Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for coronavirus disease 19 (COVID-19), has rapidly spread since December 2019 to become the focus of healthcare systems worldwide. Its highly contagious nature and significant mortality has led to its prioritization as a public health issue. The race to prevent and treat this disease has led to “off-label” prescribing of medications such as hydroxychloroquine, azithromycin, and Kaletra (lopinavir/ritonavir). Currently, there is no robust clinical evidence for the use of these drugs in the treatment of COVID-19, with most, if not all of these medications associated with the potential for QT interval prolongation, torsades de pointes, and resultant drug-induced sudden cardiac death. The aim of this document is to help healthcare providers mitigate the potential deleterious effects of drug-induced QTc prolongation.
Keywords: Drug-induced QTc prolongation, COVID-19, Guidelines, Torsades de pointes
Introduction
Since the first cases were reported in Wuhan, China, in December 2019, infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a worldwide pandemic, overwhelming health systems globally [1–3]. The rising death toll has inspired a race to identify safe and effective therapies to prevent the infection and to lessen the severity of the resultant COVID-19 respiratory illness. In the absence of proven effective pharmacological agents, a number of therapies have emerged as potential front-runners: lopinavir, a human immunodeficiency virus (HIV) type 1 aspartate protease inhibitor combined with ritonavir, as well as the re-purposed hydroxychloroquine, alone or in combination with azithromycin. While these agents have been shown to inhibit the growth of SARS-CoV-2 in vitro [4, 5], their systematic use has yet to be supported by any randomized clinical trial, though interim guidance advocates the use of these medications on a case by case basis [6].
The “off-label” use of these medications, based on anecdotal rather than definitive evidence, has brought with them their own set of hazards. At the forefront is the potential to prolong the heart rate–corrected QT interval (QTc), thereby increasing the risk of drug-induced torsades de pointes (TdP) and resultant drug-induced sudden cardiac death (SCD). Given that the prevalence of congenital long QT syndrome (LQTS) in the population is thought to be in the region of 1:2000 [7], and is generally considered to be a significantly underdiagnosed entity, we run the substantial risk of causing further harm in the absence of appropriate monitoring. The aim of this document is to highlight the areas of potential hazard with the use of such medications and to help healthcare providers circumvent and minimize their associated risks.
Pharmacodynamic and QTc-prolonging potential of “Covid-19 medications”
Antimalarial prophylactic drugs such as chloroquine and hydroxychloroquine are believed to act on the entry and post-entry stages (essential stages in viral replication) of SARS-CoV and SARS-CoV-2 infection, likely via effects on endosomal pH and the resulting under-glycosylation of angiotensin-converting enzyme 2 (ACE2) receptors that are required for viral entry into the host cell [4, 5, 8]. Based on this in vitro data, it has been hypothesized that hydroxychloroquine, more so than chloroquine, may have therapeutic efficacy in the COVID-19 pandemic. In addition, lopinavir combined with ritonavir has also been similarly touted for its reported success in inhibiting SARS-CoV in vitro [9, 10].
Based on this in vitro evidence, as well as anecdotal in vivo reports [11] of therapeutic benefit, many institutions worldwide have adopted the combination therapy of hydroxychloroquine and azithromycin or variations thereof as their first-line approach in the prevention and treatment of COVID-19. These drugs have been found to prolong the QTc interval, as identified in patient and physician databases such as crediblemeds.org [12]. This online global resource, created and maintained by AZCERT (Arizona Center for Education and Research on Therapeutics), scientifically assesses the risk of harm from medications and their relative potential to alter the QT interval, thus providing users worldwide with a reliable list of potentially hazardous medications.
Given that a substantial proportion of the world’s population, including an estimated 3 million individuals with both diagnosed and unidentified congenital LQTS, could stand to receive these medications as first-line therapy, the potential number of drug-induced SCDs attributable to the isolated use of any of these medications is not trivial. This risk could be further amplified if a number of these medications are used in combination. The institution of appropriate QTc monitoring algorithms is therefore a national and international priority.
Mitigating the potential risk of drug-induced TdP and SCD: Guidance on screening and monitoring
The subset of individuals who, due to congenital LQTS and/or by virtue of the presence of multiple risk factors [13], have exaggerated QTc prolongation at baseline (QTc > 500 ms) or inherent propensity to develop a disproportionate QTc response (delta QTc > 60 ms) are therefore most liable to the risk of adverse drug effects from these medications. To this end, if any patient is being considered for the prescription of any of the “off-label” treatments, it is suggested that:
Relevant history such as a personal history of arrhythmia/syncope, heart failure with reduced ejection fraction (HfrEF), ischemic heart disease (IHD), and family history of SCD should be elucidated.
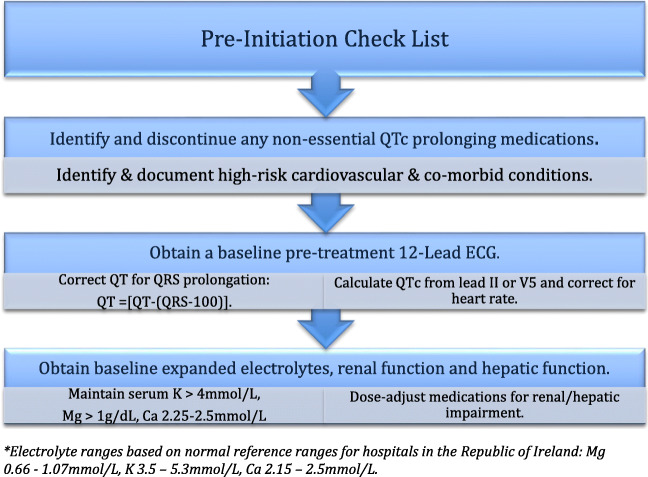
All non-essential QTc-prolonging medications should be identified and discontinued. A combined list of medications which have a known, possible, or conditional risk of causing QTc prolongation should be consulted and may be found at crediblemeds.org.
Electrolytes should be repleted to maintain potassium (K) > 4.0 mmol/L, magnesium (Mg) > 1 mg/dL, and calcium (Ca2) within normal range (2.15–2.5 mmol/L).
Baseline renal and hepatic function should be determined and monitored as inadequate dose adjustment of renal/hepatic eliminated drugs in patients with acute kidney injury, chronic kidney disease, and hepatic insufficiency may lead to higher plasma levels resulting in increased risk of drug-induced TdP/SCD.
- A baseline pre-treatment ECG should be obtained and the QTc measured by the traditional calculation from either lead II or V5 of the standard 12-lead ECG and corrected for heart rate using Bazett’s or Fridericia’s formula.
- It is suggested that, in line with AHA guidelines which advocate the over-reading and visual validation of the computer-generated QT duration [14], all clinicians should manually calculate the QT interval.
- Remember, whether by 12-lead ECG or by telemetry, if the noted QT interval is < ½ the preceding RR interval, then the QTc will always be < 460 ms.
QT is corrected for QRS prolongation in left bundle branch block (LBBB) or right bundle branch block (RBBB) morphology, non-specific intraventricular conduction delay (NICD), and RV paced rhythms such that QT = [QT − (QRS − 100)]. Chart 1.
Chart 1.
Check list
Risk stratification based on pre-treatment ECG
Acherman et al. [15] have recently published a protocol from the Mayo Clinic, Rochester, USA, outlining a “traffic light” system to triage or risk stratify patients based on their pre-treatment ECG. For clarity, this traffic light system has been adopted in these guidelines and should be applied following completion of the pre-initiation check list outlined above. Suggested monitoring based on this traffic light system is outlined below.
-
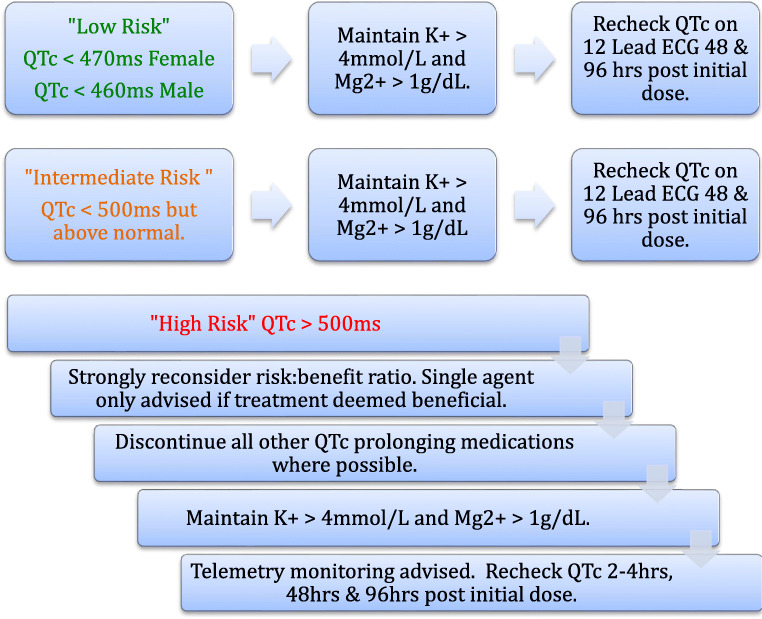
“Green Light”—low risk
If the QTc < 480 ms (adult female), < 470 ms (adult male), < 460 ms (pre-pubertal patient) then low risk of TdP is considered for treatment if it is clinically indicated.
-
“Orange Light”—intermediate risk
If the QTc < 500 ms but is greater than the low-risk thresholds, these patients are considered intermediate risk and the addition of these medications should be taken with caution.
-
“Red Light”—high risk
If the QTc is > 500 ms, these patients are considered high risk for drug-induced TdP. The overall benefit of commencing such patients on medications with the potential to further prolong the QTc needs to be strongly reconsidered and should only be undertaken with specialist input. Cessation of all other QTc-prolonging medications is strongly advised where possible and strict electrolyte optimization and ongoing monitoring undertaken.
If the treatment is still considered necessary, then telemetry is recommended for the ongoing review of QTc interval in order to avoid repeat ECGs and limit healthcare worker exposure.
Single-agent therapy should be opted for over combined therapy. Chart 2.
Chart 2.
Monitoring guidelines
Monitoring
The timing of QTc surveillance should be dictated by the determined risk for each individual, while attempting to minimize personnel exposure risk.
For those with a “red-light” status, repeat QTc measurement should be obtained around 2–4 h following first dose and then again at 48 h and 96 h (either by telemetry as recommended for red light patients, or if not available, by 12-lead ECG).
For those with an “orange light” or “green light” status, repeat measurement is suggested at 48 h and 96 h following the first dose.
- If the on-therapy QTc > 500 ms or the patient has displayed “QTc reactivity” with an increase in QTc > 60 ms, then the QTc countermeasures need to be re-examined, the degree of surveillance increased (i.e., telemetry obtained), and consideration given to stopping the offending medications based on a risk:benefit ratio.
- In high risk “red light” patients with a permanent pacemaker (PPM) or implantable cardioverter defibrillator (ICD), increasing the lower detection rate to 85 bpm should be considered.
Appropriate surveillance and risk modification aims to allow implementation of timely countermeasures and thereby hopefully circumvent the potential tragedy of iatrogenic SCD. This guidance serves to facilitate and assist decision-makers, however does not replace clinical context and judgment. Individualized interpretation is required to assess the risk:benefit ratio on a case by case basis.
Approaches in the management of drug-induced torsades de pointes
It is hoped that with appropriate risk stratification and monitoring, the incidence of drug-induced TdP will be significantly diminished. It is likely, however, given the increased use of the aforementioned medications that not all events can be avoided. In the event of drug-induced torsades de pointes, it is recommended that:
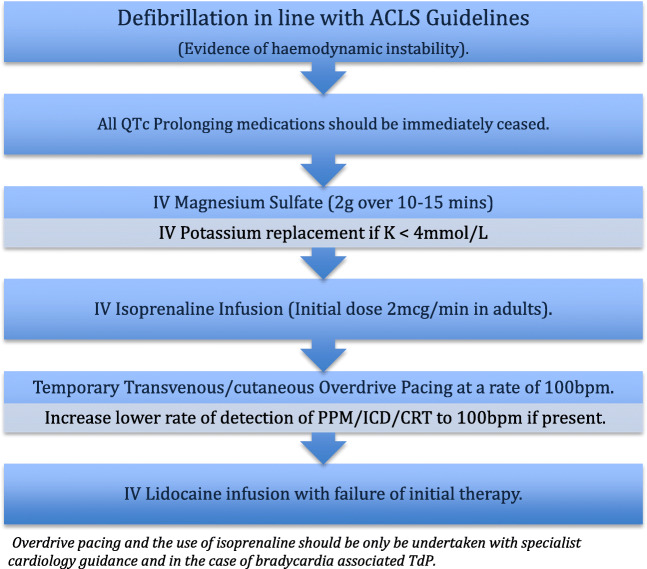
Prompt defibrillation is performed in patients with evidence of hemodynamic instability.
All QTc-prolonging medications should be ceased immediately.
Intravenous magnesium sulfate (initial dose 2 g IV over 10–15 min, followed by infusion) is first-line therapy in the stable patient [16, 17].
Intravenous potassium should be used in situations where serum K < 4 mmol/L [18].
- Temporary transvenous overdrive pacing (atrial or ventricular) at a rate of 100 beats per minute (bpm) is reserved for those who fail to respond to intravenous magnesium and TdP is associated with bradycardia [16, 19].
- In those patients with a permanent pacemaker (PPM) or implantable cardioverter defibrillator (ICD), the lower rate of detection should be increased to 100 bpm (or higher if required to achieve a rate which exceeds the patient’s native heart rate).
Isoprenaline (initial dose 2 mcg/min in adults, then titrated to achieve a heart rate of 100 bpm) can be used as a temporizing measure prior to overdrive pacing for bradycardia-associated TdP [16].
Where sedation is possible and available, transcutaneous pacing may be considered, again as a bridge to more definitive therapy.
If intensification of treatment is required where overdrive pacing or isoprenaline is not possible or deemed appropriate, intravenous lidocaine (and thereafter mexiletine) may be considered though these are less effective options. Chart 3.
Chart 3.
Management approach of drug-induced torsades de pointes
Special populations to consider during the COVID-19 pandemic
Devices: Permanent pacemakers/implantable cardioverter defibrillators
In patients with implanted devices, device modification may be considered to help reduce the risk of TdP. Specialist cardiac opinion should be sought prior to device adjustment.
Consider increasing the lower detection threshold to 85 bpm in “red light” patients prior to commencement of these medications.
In the event of drug-induced TdP with failure to respond to intravenous magnesium, be mindful of the option to increase the lower rate of detection to 100 bpm to act as “overdrive” pacing.
Clinicians are encouraged to remain cognisant of the importance of prompt therapy/device deactivation in those patients with ICDs who demonstrate severe respiratory illness due to COVID-19 and are not deemed appropriate candidates for escalation of therapy.
Brugada syndrome
COVID-19 infection can be associated with recurrent temperature spikes. Pyrexia is a known trigger for both the induction of Brugada pattern ECG abnormalities and cardiac arrest in persons known to have Brugada syndrome [20].
In patients with COVID-19 and known Brugada syndrome or those who develop a Brugada pattern on ECG with fever, aggressive temperature control measures should be instigated immediately.
Regular paracetamol administration is recommended.
Maintenance of serum K > 4 mmol/L advised.
Avoidance of “Brugada Medications” is essential—list available at www.brugadadrugs.org [21].
In the event of ventricular arrhythmia in a patient with known or suspected Brugada syndrome:
Prompt defibrillation is recommended for patients with evidence of hemodynamic instability.
Recurrent ventricular arrhythmias should be managed with amiodarone (200 mg daily after an appropriate initial loading dose) or quinidine (1–1.5 g/day of quinidine sulfate or 600–900 mg/day of hydroquinidine) divided into two to three equal daily doses [22, 23].
Intravenous isoprenaline (initial dose 2 mcg/min in adults) may be used in the event of ongoing acute ventricular arrhythmias as a temporizing measure [24].
Antiarrhythmic drugs
All antiarrhythmic drugs have the potential to be pro-arrhythmic and most are known to cause QTc prolongation. In patients who are prescribed antiarrhythmic medications for a prior history of ventricular arrhythmia, the addition of further QTc-prolonging medications needs to be heavily considered and the risk:benefit ratio strongly re-examined.
Myocarditis
COVID-19 has been described to be associated with a fulminant myocarditis as demonstrated by significantly elevated serum troponin levels and impaired LV systolic function, and in such patients, clinicians must remain mindful that the potential for malignant arrhythmia may be significantly increased, independent of the addition of any QTc-prolonging medications.
Pharmacy—the most commonly prescribed in-hospital medications with the greatest potential for QTc prolongation
Given that the list of potential QTc-prolonging medications is extensive, we felt that identifying the most commonly prescribed in-hospital medications with the greatest potential for QTc prolongation and associated TdP should be highlighted. We also highlight that the list provided by crediblemeds.org provides a reference for the extent and likelihood for QTc prolongation, with certain medications associated with greater risk than others.
The most commonly prescribed QTc-prolonging medications include [25]:
Antiarrhythmics
Antiemetics,
Antidepressants
Antipsychotics
Certain antimicrobials—particularly macrolides and fluoroquinolones.
Older, non-sedating antihistamines.
Conclusion
As the COVID-19 pandemic continues to dominate our healthcare landscape, with the death toll rising daily, it is important that healthcare providers have access to appropriate clinical guidance to help offset the potential hazards associated with the “off-label” prescribing of these medications, namely QTc prolongation and iatrogenic TdP/SCD. Thus, tactics such as this simple QTc surveillance strategy will help mitigate the potentially deleterious effects of drug-induced QTc prolongation, particularly if such medications are adopted on a national and international scale for the treatment of COVID-19.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spinelli A, Pellino G (2020) COVID-19 pandemic: perspectives on an unfolding crisis. Br J Surg 107(7):785–787 [DOI] [PMC free article] [PubMed]
- 3.Fauci AS, Lane HC, Redfield RR. Covid-19 — navigating the uncharted. N Engl J Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao X, Ye F, Zhang M et al (2020) In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis ciaa237. 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed]
- 6.Wilson KC, Chotirmall SH, Bai C et al (2020) Covid-19: interim guidance on management pending empirical evidence. American Thoracic Society-led International Task Force
- 7.Schwartz PJ, Stramba-Badiale M, Crotti L, et al. Prevalence of the congenital long QT syndrome. Circulation. 2009;120:1761–1767. doi: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F, Chan KH, Jiang Y, et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gautret P, Lagier JC, Parola P, et al Hydroxychloroquine and azithromycin as a treatment of COVID-19: preliminary results of an open-label non-randomized clinical trial. medRxiv. 2020:2020.2003.2016.20037135
- 12.Crediblemeds 2013 Combined QT drug list (all TdP risk categories). Available at: https://www.crediblemeds.org/index.php/new-drug-list. Last accessed: 19th April 2020
- 13.Tisdale JE, Heather A, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479–487. doi: 10.1161/CIRCOUTCOMES.113.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drew BJ, Ackerman MJ, Funk M, et al. Prevention of torsade de points in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55:934–947. doi: 10.1016/j.jacc.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giudicessi JR, Noseworthy PA, Friedman PA, et al Urgent guidance for navigating and circumventing the QTc prolonging and torsadogenic potential of possible pharmacotherapies for COVID-19. [published online ahead of print March 25, 2020]. Mayo Clin Proc. 10.1016/j.mayocp.2020.03.024 [DOI] [PMC free article] [PubMed]
- 16.Passman R, Kadish A. Polymorphic ventricular tachycardia, long Q-T syndrome, and torsades de pointes. Med Clin North Am. 2001;85:321–341. doi: 10.1016/S0025-7125(05)70318-7. [DOI] [PubMed] [Google Scholar]
- 17.Tzivoni D, Banai S, Schuger C, et al. Treatment of torsade de points with magnesium sulfate. Circulation. 1988;77:392–397. doi: 10.1161/01.CIR.77.2.392. [DOI] [PubMed] [Google Scholar]
- 18.Choy AM, Lang CC, Chomsky DM, et al. Normalization of acquired QT prolongation in humans by intravenous potassium. Circulation. 1997;96:2149–2154. doi: 10.1161/01.CIR.96.7.2149. [DOI] [PubMed] [Google Scholar]
- 19.Hondeghem LM, Snyders DJ. Class III antiarrhythmic agents have a lot of potential but a long way to go. Reduced effectiveness and dangers of reverse use dependence. Circulation. 1990;81:686. doi: 10.1161/01.CIR.81.2.686. [DOI] [PubMed] [Google Scholar]
- 20.Michowitz Y, Milman A, Sarquella-Brugada G, et al. Fever-related arrhythmic events in the multicenter survey on arrhythmic events in Brugada syndrome. Heart Rhythm. 2018;15:1394–1401. doi: 10.1016/j.hrthm.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 21.BrugadaDrugs.org Advisory Board 2009. Drugs to be avoided by Brugada syndrome patients. Available at: https://www.brugadadrugs.org/avoid/. Last accessed: 19th April 2020
- 22.Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Belhassen B, Glick A, Viskin S. Efficacy of quinidine in high-risk patients with Brugada syndrome. Circulation. 2004;110:1731–1737. doi: 10.1161/01.CIR.0000143159.30585.90. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe A, Fukushima Kusano F, Morita H, et al. Low-dose isoproterenol for repetitive ventricular arrhythmia in patients with Brugada syndrome. Eur Heart J. 2006;27:1579–1583. doi: 10.1093/eurheartj/ehl060. [DOI] [PubMed] [Google Scholar]
- 25.Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363–1372. doi: 10.1136/heart.89.11.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]