Abstract
Introduction
From previous studies, we hypothesized that olfactory exposure to β-caryophyllene stimulates women's libido. However, Japan's sex culture is so closed that it is difficult to test this possibility without accumulating scientific evidence. Therefore, it is necessary to measure the concentration of sex-related hormones in saliva, an experimental technique that is relatively easy to obtain research permission, and to obtain a scientific basis to convince ethics committee reviewers.
Aim
The aim of this study is to investigate whether β-caryophyllene increases salivary testosterone concentrations associated with libido and vaginal sensation during intercourse in women.
Methods
19 women in the follicular phase of the menstrual cycle participated in the study. The subjects then sat in front of the odor exposure device we had created. Each subject was exposed to dipropylene glycol for 20 minutes, followed by 3% β-caryophyllene for 20 minutes. Saliva was collected 4 times: before and after control exposure, and before and after β-caryophyllene exposure.
Main Outcome Measure
Salivary testosterone and estrogen concentrations were measured with a competition ELISA.
Results
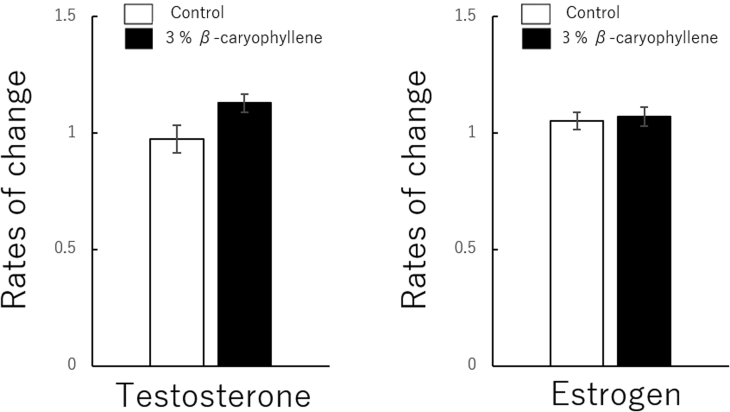
β-caryophyllene significantly increased the salivary concentration of testosterone (control vs β-caryophyllene; 0.97 ± 0.05 vs 1.13 ± 0.03, P = .00, 95% confidence interval of control: 0.84–1.09, 95% confidence interval of β-caryophyllene: 1.04–1.20) but not estrogen (control vs β-caryophyllene; 1.05 ± 0.03 vs 1.07 ± 0.04, P = .69, 95% confidence interval of control: 0.96–1.12, 95% confidence interval of β-caryophyllene: 0.98–1.15).
Strengths & Limitations
The personal preferences of the subjects and the order of exposure may have affected the results.
Conclusion
β-caryophyllene may be a remedy with fewer side effects for women with decreased libido. We believe that β-caryophyllene may be a remedy for women with decreased libido. However, this hypothesis must be tested by further clinical studies.
Wataru Tarumi, Kazuyuki Shinohara. Olfactory Exposure to β-Caryophyllene Increases Testosterone Levels in Women's Saliva. J Sex Med 2020;8:525–531.
Key Words: β-caryophyllene, Ylang-ylang, Testosterone, Sexuality, Pheromone, Aphrodisiac
Introduction
Cananga odorata, generally called ylang-ylang, is a fast-growing tree that inhabits Asian countries such as the Philippines, Malaysia, and Indonesia.1 This tree, well known for the pleasant fragrance of its flowers, was introduced into African and American countries, China, India, and Japan. The essential oil extracted from ylang-ylang flowers is used widely, not only in the food industry but also in the perfume industry and aromatherapy. Its safety in humans was approved by the Flavor and Extract Manufacturers Association of the United States.1 In Indonesia, ylang-ylang flowers are traditionally placed on the bed of a newly married couple because ylang-ylang is believed to alleviate sexual anxiety and to be associated with euphoria and sexual behavior.1
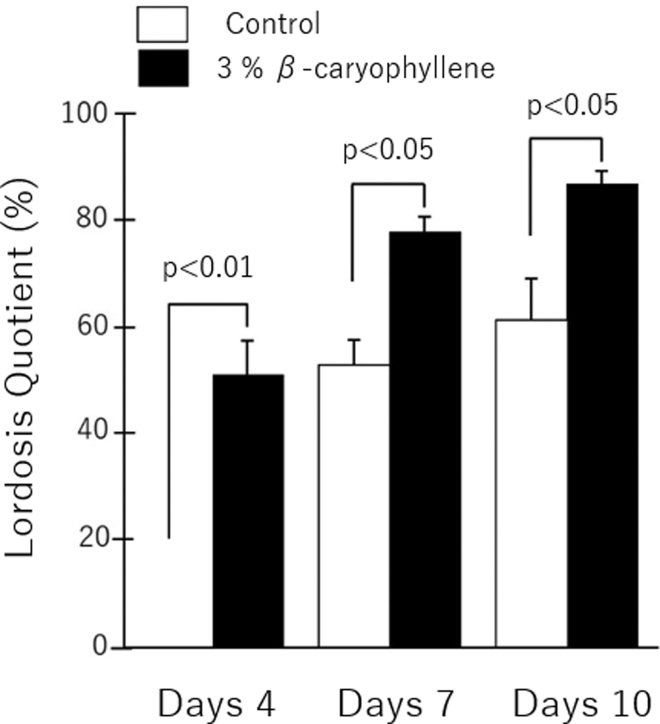
β-Caryophyllene, a major volatile constituent of ylang-ylang, accounts for 15.05–33.30% of ylang-ylang,2 and may confer the anxiolytic potency long attributed to ylang-ylang in Indonesia, may be derived from this compound.3 Our previous study showed that exposure of female rodents to β-caryophyllene increased the lordosis quotient (Figure 1).4 Taking previous studies into consideration, we hypothesized that the β-caryophyllene in ylang-ylang induces sexual behavior in humans also. To reinforce the hypothesis, we examined changes in salivary testosterone and estrogen concentrations in women exposed to β-caryophyllene. Salivary hormone concentrations reflect blood concentrations, and their measurement is often used for easy detection of physiological phenomena.5, 6, 7, 8
Figure 1.
β-Caryophyllene induces sexual behavior (lordosis) in female rodents. The lordosis quotient is calculated as the rate of lordosis in female rodents ÷ the number of mountings by male rodents × 100.
This figure is adapted from our previous review paper submitted to the Japanese Journal of Taste and Smell Research (in Japanese).
Testosterone concentration gradually decreases with aging in women. Estrogen and progesterone concentrations rapidly decrease just after menopause, around 50 years of age.9, 10, 11 Postmenopausal women are likely to suffer from impaired genital congestion and vaginal lubrication during sexual arousal, resulting in vaginal atrophy and dyspareunia.12, 13, 14 Estrogen supplementation can alleviate these symptoms.15, 16, 17, 18, 19 However, estrogen itself does not increase libido and frequency of coitus; testosterone, instead, plays an important role in libido and vaginal sensation during intercourse.20, 21, 22 Therefore, we thought that if olfactory exposure of women to β-caryophyllene increased salivary testosterone concentration, this would indirectly support the hypothesis that β-caryophyllene can induce female sexual behaviors.
Readers may wonder why we did not directly assess the frequency of female sexual behavior. The reason is that human sexual behavior is one of the least studied fields in biological, psychological, and sociological research: its negative image makes researchers hesitant to study it, thus preventing the development of this field.23 Being affected by social environment, human sexual behavior and awareness reflect regional sexual culture. Large-scale changes in cultural and ethical backgrounds in recent years have increased openness, creating opportunities to study human sexual behavior if enough evidence can be gathered.24, 25, 26, 27, 28 In this context, before planning a clinical study of the association between β-caryophyllene and female sexual behavior, we designed this study to obtain scientific evidence sufficient for conducting the clinical study.
Materials and methods
Subjects
19 women (mean = 23.47 years, SD = 3.76) in the follicular phase of the menstrual cycle (within 5 to 10 days of the onset of menstruation) participated in the study. We determined this number from the results of our previous study, in which the scent of jasmine absolute increased the salivary testosterone concentration the most among essential oils (control vs jasmine absolute; 1.00 ± 0.22 vs 1.15 ± 0.13; data are means ± standard deviations. P < .05). In the R Commander interface for R statistical software, we entered the difference in the mean values between the 2 groups (0.15), the standard deviation common to the 2 groups (0.22), the α error (0.05), and the power (0.80) to determine the subject number as 19. No subjects were taking any regular medication or suffering from any mental disorders or gynecological disease. None of the women were smokers or taking the pill, and their BMI was between 20–24. Also, no one was attending the hospital for diabetes, high blood pressure, or sexual dysfunction. We asked the subjects to self-report the beginning of menstruation and to use an ovulation detection kit to confirm that they had a normal menstrual cycle (28–35 days). After explaining the objectives of the experiment to each subject, we obtained written informed consent from all subjects. The study was performed according to the Helsinki Declaration. This study was approved by the Ethics Committee of the Nagasaki University School of Medicine (approval number 13121970).
Chemicals and Apparatus for Odorant Exposure
We used an apparatus for odorant exposure that had been used in a previous study.7 It consists of an air pump, a flow meter, a glass bottle, a silicone tube, and a glass funnel. The bottle was filled with 15 mL of 3% β-caryophyllene (Sigma Aldrich, MO) or solvent (dipropylene glycol) as a control. The exposure concentration of 3% was selected because of its lack of toxicity and skin irritation.29 The flow rate was set at 2.0 L/min with a flow meter. Air was sent to the bottle, and then airflow from the bottle was delivered through the silicone tube close to the subjects’ nostrils. The glass funnel was used to reduce the airflow stress that subjects might experience during the experiment.
Procedure
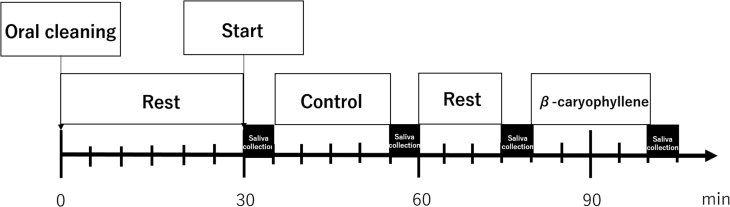
We asked each subject to rinse her mouth on arrival at the laboratory and then rest for 30 min to stabilize intraoral hormone concentrations. Each subject was then exposed to dipropylene glycol for 20 min, followed by 3% β-caryophyllene for 20 min. Saliva was collected 4 times: before and after control exposure, and before and after β-caryophyllene exposure. The odor exposure schedule is shown in Figure 2. The saliva was stored at −80°C until analysis.
Figure 2.
Schematic representation of the odor exposure schedule.
Measures of Salivary Testosterone and Estrogen Concentrations
Saliva samples were completely thawed and then centrifuged at 1,500 × g for 15 min at room temperature. Salivary testosterone and estrogen concentrations were measured with a competition ELISA kit (Salimetrics LLC, Carlsbad, CA, USA) according to the protocol recommended by the manufacturer. For testosterone, the intra-assay coefficient of variation was 1.6%, and the sensitivity was 0.67 pg/mL. For estrogen, the intra-assay coefficient of variation was 2.1%, and the sensitivity was 0.1 pg/mL.
Analysis
Hormones are secreted according to the circadian rhythm.30 Therefore, the variation in baseline hormone concentrations (testosterone; means = 97.6 pg/ml, range = 59.1–125.9 pg/ml, SD = 25.2 pg/ml, estrogen; means = 2.07 pg/ml, range = 1.2–3.2 pg/ml, SD = 0.6 pg/ml) is greater between subjects. To minimize the impact of rhythm and variance of data on the results, we determined the rate of change in salivary testosterone and estrogen concentrations as the concentration after exposure to the odor divided by that before exposure. To show the effect of β-caryophyllene on salivary testosterone and estrogen concentrations, we compared the rates of change of concentrations between the control and β-caryophyllene groups. The quantitative values are expressed as means ± standard errors. Statistical analysis was performed using paired t-tests between groups, and significant difference was set at P < .05.
Results
Exposure of women in the follicular phase of the menstrual cycle to β-caryophyllene significantly increased the salivary concentration of testosterone (control vs β-caryophyllene; 0.97 ± 0.05 vs 1.13 ± 0.03; data are means ± standard errors. P = .00, t = −3.25, 95% confidence interval of the control: 0.84–1.09, 95% confidence interval of β-caryophyllene: 1.04–1.20) but not estrogen (control vs β-caryophyllene; 1.05 ± 0.03 vs 1.07 ± 0.04; data are means ± standard errors. P = .69, t = −0.40, 95% confidence interval of the control: 0.96–1.12, 95% confidence interval of β-caryophyllene: 0.98–1.15) (Figure 3).
Figure 3.
Effect of β-caryophyllene on salivary testosterone and estrogen concentrations in women. Concentrations are presented as the rate of change (concentration after exposure ÷ concentration before exposure). Data are means ± standard errors. Statistical analysis was performed using paired t-tests between groups, with significance at P < .05.
Discussion
Exposure to β-caryophyllene increased the salivary testosterone concentration in women. Testosterone is a hormone produced mainly in the gonads, and its increase is associated with an increase in human sexual desire.20, 21, 22 Indonesian folklore tells us that ylang-ylang, which contains up to 33% β-caryophyllene, is associated with euphoria and sexual behavior.1 We intend to conduct a study to test this folklore and conducted this preliminary study in preparation. The results of this study will provide scientific significance to our future study “Induction of human sexual behavior by β-caryophyllene.”
Previous studies show regulation of the reproductive endocrine system by olfactory stimulation. For example, female scent increases the salivary testosterone concentration in men.31 Women's tears contain a chemical that reduces sexual arousal and salivary testosterone concentration in men.32 Salivary estrogen and cortisol concentrations and anxiety in women are regulated by saffron odor.33 These physiological responses occur within 20 min after olfactory stimulation. The present study aimed to explore the feasibility of our future study to verify the relationship between β-caryophyllene and human sexual behavior. Therefore, the time from exposure to β-caryophyllene to the expression of the physiological response can be meaningful information for developing an experimental protocol of a clinical study. The salivary testosterone concentration increased within 20 min after olfactory stimulation in the present study, as with the previous studies. This is a valid result in the context of the previous studies.
In the previous paragraphs, we discussed the possibility that olfactory stimulation increases testosterone in human saliva within 20 minutes. However, we have not been able to examine how long this olfactory stimulus–induced increase in testosterone is sustained. There is a previous study that provides useful information in testing this issue: Cerda-Molina et al reported that the increase in salivary testosterone levels in men who smelled the external genitalia of women during the ovulatory period persisted for more than 60 minutes.34 Therefore, it is possible that the effect of β-caryophyllene we reported may also persist for about 60 minutes. Also, we have not examined what the physiological implications of increased testosterone are. We believe that olfactory exposure to β-caryophyllene increases testosterone levels in women and activates libido. However, the present results are insufficient and premature to write a conclusion on these issues.
Although we assumed that testosterone is associated with human sexual behavior, we need to verify our results carefully when designing a clinical study, as β-caryophyllene may affect mental and physiological functions via other mechanisms. For example, we did not examine hormone kinetics other than testosterone and estrogen. Furthermore, β-caryophyllene may affect the function of the central nervous system, independent of its endocrine function.35 To verify these 2 physiological functions separately, we will first need to conduct basic in vitro or animal experiments.
Our results reveal that β-caryophyllene increased the testosterone concentration, which was associated with female sexual behavior. Our previous study showed that it promoted sexual behavior in female rodents. Sexual behavior in humans and rodents may not be supported by the same molecular basis. Nevertheless, these results suggest that β-caryophyllene can work as an aphrodisiac-like pheromone, common to humans and animals. We believe that β-caryophyllene will help solve the issue of sexless marriage in humans and increase populations of rare animals by induction of mating behavior.
In recent years, salivary biomarkers have been increasingly used in studies related to human behavior and health assessment.36,37 Monitoring biomarkers in saliva has distinct advantages over monitoring biomarkers in other biological fluids (urine, serum, plasma). For example, saliva sampling is relatively noninvasive and allows the collection of samples in infants, children, and the elderly. In addition, estrogen and testosterone concentrations in saliva accurately reflect the concentration of those hormones in the blood.38, 39, 40, 41, 42, 43, 44, 45 During a woman's menstrual cycle, blood estrogen concentrations show 2 peaks; these 2 peaks can likewise be detected by measuring estrogen concentrations secreted in saliva.38 In addition, previous studies have shown that salivary testosterone concentrations may be a biomarker that functions equivalently to free testosterone concentrations in serum.39, 40, 41, 42, 43, 44, 45 Furthermore, unless visibly contaminated with blood, human saliva is not considered a class II biohazard and provides administrative and safety benefits to researchers and institutions.37 For more than one reason, we used saliva samples to facilitate the detection of biological phenomena.
In 2019, a meta-analysis of testosterone therapy for hypoactive sexual desire disorder (HSDD) in menopausal women was conducted, and a position statement was made in response.46 These are important steps toward the development of effective treatment strategies for HSDD in women. We believe that β-caryophyllene will help develop this therapeutic strategy. However, the participants in this study may have a different biological basis compared with those on testosterone therapy. This is because the participants in this study were health subjects. Therefore, it is difficult to determine whether β-caryophyllene is effective in the treatment of HSDD; the effects of β-caryophyllene on HSDD will need to be tested in further clinical studies. At that time, to test the characteristics of β-caryophyllene on HSDD, we will evaluate its effects not only by blood hormones but also by psychological questionnaires such as the Female Sexual Function Index and the Female Sexual Distress Scale-Revised.
In women, the clitoris and vagina are important organs for physiological sexual function.47 Sexual arousal is characterized by a subjective sense of sexual arousal and pleasure accompanied by vascular congestion of the pelvis, lubrication of the vagina, and swelling of the external genitalia.48 Sexual arousal in women is mediated by a combination of vasoconstriction and neuromuscular events.47 This event indicates an erection of the clitoris, and it requires relaxation of the clitoral smooth muscle.49 In recent years, attention has been focused on the hormonal control mechanisms that control the proper function of the clitoris. Testosterone and estrogen were reported to control the relaxing function and the contractile function of the clitoris, respectively.50 A study showed that β-caryophyllene increased testosterone in women. Considering these results and previous studies in an integrated manner, β-caryophyllene may also act on the erectile mechanism of the female clitoris. This suggests that β-caryophyllene may be a simple treatment for menopausal women to enjoy their sex lives, considering that the amount of circulating testosterone in menopausal women is less than half that of premenopausal women.9,10
This study has limitations regarding experimental methods that should be taken into consideration in future studies. For example, the inherent aromas of β-caryophyllene and dipropylene glycol prevented us from conducting a blinded study. Preference for odors varies among individuals. Although preference is usually developed through past experience and learning, it is also affected by variations within olfactory receptor genes.51 Therefore, the personal preferences of the subjects may have affected the results. In addition, the subjects were always exposed to the control odor first, followed by β-caryophyllene, to prevent any persistent effects of the essential oil from affecting the results of the control; therefore, the order of exposure may have affected the results. To apply these results to future studies of human sexual behavior, these limitations need to be addressed.
In conclusion, this study showed that β-caryophyllene increased the salivary testosterone concentration in women. We do not yet fully understand the meaning of these results; however, in consideration of previous studies, olfactory stimulation with β-caryophyllene may be useful in understanding female sexual behavior and awareness.
Statement of authorship
Category 1
-
(a)Conception and Design
- Wataru Tarumi; Kazuyuki Shinohara
-
(b)Acquisition of Data
- Wataru Tarumi
-
(c)Analysis and Interpretation of Data
- Wataru Tarumi
Category 2
-
(a)Drafting the Article
- Wataru Tarumi
-
(b)Revising It for Intellectual Content
- Wataru Tarumi
Category 3
-
(a)Final Approval of the Completed Article
- Wataru Tarumi; Kazuyuki Shinohara
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Funding: This work was partly supported by JSPS KAKENHI grant number 18K13116 to WT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tan L.T., Lee L.H., Yin W.F. Traditional Uses, Phytochemistry, and Bioactivities of Cananga odorata (Ylang-Ylang) Evid Based Complement Alternat Med. 2015;2015:896314. doi: 10.1155/2015/896314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin X.W., Hao C.Y., He S.Z. Volatile organic compound emissions from different stages of Cananga odorata flower development. Molecules. 2014;19:8965–8980. doi: 10.3390/molecules19078965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galdino P.M., Nascimento M.V., Florentino I.F. The anxiolytic-like effect of an essential oil derived from Spiranthera odoratissima A. St. Hil. leaves and its major component, β-caryophyllene, in male mice. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:276–284. doi: 10.1016/j.pnpbp.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Tarumi W., Shinohara K. The potential for aromatherapy (in Japanese), Japanese. J Of. Taste. And. Smell. Res (in Japanese) 2017;24:59–66. [Google Scholar]
- 5.Dabbs J.M., Jr. Salivary testosterone measurements in behavioral studies. Ann NY Acad Sci. 1993;694:177–183. doi: 10.1111/j.1749-6632.1993.tb18351.x. [DOI] [PubMed] [Google Scholar]
- 6.Diver M. Laboratory measurement of testosterone. Front Horm Res. 2009;37:21–31. doi: 10.1159/000175841. [DOI] [PubMed] [Google Scholar]
- 7.Shinohara K., Doi H., Kumagai C. Effects of essential oil exposure on salivary estrogen concentration in perimenopausal women. Neuro Endocrinol Lett. 2017;37:567–572. [PubMed] [Google Scholar]
- 8.Vining R.F., McGinley R.A. Hormones in saliva. Crit Rev Clin Lab Sci. 1986;23:95–146. doi: 10.3109/10408368609165797. [DOI] [PubMed] [Google Scholar]
- 9.Hughes C.L., Jr., Wall L.L., Creasman W.T. Reproductive hormone levels in gynecologic oncology patients undergoing surgical castration after spontaneous menopause. Gynecol Oncol. 1991;40:42–45. doi: 10.1016/0090-8258(91)90083-h. [DOI] [PubMed] [Google Scholar]
- 10.Judd H.L. Hormonal dynamics associated with the menopause. Clin Obstet Gynecol. 1976;19:775–788. doi: 10.1097/00003081-197612000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Reid R.L., Fortier M.P. Menopausal hormone therapy and quality of life: too many pyjamas. J Obstet Gynaecol Can. 2014;36:953–954. doi: 10.1016/S1701-2163(15)30405-9. [DOI] [PubMed] [Google Scholar]
- 12.Dennerstein L., Dudley E., Burger H. Are changes in sexual functioning during midlife due to aging or menopause? Fertil Steril. 2001;76:456–460. doi: 10.1016/s0015-0282(01)01978-1. [DOI] [PubMed] [Google Scholar]
- 13.Kingsberg S.A. Postmenopausal sexual functioning: a case study. Int J Fertil Womens Med. 1998;4:122–128. [PubMed] [Google Scholar]
- 14.McCoy N.L., Davidson J.M. A longitudinal study of the effects of menopause on sexuality. Maturitas. 1985;7:203–210. doi: 10.1016/0378-5122(85)90041-6. [DOI] [PubMed] [Google Scholar]
- 15.Freedman M.A. Quality of life and menopause: the role of estrogen. J Womens Health (Larchmt) 2002;11:703–718. doi: 10.1089/15409990260363661. [DOI] [PubMed] [Google Scholar]
- 16.Miller M.M., Franklin K.B. Theoretical basis for the benefit of postmenopausal estrogen substitution. Exp Gerontol. 1999;34:587–604. doi: 10.1016/s0531-5565(99)00032-7. [DOI] [PubMed] [Google Scholar]
- 17.Sarrel P.M. Sexuality and menopause. Obstet Gynecol. 1990;75:26–30. [PubMed] [Google Scholar]
- 18.Semmens J.P., Wagner G. Estrogen deprivation and vaginal function in postmenopausal women. JAMA. 1982;248:445–448. [PubMed] [Google Scholar]
- 19.Shulman L.P., Yankov V., Uhl K. Safety and efficacy of a continuous once-a-week 17β-estradiol/levonorgestrel transdermal system and its effects on vasomotor symptoms and endometrial safety in postmenopausal women: the results of two multicenter, double-blind, randomized, controlled trials. Menopause. 2002;9:195–207. doi: 10.1097/00042192-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Davis S.R., Moreau M., Kroll R. Testosterone for low libido in postmenopausal women not taking estrogen. N Engl J Med. 2008;359:2005–2017. doi: 10.1056/NEJMoa0707302. [DOI] [PubMed] [Google Scholar]
- 21.Sherwin B.B., Gelfand M.M., Brender W. Androgen enhances sexual motivation in females: a prospective, crossover study of sex steroid administration in the surgical menopause. Psychosom Med. 1985;47:339–351. doi: 10.1097/00006842-198507000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Sherwin B.B., Gelfand M.M. The role of androgen in the maintenance of sexual functioning in oophorectomized women. Psychosom Med. 1987;49:397–409. doi: 10.1097/00006842-198707000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Wellings K., Field J., Wadsworth J. Sexual lifestyles under scrutiny. Nature. 1990;348:276–278. doi: 10.1038/348276a0. [DOI] [PubMed] [Google Scholar]
- 24.Bajos N., Wadsworth J., Ducot B.L. Sexual behaviour and HIV epidemiology: comparative analysis in France and Britain. AIDS. 1995;9:735–743. [PubMed] [Google Scholar]
- 25.Konings E., Bantebya G., Caraël M. Validating population surveys for the measurement of HIV/STD prevention indicators. AIDS. 1995;9:375–382. [PubMed] [Google Scholar]
- 26.Meston C.M., Trapnell P.D., Gorzalka B.B. Ethnic and gender differences in sexuality: variations in sexual behavior between Asian and non-Asian university students. Arch Sex Behav. 1996;25:33–72. doi: 10.1007/BF02437906. [DOI] [PubMed] [Google Scholar]
- 27.AIDS and sexual behaviour in France. ACSF investigators. Nature. 1992;360:407–409. doi: 10.1038/360407a0. [DOI] [PubMed] [Google Scholar]
- 28.Strassberg D.S., Lowe K. Volunteer bias in sexuality research. Arch Sex Behav. 1995;24:369–382. doi: 10.1007/BF01541853. [DOI] [PubMed] [Google Scholar]
- 29.Robert T., Rodney Y. second ed. Churchill Livingstone; New York: 2014. Essential oil safety. [Google Scholar]
- 30.Fukui H., Yamashita M. The effects of music and visual stress on testosterone and cortisol in men and women. Neuro Endocrinol Lett. 2003;24:173–180. [PubMed] [Google Scholar]
- 31.Miller S.L., Maner J.K. Scent of a woman: men's testosterone responses to olfactory ovulation cues. Psychol Sci. 2010;21:276–283. doi: 10.1177/0956797609357733. [DOI] [PubMed] [Google Scholar]
- 32.Gelstein S., Yeshurun Y., Rozenkrantz L. Human tears contain a chemosignal. Science. 2011;331:226–230. doi: 10.1126/science.1198331. [DOI] [PubMed] [Google Scholar]
- 33.Fukui H., Toyoshima K., Komaki R. Psychological and neuroendocrinological effects of odor of saffron (Crocus sativus) Phytomedicine. 2011;18:726–730. doi: 10.1016/j.phymed.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Cerda-Molina A.L., Hernández-López L., de la O.C.E. Changes in men's salivary testosterone and cortisol levels, and in sexual desire after smelling female Axillary and Vulvar scents. Front Endocrinol. 2013;28:159. doi: 10.3389/fendo.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottfried J.A. Smell: central nervous processing. Adv Otorhinolaryngol. 2006;63:44–69. doi: 10.1159/000093750. [DOI] [PubMed] [Google Scholar]
- 36.Kirschbaum C., Wüst S., Strasburger C.J. 'Normal' cigarette smoking increases free cortisol in habitual smokers. Life Sci. 1992;50:435–442. doi: 10.1016/0024-3205(92)90378-3. [DOI] [PubMed] [Google Scholar]
- 37.Shirtcliff E.A., Granger D.A., Schwartz E. Use of salivary biomarkers in Biobehavioral research: Cotton-Based sample collection methods can Interfere with salivary immunoassay results. Psychoneuroendocrinology. 2001;26:165–173. doi: 10.1016/s0306-4530(00)00042-1. [DOI] [PubMed] [Google Scholar]
- 38.Lipson S.F., Ellison P.T. Comparison of salivary steroid Profiles in Naturally Occurring Conception and non-Conception cycles. Hum Reprod. 1996;11:2090–2096. doi: 10.1093/oxfordjournals.humrep.a019055. [DOI] [PubMed] [Google Scholar]
- 39.Morley J.E., Perry H.M., 3rd, Patrick P. Validation of salivary testosterone as a Screening test for male hypogonadism. Aging Male. 2006;9:165–169. doi: 10.1080/13685530600907993. [DOI] [PubMed] [Google Scholar]
- 40.Goncharov N., Katsya G., Dobracheva A. Diagnostic significance of free salivary testosterone measurement using a direct luminescence immunoassay in healthy men and in patients with disorders of androgenic status. Aging Male. 2006;9:111–122. doi: 10.1080/13685530600713060. [DOI] [PubMed] [Google Scholar]
- 41.Arregger A.L., Contreras L.N., Tumilasci O.R. Salivary testosterone: a reliable approach to the diagnosis of male hypogonadism. Clin Endocrinol. 2007;67:656–662. doi: 10.1111/j.1365-2265.2007.02937.x. [DOI] [PubMed] [Google Scholar]
- 42.Cardoso E.M., Contreras L.N., Tumilasci E.G. Salivary testosterone for the diagnosis of androgen deficiency in end-stage renal disease. Nephrol Dial Transpl. 2011;26:677–683. doi: 10.1093/ndt/gfq439. [DOI] [PubMed] [Google Scholar]
- 43.Vittek J., L'Hommedieu D.G., Gordon G.G. Direct radioimmunoassay (RIA) of salivary testosterone: correlation with free and total serum testosterone. Life Sci. 1985;37:711–716. doi: 10.1016/0024-3205(85)90540-5. [DOI] [PubMed] [Google Scholar]
- 44.Wang C., Plymate S., Nieschlag E. Salivary testosterone in men: further evidence of a direct correlation with free serum testosterone. J Clin Endocrinol Metab. 1981;53:1021–1024. doi: 10.1210/jcem-53-5-1021. [DOI] [PubMed] [Google Scholar]
- 45.Fiers T., Delanghe J., T'Sjoen G. A critical evaluation of salivary testosterone as a method for the assessment of serum testosterone. Steroids. 2014;86:5–9. doi: 10.1016/j.steroids.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Vignozzi L., Reisman Y. Testosterone in women: are we closing the gender gap? Nat Rev Urol. 2020;17:67–68. doi: 10.1038/s41585-019-0266-3. [DOI] [PubMed] [Google Scholar]
- 47.Goldstein I., Berman J.R. Vasculogenic female sexual dysfunction: vaginal engorgement and clitoral erectile insufficiency syndromes. Int J Impotqnce Res. 1998;10:S84. [PubMed] [Google Scholar]
- 48.Scjoavo R.C., Segraves R.T. The biology of sexual function. Psychiat Clin North Am. 1995;18 [PubMed] [Google Scholar]
- 49.Park J.K., Kim S.Z., Kim S.H. Renin angiotensin system of rabbit clitoral cavernosum: interaction with nitric oxide. J Urol. 2000;164:556–561. [PubMed] [Google Scholar]
- 50.Comeglio P., Cellai I., Filippi S. Differential effects of testosterone and estradiol on clitoral function: an experimental study in Rats. J Sex Med. 2016;13:1858–1871. doi: 10.1016/j.jsxm.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Jaeger S.R., McRae J.F., Bava C.M. A Mendelian trait for olfactory sensitivity affects odor experience and food selection. Curr Biol. 2013;23:1601–1605. doi: 10.1016/j.cub.2013.07.030. [DOI] [PubMed] [Google Scholar]