Abstract
Aims/hypothesis
Hyperglycaemia is associated with an elevated risk of mortality in community-acquired pneumonia, stroke, acute myocardial infarction, trauma and surgery, among other conditions. In this study, we examined the relationship between fasting blood glucose (FBG) and 28-day mortality in coronavirus disease 2019 (COVID-19) patients not previously diagnosed as having diabetes.
Methods
We conducted a retrospective study involving all consecutive COVID-19 patients with a definitive 28-day outcome and FBG measurement at admission from 24 January 2020 to 10 February 2020 in two hospitals based in Wuhan, China. Demographic and clinical data, 28-day outcomes, in-hospital complications and CRB-65 scores of COVID-19 patients in the two hospitals were analysed. CRB-65 is an effective measure for assessing the severity of pneumonia and is based on four indicators, i.e. confusion, respiratory rate (>30/min), systolic blood pressure (≤90 mmHg) or diastolic blood pressure (≤60 mmHg), and age (≥65 years).
Results
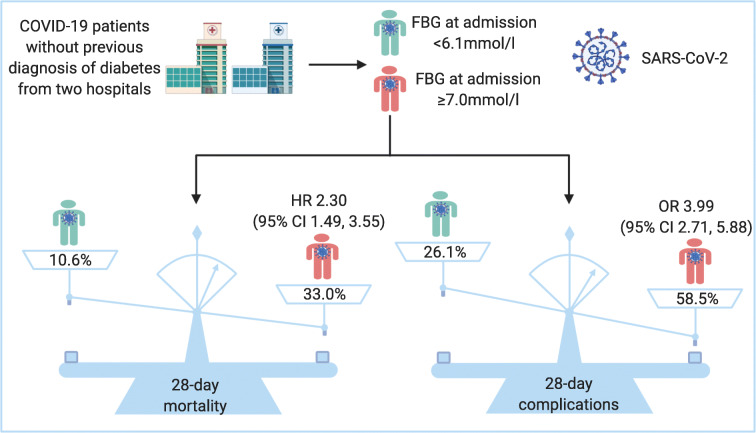
Six hundred and five COVID-19 patients were enrolled, including 114 who died in hospital. Multivariable Cox regression analysis showed that age (HR 1.02 [95% CI 1.00, 1.04]), male sex (HR 1.75 [95% CI 1.17, 2.60]), CRB-65 score 1–2 (HR 2.68 [95% CI 1.56, 4.59]), CRB-65 score 3–4 (HR 5.25 [95% CI 2.05, 13.43]) and FBG ≥7.0 mmol/l (HR 2.30 [95% CI 1.49, 3.55]) were independent predictors for 28-day mortality. The OR for 28-day in-hospital complications in those with FBG ≥7.0 mmol/l and 6.1–6.9 mmol/l vs <6.1 mmol/l was 3.99 (95% CI 2.71, 5.88) or 2.61 (95% CI 1.64, 4.41), respectively.
Conclusions/interpretation
FBG ≥7.0 mmol/l at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes. Glycaemic testing and control are important to all COVID-19 patients even where they have no pre-existing diabetes, as most COVID-19 patients are prone to glucose metabolic disorders.
Graphical abstract
Keywords: Coronavirus disease 2019, Fasting blood glucose, Mortality
Introduction
Since December 2019, a pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), dubbed coronavirus disease 2019 (COVID-19) hit Wuhan, Hubei province, China [1]. As the infection quickly spread around the globe to an alarming level, the WHO unprecedentedly declared a pandemic on 11 March 2020 [2]. As of 6 June 2020, the number of affected countries and regions has soared to 216, with more than 6,600,000 cases of COVID-19 confirmed worldwide [3]. A total of 392,000 patients have died of COVID-19, according to the WHO [3]. The crude fatality ratio (CFR) stood at 5.9% as of 6 June 2020 [3], whereas, at the inception of the outbreak, the overall CFR was 17.3%, as reported by the WHO–China joint mission [1], and even moderately ill patients may progress to death [1]. Therefore, it is crucial to assess the prognosis of the illness early and start intervention promptly.
As we know, diabetes is an established risk factor for significantly elevated mortality rates in a wide array of acute or chronic diseases, such as cardiovascular diseases, cerebrovascular diseases, cancer and infections due to poor glycaemic control and chronic hyperglycaemic state [4–7]. In addition, one study showed that fasting hyperglycaemia was strongly correlated with mortality in patients with or without diabetes [7]. Evidence indicated that a chronic hyperglycaemic state was associated with impaired immunity [6] and hyperglycaemia is an independent predictor for lower respiratory tract infection and poor prognosis [6, 8–10]. In particular, a few previous studies have shown that hyperglycaemia was a risk factor for high morbidity and mortality from severe acute respiratory syndrome (SARS) [11] and Middle East respiratory syndrome (MERS) [12].
Recently, a descriptive study suggested that diabetes and/or acute uncontrolled hyperglycaemia (defined as blood glucose measurements >10 mmol/l twice within any 24 h period) were associated with an increased length of hospital stay and higher mortality due to COVID-19 [13]. Furthermore, well-controlled blood glucose (glycaemic variability within 3.9–10.0 mmol/l) was reportedly associated with markedly lower mortality compared with individuals with poorly controlled blood glucose (upper limit of glycaemic variability exceeding 10.0 mmol/l) in patients with pre-existing type 2 diabetes during hospitalisation for COVID-19 [14]. However, direct correlation between fasting blood glucose (FBG) level at admission and clinical outcomes of COVID-19 patients without diagnosed diabetes has not been well established. Therefore, in this study, we examined the association between FBG on admission and the 28-day mortality of COVID-19 patients without previously diagnosed diabetes in two hospitals.
Methods
Study design and participants
We conducted a retrospective study of COVID-19 patients admitted to Wuhan Union West Hospital and Wuhan Red Cross Hospital to ascertain whether FBG was an independent predictor for 28-day mortality in patients with COVID-19 without a previous diagnosis of diabetes. COVID-19 infection was laboratory-confirmed in accordance with the interim guidance formulated by the WHO [15]. The aforementioned hospitals were two mandatorily designated hospitals for the treatment of COVID-19 patients in China. The institutional ethics committees of Wuhan Union Hospital (No. 0036) reviewed and approved this study protocol. No patients or medical staff involved in patient care took part in the study design and statistical analyses.
All consecutive patients included in this study had a definitive outcome (died, discharged or still hospitalised) within 28 days, with the time period spanning from 24 January 2020 to 10 February 2020. All patients received standard treatment, including antiviral therapy, respiratory support (nasal cannulation, mask oxygenation, high-flow nasal cannula oxygen therapy, non-invasive positive pressure ventilation or invasive mechanical ventilation), symptomatic and supportive treatment and antimicrobial therapy, as appropriate, to prevent or treat secondary infections, which was in accordance with the COVID-19 diagnosis and treatment protocols released by the National Health Commission of the People’s Republic of China [16]. This retrospective project did not interfere with the course of medical management.
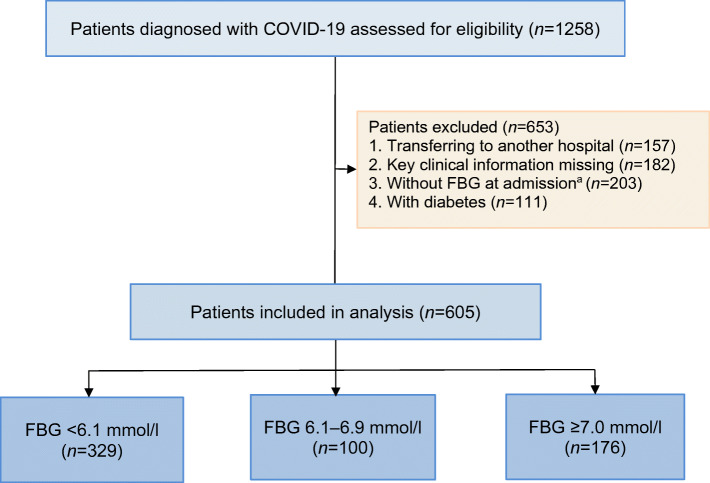
A total of 1258 confirmed COVID-19 patients were admitted into the two hospitals. Of these, 653 patients were ruled out for one of the following reasons: (1) no definitive 28-day outcome since they were transferred to another hospital; (2) missing key clinical information (e.g. demographic or clinical data); (3) no FBG data available at admission (for one of the following reasons: [a] patients had a blood glucose measurement taken before admission; [b] patients were tested for blood glucose 24 h after admission; [c] patients received a random blood glucose test but were not tested for FBG; [d] patients did not receive a blood glucose test since this was not routinely conducted for every COVID-19 patient); (4) having previously diagnosed diabetes. The flow diagram of patient selection is detailed in Fig. 1.
Fig. 1.
Flow diagram of patient selection. aReasons for no FBG measurement at admission: 13 patients had a blood glucose measurement taken before admission; 25 patients were tested for blood glucose 24 h after admission; 84 patients received a random blood glucose test but were not tested for FBG; and 81 patients did not receive a blood glucose test since this was not routinely conducted for every COVID-19 patient
Patients were discharged when they met the following discharge criteria: (1) body temperature returned to normal, lasting for more than 3 days; (2) respiratory symptoms significantly improved; (3) imaging examinations revealed that acute exudative lesions were significantly improved; (4) two real-time RT-PCR tests for the presence of SARS-CoV-2 virus yielded negative results (with two samples of respiratory specimens taken over 24 h apart) [16].
Data collection
We obtained data from the electronic records of the relevant departments. The following data were collected: demographics, clinical data (symptoms, past medical history, admission FBG and in-hospital complications) and the data on 28-day outcomes.
Past medical histories were obtained from hospital databases or by self-reporting, including diabetes, hypertension, chronic lung disease, chronic heart disease, chronic liver disease, chronic kidney disease, cerebrovascular disease and carcinoma, which were diagnosed according to standard criteria. The common complications that developed after hospitalisation included acute respiratory distress syndrome (ARDS), acute cardiac injury, acute kidney injury, acute liver injury, cerebrovascular accident, coagulopathy and secondary infection.
Definition and measurement of FBG levels at admission
Complications were defined as the occurrence of one or more condition(s) (Table 1) that developed after hospitalisation. ARDS was defined according to WHO clinical management interim guidance [17]. Acute cardiac injury was defined as new electrocardiographic and echocardiographic abnormalities detected, or serum level of cardiac biomarkers (cardiac troponin I, cardiac troponin T, or hype-sensitive troponin I) above the upper limit of normal (ULN) [18]. Acute kidney injury was defined as an elevation of serum creatinine by 26.5 μmol/l or higher within 48 h, or serum creatinine increased to 1.5 times baseline or higher within the previous 7 days [19]. Acute liver injury was defined as alanine aminotransferase or aspartate aminotransferase levels two times above the ULN. Cerebrovascular accident was defined as the occurrence of cerebral haemorrhage or cerebral infarction during hospitalisation [20]. Coagulopathy was defined as prothrombin time prolonged by 3 s or activated partial thromboplastin time prolonged by 5 s [21]. Secondary infection was defined as the occurrence of symptoms or signs of nosocomial infection and new pathogens detected in patients’ specimens (e.g. sputum, blood) taken more than 48 h after admission [18].
Table 1.
Baseline characteristics of COVID-19 patients without previous diagnosis of diabetes within 28 days after admission
| Variables | Total (n = 605) |
Non-survivor (n = 114) |
Survivor (n = 491) |
p value |
|---|---|---|---|---|
| Hospital | ||||
| Wuhan Red Cross Hospital | 157 (26.0) | 33 (21.0) | 124 (79.0) | 0.4178 |
| Wuhan Union West Hospital | 448 (74.0) | 81 (18.1) | 367 (81.9) | |
| Age, years | ||||
| Median (IQR) | 59.0 (47.0, 68.0) | 66.0 (61.0, 72.0) | 56.0 (43.0, 65.0) | <0.0001 |
| <65, n (%) | 408 (67.4) | 49 (43.0) | 359 (73.1) | <0.0001 |
| ≥65, n (%) | 197 (32.6) | 65 (57.0) | 132 (26.9) | |
| Sex | ||||
| Female, n (%) | 283 (46.8) | 36 (31.6) | 247 (50.3) | 0.0003 |
| Male, n (%) | 322 (53.2) | 78 (68.4) | 244 (49.7) | |
| Onset symptoms | ||||
| Fever, n (%) | 463/530 (87.4) | 88 (85.4) | 375 (87.8) | 0.5132 |
| Cough, n (%) | 404/555 (72.8) | 66 (66.7) | 338 (74.1) | 0.1308 |
| Expectoration, n (%) | 217/521 (41.7) | 43 (43.4) | 174 (41.2) | 0.6892 |
| Muscular soreness, n (%) | 129/504 (25.6) | 23 (24.5) | 106 (25.9) | 0.7813 |
| Fatigue, n (%) | 300/528 (56.8) | 58 (58.6) | 242 (56.4) | 0.6936 |
| Diarrhoea, n (%) | 91/512 (17.8) | 15 (15.3) | 76 (18.4) | 0.4774 |
| Past history of disease | 208 (34.4) | 55 (48.3) | 153 (31.2) | 0.0005 |
| Hypertension, n (%) | 139/543 (25.6) | 34 (29.8) | 105 (24.5) | 0.2447 |
| Chronic lung disease, n (%) | 18 (3.0) | 4 (3.5) | 14 (2.9) | 0.7587 |
| Chronic heart disease, n (%) | 55 (9.1) | 13 (11.4) | 42 (8.6) | 0.3404 |
| Chronic liver disease, n (%) | 16 (2.6) | 3 (2.6) | 13 (2.7) | >0.9999 |
| Chronic kidney disease, n (%) | 16 (2.6) | 6 (5.3) | 10 (2.0) | 0.0531 |
| Cerebrovascular disease, n (%) | 16 (2.6) | 7 (6.1) | 9 (1.8) | 0.0098 |
| Carcinoma, n (%) | 29 (4.8) | 9 (7.9) | 20 (4.1) | 0.0853 |
| CRB-65 score | <0.0001 | |||
| 0, n (%) | 334 (55.2) | 27 (23.7) | 307 (62.5) | |
| 1–2, n (%) | 261 (43.1) | 80 (70.2) | 181 (36.9) | |
| 3–4, n (%) | 10 (1.7) | 7 (6.1) | 3 (0.6) | |
| Admission FBG | <0.0001 | |||
| <6.1 mmol/l, n (%) | 329 (54.4) | 35 (30.7) | 294 (59.9) | |
| 6.1–6.9 mmol/l, n (%) | 100 (16.5) | 21 (18.4) | 79 (16.1) | |
| ≥7.0 mmol/l, n (%) | 176 (29.1) | 58 (50.9) | 118 (24.0) | |
| Complications | 237 (39.2) | 114 (100.0) | 123 (25.1) | <0.0001 |
| ARDS, n (%) | 142 (23.5) | 107 (93.9) | 35 (7.1) | <0.0001 |
| Acute cardiac injury, n (%) | 80 (13.2) | 63 (55.3) | 17 (3.5) | <0.0001 |
| Acute kidney injury, n (%) | 76 (12.6) | 65 (57.0) | 11 (2.2) | <0.0001 |
| Acute liver injury, n (%) | 167 (27.6) | 95 (83.3) | 72 (14.7) | <0.0001 |
| Cerebrovascular accident, n (%) | 3 (0.5) | 3 (2.6) | 0 | 0.0065 |
| Coagulopathy, n (%) | 96 (15.9) | 85 (74.6) | 11 (2.2) | <0.0001 |
| Secondary infection, n (%) | 79 (13.1) | 69 (60.5) | 10 (2.0) | <0.0001 |
| With complications | ||||
| <6.1 mmol/l, n (%) | 86 (14.2) | 35 (30.7) | 51 (10.4) | |
| 6.1–6.9 mmol/l, n (%) | 48 (7.9) | 21 (18.4) | 27 (5.5) | |
| ≥7.0 mmol/l, n (%) | 103 (17.0) | 58 (50.9) | 45 (9.2) | |
| Without complications | ||||
| <6.1 mmol/l, n (%) | 243 (40.2) | 0 | 243 (49.5) | |
| 6.1–6.9 mmol/l, n (%) | 52 (8.6) | 0 | 52 (10.6) | |
| ≥7.0 mmol/l, n (%) | 73 (12.1) | 0 | 73 (14.9) | |
Data are median (IQR) or n (%)
p values were calculated by using χ2 test, Cochran–Mantel–Haenszel χ2 test, Fisher’s exact test or Wilcoxon rank-sum test, as appropriate
Seventy-five patients (12.4%) had missing information on onset symptoms of fever; 50 (8.3%) on cough; 84 (13.9%) on expectoration; 101 (16.7%) on muscular soreness; 77 (12.7%) on fatigue; 93 (15.4%) on diarrhoea; and 62 (10.2%) on hypertension
For the test of FBG levels at admission, blood samples were collected after an overnight fast lasting at least 8 h within 24 h after admission, according to the WHO guidelines. Serum concentrations of FBG were measured by using an automatic biochemical analyser (Beckman Coulter AU5800 Analyzer, USA).
Assessment of pneumonia severity
CRB-65 is a generally accepted tool used for assessing the severity of pneumonia because of its simplicity and effectiveness. It is based on confusion, respiratory rate (>30/min), systolic blood pressure (≤90 mmHg) or diastolic blood pressure (≤60 mmHg), and age (≥65 years) [22, 23]. Given that pneumonia is the major clinical feature of hospitalised COVID-19 patients [24], and all participants enrolled had pneumonia, as confirmed by chest computed tomography (CT) scans, CRB-65 was used in this study to assess the severity of COVID-19. CRB-65 measures the severity of pneumonia on a 0 to 4 scale, and we grouped scores into three risk levels (CRB-65 score of 0; CRB-65 score of 1–2; CRB-65 score of 3–4) according to Ewig et al [23] and Lepper et al [9]. CRB-65 score 0, 1–2 and 3–4 are, respectively, representative of mild, moderate and severe pneumonia.
Outcome measures
All patients were categorised into three groups according to WHO guidelines in terms of admission FBG (<6.1, 6.1–6.9, and ≥7.0 mmol/l). Two outcome measures were examined: the independent risk factors for 28-day mortality and percentage differences in in-hospital complications between different FBG groups.
Statistical analysis
Descriptive statistics were used to describe patient baseline data. Categorical variables were presented as numbers with percentage proportions, and continuous variables were expressed as mean ± SD if they were normally distributed or as median (IQR) if they were not. Proportions for categorical variables were compared using the χ2 test, Cochran–Mantel–Haenszel χ2 test or Fisher’s exact test. Means of continuous variables were compared using independent group t test when the data were normally distributed. Otherwise, the Wilcoxon rank-sum test was used for medians.
For the analysis of mortality, we conducted a univariable Cox regression analysis to assess the effects of age, sex, onset symptoms, past medical history, CRB-65 score, and admission FBG on the 28-day mortality. Variables with p < 0.05 were regarded as potential risk factors and were included in multivariable Cox regression analysis by using the stepwise bidirectional selection (significance level for entry = 0.05, significance level to stay = 0.1). We conducted subgroup analysis by using Kaplan–Meier curves to assess associations between FBG or severity of pneumonia and mortality within 28 days, and tested linear trends across different groups of different FBG levels. Then we carried out a test for interaction of FBG levels and severity of pneumonia and stratified analyses according to severity of pneumonia.
Finally, univariable logistic analysis was used to assess the association between different FBG levels and in-hospital complications.
A two-sided p value <0.05 was considered to be statistically significant. All statistical analyses were performed using SAS software (version 9.4; USA).
Results
Patient characteristics
Up to 10 February 2020, 1258 patients were admitted to Wuhan Union West Hospital or Wuhan Red Cross Hospital for confirmed COVID-19. After excluding 157 patients who were re-directed to other hospitals, 182 patients missing key clinical information, 203 patients without FBG at admission and 111 patients with previous history of diabetes, a total of 605 patients without a previous diagnosis of diabetes (448 from Wuhan Union West Hospital and 157 from Wuhan Red Cross Hospital) were included in the analysis (Fig. 1).
Baseline data of continuous and categorical variables among the groups of survivors and non-survivors are shown in Table 1. The median age of participants was 59.0 years (IQR 47.0, 68.0), and 322 (53.2%) were men. Two hundred and eight patients (34.4%) had one or more past diseases, of which hypertension was the most common comorbidity. At admission, the most common symptoms were fever, followed by cough and fatigue. Three hundred and thirty four patients (55.2%) had a CRB-65 score of 0; 261 (43.1%) had a CRB-65 score of 1–2; 10 (1.7%) had a CRB-65 score of 3–4. The patients were categorised in terms of their glucose levels into three groups: patients with FBG <6.1 mmol/l (n = 329, 54.4%), patients with FBG 6.1–6.9 mmol/l (n = 100, 16.5%) and patients with FBG ≥7.0 mmol/l (n = 176, 29.1%). One hundred and fourteen patients (18.8%) died within 28 days during hospitalisation. Among 605 patients, 237 (39.2%) developed one or more in-hospital complications (Table 1).
Comparison between non-survivors and survivors
Compared with survivors, more non-survivors were found among older people (median age 66.0 years vs 56.0 years, p < 0.0001), male (68.4% vs 49.7%, p = 0.0003), patients with a past medical history (48.3% vs 31.2%, p = 0.0005). In terms of past medical history, cerebrovascular disease (6.1% vs 1.8%, p = 0.0098) was significantly higher in non-survivors than in survivors. In non-survivors, the percentages were higher in patients having CRB-65 score of 3–4 and FBG ≥7.0 mmol/l at admission (Table 1).
Factors associated with in-hospital 28-day mortality
The univariable Cox regression analysis showed that age, male sex, chronic kidney disease, cerebrovascular disease, CRB-65 score and FBG were associated with in-hospital 28-day mortality. The multivariable Cox regression analysis further suggested that age (HR 1.02 [95% CI 1.00, 1.04]), male sex (HR 1.75 [95% CI 1.17, 2.60]), CRB-65 score 1–2 (HR 2.68 [95% CI 1.56, 4.59]), CRB-65 score 3–4 (HR 5.25 [95% CI 2.05, 13.43]) and FBG ≥7.0 mmol/l (HR 2.30 [95% CI 1.49, 3.55]) were independent predictors for mortality (Table 2).
Table 2.
Univariable and multivariable analyses of various indicators for death within 28 days in all participants
| Univariable analysis HR (95% CI) | p value | Multivariable analysis HR (95% CI) | p value | ||
|---|---|---|---|---|---|
| Hospital | |||||
| Wuhan Red Cross Hospital | 1 (ref) | ||||
| Wuhan Union West Hospital | 0.85 (0.57, 1.28) | 0.4347 | |||
| Age, years | 1.05 (1.03, 1.06) | <0.0001 | 1.02 (1.00, 1.04) | 0.0252 | |
| Sex | Female | 1 (ref) | 1 (ref) | ||
| Male | 2.03 (1.37, 3.01) | 0.0004 | 1.75 (1.17, 2.60) | 0.0060 | |
| Onset symptoms | |||||
| Fever | No | 1 (ref) | |||
| Yes | 0.84 (0.48, 1.44) | 0.5191 | |||
| Cough | No | 1 (ref) | |||
| Yes | 0.71 (0.47, 1.08) | 0.1125 | |||
| Expectoration | No | 1 (ref) | |||
| Yes | 1.06 (0.71, 1.58) | 0.7788 | |||
| Muscular soreness | No | 1 (ref) | |||
| Yes | 0.92 (0.57, 1.47) | 0.7256 | |||
| Fatigue | No | 1 (ref) | |||
| Yes | 1.07 (0.72, 1.60) | 0.7386 | |||
| Diarrhoea | No | 1 (ref) | |||
| Yes | 0.83 (0.48, 1.44) | 0.5124 | |||
| Past history of disease | |||||
| Hypertension | Without | 1 (ref) | |||
| With | 1.27 (0.85, 1.89) | 0.2493 | |||
| Chronic lung disease | Without | 1 (ref) | |||
| With | 1.12 (0.41, 3.03) | 0.8285 | |||
| Chronic heart disease | Without | 1 (ref) | |||
| With | 1.34 (0.75, 2.39) | 0.3151 | |||
| Chronic liver disease | Without | 1 (ref) | |||
| With | 0.95 (0.30, 2.99) | 0.9296 | |||
| Chronic kidney disease | Without | 1 (ref) | |||
| With | 2.28 (1.00, 5.19) | 0.0496 | |||
| Cerebrovascular disease | Without | 1 (ref) | |||
| With | 2.82 (1.31, 6.05) | 0.0080 | |||
| Carcinoma | Without | 1 (ref) | |||
| With | 1.81 (0.92, 3.58) | 0.0876 | |||
| CRB-65 score | 0 | 1 (ref) | 1 (ref) | ||
| 1–2 | 4.35 (2.81, 6.72) | <0.0001 | 2.68 (1.56–4.59) | 0.0003 | |
| 3–4 | 13.80 (5.99, 31.80) | <0.0001 | 5.25 (2.05–13.43) | 0.0005 | |
| Admission FBG, mmol/l | <6.1 | 1 (ref) | 1 (ref) | ||
| 6.1–6.9 | 2.06 (1.20, 3.54) | 0.0087 | 1.71 (0.99, 2.94) | 0.0524 | |
| ≥7.0 | 3.54 (2.33, 5.38) | <0.0001 | 2.30 (1.49, 3.55) | 0.0002 | |
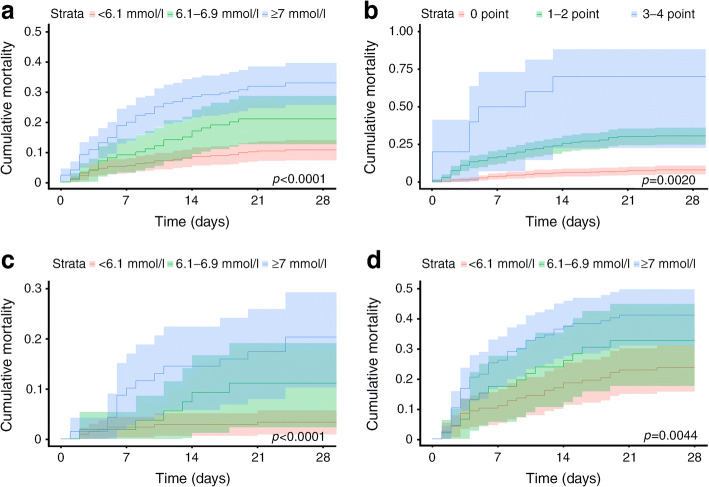
The cumulative death rate within 28 days in all COVID-19 participants stratified in terms of FBG and CRB-65 score at admission overall is shown in Fig. 2a (ptrend < 0.0001) and Fig. 2b (ptrend = 0.0020). Compared with patients with FBG <6.1 mmol/l, mortality within 28 days was higher in those with FBG of 6.1–6.9 mmol/l (crude HR 2.06 [95% CI 1.20, 3.54]) and ≥7.0 mmol/l (crude HR 3.54 [95% CI 2.33, 5.38]), respectively (Table 2). Compared with patients with FBG of 6.1–6.9 mmol/l, mortality within 28 days was higher in those with FBG ≥7.0 mmol/l (crude HR 1.72 [95% CI 1.05, 2.84]). Meanwhile, compared with patients with CRB-65 score of 0, mortality within 28 days was higher in those with CRB-65 of 1–2 (crude HR 4.35 [95% CI 2.81, 6.72]) and 3–4 (crude HR 13.80 [95% CI 5.99, 31.80]), respectively (Table 2). Compared with patients with CRB-65 score of 1–2, mortality within 28 days was higher in those with CRB-65 of 3–4 (crude HR 3.18 [95% CI 1.46, 6.89]).
Fig. 2.
Kaplan–Meier survival curves (showing cumulative mortality and 95% CI) for COVID-19 patients stratified in terms of FBG or CRB-65 score at admission. (a) Kaplan–Meier survival curves of all COVID-19 patients stratified by FBG; (b) Kaplan–Meier survival curves of all COVID-19 patients stratified by CRB-65 score; (c) Kaplan–Meier survival curves of COVID-19 patients with a CRB-65 score of 0, stratified by FBG; (d) Kaplan–Meier survival curves of COVID-19 patients with a CRB-65 score of >0, stratified by FBG
FBG and CRB-65 score at admission
CRB-65 score is a measure of pneumonia severity. The interaction between trends across FBG and CRB-65 scores did not reach statistical significance (p = 0.1112 for crude and 0.2243 for adjusted analyses). Higher FBG levels were associated with increased mortality in the group with a CRB-65 score of 0 (Fig. 2c, ptrend < 0.0001) and with a CRB-65 score of >0 (Fig. 2d, ptrend = 0.0044). In patients with a CRB-65 score of 0, mortality within 28 days was higher in the group with FBG ≥7.0 mmol/l (crude HR 6.57 [95% CI 2.65, 16.27]) and with FBG 6.1–6.9 mmol/l (crude HR 3.42 [95% CI 1.51, 10.19]) when compared with the group with FBG <6.1 mmol/l, respectively. No statistically significant difference was found between the groups with FBG ≥7.0 mmol/l (crude HR 1.92 [95% CI 0.74, 4.99]) and FBG of 6.1–6.9 mmol/l. In patients with a CRB-65 score of >0, mortality within 28 days was higher in the group with FBG ≥7.0 mmol/l (crude HR 1.99 [95% CI 1.24, 3.20]) compared with FBG <6.1 mmol/l. No statistically significant difference was found between the groups with FBG of 6.1–6.9 mmol/l and FBG <6.1 mmol/l (crude HR 1.44 [95% CI 0.77, 2.70]), or between the groups with FBG ≥7.0 mmol/l and FBG of 6.1–6.9 mmol/l group (crude HR 1.38 [95% CI 0.77, 2.48]).
FBG at admission and complications within 28 days
Then, we analysed the relationship between the FBG and complications in COVID-19 patients. The number of patients who had complications within 28 days in the groups with FBG <6.1 mmol/l, 6.1–6.9 mmol/l and ≥7.0 mmol/l was 86 (14.2%), 48 (7.9%) and 103 (17.0%), respectively. The number of patients without complications within 28 days in the groups with FBG <6.1 mmol/l, 6.1–6.9 mmol/l and ≥7.0 mmol/l was 243 (40.2%), 52 (8.6%), and 73 (12.1%), respectively (Table 1). Compared with patients with admission FBG <6.1 mmol/l, patients with admission FBG ≥7.0 mmol/l (OR 3.99 [95% CI 2.71, 5.88]) and 6.1–6.9 mmol/l (OR 2.61 [95% CI 1.64, 4.41]) had higher levels of in-hospital complications.
Discussion
The ongoing COVID-19 pandemic is taking a heavy toll worldwide and effective measures have to be taken to minimise its impact and to lower mortality. Previous studies have shown that diabetes and acute uncontrolled hyperglycaemia (defined as blood glucose >10 mmol/l twice within any 24 h period) are related to morbidity and/or mortality from COVID-19 [13, 21, 25]. Nonetheless, so far, no research effort has been directed at whether the admission FBG level is an independent predictor of mortality in COVID-19 patients without previously diagnosed diabetes. This two-centre retrospective study shows, for the first time, that elevated FBG (≥7.0 mmol/l) at admission is independently associated with increased 28-day mortality and percentages of in-hospital complications in COVID-19 patients without previous diagnosis of diabetes.
Our multivariable Cox regression analysis showed that FBG, CRB-65 score, age and sex were independently associated with 28-day mortality in COVID-19 patients without previous diagnosis of diabetes. In a study involving 191 COVID-19 patients, advanced age, a high Sequential Organ Failure Assessment (SOFA) score and a D-dimer level greater than 1 μg/l were risk factors for mortality [21]. Because of differences in patient composition, their study failed to reveal any significant difference for sex, although the proportion of male patients in the non-survivor group was higher than in the surviving group (68.4% vs 49.7%). Hyperglycaemia and/or diabetes were identified to be risk factors for morbidity and mortality caused by infection with community-acquired pneumonia (CAP), SARS and MERS [9–12]. Consistent with the studies on CAP [9, 10], our results indicate that admission FBG is a significant prognostic factor for COVID-19. Another analysis of COVID-19 patients with ARDS showed that FBG is related to the occurrence of ARDS, but not associated with the death of patients with ARDS [26]. We have, for the first time, performed a stratified analysis of different FBG levels in a larger population and demonstrated that FBG ≥7.0 mmol/l is critical to the prognosis of patients with COVID-19. Our data also show that non-survivors are more likely to have serious complications. In addition, we demonstrate that the incidence of in-hospital complications increases with the elevation of FBG. The CRB-65 score is a quick and convenient indicator for judging the severity of pneumonia [27]; we have also shown that FBG ≥7.0 mmol/l is associated with increased mortality in participants, regardless of whether the patient’s CRB-65 score is 0 or greater.
Patients with FBG >6.1 mmol/l accounted for 45.6% (276/605) and a total of 29.1% (176/605) patients had blood glucose ≥7.0 mmol/l. These results indicate that our study included both undiagnosed diabetic patients and non-diabetic patients with hyperglycaemia caused by an acute blood-glucose disorder. Similarly to a previous study, COVID-19 patients might suffer from stress hyperglycaemia [10] and critically ill patients may develop acute insulin resistance, manifested by hyperglycaemia and hyperinsulinaemia [28]. Patients with conditions not related to diabetes, such as severe sepsis, systemic inflammatory response syndrome (SIRS) and traumatic brain injury tend to have hyperglycaemia [28]. Hyperglycaemia at admission to the intensive care unit (ICU) is directly related to increased mortality or morbidity [29]. At the same time, drugs, such as antibiotics and corticosteroids, could also elevate serum glucose concentration [30, 31]. In particular, corticosteroids may impair glucose tolerance by promoting gluconeogenesis in the liver, reducing glucose uptake and utilisation in peripheral tissues and increasing the effects of other glycaemic hormones [31]. It is recommended that close attention be paid to hospitalised patients, regardless of whether they have been previously diagnosed as having diabetes.
Although insulin treatment for critically ill patients has become a standard of care, efficacy might well vary with different ICUs. A study published in 2001 reported that intensive insulin therapy with blood glucose maintained at or below 6 mmol/l reduced morbidity and mortality in critically ill patients in surgical ICUs [32]. However, subsequent studies on intensive insulin therapy of critically ill patients in medical ICUs showed that this treatment could reduce morbidity but not mortality [33]. The debate over this issue remains, and a study published in 2009 found that intensive glycaemic control could increase mortality in adults in ICUs, but increasing target glucose levels to 10 mmol/l could reduce mortality [34]. A recent review separately examined surgical and medical patients and suggested that, to treat hyperglycaemia, insulin therapy should be used to maintain the glucose level between 8 mmol/l and 10 mmol/l. This treatment could reduce the mortality and morbidity resulting from high FBG and lower the occasional risk of hypoglycaemia associated with intensive insulin therapy [35].
This study has several limitations. First, this was a retrospective study. Second, we did not cover HbA1c, a long-term glycaemic control indicator that helps distinguish patients with poor long-term glycaemic control from those with stress hyperglycaemia. Finally, because our results were premised only on the glucose levels at admission, we might have underestimated the risks associated with hyperglycaemia (assuming that patients with the highest glucose levels are more likely to be treated in the hospital), and we did not have sufficient data to study the effect of glucose-lowering treatment (e.g. insulin, metformin) on the outcome of our patients. However, we believe that acute hyperglycaemia is more important than long-term glycaemic control in predicting the prognosis of hospitalised COVID-19 patients.
In conclusion, FBG ≥7.0 mmol/l at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes. Glycaemic testing and control should be recommended for all COVID-19 patients even if they do not have pre-existing diabetes, as most COVID-19 patients are prone to glucose metabolic disorders. During a pandemic of COVID-19, FBG can facilitate the assessment of prognosis and early intervention of hyperglycaemia to help improve the overall outcomes in treatment of COVID-19.
Acknowledgements
We are indebted to the medical workers who are fighting COVID-19 on the front line of patient care and all the people in Hubei, especially those in Wuhan, who have sacrificed so much in the battle with the COVID-19 epidemic. The graphical abstract was created in Biorender.com.
Data availability
The dataset generated during the current study is available from the corresponding author on reasonable request.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82041018; No. 81770096) and Major Projects of the National Science and Technology (No. 2019ZX09301001).
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
YJ and TZ designed the study. SW, PM, SZ, SS, YM, JX, FW, HL and NX collected the epidemiological and clinical data. ZW and MX analysed data. PM, SZ, ZW, LD and ZY interpreted the analyses. SW, PM, SZ and SS drafted the manuscript. All authors participated in the discussion of the initial manuscript and provided important suggestions on revision. TZ and YJ revised the final manuscript. All authors approved the final version. YJ is responsible for the integrity of the work as a whole.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- COVID-19
Coronavirus disease 2019
- CFR
Crude fatality ratio
- FBG
Fasting blood glucose
- ICU
Intensive care unit
- MERS
Middle East respiratory syndrome
- SARS
Severe acute respiratory syndrome
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- ULN
Upper limit of normal
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sufei Wang, Pei Ma, Shujing Zhang, Siwei Song and Zhihui Wang contributed equally to this work.
Contributor Information
Tianshu Zeng, Email: tszeng@126.com.
Yang Jin, Email: whuhjy@126.com.
References
- 1.WHO (2020) Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) Available from www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. Accessed 30 Mar 2020
- 2.WHO (2020) Rolling updates on coronavirus disease (COVID-19). Available from www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Accessed 11 Apr 2020
- 3.WHO (2020) Coronavirus disease (COVID-19) pandemic. Available from www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 7 June 2020
- 4.Bragg F, Holmes MV, Iona A, et al. Association between diabetes and cause-specific mortality in rural and urban areas of China. JAMA. 2017;317(3):280–289. doi: 10.1001/jama.2016.19720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregg EW, Cheng YJ, Srinivasan M, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet. 2018;391(10138):2430–2440. doi: 10.1016/s0140-6736(18)30314-3. [DOI] [PubMed] [Google Scholar]
- 6.Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016;4(2):148–158. doi: 10.1016/s2213-8587(15)00379-4. [DOI] [PubMed] [Google Scholar]
- 7.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrlich SF, Quesenberry CP, Jr, Van Den Eeden SK, Shan J, Ferrara A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2010;33(1):55–60. doi: 10.2337/dc09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lepper PM, Ott S, Nuesch E et al (2012) Serum glucose levels for predicting death in patients admitted to hospital for community acquired pneumonia: prospective cohort study. BMJ 344:e3397. 10.1136/bmj.e3397 [DOI] [PMC free article] [PubMed]
- 10.McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28(4):810–815. doi: 10.2337/diacare.28.4.810. [DOI] [PubMed] [Google Scholar]
- 11.Yang JK, Feng Y, Yuan MY, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 12.Alanazi KH, Abedi GR, Midgley CM, et al. Diabetes mellitus, hypertension, and death among 32 patients with MERS-CoV infection, Saudi Arabia. Emerg Infect Dis. 2020;26(1):166–168. doi: 10.3201/eid2601.190952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bode B, Garrett V, Messler J et al (2020) Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol:193229682092446. 10.1177/1932296820924469 [DOI] [PMC free article] [PubMed]
- 14.Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO (2020) Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases. Available from www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117. Accessed 31 Mar 2020
- 16.National Health Commission of the People’s Republic of China (2020) Diagnosis and treatment plan of coronavirus disease 2019 (COVID-19) (trial version 6) Available from www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf. Accessed 31 Mar 2020 [document in Chinese]
- 17.WHO (2020) Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. Available from https://apps.who.int/iris/handle/10665/331446. Accessed 30 Mar 2020
- 18.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 20.Schneider GT, Maier SAN. Cerebrovascular accident. In: Weissbrod PA, Francis DO, editors. Neurologic and neurodegenerative diseases of the larynx. Cham: Springer; 2020. pp. 215–228. [Google Scholar]
- 21.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ewig S, Bauer T, Richter K, et al. Prediction of in-hospital death from community-acquired pneumonia by varying CRB-age groups. Eur Respir J. 2013;41(4):917–922. doi: 10.1183/09031936.00065212. [DOI] [PubMed] [Google Scholar]
- 23.Ewig S, Birkner N, Strauss R, et al. New perspectives on community-acquired pneumonia in 388 406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax. 2009;64(12):1062–1069. doi: 10.1136/thx.2008.109785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/s1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu C, Chen X, Cai Y et al (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed]
- 27.Bauer TT, Ewig S, Marre R, Suttorp N, Welte T. CRB-65 predicts death from community-acquired pneumonia. J Intern Med. 2006;260(1):93–101. doi: 10.1111/j.1365-2796.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 28.Bar-Or D, Rael LT, Madayag RM, et al. Stress hyperglycemia in critically ill patients: insight into possible molecular pathways. Front Med (Lausanne) 2019;6:54. doi: 10.3389/fmed.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitcomb BW, Pradhan EK, Pittas AG, Roghmann MC, Perencevich EN. Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations. Crit Care Med. 2005;33(12):2772–2777. doi: 10.1097/01.ccm.0000189741.44071.25. [DOI] [PubMed] [Google Scholar]
- 30.Burcelin R, Amar J. Diabetes: antibiotics or prodiabetics? Nat Rev Endocrinol. 2015;11(7):385–386. doi: 10.1038/nrendo.2015.75. [DOI] [PubMed] [Google Scholar]
- 31.Johns EC, Reynolds RM. Topical glucocorticoids and risk of type 2 diabetes mellitus. Nat Rev Endocrinol. 2019;15(7):379–380. doi: 10.1038/s41574-019-0212-8. [DOI] [PubMed] [Google Scholar]
- 32.Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 33.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 34.Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 35.Stapleton RD, Heyland DK (2020) UpToDate: glycemic control and intensive insulin therapy in critical illness. Available from www.uptodate.com/contents/glycemic-control-and-intensive-insulin-therapy-in-critical-illness. Accessed 30 Mar 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated during the current study is available from the corresponding author on reasonable request.