Abstract
Respiratory syncytial virus (RSV) can cause recurrent infection in people because it does not stimulate a long-lived immunological memory. There is an urgent need to develop a safe and efficacious vaccine against RSV that would induce immunological memory without causing immunopathology following natural RSV infection. We have previously generated two recombinant live attenuated influenza vaccine (LAIV) viruses that encode immunodominant T-cell epitopes of RSV M2 protein in the neuraminidase or NS1 genes. These chimeric vaccines afforded protection against influenza and RSV infection in mice, without causing pulmonary eosinophilia or inflammatory RSV disease. The current study assessed the formation of influenza-specific and RSV-specific CD4 and CD8 T-cell responses in the lungs of mice, with special attention to the lung tissue-resident memory T cell subsets (TRM). The RSV epitopes did not affect influenza-specific CD4 effector memory T cell (Tem) levels in the lungs. The majority of these cells formed by LAIV or LAIV-RSV viruses had CD69+CD103- phenotype. Both LAIV+NA/RSV and LAIV+NS/RSV recombinant viruses induced significant levels of RSV M282 epitope-specific lung-localized CD8 Tem cells expressing both CD69 and CD103 TRM markers. Surprisingly, the CD69+CD103+ influenza-specific CD8 Tem responses were augmented by the addition of RSV epitopes, possibly as a result of the local microenvironment formed by the RSV-specific memory T cells differentiating to TRM in the lungs of mice immunized with LAIV-RSV chimeric viruses. This study provides evidence that LAIV vector-based vaccination can induce robust lung-localized T-cell immunity to the inserted T-cell epitope of a foreign pathogen, without altering the immunogenicity of the viral vector itself.
Keywords: Respiratory syncytial virus, T cell immunity, Live attenuated influenza vaccine, Viral vector, Tissue-resident memory T cells
Highlights
-
•
Two LAIV-RSV vaccine viruses induced RSV M282-specific effector memory CD8 T cells producing both IFNγ and TNFα cytokines.
-
•
The inserted RSV epitopes did not affect influenza-specific CD4 Tem levels in the lungs of immunized mice.
-
•
LAIV-RSV viruses induced RSV M282-specific lung-localized CD8 Tem cells expressing both CD69 and CD103 TRM markers.
-
•
The magnitude of RSV M282-specific CD8 Tem responses correlates with protection against RSV-induced lung pathology.
-
•
The addition of RSV epitopes into the LAIV strain augmented CD69+CD103+ influenza-specific CD8 Tem responses in the lungs.
1. Introduction
While there are currently a wide variety of vaccines against influenza A and B viruses, no vaccine against other respiratory infections has yet been licensed, despite attempts over many years to develop such vaccines (Tannock et al., 2020). In particular, there is an urgent need to develop a safe and efficacious vaccine against respiratory syncytial virus (RSV). RSV infection causes lower respiratory disease in infants and young children, with an estimated annual global burden of 33.1 million episodes of acute lower respiratory tract infection and over 100,000 deaths (Shi et al., 2017). The development of an RSV vaccine suffered a major setback in the 1960s, when an alum-adjuvanted formalin-inactivated RSV (FI-RSV) vaccine resulted in enhanced respiratory disease (ERD) in infants and toddlers after subsequent exposure to natural RSV infection (Kim et al., 1969). Development of an RSV vaccine is more problematic than an influenza vaccine because of striking differences in the virology and host response to these two pathogens. Influenza infection induces mucosal IgA and systemic IgG antibodies, as well as broadly reactive T-cell responses, which help to protect against re-infection by neutralizing the virus, or ameliorate the disease by killing infected cells. RSV, in contrast, can re-infect an individual even in the presence of high levels of virus-specific neutralizing antibodies (Hall et al., 1991). This is because RSV can modulate the host immune system, impairing B-cell memory and reducing T-cell generation and functionality (reviewed in (Ascough et al., 2018)). FI-RSV vaccination has been shown to induce T helper type 2 (Th2)-biased CD4 T cells with low levels of cytotoxic CD8 T cells and less protective antibodies, causing ERD in animal models after RSV challenge, accompanied by high pulmonary eosinophilic infiltrates (Collins et al., 2013; Graham et al., 1993; Knudson et al., 2015). The ability of RSV M2-specific CD8 T cells to protect against a secondary infection and inhibit severe pulmonary eosinophilia by reducing the number of Th2 CD4 T cells in the lungs after RSV challenge (Graham et al., 1991; Olson and Varga, 2007; Srikiatkhachorn and Braciale, 1997) provided the rationale for designing a new class of CD8 T-cell-based RSV vaccines. However, excessive generation of RSV M2 epitope-specific systemic memory CD8 T cells can result in exacerbated morbidity and mortality following RSV infection, indicating that caution is needed in designing T-cell-based RSV vaccines (Schmidt et al., 2018). Importantly, Schmidt et al. demonstrated that a booster immunization with M282 RSV epitope, delivered directly to the lungs by a recombinant influenza virus expressing this epitope, generated RSV-specific lung-localized memory CD8 T cells, which were associated with reduced immunopathology following RSV challenge (Schmidt et al., 2018). These data suggest that the site of administration of T-cell-based RSV vaccines is crucial. For example, these epitopes can be delivered to the respiratory tract by live attenuated influenza vaccine (LAIV) viruses (Isakova-Sivak et al., 2016a), which are known to be effective inducers of cross-reactive T-cell responses (Isakova-Sivak et al., 2020; Korenkov et al., 2018; Mohn et al., 2017). In addition, such bivalent LAIV-RSV vaccines could be used for simultaneous prophylaxis of the two respiratory pathogens.
We have previously generated two prototype LAIV-RSV viral vectored vaccines carrying several conserved T-cell epitopes of RSV inserted into the neuraminidase (NA) or NS1 gene of H7N9 LAIV virus. These vaccines induced functional cytotoxic T-cell immune responses, targeted at both influenza virus and the RSV M282 epitope (Isakova-Sivak et al., 2016b; Kotomina et al., 2018). Subsequently, we demonstrated that these recombinant viruses were able to protect vaccinated mice against virulent influenza virus and, most importantly, RSV, without causing inflammatory disease (Kotomina et al., 2019). In the latter experiment, RSV M282 epitope-specific T-cell recall responses were measured in lungs and bronchoalveolar lavage (BAL) five days after RSV challenge; levels in the LAIV-RSV groups were significantly higher than those in groups given the LAIV vector or FI-RSV (Kotomina et al., 2019). The precise mechanisms of immune protection afforded by such LAIV-vectored RSV vaccines still need to be established. Of particular interest is whether LAIV-delivered RSV epitopes can induce memory T-cell responses that persist at the site of infection of the target pathogen. For the majority of respiratory pathogens, the establishment of lung tissue-resident memory CD8 T cells (TRM) can provide a first line of adaptive cellular defense on exposure to the same or similar pathogen, because CD8 T cells are targeted primarily to the relatively conserved viral proteins (reviewed in (Takamura, 2017; Topham and Reilly, 2018)). A recent study found that LAIV, but not inactivated influenza vaccine (IIV), can efficiently promote robust long-lived influenza-specific TRM responses that provide protection against heterologous influenza virus challenge, independent of circulating T cells and neutralizing antibodies (Zens et al., 2016). The current study was undertaken to obtain a deeper understanding of how influenza-specific and RSV-specific memory T-cell immunity is established in the lungs after intranasal (i.n.) immunization with the recombinant LAIV-RSV viruses, and in particular whether the insertion of an immunodominant foreign CD8 T-cell epitope can affect anti-influenza T-cell immunity in the lungs.
2. Materials and methods
2.1. Cells, viruses, peptides
Hep-2 cells (ATCC CCL-23) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Capricorn, Germany) and antibiotic-antimycotic solution (AA) (Thermo Fisher Scientific, USA).
Recombinant H7N9 LAIV-RSV viruses were previously generated by means of reverse genetics, on A/Leningrad/134/17/57 backbone (Kotomina et al., 2018). These viruses expressed the RSV cassette containing human RSV A2 strain M2-1 fragment (amino acids 70 to 101 and 114 to 146) in the C-terminal NA [LAIV+NA/RSV] or NS1 truncated to 126 amino acids [LAIV+NS/RSV] open reading frames. The viruses were propagated in 10–11-day-old chicken embryos for 2 days at 33 °C and stored in aliquots at −70 °C.
RSV A2 strain virus was obtained from the Smorodintsev Research Institute of Influenza (Saint Petersburg, Russia) and propagated in Hep-2, as described previously (Kotomina et al., 2019). To prepare the FI-RSV vaccine, formalin was added to the RSV-containing supernatant to a concentration of 1:4000, incubated for 72 h at 37 °C and centrifuged at 30 000 g and 4 °C for 1 h. The pellet was suspended in Dulbecco's phosphate-buffered saline (PBS), and stored in aliquots at −70 °C. The RSV titer was determined by plaque assay in 6-well plates seeded with Hep-2 cells. Serially diluted RSV was inoculated onto the cell monolayer, and incubated for 2 h at 37 °C. The cells were then covered with an overlay containing DMEM and 0.9% agarose (Thermo, USA). After 5 days' incubation, the cells were fixed in 1% formaldehyde and the immune plaques were developed using primary anti-RSV F monoclonal antibody (MAB 8599, EMD Millipore Corp., USA), secondary horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody (Southern Biotech, USA) and 3,3′diaminobenzidine (DAB) substrate (Thermo Scientific, USA). The RSV titer was expressed in plaque-forming units (PFU) per ml.
RSV strain A2 matrix protein peptide M282–90 (SYIGSINNI) was chemically synthesized by Almabion Ltd (Russia) with a purity of more than 80%, as measured by high-performance liquid chromatography. The peptide was reconstituted in dimethyl sulfoxide at a concentration of 1 mM and stored at −70 °C in single-use aliquots.
2.2. Mouse immunization and challenge
Female BALB/c mice aged 6–8 weeks were purchased from Stolbovaya Laboratory Animal Breeding Nursery (Moscow region, Russia). Mice were housed at the Animal Facility of the Institute of Experimental Medicine. The protocol was approved by the Local Ethics Committee of the Institute of Experimental Medicine (No. 3/19 of 25 April 2019). Immunization and bleeding procedures were performed under light ether anesthesia.
Immunization procedures, as well as influenza virus and RSV challenge were performed as previously described (Kotomina et al., 2019). Briefly, groups of mice were given i.n. immunization with either H7N9 LAIV or one of the LAIV-RSV vaccines [LAIV+NA/RSV and LAIV+NS/RSV], at a dose of 106 EID50 in a volume of 50 μl, twice at a three-week interval. A control group received two i.n. doses of PBS. There was an additional vaccine group (FI-RSV, n = 10), in which mice were given two 100-μl intramuscular injections of 2 μg of formalin-inactivated purified RSV with AlumVax Hydroxide adjuvant formulation (50 μg) (OzBiosciences, France) at a two-week interval. Three weeks after the second immunization five mice from each group were infected intranasally with 1 × 105 PFU of RSV A2. They were euthanized on day 5 after RSV infection and lungs were collected for virological and histopathological studies. Lung RSV titers were determined as described by (Kotomina et al., 2019) and expressed as PFU per gram of lung tissue.
2.3. Systemic T-cell immune responses
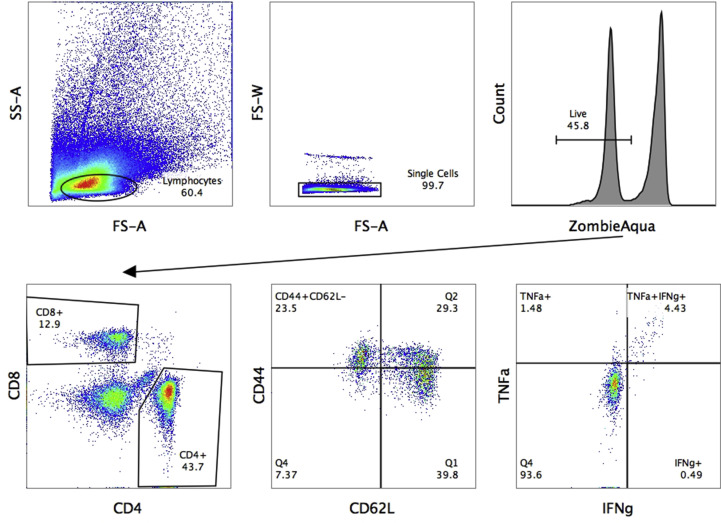
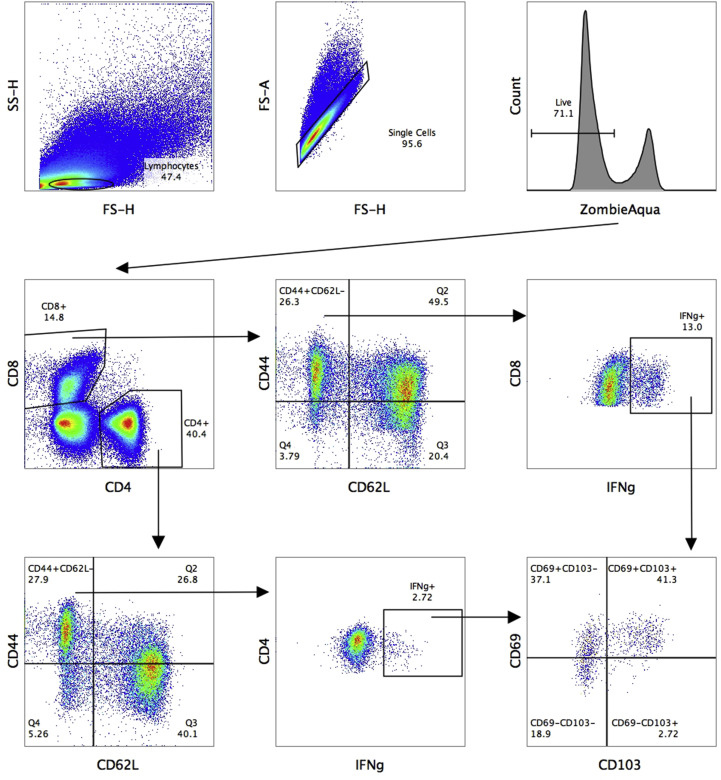
On day 7 after the second immunization, spleens were collected from five mice and single splenocytes were isolated in conditioned media (RPMI-1640, Capricorn Scientific, Germany) with AA solution (Thermo Fisher Scientific, USA), 25 mM Hepes (Gibco, USA) and 50 μM 2-mercaptoethanol (Sigma, USA), using 70-μm cell strainers (BD Biosciences, USA). Red blood cells were then lysed with ammonium-chloride-potassium lysing buffer. For intracellular cytokine staining (ICS), 2 × 106 cells were plated into U-bottom well microplates in 50 μl of conditioned media; 50 μl of sucrose-purified influenza virus was added for LAIV-stimulation to a final multiplicity of infection (MOI) of 3.0. Samples for non-peptide and peptide stimulation received 50 μl of conditioned media and were placed in a CO2-incubator for 1 h, after which 50 μl of conditioned media was added with 30% FBS, to give a final FBS concentration of 10%. After 16–18 h incubation in a CO2-incubator, 50 μl of conditioned media with GolgiPlug™ solution (BD Biosciences, USA) was added to a final dilution of 1:1000; 1 μM of RSV M282 peptide was added to the peptide stimulation group, and incubated for an additional 5 h. Samples were then stained for 20 min at 4 °C in the dark with live/dead fixable stain (ZombieAqua, Invitrogen) and surface antibody-conjugates anti-CD4 (RM4-5) and anti-CD62L (MEL-14) (from BioLegend, USA), and anti-CD8 (53–6.7) and anti-CD44 (IM7) (from Thermo). A Cytofix/Cytoperm kit (BD Biosciences, USA) was used for fixation/permeabilization, then samples were stained with intracellular cytokine antibody anti-IFNγ (XMG1.2) and anti-TNFα (MP6-XT22) (from BioLegend) for 20 min at 4 °C in the dark. Samples were fixed with 1% paraformaldehyde and analyzed by Navios flow cytometer (Beckman Coulter). Positive controls with phorbol myristate acetate (PMA) stimulation (Sigma), non-stimulated controls and isotype controls were also prepared. The number of IFNγ- and TNFα-positive cells in stimulated groups were counted, and the spontaneous cytokine secretion level of non-stimulated controls subtracted. Gating strategy for measuring IFNγ- and/or TNFα-secreting CD8 Tem cells in mouse spleens is shown in Fig. S1.
2.4. T-cell immune responses in the lungs
On day 7 after the second immunization, the lungs of five mice from each group were perfused with 10 ml of PBS through the right ventricle. The perfused lungs were cut into small pieces and homogenized using 5-mm beads in TissueLyser LT (QIAGEN, Germany), then digested with collagenase (Sigma, USA) and DNAse I (Sigma, USA) for 30 min at 37 °C. The cells were isolated using 70-μm cell strainers (BD Biosciences, USA), and red blood cells were lysed by ammonium-chloride-potassium lysing buffer. To determine the numbers of live and dead cells, staining with ZombieAqua (BioLegend, USA) was used for 20 min at room temperature in the dark. Lung lymphocytes were phenotyped by staining with surface fluorescent antibodies anti-CD4 (RM4-5), anti-CD62L (MEL-14) and anti-CD103 (2E7) (from BioLegend, USA), anti-CD69 (H1.2F3), anti-CD8 (53–6.7) and anti-CD44 (IM7) (from Thermo, USA) for 20 min at room temperature in the dark. Samples were fixed by 1% paraformaldehyde and analyzed with a Navios flow cytometer (Beckman Coulter). Gating strategy for phenotyping IFNγ-secreting CD4 and CD8 T cells in the lungs is shown in Fig. S2. For ICS, 2 × 106 lung-derived cells were used with the protocol described above, with additional staining with surface antibody-conjugates anti-CD103 (2E7) (BioLegend) and anti-CD69 (H1.2F3) (Thermo). For lung ICS, only intracellular cytokine antibody anti-IFNγ (XMG1.2) was used.
2.5. Histopathological studies
Histopathological changes in the lungs were analyzed in five mice from each test group on day 5 after RSV challenge. We used the method described by (Albert et al., 2011) to detect eosinophils in tissues. Briefly, 5-μm paraffin-embedded lung sections were rehydrated, stained for 1 h in a 1% ethanolic solution of Congo Red (Sigma), counterstained in Karazzi's haematoxylin for 10 min, and incubated for 10 min with Alcian Blue 8GX stain at pH 2.5 to detect mucus in the bronchial lumen. The degree of RSV-induced pathology was evaluated as described by (Matsuse et al., 2000). Briefly, the lung sections were reviewed and evaluated for epithelial damage, peribronchovascular cell infiltrate, and interstitial-alveolar cell infiltrate. Epithelial damage was scored as: 0 (no damage); 1 (increased cytoplasm of epithelial cells without desquamation); 2 (epithelial desquamation without bronchial exudate composed of inflammatory cells); or 3 (bronchial exudate composed of desquamate epithelial cells and inflammatory cells). Peribronchovascular cell infiltrate was scored as: 0 (no infiltrate); 1 (infiltrate up to 4 cells); 2 (infiltrate 5–10 cells); or 3 (infiltrate >10 cells). Interstitial-alveolar cell infiltrate was scored as: 0 (no infiltrate); 1 (mild, generalized increase in cell mass of the alveolar septa without thickening of the septa or significant air space consolidation); 2 (dense septal infiltrate with thickening of septa); or 3 (significant alveolar consolidation in addition to interstitial inflammation). Eosinophils were counted in 5–10 perivascular areas of each section.
2.6. Statistical analyses
Data were analyzed with the GraphPad Prism 6.0 software (GraphPad Software Inc). The statistical significance of virological and immunological outcomes was determined by one-way or two-way ANOVA followed by Holm-Sidak's multiple comparisons test. P values less than 0.05 were considered statistically significant.
3. Results
3.1. Recombinant LAIV-RSV viruses induce protective immunity against influenza virus and RSV
Consistent with our previous report (Kotomina et al., 2019), the vaccine viruses demonstrated distinct patterns of replication in the upper respiratory tract (URT): high levels of infectious virus were seen in the nasal turbinates of LAIV- and LAIV+NA/RSV-infected mice, whereas no viable virus was detected in the LAIV+NS/RSV group; nevertheless, all three live vaccines induced similar levels of virus-specific serum IgG antibody after two immunizations and fully protected mice against pulmonary replication of virulent homologous influenza virus (data not shown).
3.2. Recombinant LAIV-RSV viruses induce robust systemic RSV M282-specific CD8 memory T-cell responses
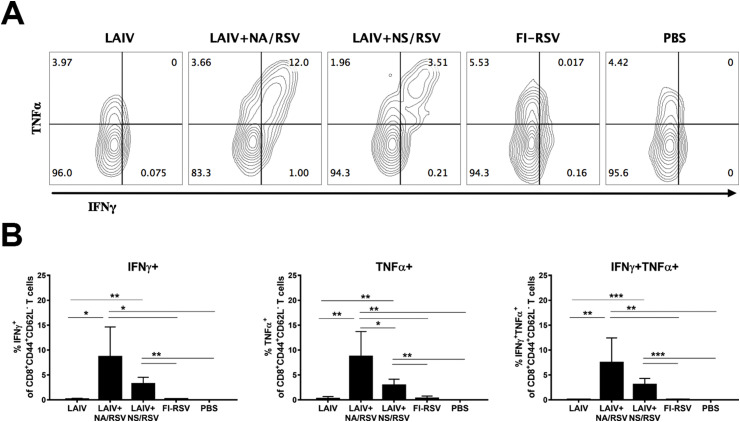
The two LAIV-RSV vaccines induced high levels of M282-specific IFNγ-secreting CD8 Tem cells (Fig. 1 ), reflecting our earlier findings that these vaccines induce systemic CD8 T-cell responses (Kotomina et al., 2018). It is noteworthy that the majority of the IFNγ-secreting CD8 Tem cells were also positive for TNFα cytokine (Fig. 1). These data are in accordance with the T-cell recall responses seen in BAL and lung tissues of LAIV-RSV-immunized mice after RSV challenge (Kotomina et al., 2019). Interestingly, the LAIV+NA/RSV recombinant virus induced significantly higher levels of cytokine-secreting CD8 T cells specific to the RSV epitope than the LAIV+NS/RSV candidate, suggesting that the replication capacity of the recombinant virus has an impact on the vaccine's immunogenicity. The FI-RSV vaccine was unable to induce systemic CD8 T-cell responses to the RSV M282 epitope, which is in line with other studies (Krause et al., 2011; Olson and Varga, 2007) (Fig. 1).
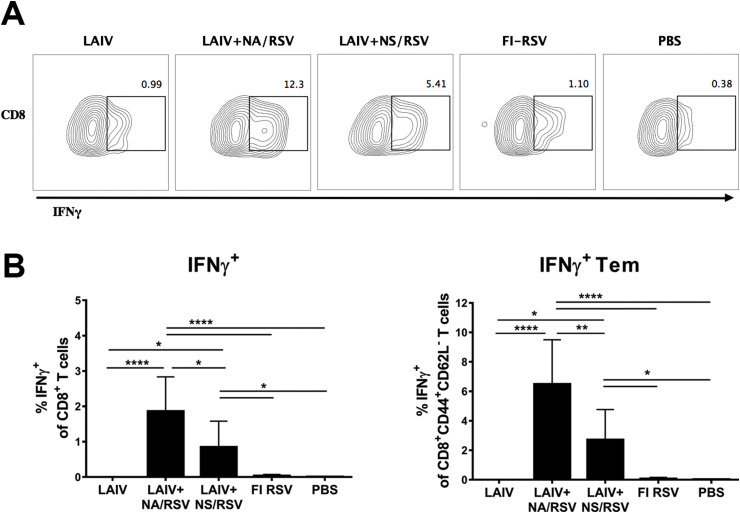
Fig. 1.
Systemic RSV M282-specific effector memory CD8 T-cell responses, measured in mouse spleen on day 7 after the second immunization. A. Representative flow cytometry plots for IFNγ and TNFα expression by Tem cells. B. Percentages of CD8 Tem cells expressing one or both cytokines. Data were analyzed with multiple t tests with Holm-Sidak's correction.
3.3. Recombinant LAIV-RSV viruses induce robust CD8 T-cell responses and increase the proportion of CD69+CD103+ CD4 and CD8 effector memory T cells in the lungs
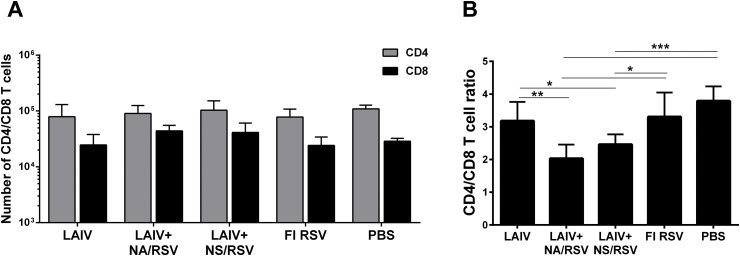
Since the LAIV-RSV vaccine candidates are delivered intranasally, i.e. at the site of entry of influenza virus and RSV, it was important to ascertain the magnitude of the induced T-cell responses directly in the lungs. No significant differences were observed in the numbers of CD4 and CD8 cells in the lungs of immunized and control mice on day 7 after the second vaccine dose (Fig. 2 A). However there were significant differences in the relative proportions of these T cells. The CD8 to CD4 ratio was significantly higher in the LAIV-RSV vaccine groups than in the control group, suggesting that robust CD8 lung-localized T-cell responses were induced by both recombinant viruses (Fig. 2B). The CD8 response in the LAIV+NA/RSV group was even more pronounced, showing a significant difference with the CD8/CD4 ratio in both the LAIV and FI-RSV groups.
Fig. 2.
CD4 and CD8 T cells in the lungs of immunized mice. A. Total number of CD4 and CD8 T cells in the lungs on day 7 after the second immunization. Data were analyzed with two-way ANOVA. B. Ratio of CD8 to CD4 T cells in the lungs. Data were analyzed with one-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001).
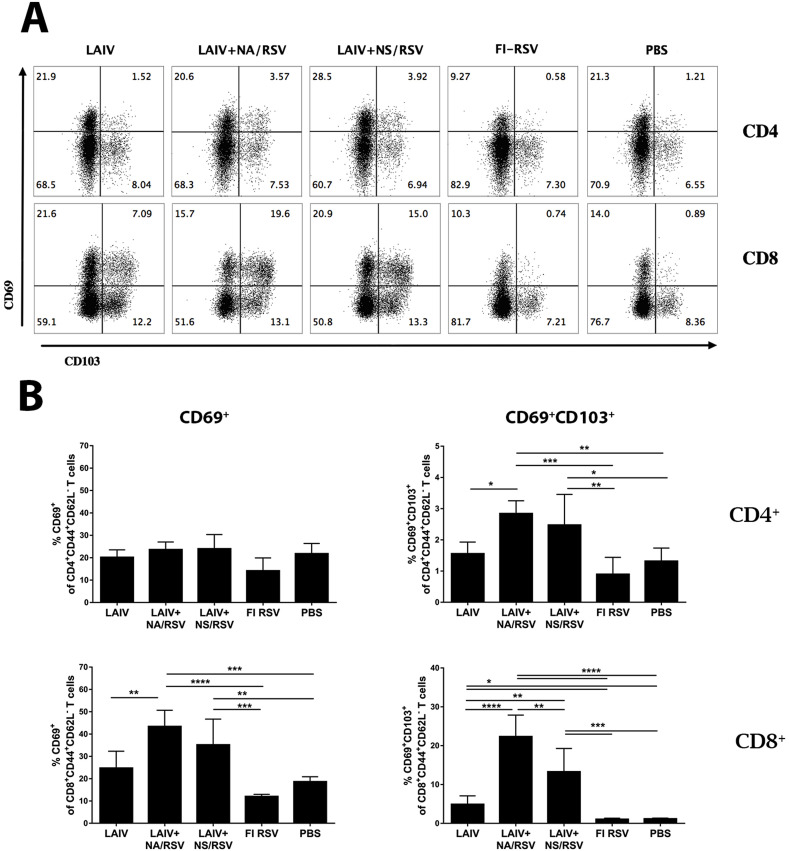
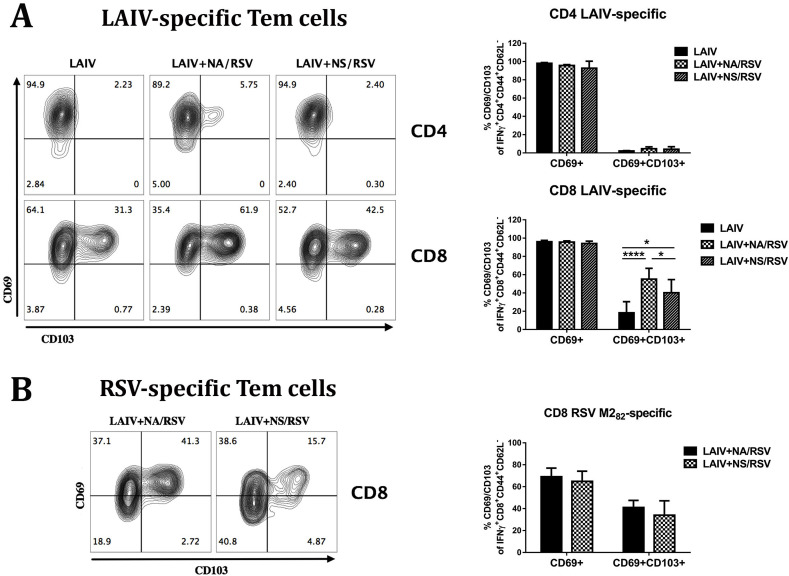
CD103, a mucosal lymphocyte surface marker integrin αEβ7, and CD69 have been widely accepted as the common markers of tissue-resident cells in mucosal and some non-mucosal tissues (Liu et al., 2018). Seven days after the second immunization, the levels of effector memory CD4 T cells expressing CD69 early activation marker were not significantly higher in the lungs of immunized mice than in those of the control group (Fig. 3 B). However, significant levels of CD4 Tem cells, expressing both CD69 and CD103 markers associated with tissue residence, were identified in the lungs of mice immunized with either of the LAIV-RSV vaccine candidates (Fig. 3B). This is somewhat surprising because CD103 is usually expressed only by some subsets of CD8 TRM (Mueller and Mackay, 2016). Nevertheless, several studies have identified distinct populations of CD4 T cells that carry typical TRM markers in both mouse and human lungs, with the CD4 TRM cells usually carrying less CD103 or expressing CD11a instead of CD103 (reviewed in (Liu et al., 2018)). In this study, the CD69+CD103+ CD4 Tem cells were induced only by the recombinant viruses carrying RSV epitopes, and not by LAIV alone, which suggests that the RSV cassette is involved in the formation of CD4 TRM cells after prime-boost immunization.
Fig. 3.
Expression of CD69 and CD103 TRM markers by CD4 and CD8 T cells in the lungs of immunized mice. A. Representative flow cytometry plots for CD69 and CD103 expression by CD4 and CD8 Tem cells. B. Percentages of CD69+ (left panel) and CD69+CD103+ (right panel) CD4 and CD8 Tem cells. Data were analyzed with one-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Similarly, the proportion of CD8 Tem cells possessing either CD69 or both CD69 and CD103 surface markers in the lungs was significantly increased by immunization with a recombinant LAIV-RSV virus (Fig. 3). Although LAIV provoked the development of CD8 T cells with dual TRM phenotype in the lungs, the proportion of these cells among all CD44+CD62L− effector memory CD8 T cells was relatively low and substantially lower than in the LAIV-RSV groups. Our data suggest that the inserted CD8 RSV epitope is immunodominant, and capable of generating resident memory CD8 T cells in the lungs after local delivery to the mucosal sites. This is in agreement with other studies involving intranasal delivery of RSV M282 epitope using different viral vectors (Morabito et al., 2017; Schmidt et al., 2018).
3.4. Recombinant LAIV-RSV viruses induce memory T cells specific to LAIV and RSV which also express tissue resident markers
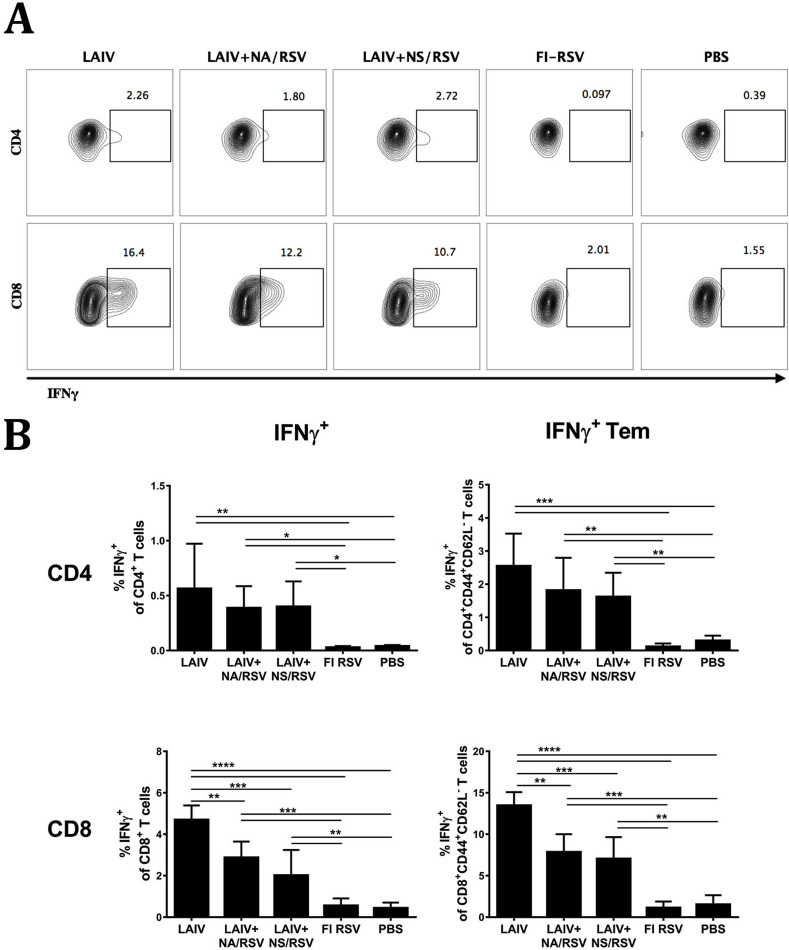
As expected, the LAIV and both LAIV-RSV vaccines induced robust influenza virus-specific CD4 and CD8 T-cell responses in the lungs, with similar levels of IFNγ-positive CD4 T cells (Fig. 4 ). Influenza-specific CD8 T-cell responses in the lungs were more pronounced in mice immunized with LAIV than in the two LAIV-RSV vaccine groups. This suggests that the insertion of the RSV epitopes in the LAIV virus can change the distribution of CD8 T-cell specificity in the lungs after intranasal immunization (Fig. 4). Indeed, high levels of RSV M282-specific IFNγ-secreting CD8 T cells were observed in the lungs of mice immunized with LAIV-RSV, confirming that the RSV peptide is highly immunogenic and efficiently establishes RSV-specific effector memory in the lungs (Fig. 5 ). The RSV-specific CD8 T-cell responses were more robust in the LAIV+NA/RSV group than in the LAIV+NS/RSV group, which is in line with the systemic CD8 responses measured in spleens (Fig. 1). Since influenza-specific CD8 T-cell responses are identical in these vaccine groups (Fig. 4), the differences in the expression of the RSV cassette by these two vaccine viruses could be the reason for diverse RSV-specific responses.
Fig. 4.
Influenza-specific IFNγ-producing T cells in the lungs. A. Representative flow cytometry plots for IFNγ expression by CD4 and CD8 Tem cells. B. Percentages of IFNγ-positive T cells among total CD4 or CD8 T-cell populations in the lungs (left panel) or among the corresponding effector memory subset (right panel). Data were analyzed with one-way ANOVA with Holm-Sidak's multiple comparisons test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Fig. 5.
RSV-specific IFNγ-producing T cells in the lungs. A. Representative flow cytometry plots for IFNγ expression by CD8 Tem cells. B. Percentages of IFNγ-positive T cells among total CD8 T-cell population in the lungs (left graph) or among the corresponding effector memory subset (right graph). Data were analyzed with one-way ANOVA with Holm-Sidak's multiple comparisons test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
The vast majority of influenza virus-specific CD4 T cells had CD69+CD103- phenotype, which is in line with previous findings that the CD4 TRM cells usually express CD11a instead of CD103 marker (Liu et al., 2018) (Fig. 6 A). Since influenza-specific CD4 in the lungs do not have CD103, the CD103-positive CD4 Tem found in the lungs of LAIV-RSV immunized mice (Fig. 3B) might originate from the RSV M2131-145 CD4 epitope inserted into the LAIV genome within the RSV cassette (Isakova-Sivak et al., 2016b; Kotomina et al., 2018). Strikingly, although the majority of the influenza-specific CD8 Tem cells in the lungs of LAIV- and LAIV-RSV-vaccinated mice expressed CD69 marker, the second canonical TRM marker CD103 was present at much higher frequencies in the LAIV-RSV groups than in the LAIV group (Fig. 6A). Given that the proportion of the IFNγ-secreting influenza-specific CD8 Tem cells in the lungs of immunized mice was significantly higher in the LAIV group than the LAIV-RSV groups, it can be speculated that the RSV insert had a significant impact on influenza-specific CD8 TRM differentiation or maintenance. This suggestion is supported by the evidence that there were more influenza-specific CD8 TRM in the LAIV+NA/RSV group than in the LAIV+NS/RSV group, and the NA-modified recombinant virus had higher RSV-specific CD8 responses than the NS-modified LAIV-RSV candidate, both in spleen (Fig. 1) and in the lungs (Fig. 5). Nevertheless, the RSV M282 epitope-specific CD8 TRM levels were similar in the two LAIV-RSV groups (Fig. 6B). Importantly, only 65–70% of lung-localized RSV-specific CD8 Tem cells possessed the CD69 marker, whereas almost all influenza-specific CD8 Tem were CD69-positive (Fig. 6A).
Fig. 6.
The expression of TRM markers by LAIV virus-specific and RSV M282 epitope-specific Tem cells in the lungs of mice immunized with LAIV or LAIV-RSV candidates. A. Expression of CD69 and CD103 markers by influenza-specific CD4 and CD8 Tem cells induced by immunization with indicated vaccine. B. Expression of CD69 and CD103 markers by RSV-specific CD8 Tem cells induced by immunization with indicated vaccine. Data were analyzed by 2-way ANOVA with Holm-Sidak's multiple comparison test (*p < 0.05, ****p < 0.0001).
3.5. Induced RSV-specific T cells protect mice against RSV without immunopathology
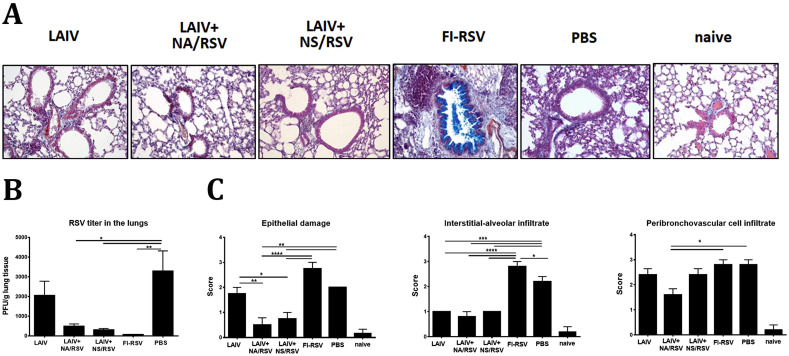
After challenge with live RSV A2 strain, the infectious virus was detected at significantly lower levels in both LAIV-RSV groups, compared with the PBS and LAIV only groups (Fig. 7 B). No RSV plaques were seen in the lungs of mice in the FI-RSV group, which is in line with previously published studies. A comparative histopathological study of the preparations of the lungs of mice on day 5 after challenge with live RSV A2 revealed the presence of homogeneous changes in the peribronchiolar and perivascular zones, as well as alveolar interstitium, atelectasis and emphysema, to different degrees in the different groups of mice. In the control groups, the areas of pulmonary tissue collapse accounted for 1/5 to 1/3 of the histological section area, and were often accompanied by emphysematous dilated alveoli along the periphery of the lung lobes, indicating uneven air filling in the alveoli. In both LAIV-RSV groups, damage to the bronchiolar epithelium and signs of alveolar-interstitial cell infiltration were minimal (Fig. 7C). These vaccines protected mice against both damage to lung tissue by RS virus and reduced respiratory function. In the FI-RSV group, overproduction of mucus (stained with Alcian blue) in the bronchi and bronchioles, bronchiolelectasis, peribronchiolar and perivascular infiltration with mononuclear cells, and eosinophilic leukocytes (inclusions in the cytoplasm stained with Congo red) were noted. These pathological changes were not observed in the other test groups. Overall, histopathological evaluation of lung sections of RSV-challenged mice revealed a high degree of protection by LAIV-RSV vaccines, with evidence of slightly higher protection against pulmonary pathology in the LAIV+NA/RSV group than the LAIV+NS/RSV group. These findings indicate that the more robust RSV-specific CD8 TRM responses induced by the LAIV+NA/RSV vaccine could have played a role in better protection against RSV disease.
Fig. 7.
Assessment of RSV replication and histopathological examination of lungs of mice five days after challenge with RSV. A. Representative photographs of the lung sections simultaneously stained with Congo Red, haematoxylin and Alcian blue. B. Replication of RSV A2 strain in the lungs of immunized mice at day 5 after RSV challenge. C. Scores of pathological changes. Data were analyzed with one-way ANOVA with Holm-Sidak's multiple comparisons test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
4. Discussion
RS virus is able to cause recurrent infection in people because it does not produce a long-lived immunological memory. In particular, it has been shown that RSV infection leads to suboptimal activation of dendritic cells, which subsequently leads to impaired immunological synapse formation, causing insufficient activation of naïve lymphocytes, failure of differentiation, and absence of long-lived T-cell memory in the tissue (Gonzalez et al., 2008). In contrast, infection with influenza virus produces a long-lived protective immune response, with the antigen being presented in an optimal way for formation of immunological memory. LAIV virus replicates in the upper respiratory tract and generates an immune response similar to that to natural influenza infection, including antibody- and cell-mediated immunity. It is thus a promising viral vector for the delivery of immunogenic epitopes of other respiratory viruses, including RSV, to the site of virus infection. We hypothesized that individual RSV epitopes delivered to the site of infection by LAIV, in the absence of the other RSV proteins, could activate dendritic cells in an optimal way, inducing various T cell memory populations. We had previously generated two LAIV-RSV recombinant viruses, which carry several immunogenic epitopes of RSV M2 protein. These viruses have been shown to induce a functional cytotoxic T-cell response and to protect mice against both influenza and RSV challenge without causing immunopathology and eosinophil infiltration (Kotomina et al., 2018, 2019). In accordance with other studies, truncation of NS1 protein in the LAIV+NS/RSV candidate led to the inability to cause a productive infection in the respiratory tract, without affecting the immunogenicity against influenza (Ferko et al., 2001; Talon et al., 2000; Zhou et al., 2010).
For viruses like RSV, it is important to establish the precise mechanisms of the development and maintenance of virus-specific immunity, especially in light of the severe immunopathology caused by the excessive generation of RSV M2 epitope-specific systemic memory CD8 T cells after natural RSV infection (Schmidt et al., 2018). Our previous studies demonstrated that our LAIV-RSV vaccine candidates generate systemic RSV M282-specific CD8 T cells and a robust recall response in the BAL and lungs of immunized mice following RSV challenge, which accelerated viral clearance without exacerbation of disease (Kotomina et al., 2018, 2019). However, we have not previously measured the magnitude of different memory T-cell subsets, such as effector memory and tissue-resident memory, which have RSV- or LAIV-specificity. Since the vaccines being developed are assumed to be effective against both influenza and RSV, it is important to understand the balance between T-cell responses to both pathogens after vaccination.
The current study found that both vectored LAIV-RSV vaccine candidates generated significant levels of both IFNγ- and TNFα-secreted RSV M2-specific CD8 Tem cells, most of which were double cytokine producers. This is in accordance with the recall response data from the previous study (Kotomina et al., 2019). Importantly, in contrast to the study by (Schmidt et al., 2018), these functional systemic RSV-specific double cytokine-producing CD8 memory T cells did not cause immunopathology following RSV infection.
A number of recent experimental studies reported the establishment of a specialized subset of memory T cells that remain in the tissue and do not re-enter the circulation following the resolution of infection (reviewed by (Nolz, 2015; Takamura, 2017)). These resident memory T cells can quickly respond to the invading pathogen, either by secreting cytokines and chemokines to recruit immune cells to the infected tissue (McMaster et al., 2015) or by rapid proliferation and killing of the infected cells (Schenkel and Masopust, 2014), thus providing local protection immediately after infection. Such TRM in the lungs are commonly identified by the expression of CD69, an early activation marker, and the αE integrin (CD103) chain of αEβ7 which binds E-cadherin and helps to retain TRM specifically in tissues (Cepek et al., 1994). A recent study found that immunization with LAIV could efficiently induce lung-localized TRM (Zens et al., 2016). We therefore assessed the levels of Tem cells (CD44+CD62L−) with surface markers TRM CD69 and CD103 in the lungs of mice immunized with LAIV, LAIV-RSV candidates and FI-RSV. Phenotyping of lung lymphocytes showed that both the vector itself and the vectored vaccines induced CD8 TRM with the double phenotype CD69+CD103+; however, the vectored vaccines generated a significantly higher level of TRM than LAIV. Unexpectedly, we found that the vectored vaccines also stimulated the formation of CD4 Tem cells with CD69+CD103+ phenotype. This can be explained by the inclusion in the RSV construct of the CD4 epitope of the M2-1 RSV protein amino acids 131 to 146, which is specific for Th1 and stimulates the production of interferon in BALB/c mice (McDermott et al., 2014). The response to this peptide was not assessed in the current study, since our early experiments showed an absence of detectable systemic CD4 T-cell responses to this epitope after vaccination with the LAIV-RSV vaccine candidates (unpublished data). However, in the light of the current study, it is worthwhile to estimate the level of RSV М2131-146-specific CD4 T-cell responses in the lungs. The induction of such CD4 TRM could play a significant role in keeping a balance of RSV-specific CD4/CD8 T-cell responses after immunization with LAIV-RSV vaccine, thus preventing lung immunopathology. In addition, CD103-expressing CD4 T cells are not usually detected in the lungs (Mueller and Mackay, 2016); the CD4 TRM are rather localized in the interstitium of the lung and express CD49b as a typical marker (Richter et al., 2007). Therefore, in future experiments, both CD103 and CD49b markers should be used to assess RSV-specific CD4 T-cell responses in the lungs of mice immunized with LAIV-RSV vaccine candidates.
The LAIV vector itself induced rather weak TRM responses, which is in contrast to a previous study that found high levels of CD103-positive CD8 TRM in mice immunized with commercial LAIV (Zens et al., 2016). In that study, a quadrivalent vaccine formulation was used, including two influenza B viruses (FluMist) at a dose of 105.5-106.5 FFU per strain, whereas in our study all vaccines were administered as a single strain at a dose of 106.0 EID50. The antigen load could have played a role in the formation of memory T-cell responses in the lungs of immunized mice. In addition, Zens et al. used C57BL6/J mice, which differ significantly from BALB/c mice in the development of T-cell immune responses to influenza (Califano et al., 2018; Sellers et al., 2012).
We further estimated the levels of influenza virus-specific CD4 and CD8 TRM cells in the lungs of immunized mice, as well as RSV M282 epitope-specific CD8 TRM. Specificity was determined by IFNγ secretion upon in vitro stimulation of isolated lung cells with the corresponding antigen. For maximal influenza epitope coverage we used whole virus for lymphocyte stimulation. As expected, the LAIV and both LAIV-RSV groups induced strong virus-specific CD4 and CD8 Tem responses after vaccination. While CD4 T-cell responses to influenza were similar in the LAIV and LAIV-RSV groups, the influenza-specific CD8 Tem cell levels were significantly higher in the LAIV group than in the LAIV-RSV groups. Interestingly, the vast majority of influenza-specific CD4 T cells in the lungs possessed the CD69+CD103- phenotype, with no differences between the LAIV and LAIV-RSV groups, further indicating that the CD69+CD103+ CD4 memory T cells found in the LAIV-RSV groups are not specific for influenza. Surprisingly, 40–60% of influenza-specific CD8 Tem cells induced by LAIV-RSV chimeric viruses were positive for both TRM markers CD69 and CD103, whereas the corresponding proportion in the LAIV group was significantly lower. These data suggest that the robust TRM response to the inserted RSV immunodominant epitope could have boosted the influenza-specific TRM responses as well. Indeed, the recombinant vaccines induced high levels of RSV-specific CD8 Tem, about 75% of which carried CD69, while at last half had the “dual” TRM CD69+CD103+ phenotype. There was a clear dependence on the magnitude of induced RSV-specific and influenza-specific CD8 TRM responses, with higher frequencies in the LAIV+NA/RSV group, compared with LAIV+NS/RSV group. A possible explanation for the co-stimulatory effect of the RSV epitope on TRM formation to influenza virus epitope(s) is that differentiation of effector CD8+ T cells into tissue-resident memory CD8+ T cell populations occurs in response to cytokines, such as transforming growth factor (TGF-β) and, to a lesser extent, IL-33 and tumor necrosis factor alpha (TNFα) (Mackay et al., 2013; Nolz, 2015; Wakim et al., 2015; Zhang and Bevan, 2013). It is possible that the local microenvironment formed by the RSV-specific memory T cells in the lungs can increase influenza-specific CD8 TRM formation, which is relevant since all these epitopes are expressed together within an infected cell and can be simultaneously processed through the MHC-I pathway.
About 25% of the RSV-specific lung-localized Tem did not have TRM markers and were probably migratory Tem cells. The major limitation of our study is that we did not use an intravascular antibody-labeling procedure to differentiate the population of T cells that reside permanently in the lungs and those that transit the tissue and re-enter the bloodstream (Anderson et al., 2014). However, the identification of virus-specific CD8 T cell subsets expressing both CD69 and CD103 markers suggest that they remain in lung tissue (Liu et al., 2018). It should be noted that the localization of TRMs in peripheral tissue is important and depends directly on their phenotype. Thus, two “lines of defense” can be distinguished: the first is TRM cells located directly in the respiratory tract, which are the first to encounter infection. They possess CD103 marker (receptor for E-cadherin), which determines their location within the epithelial cells of the respiratory tract, and this is probably why the proportion of CD69+CD103+ CD8 T cells is relatively low. The second line of defense against reinfection is TRMs located in the interstitium of the lungs and basal membranes of the epithelium of the respiratory tract. These possess CD49a marker, the VLA-1 receptor that binds to type I and IV collagen (Takamura, 2017). In the current study, we observed a decrease in RSV titer at only one time point (5 days post challenge), while it would be important to assess the impact of the CD8 TRM levels on the kinetics of RS virus clearance from the lungs of immunized mice.
Overall, the results of our study indicate that the designed LAIV-RSV recombinant viruses carrying immunodominant T-cell epitopes of RSV are capable of inducing balanced cell-mediated immune responses in the lungs that afford strong protection against both influenza and RSV without causing pathological changes in the lung tissues. The LAIV vector not only delivered the RSV epitopes to the site of viral entry, but also correctly presented these epitopes to the immune system. This is the first evidence that LAIV vector-based vaccination can induce robust lung-localized T-cell immunity to the inserted T-cell epitope of a foreign pathogen, without altering the immunogenicity of the viral vector itself. Since the LAIV-RSV vaccine is also designed for the elderly as a risk group for RSV infection, it is important to evaluate the immunogenicity of the recombinant viruses in aged mice as well.
H7N9 pandemic LAIV was used in this study for modelling purposes, as this strain is known to replicate well in the upper (but not lower) respiratory tract, inducing high virus-specific neutralizing antibody titers, as well as cell-mediated immunity (Stepanova et al., 2019), but any other LAIV strain could be used for this purpose. It is of note that the insertion of foreign epitopes in the NA other than N9 might yield slightly different immunogenicity to the target pathogen, whereas insertion in the NS gene of LAIV virus is universal and can be combined with any other HA and NA genes of LAIV viral vector. Our results provide a rationale for generating a universal multivalent vaccine against various pathogens that naturally target the human respiratory tract, such as RSV, parainfluenza viruses, adenoviruses, coronaviruses, metapneumoviruses, and rhinoviruses.
To conclude, the LAIV-RSV constructs evaluated in this study were generated as a proof-of-concept for detailed assessment of the establishment of CD4 and CD8 T-cell subsets in mice following intranasal immunization. The promising data obtained allow the further development of T cell-based RSV vaccines by incorporating human RSV epitopes into the LAIV genome. RSV human challenge models will help to accelerate this vaccine development (Habibi and Chiu, 2017). In particular, new data are emerging regarding RSV epitopes for CD4 airway-resident T cells (Guvenel et al., 2020) and CD8 resident memory T cells (Jozwik et al., 2015) with a defined immunodominance hierarchy. In addition, a combination of B-cell and T-cell RSV epitopes can be considered for RSV vaccine design (Retamal-Diaz et al., 2019). It is desirable that the safe and immunogenic RSV vaccine should contain a B-cell epitope with neutralizing activity, a CD4 epitope (most likely Th1, so that there is no possible bias towards Th2 and Th17, which are associated with asthma) and CD8 epitopes, both highly immunogenic and less dominant (Ruckwardt et al., 2010).
Funding
The current research was supported by Russian Science Foundation Grant №17-75-20054.
Declaration of competing interest
All authors have no conflicts to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.antiviral.2020.104864.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
Gating strategy for measuring IFNγ- and/or TNFα-secreting CD8 Tem cells in mouse spleens using an ICS assay.
Fig. S2.
Gating strategy for phenotyping IFNγ-secreting CD4 and CD8 T cells in the lungs.
References
- Albert E.J., Duplisea J., Dawicki W., Haidl I.D., Marshall J.S. Tissue eosinophilia in a mouse model of colitis is highly dependent on TLR2 and independent of mast cells. Am. J. Pathol. 2011;178:150–160. doi: 10.1016/j.ajpath.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K.G., Mayer-Barber K., Sung H., Beura L., James B.R., Taylor J.J., Qunaj L., Griffith T.S., Vezys V., Barber D.L., Masopust D. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat. Protoc. 2014;9:209–222. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascough S., Paterson S., Chiu C. Induction and subversion of human protective immunity: contrasting influenza and respiratory syncytial virus. Front. Immunol. 2018;9:323. doi: 10.3389/fimmu.2018.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Califano D., Furuya Y., Metzger D.W. Effects of influenza on alveolar macrophage viability are dependent on mouse genetic strain. J. Immunol. 2018;201:134–144. doi: 10.4049/jimmunol.1701406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepek K.L., Shaw S.K., Parker C.M., Russell G.J., Morrow J.S., Rimm D.L., Brenner M.B. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- Collins P.L., Fearns R., Graham B.S. Respiratory syncytial virus: virology, reverse genetics, and pathogenesis of disease. Curr. Top. Microbiol. Immunol. 2013;372:3–38. doi: 10.1007/978-3-642-38919-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferko B., Stasakova J., Sereinig S., Romanova J., Katinger D., Niebler B., Katinger H., Egorov A. Hyperattenuated recombinant influenza A virus nonstructural-protein-encoding vectors induce human immunodeficiency virus type 1 Nef-specific systemic and mucosal immune responses in mice. J. Virol. 2001;75:8899–8908. doi: 10.1128/JVI.75.19.8899-8908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez P.A., Prado C.E., Leiva E.D., Carreno L.J., Bueno S.M., Riedel C.A., Kalergis A.M. vol. 105. 2008. Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells; pp. 14999–15004. (Proceedings of the National Academy of Sciences of the United States of America). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B.S., Bunton L.A., Wright P.F., Karzon D.T. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Invest. 1991;88:1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B.S., Henderson G.S., Tang Y.W., Lu X., Neuzil K.M., Colley D.G. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J. Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- Guvenel A., Jozwik A., Ascough S., Ung S.K., Paterson S., Kalyan M., Gardener Z., Bergstrom E., Kar S., Habibi M.S., Paras A., Zhu J., Park M., Dhariwal J., Almond M., Wong E.H., Sykes A., Del Rosario J., Trujillo-Torralbo M.B., Mallia P., Sidney J., Peters B., Kon O.M., Sette A., Johnston S.L., Openshaw P.J., Chiu C. Epitope-specific airway-resident CD4+ T cell dynamics during experimental human RSV infection. J. Clin. Invest. 2020;130:523–538. doi: 10.1172/JCI131696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi M.S., Chiu C. Controlled human infection with RSV: the opportunities of experimental challenge. Vaccine. 2017;35:489–495. doi: 10.1016/j.vaccine.2016.08.086. [DOI] [PubMed] [Google Scholar]
- Hall C.B., Walsh E.E., Long C.E., Schnabel K.C. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- Isakova-Sivak I., Grigorieva E., Rudenko L. Insights into current clinical research on the immunogenicity of live attenuated influenza vaccines. Expert Rev. Vaccines. 2020:1–13. doi: 10.1080/14760584.2020.1711056. [DOI] [PubMed] [Google Scholar]
- Isakova-Sivak I., Tretiak T., Rudenko L. Cold-adapted influenza viruses as a promising platform for viral-vector vaccines. Expet Rev. Vaccine. 2016;15:1241–1243. doi: 10.1080/14760584.2016.1208088. [DOI] [PubMed] [Google Scholar]
- Isakova-Sivak I.N., Korenkov D.A., Fedorova E.A., Tretiak T.S., Matyushenko V.A., Smolonogina T.A., Rudenko L.G. Analysis of immune epitopes of respiratory syncytial virus for designing of vectored vaccines based on influenza virus platform. Bull. Exp. Biol. Med. 2016;161:533–537. doi: 10.1007/s10517-016-3454-7. [DOI] [PubMed] [Google Scholar]
- Jozwik A., Habibi M.S., Paras A., Zhu J., Guvenel A., Dhariwal J., Almond M., Wong E.H.C., Sykes A., Maybeno M., Del Rosario J., Trujillo-Torralbo M.B., Mallia P., Sidney J., Peters B., Kon O.M., Sette A., Johnston S.L., Openshaw P.J., Chiu C. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat. Commun. 2015;6:10224. doi: 10.1038/ncomms10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.W., Canchola J.G., Brandt C.D., Pyles G., Chanock R.M., Jensen K., Parrott R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Knudson C.J., Hartwig S.M., Meyerholz D.K., Varga S.M. RSV vaccine-enhanced disease is orchestrated by the combined actions of distinct CD4 T cell subsets. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1004757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenkov D., Isakova-Sivak I., Rudenko L. Basics of CD8 T-cell immune responses after influenza infection and vaccination with inactivated or live attenuated influenza vaccine. Expert Rev. Vaccines. 2018;17:977–987. doi: 10.1080/14760584.2018.1541407. [DOI] [PubMed] [Google Scholar]
- Kotomina T., Isakova-Sivak I., Matyushenko V., Kim K.H., Lee Y., Jung Y.J., Kang S.M., Rudenko L. Recombinant live attenuated influenza vaccine viruses carrying CD8 T-cell epitopes of respiratory syncytial virus protect mice against both pathogens without inflammatory disease. Antivir. Res. 2019;168:9–17. doi: 10.1016/j.antiviral.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotomina T., Korenkov D., Matyushenko V., Prokopenko P., Rudenko L., Isakova-Sivak I. Live attenuated influenza vaccine viral vector induces functional cytotoxic T-cell immune response against foreign CD8+ T-cell epitopes inserted into NA and NS1 genes using the 2A self-cleavage site. Hum. Vaccines Immunother. 2018;14:2964–2970. doi: 10.1080/21645515.2018.1502529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause A., Xu Y., Ross S., Wu W., Joh J., Worgall S. Absence of vaccine-enhanced RSV disease and changes in pulmonary dendritic cells with adenovirus-based RSV vaccine. Virol. J. 2011;8:375. doi: 10.1186/1743-422X-8-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ma C., Zhang N. Tissue-specific control of tissue-resident memory T cells. Crit. Rev. Immunol. 2018;38:79–103. doi: 10.1615/CritRevImmunol.2018025653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay L.K., Rahimpour A., Ma J.Z., Collins N., Stock A.T., Hafon M.L., Vega-Ramos J., Lauzurica P., Mueller S.N., Stefanovic T., Tscharke D.C., Heath W.R., Inouye M., Carbone F.R., Gebhardt T. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- Matsuse H., Behera A.K., Kumar M., Rabb H., Lockey R.F., Mohapatra S.S. Recurrent respiratory syncytial virus infections in allergen-sensitized mice lead to persistent airway inflammation and hyperresponsiveness. J. Immunol. 2000;164:6583–6592. doi: 10.4049/jimmunol.164.12.6583. [DOI] [PubMed] [Google Scholar]
- McDermott D.S., Knudson C.J., Varga S.M. Determining the breadth of the respiratory syncytial virus-specific T cell response. J. Virol. 2014;88:3135–3143. doi: 10.1128/JVI.02139-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster S.R., Wilson J.J., Wang H., Kohlmeier J.E. Airway-resident memory CD8 T cells provide antigen-specific protection against respiratory virus challenge through rapid IFN-gamma production. J. Immunol. 2015;195:203–209. doi: 10.4049/jimmunol.1402975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn K.G.I., Zhou F., Brokstad K.A., Sridhar S., Cox R.J. Boosting of cross-reactive and protection-associated T cells in children after live attenuated influenza vaccination. J. Infect. Dis. 2017;215:1527–1535. doi: 10.1093/infdis/jix165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito K.M., Ruckwardt T.R., Redwood A.J., Moin S.M., Price D.A., Graham B.S. Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung. Mucosal Immunol. 2017;10:545–554. doi: 10.1038/mi.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S.N., Mackay L.K. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- Nolz J.C. Molecular mechanisms of CD8(+) T cell trafficking and localization. Cell. Mol. Life Sci.: CMLS. 2015;72:2461–2473. doi: 10.1007/s00018-015-1835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M.R., Varga S.M. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J. Immunol. 2007;179:5415–5424. doi: 10.4049/jimmunol.179.8.5415. [DOI] [PubMed] [Google Scholar]
- Retamal-Diaz A., Covian C., Pacheco G.A., Castiglione-Matamala A.T., Bueno S.M., Gonzalez P.A., Kalergis A.M. Contribution of resident memory CD8(+) T cells to protective immunity against respiratory syncytial virus and their impact on vaccine design. Pathogens. 2019;8 doi: 10.3390/pathogens8030147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M., Ray S.J., Chapman T.J., Austin S.J., Rebhahn J., Mosmann T.R., Gardner H., Kotelianski V., deFougerolles A.R., Topham D.J. Collagen distribution and expression of collagen-binding alpha1beta1 (VLA-1) and alpha2beta1 (VLA-2) integrins on CD4 and CD8 T cells during influenza infection. J. Immunol. 2007;178:4506–4516. doi: 10.4049/jimmunol.178.7.4506. [DOI] [PubMed] [Google Scholar]
- Ruckwardt T.J., Luongo C., Malloy A.M., Liu J., Chen M., Collins P.L., Graham B.S. Responses against a subdominant CD8+ T cell epitope protect against immunopathology caused by a dominant epitope. J. Immunol. 2010;185:4673–4680. doi: 10.4049/jimmunol.1001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel J.M., Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M.E., Knudson C.J., Hartwig S.M., Pewe L.L., Meyerholz D.K., Langlois R.A., Harty J.T., Varga S.M. Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers R.S., Clifford C.B., Treuting P.M., Brayton C. Immunological variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Vet. Pathol. 2012;49:32–43. doi: 10.1177/0300985811429314. [DOI] [PubMed] [Google Scholar]
- Shi T., McAllister D.A., O'Brien K.L., Simoes E.A.F., Madhi S.A., Gessner B.D., Polack F.P., Balsells E., Acacio S., Aguayo C., Alassani I., Ali A., Antonio M., Awasthi S., Awori J.O., Azziz-Baumgartner E., Baggett H.C., Baillie V.L., Balmaseda A., Barahona A., Basnet S., Bassat Q., Basualdo W., Bigogo G., Bont L., Breiman R.F., Brooks W.A., Broor S., Bruce N., Bruden D., Buchy P., Campbell S., Carosone-Link P., Chadha M., Chipeta J., Chou M., Clara W., Cohen C., de Cuellar E., Dang D.A., Dash-Yandag B., Deloria-Knoll M., Dherani M., Eap T., Ebruke B.E., Echavarria M., de Freitas Lazaro Emediato C.C., Fasce R.A., Feikin D.R., Feng L., Gentile A., Gordon A., Goswami D., Goyet S., Groome M., Halasa N., Hirve S., Homaira N., Howie S.R.C., Jara J., Jroundi I., Kartasasmita C.B., Khuri-Bulos N., Kotloff K.L., Krishnan A., Libster R., Lopez O., Lucero M.G., Lucion F., Lupisan S.P., Marcone D.N., McCracken J.P., Mejia M., Moisi J.C., Montgomery J.M., Moore D.P., Moraleda C., Moyes J., Munywoki P., Mutyara K., Nicol M.P., Nokes D.J., Nymadawa P., da Costa Oliveira M.T., Oshitani H., Pandey N., Paranhos-Baccala G., Phillips L.N., Picot V.S., Rahman M., Rakoto-Andrianarivelo M., Rasmussen Z.A., Rath B.A., Robinson A., Romero C., Russomando G., Salimi V., Sawatwong P., Scheltema N., Schweiger B., Scott J.A.G., Seidenberg P., Shen K., Singleton R., Sotomayor V., Strand T.A., Sutanto A., Sylla M., Tapia M.D., Thamthitiwat S., Thomas E.D., Tokarz R., Turner C., Venter M., Waicharoen S., Wang J., Watthanaworawit W., Yoshida L.M., Yu H., Zar H.J., Campbell H., Nair H., Network R.S.V.G.E. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikiatkhachorn A., Braciale T.J. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J. Exp. Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova E.A., Kotomina T.S., Matyushenko V.A., Smolonogina T.A., Shapovalova V.S., Rudenko L.G., Isakova-Sivak I.N. Amino acid substitutions N123D and N149D in hemagglutinin molecule enhance immunigenicity of live attenuated influenza H7N9 vaccine strain in experiment. Bull. Exp. Biol. Med. 2019;166:631–636. doi: 10.1007/s10517-019-04407-1. [DOI] [PubMed] [Google Scholar]
- Takamura S. Persistence in temporary lung niches: a survival strategy of lung-resident memory CD8(+) T cells. Viral Immunol. 2017;30:438–450. doi: 10.1089/vim.2017.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon J., Salvatore M., O'Neill R.E., Nakaya Y., Zheng H., Muster T., Garcia-Sastre A., Palese P. Influenza A and B viruses expressing altered NS1 proteins: a vaccine approach. Proc. Natl. Acad. Sci. U.S.A. 2000;97:4309–4314. doi: 10.1073/pnas.070525997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G.A., Kim H., Xue L. Why are vaccines against many human viral diseases still unavailable; an historic perspective? J. Med. Virol. 2020;92:129–138. doi: 10.1002/jmv.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham D.J., Reilly E.C. Tissue-resident memory CD8(+) T cells: from phenotype to function. Front. Immunol. 2018;9:515. doi: 10.3389/fimmu.2018.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim L.M., Smith J., Caminschi I., Lahoud M.H., Villadangos J.A. Antibody-targeted vaccination to lung dendritic cells generates tissue-resident memory CD8 T cells that are highly protective against influenza virus infection. Mucosal Immunol. 2015;8:1060–1071. doi: 10.1038/mi.2014.133. [DOI] [PubMed] [Google Scholar]
- Zens K.D., Chen J.K., Farber D.L. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight. 2016;1 doi: 10.1172/jci.insight.85832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Bevan M.J. Transforming growth factor-beta signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. 2013;39:687–696. doi: 10.1016/j.immuni.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Li Y., Belser J.A., Pearce M.B., Schmolke M., Subba A.X., Shi Z., Zaki S.R., Blau D.M., Garcia-Sastre A., Tumpey T.M., Wentworth D.E. NS-based live attenuated H1N1 pandemic vaccines protect mice and ferrets. Vaccine. 2010;28:8015–8025. doi: 10.1016/j.vaccine.2010.08.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.