Clin Pathol. 2016;145:55–61. doi: 10.1093/ajcp/aqv013. [DOI] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Research Network Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutt A, Salvesen HB, Chen TH, et al. Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc Natl Acad Sci USA. 2008;105:8713–8717. doi: 10.1073/pnas.0803379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konecny GE, Finkler N, Garcia AA, et al. Second-line dovitinib (TKI258) in patients with FGFR2-mutated or FGFR2-non-mutated advanced or metastatic endometrial cancer: A non-randomised, open-label, two-group, two-stage, phase 2 study. Lancet Oncol. 2015;16:686–694. doi: 10.1016/S1470-2045(15)70159-2. [DOI] [PubMed] [Google Scholar]
- 13.Reis-Filho JS, Simpson PT, Turner NC, et al. FGFR1 emerges as a potential therapeutic target for lobular breast carcinomas. Clin Cancer Res. 2006;12:6652–6662. doi: 10.1158/1078-0432.CCR-06-1164. [DOI] [PubMed] [Google Scholar]
- 14.Deng N, Goh LK, Wang H, et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012;61:673–684. doi: 10.1136/gutjnl-2011-301839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Zhang L, Su X, et al. Translating the therapeutic potential of AZD4547 in FGFR1-amplified non-small cell lung cancer through the use of patient-derived tumor xenograft models. Clin Cancer Res. 2012;18:6658–6667. doi: 10.1158/1078-0432.CCR-12-2694. [DOI] [PubMed] [Google Scholar]
- 16.Xie L, Su X, Zhang L, et al. FGFR2 gene amplification in gastric cancer predicts sensitivity to the selective FGFR inhibitor AZD4547. Clin Cancer Res. 2013;19:2572–2583. doi: 10.1158/1078-0432.CCR-12-3898. [DOI] [PubMed] [Google Scholar]
- 17.Loriot Y, Necchi A, Park SH, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381:338–348. doi: 10.1056/NEJMoa1817323. [DOI] [PubMed] [Google Scholar]
- 18.Gavine PR, Mooney L, Kilgour E, et al. AZD4547: An orally bioavailable, potent, and selective inhibitor of the fibroblast growth factor receptor tyrosine kinase family. Cancer Res. 2012;72:2045–2056. doi: 10.1158/0008-5472.CAN-11-3034. [DOI] [PubMed] [Google Scholar]
- 19.Lesca E, Lammens A, Huber R, et al. Structural analysis of the human fibroblast growth factor receptor 4 kinase. J Mol Biol. 2014;426:3744–3756. doi: 10.1016/j.jmb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Paik PK, Shen R, Berger MF, et al. A Phase Ib open-label multicenter study of AZD4547 in patients with advanced squamous cell lung cancers. Clin Cancer Res. 2017;23:5366–5373. doi: 10.1158/1078-0432.CCR-17-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saka H, Kitagawa C, Kogure Y, et al. Safety, tolerability and pharmacokinetics of the fibroblast growth factor receptor inhibitor AZD4547 in Japanese patients with advanced solid tumours: A phase I study. Invest New Drugs. 2017;35:451–462. doi: 10.1007/s10637-016-0416-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andre F, Ranson M, Dean E, et al: Results of a phase I study of AZD4547, an inhibitor of fibroblast growth factor receptor (FGFR), in patients with advanced solid tumors. Cancer Res 73, 2013 (8 suppl; abstr LB-145)
- 23.Van Cutsem E, Bang YJ, Mansoor W, et al. A randomized, open-label study of the efficacy and safety of AZD4547 monotherapy versus paclitaxel for the treatment of advanced gastric adenocarcinoma with FGFR2 polysomy or gene amplification. Ann Oncol. 2017;28:1316–1324. doi: 10.1093/annonc/mdx107. [DOI] [PubMed] [Google Scholar]
- 24. Seckl M, Badman PD, Liu X, et al: RADICAL trial: A phase Ib/IIa study to assess the safety and efficacy of AZD4547 in combination with either anastrozole or letrozole in ER positive breast cancer patients progressing on these aromatase inhibitors (AIs). J Clin Oncol 35, 2017 (15_suppl; abstr 1059) [Google Scholar]
- 25.Lih CJ, Harrington RD, Sims DJ, et al. Analytical validation of the next-generation sequencing assay for a nationwide signal-finding clinical trial: Molecular analysis for therapy choice clinical trial. J Mol Diagn. 2017;19:313–327. doi: 10.1016/j.jmoldx.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aggarwal C, Redman MW, Lara PN, Jr, et al. SWOG S1400D ( NCT02965378), a phase II study of the fibroblast growth factor receptor inhibitor AZD4547 in previously treated patients with fibroblast growth factor pathway-activated stage IV squamous cell lung cancer (Lung-MAP substudy) J Thorac Oncol. 2019;14:1847–1852. doi: 10.1016/j.jtho.2019.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aggarwal C, Redman MW, Lara P, et al: Phase II study of the FGFR inhibitor AZD4547 in previously treated patients with FGF pathway-activated stage IV squamous cell lung cancer (SqNSCLC): LUNG-MAP sub-study SWOG S1400D. J Clin Oncol 35, 2017 (15_suppl; abstr 9055) [Google Scholar]
- 28.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Formisano L, Stauffer KM, Young CD, et al. Association of FGFR1 with ERα maintains ligand-independent ER transcription and mediates resistance to estrogen deprivation in ER+ breast cancer. Clin Cancer Res. 2017;23:6138–6150. doi: 10.1158/1078-0432.CCR-17-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanker AB, Garrett JT, Estrada MV, et al. HER2-overexpressing breast cancers amplify FGFR signaling upon acquisition of resistance to dual therapeutic blockade of HER2. Clin Cancer Res. 2017;23:4323–4334. doi: 10.1158/1078-0432.CCR-16-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeselsohn R, Buchwalter G, De Angelis C, et al. ESR1 mutations—a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol. 2015;12:573–583. doi: 10.1038/nrclinonc.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson DR, Wu YM, Vats P, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45:1446–1451. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toy W, Shen Y, Won H, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45:1439–1445. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner N, Grose R. Fibroblast growth factor signalling: From development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 35.Wöhrle S, Bonny O, Beluch N, et al. FGF receptors control vitamin D and phosphate homeostasis by mediating renal FGF-23 signaling and regulating FGF-23 expression in bone. J Bone Miner Res. 2011;26:2486–2497. doi: 10.1002/jbmr.478. [DOI] [PubMed] [Google Scholar]
- 36.Dieci MV, Arnedos M, Andre F, et al. Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer Discov. 2013;3:264–279. doi: 10.1158/2159-8290.CD-12-0362. [DOI] [PubMed] [Google Scholar]
- 37.Facchinetti F, Hollebecque A, Bahleda R, et al. Facts and new hopes on selective FGFR inhibitors in solid tumors. Clin Cancer Res. 2020;26:764–774. doi: 10.1158/1078-0432.CCR-19-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bahleda R, Italiano A, Hierro C, et al. Multicenter phase I study of erdafitinib (JNJ-42756493), oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced or refractory solid tumors. Clin Cancer Res. 2019;25:4888–4897. doi: 10.1158/1078-0432.CCR-18-3334. [DOI] [PubMed] [Google Scholar]
- 39.Javle M, Lowery M, Shroff RT, et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol. 2018;36:276–282. doi: 10.1200/JCO.2017.75.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nogova L, Sequist LV, Perez Garcia JM, et al. Evaluation of BGJ398, a fibroblast growth factor receptor 1-3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: Results of a global phase I, dose-escalation and dose-expansion study. J Clin Oncol. 2017;35:157–165. doi: 10.1200/JCO.2016.67.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pal SK, Rosenberg JE, Hoffman-Censits JH, et al. Efficacy of BGJ398, a fibroblast growth factor receptor 1-3 inhibitor, in patients with previously treated advanced urothelial carcinoma with FGFR3 alterations. Cancer Discov. 2018;8:812–821. doi: 10.1158/2159-8290.CD-18-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smyth EC, Turner NC, Peckitt C, et al: Phase II multicenter proof of concept study of AZD4547 in FGFR amplified tumours. J Clin Oncol 33, 2015 (15_suppl; abstr 2508) [Google Scholar]
- 43.Pearson A, Smyth E, Babina IS, et al. High-level clonal FGFR amplification and response to FGFR inhibition in a translational clinical trial. Cancer Discov. 2016;6:838–851. doi: 10.1158/2159-8290.CD-15-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reynisdottir I, Arason A, Einarsdottir BO, et al. High expression of ZNF703 independent of amplification indicates worse prognosis in patients with luminal B breast cancer. Cancer Med. 2013;2:437–446. doi: 10.1002/cam4.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holland DG, Burleigh A, Git A, et al. ZNF703 is a common luminal B breast cancer oncogene that differentially regulates luminal and basal progenitors in human mammary epithelium. EMBO Mol Med. 2011;3:167–180. doi: 10.1002/emmm.201100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones BH, Anthoney DA, Jones RJ, et al. FIESTA: A phase Ib and pharmacokinetic trial of AZD4547 in combination with gemcitabine and cisplatin. J Clin Oncol. 2016;34 (15_suppl; abstr 4521) [Google Scholar]
- 48. Lindsay C, Thistlethwaite F, Gupta A, et al: FGFR inhibitor and chemotherapy in gastric cancer (FACING): Phase I results from an ECMC combinations alliance phase I/II trial of AZD4547 in combination with cisplatin and capecitabine (CX). Ann Oncol 25:iv155, 2014. [Google Scholar]
- 49.Packer LM, Geng X, Bonazzi VF, et al. PI3K inhibitors synergize with FGFR inhibitors to enhance antitumor responses in FGFR2mutant endometrial cancers. Mol Cancer Ther. 2017;16:637–648. doi: 10.1158/1535-7163.MCT-16-0415. [DOI] [PubMed] [Google Scholar]