Abstract
Breast cancer and ovarian cancer are closely related. The major common risk factors of these 2 types of cancer are likely genetic factors. However, few studies have shown any common characteristics in patients who have both types of these 2 cancers. The purpose of this retrospective study is to explore the clinical characteristics and survival outcomes of patients with both primary breast cancer and primary ovarian cancer.
A cohort of patients who had a history of both primary breast cancer and primary ovarian cancer were enrolled, and they received treatment in the Peking Union Medical College Hospital between January 1, 2010, and December 31, 2018. Both descriptive statistics analysis and survival analysis were performed for analysis.
A total of 114 patients with both primary breast cancer and primary ovarian cancer were included in the study. The median (range) follow-up was 129.5 (20–492) months. The average interval time between the diagnosis of 2 types of cancer was 79.4 months in patients having ovarian cancer firstly and was 115.9 months in patients having breast cancer firstly. The 5- and 10-year overall survival (OS) rates were 91.5% and 81.7% for patients with ovarian cancer following breast cancer, respectively, and 90.6% and 87.5% for patients with breast cancer following ovarian cancer, respectively. Multivariate analysis revealed that independent predictors of OS were the age of diagnosis of the first tumor and the time interval between two types of tumor in patients with ovarian cancer following breast cancer.
Most breast cancer or ovarian cancer occurred within 5 years after being diagnosed with the first tumor, and the interval time was significantly shorter in patients with previous ovarian cancer. The prognosis is likely positively correlated to the interval time between the occurrences of two types of cancer.
Keywords: breast cancer, clinical characteristics, ovarian cancer, prognosis
1. Introduction
Breast cancer and ovarian cancer are among the most common malignant tumors for women worldwide. Ovarian cancer was the fifth common cause of cancer mortality among women, with 22,530 new cases and 13,980 deaths in the United States (US) in 2009.[1] Additionaly, there were approximately 268,600 new cases and 41,760 deaths caused by invasive breast cancer among the US women in 2019.[2] In China, breast cancer has the highest incidence among all cancers and ranks the sixth common cause of cancer death in females.[3] The incidence of breast cancer is increasing in recent decades, but the prognosis has also improved thanks to the maturity of treatment and the promotion of screening. The second primary cancer of breast cancer patients gradually attracts attention. Several population-based cancer registry studies and multicenter cancer registry studies had shown women diagnosed with previous breast cancer had a higher risk of developing a second primary cancer compared to the overall population.[4–7] Among them, endometrial cancer and ovarian cancer were the most common.[8,9] Similarly, patients diagnosed with ovarian cancer are more likely to suffer from second primary breast cancer. The incidence of breast tumorigenesis was 7.8% within 10 years after ovarian cancer surgery according to previous research.[10] The occurrence of multiple cancers is related to many factors like common etiology between cancers (i.e. genetic, environmental or hormonal factors).[11] Until now, many studies have shown that the occurrences of breast cancer and ovarian cancer was associated with some common predisposition genes such as BRCA1 and BRCA2.[12] However, the clinical features and prognosis of patients with both of the two cancers are still rarely analyzed. Our retrospective analysis aims at estimating the clinical characteristics and survival outcomes of women with both primary breast cancer and primary ovarian cancer, and comparing the characteristics of these two types of cancer from a new perspective.
2. Methods
2.1. Data source
With the approval of the Peking Union Medical College Hospital Ethics Committee, we reviewed our medical records to identify all consecutive patients who were diagnosed or treated in Peking Union Medical College Hospital between January 1, 2010, and December 31, 2018. Informed written consents were obtained from all patients. Women with both primary breast and ovarian cancer were eligible for our study. Cases with breast or ovary metastatic tumors were excluded. Additionally, patients who had insufficient data were excluded from the study, as shown in Figure 1. Information extracted from the medical records or collected through telephone interviews, included date of both first and second tumor diagnosis, dates of recurrence or metastasis, date of last follow-up, current vital status (living or deceased), causes of death, family history and clinicopathological characteristics (tumor grade, stage, histological subtype, and associated treatment). It is worth mentioning that patients whose close blood relative had a history of breast cancer, ovarian cancer, prostate cancer, pancreatic cancer, or melanoma were defined as positive of family history.
Figure 1.
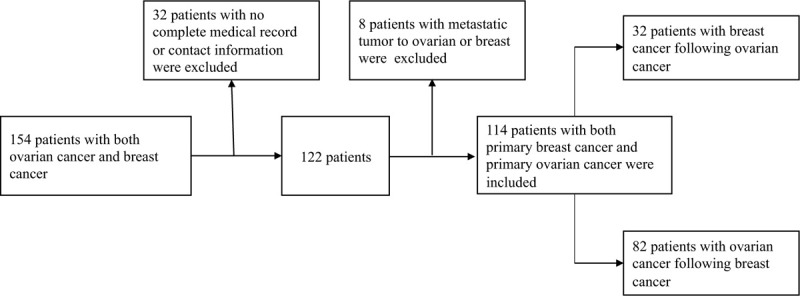
Screening flow chart of patients had both primary breast cancer and primary ovarian cancer.
2.2. Outcome of interests
The primary outcome of interest was patients’ overall survival (OS), defined as the time from the date of diagnosis of the first tumor to the date of death or the most recent follow up. The cause of death was divided into breast cancer-specific death, ovarian cancer-specific death, and other causes of death. Secondary outcome measures included time to recurrence or progress of the disease for both ovarian cancer-specific or breast cancer-specific, defined as ovarian cancer-progress free survival (OCPFS) and breast cancer-progress free survival (BCPFS), respectively.
2.3. Statistical analysis
We performed the χ2-test and Fisher exact test for categorical variable analysis, and compared continuous variables between groups using Student's t-test and Mann-Whitney U-test. As for the survival analysis, univariate analysis was estimated by the Kaplan-Meier method and compared with the log-rank test. Cox proportional hazards models were fit to determine the independent risk factors of the outcome. All statistical analysis was conducted using SPSS statistical software version 22, with P < .05 considered to be statistically significant.
3. Results
From January 1, 2010, to December 31, 2018, a total of 154 patients visiting Peking Union Medical College Hospital had a history of both breast tumor and ovarian tumor. The study excluded 32 patients due to lack of detailed medical records or contact information. Another 8 patients were excluded because of breast or ovatian metastasis from other organs. Finally, a total of 114 patients with a history of both primary ovarian cancer and primary breast cancer were included. Among these patients, 32 had breast cancer following ovarian cancer and the other 82 had ovarian cancer following breast cancer, as shown in Figure 1. We divided patients into two groups according to the sequence of tumor occurrence and the demographics were presented in Table 1 . The two groups had no significant differences in breast cancer pathological types, stages, tumor grades, treatment methods. The 2 groups had no significant differences in pathological types, tumor grades, and treatment methods for ovarian cancer, either. As for the stages of ovarian cancer, patients who had ovarian cancer first were more likely to have Stage I and II (a total of 65.6%), while patients with ovarian cancer following breast cancer were more likely to have a later stage of ovarian cancer, with stage III patients taking up 64.6%. The vast majority of women underwent surgery for breast cancer in the entire cohort (112/114, 98.2%), while 66.7% and 27.2% were treated with chemotherapy and radiotherapy, respectively. In terms of ovarian cancer, most patients had surgery (97.4%) and chemotherapy (82.5%), and only 2 patients (1.8%) received radiotherapy. A total of 52 (45.6%) patients had a family history of related tumors, of which 37.5% were patients who had ovarian cancer as the first tumor and 48.8% of those diagnosed with prior breast cancer.
Table 1.
Demographic and clinicopathological characteristics of ovarian cancer and breast cancer patients.

The median follow-up time was 129.5 months (range, 20–492 months). Among patients with breast cancer following ovarian cancer, the median age at first tumor (ovarian cancer) diagnosis was 51.5 years (range, 18–73 years), and the median age of breast cancer diagnosis was 56.5 years (range, 30–78 years). As for the patients who had breast cancer first, the median diagnosis ages were 47.5 years (range, 27–71 years) and 56 years (range, 30–84 years) for breast cancer and ovarian cancer respectively. The distribution was shown in Figure 2. The diagnosis time intervals from the first tumor to the second tumor were statistically different in these two groups (P = .026) as shown in Table 2 and the distribution was shown in Figure 3. For patients who had ovarian cancer first, most of them were diagnosed of breast cancer over 5 years after the diagnosis of ovarian cancer (21/32, 65.6%), and also quite a few of them got breast cancer 10 years after the diagnosis of ovarian cancer (10/32, 31.3%). The average interval between the 2 cancers was 79.4 months (median 37.5 months, range 0–336 months). But when it comes to the patients who got breast cancer first, 41.5% were diagnosed with ovarian cancer within 5 years after the diagnosis of breast cancer, and as the length of time after the diagnosis of breast cancer increased, the number of patients diagnosed with ovarian cancer decreased. The average interval between the two cancers of this group was 115.9 months (median 84 months, range 5–480 months).
Figure 2.
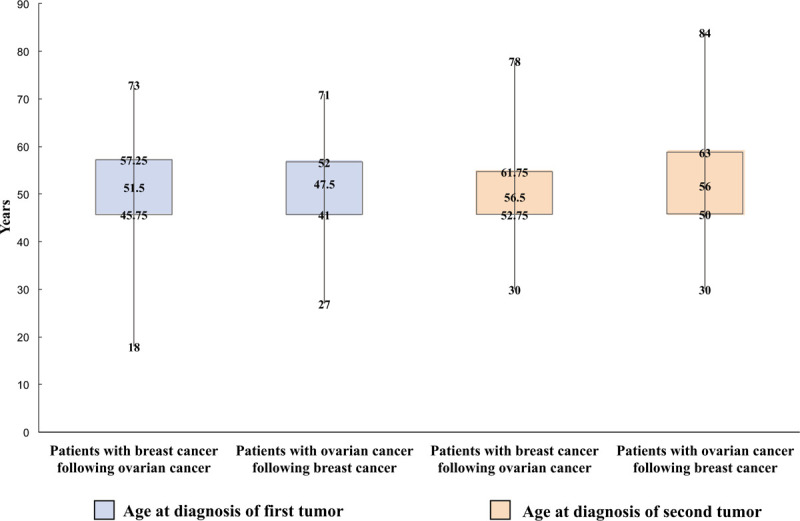
Distribution of ages at diagnosis of 2 primary cancers.
Table 1 (Continued).
Demographic and clinicopathological characteristics of ovarian cancer and breast cancer patients.

Figure 3.
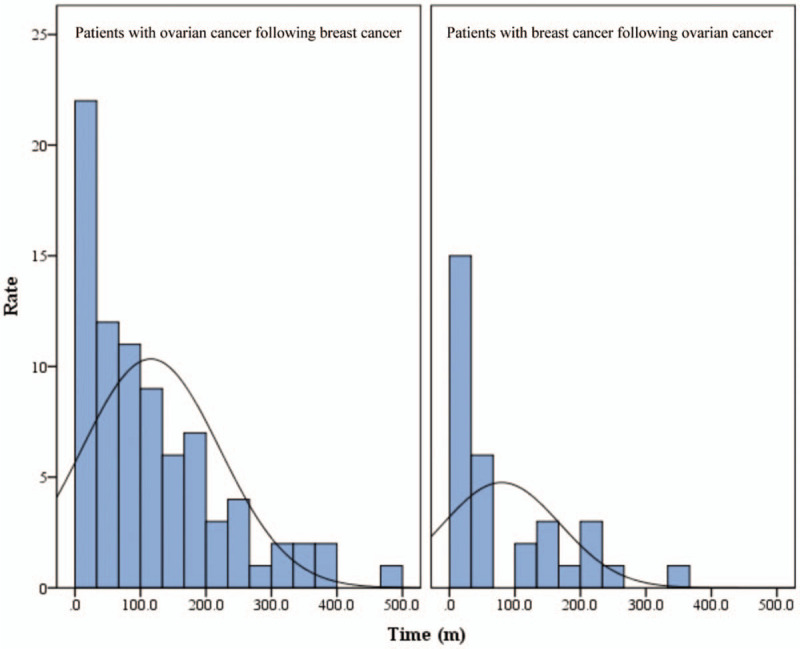
Distribution of time intervals between 2 primary cancers.
During the follow-up, 35 women died of ovarian cancer, 3 died of breast cancer, and 1 died of other causes (accidental death). The 5-year OS rate of patients with ovarian cancer following breast cancer was 91.5%, and the 10-year OS rate was 81.7%. For patients with ovarian cancer first, the 5-year and 10-year OS rate was 90.6% and 87.5% respectively, shown as Table 3. But no significant statistical difference of OS between the 2 groups was seen through survival analysis (P = .175), shown as Fig. 4. The breast cancer-specific survival (BCSS) did not differ significantly among 2 groups but the 10-year ovarian cancer-specific survival (OCSS) rate was lower in patients with breast cancer first (82.9% vs % 87.5%, P < .001). On the other hand, the BCPFS and OCPFS were significantly different between the two groups (P = .007, P < .001), shown as Figure 5. Five-year BCPFS was 81.3% in patients with breast cancer following ovarian cancer and was 91.5% in patients with ovarian cancer following breast cancer. Meanwhile, the 5-year OCPFS of these two groups were 78.1% and 37.8%, respectively. For patients who were diagnosed with ovarian cancer after a history of breast cancer, we performed univariate and multivariate survival analysis of OS, BCPFS as well as OCPFS, as shown in Table 4. The multivariate survival analysis showed that independent predictors of OS were age at diagnosis of the first tumor (P = .029) and the time intervals between two cancers (P < .001). The age of diagnosis of the first tumor and the interval time between two cancers had a positive correlation with poor prognosis (Fig. 6). Except for the stage of ovarian cancer which was a independent risk factor of OCPFS (P = .030), family history, age at diagnosis, breast cancer grade, breast cancer stage, and ovarian cancer grade were not significantly related to BCPFS and OCPFS after performing cox regression. As for the patients with breast cancer following ovarian cancer, because of the small sample size and few number of events, we only performed univariate analysis, which showed that ovarian cancer stage had an impact on OS (P = .026). In this group both of ovarian cancer stage (P = .003) and the time intervals (P = .003) had influence on OCPFS, shown as Table 5.
Table 2.
Distribution of age at diagnosis and time intervals between 2 primary tumors.

Figure 4.
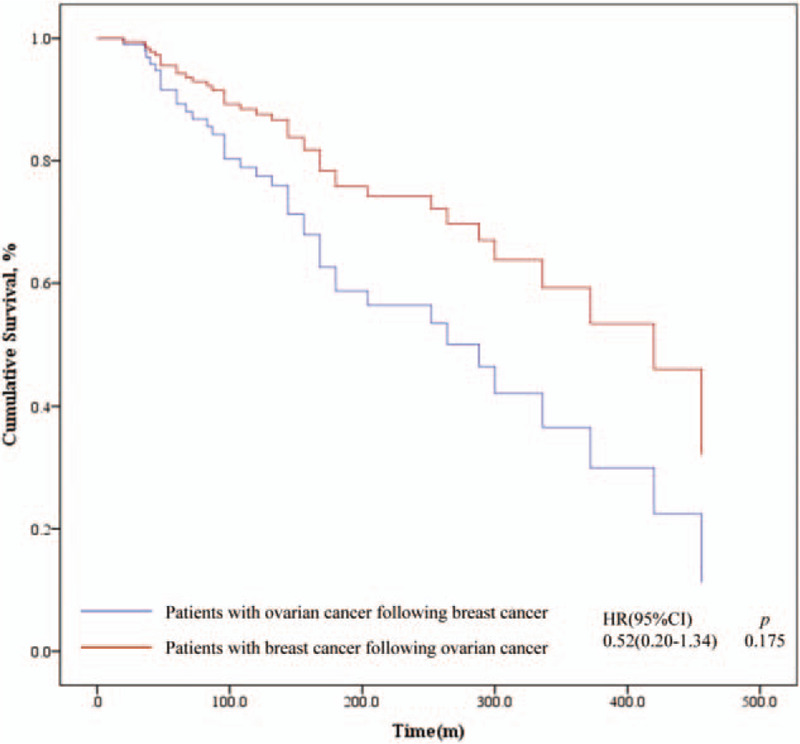
Survival analysis of overall survival (OS) according to the tumor diagnosis sequence.
Figure 5.
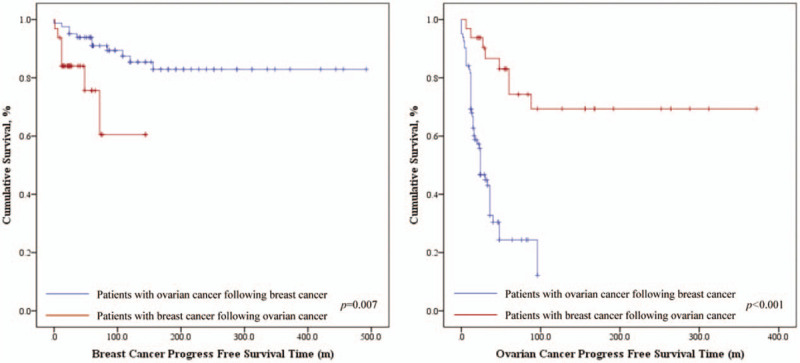
Kaplan-Meier survival curves of breast cancer progress free survival (BCPFS) and ovarian cancer progress free survival (OCPFS) according to the tumor diagnosis sequence.
Table 3.
Survival rate of patients.

Figure 6.
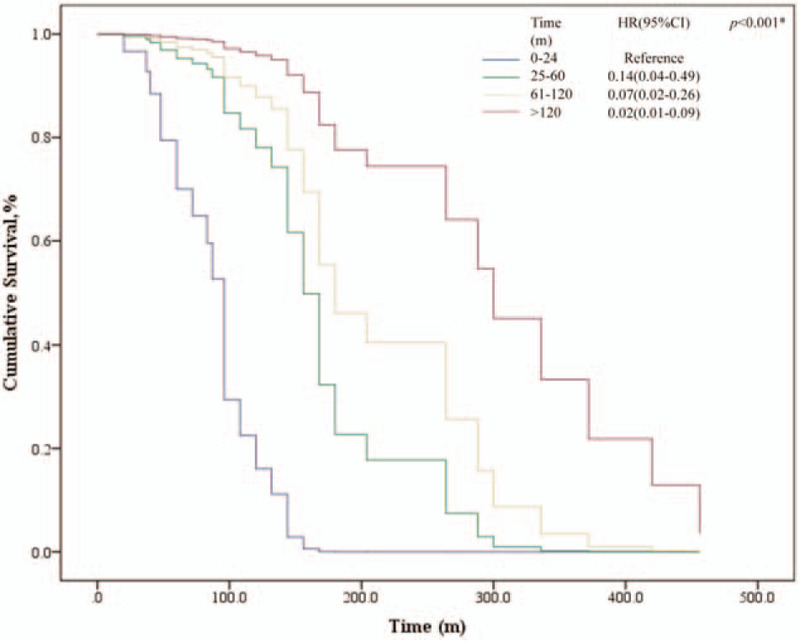
Survival analysis of overall survival (OS) according to the time intervals between two primary tumors in patients with ovarian cancer following breast cancer.
Table 4.
Univariate and Multivariate analysis of patients with ovarian cancer following breast cancer.

Table 5.
Univariate analysis of patients with breast cancer following ovarian cancer.

4. Discussion
Breast cancer has the highest incidence and causes a lot death of cancer in women worldwide, and ovarian cancer is also a significant source of morbidity and mortality.[1] Although breast and ovarian cancer usually present as distinct clinical entities, the recent explosion of researches had uncovered many correlations, particularly with respect to genetic and epigenetic alterations.[13] However, to our best knowledge, few studies have evaluated the clinical features and survival outcomes of patients with both breast cancer and ovarian cancer, especially the comparison between patients who had breast cancer first versus who got ovarian cancer first.
In our study, patients with a history of both primary breast cancer and primary ovarian cancer did not show significant difference in their age at diagnosis, family history, tumor pathology, tumor grade, and treatment measures. Although no significant difference in breast cancer stages had been seen, significant difference in the stages of ovarian cancer was observed between these two groups (P = .004). From literature, regardless of whether or not associated with BRCA gene mutation, most of the ovarian cancer patients were diagnosed with advanced and high-grade tumors.[14,15] This phenomenon was similar to the patients with ovarian cancer following breast cancer in our study. But when it comes to patients who were diagnosed with breast cancer following ovarian cancer, it is worth noting that 65.5% of them were stage I and II of ovarian cancer. We infer that because only when ovarian cancer patients achieve sustained remission and survival for a period of time can they have the condition to develop breast cancer.
We also found the interval times between the two tumors were different in two groups (P = .026). For patients who were diagnosed with ovarian cancer first, available studies have shown that the median diagnosis time from ovarian cancer to breast cancer was 50.5 to 108 months,[16–18] while researches showed the mean interval time from breast cancer to ovarian cancer was 84 to 97.2 months.[9,19] Our results were similar to those in the existing literature, but the interval was significantly shorter in the ovarian cancer first group (79.4 m vs 115.9 m, P = .026). Most patients developed breast cancer within 5 years after being diagnosed with ovarian cancer (65.6%), while only 41.5% of patients with breast cancer first were diagnosed ovarian cancer within 5 years. On the other hand, after 10 years of the initial tumor diagnosis, there were still many patients acquiring breast cancer or ovarian cancer (34.4% vs 35.4%). Previous studies have suggested that patients who had a history of ovarian cancer had low risk of developing breast cancer,[16,20] and the prognosis of these patients was optimistic. And most of the deaths were owing to ovarian cancer of these women.[17] So previous studies supports the use of less aggressive risk-reduction strategies of breast cancer after a diagnosis of ovarian cancer. From our results, it can be seen that patients with early-stage ovarian cancer and better tumor grade tended to develop breast cancer. Therefore, as suggested by Peters et al,[21] we believed that ovarian cancer patients with early-stage disease or more favorable advanced disease should have proper breast cancer screening, as well as patients sustained remission >10 years. Kelly et al[19] had reported that 10.2% of BRCA-positive breast cancer patients had recurrent ovarian cancer, and they also have the actuarial cumulative risk of developing ovarian cancer after breast cancer in BRCA1 mutation carriers was 4.5% at 5 years after the diagnosis of breast cancer and 12.7% at 10 years; for BRCA2 carriers, it was 5.3% at 5 years and 6.8% at 10 years. Bergfeldt et al[9] reported that even without a family history, breast cancer patients are also at high risk for ovarian cancer, especially for young patients. On the other hand, it is worth noting that according to our data, the most death of patients with ovarian cancer following breast cancer were owing to ovarian cancer, and it was not significantly correlated to breast cancer-related treatment. Therefore, regardless of genetic mutations, patients should be screened for ovarian cancer after the first diagnosis of breast cancer, even after 10 years. And for patients have gene mutations, as recommended by Kelly et al[19] prophylactic oophorectomy should be considered according to the individual situation.
Studies have shown that the 5-year survival rate of breast cancer in China had reached 79% to 85%[22] but the 5-year survival of ovarian cancer was only about 46%.[23–25] However, studies showed the survival rates of women with breast cancer following ovarian cancer differ from those of patients with ovarian cancer who did not develop breast cancer,[16,17] the 5- and 10-year OS rates were 58.3% and 50% for BRCA mutation associated ovarian cancer women who developed breast cancer but 30.9% and 13.8% for those who did not have breast cancer.[16] Our study showed that the 5- and 10-year OS rates for patients with breast cancer following ovarian cancer were 90.6% and 87.5%, higher than previous research findings. We suggested it was because patients with a better prognosis of ovarian cancer had the opportunity to develop breast cancer, while patients with a poorer prognosis died before developing the second primary tumor. In addition, we found that the longer the interval between the diagnosis of the 2 cancers in patients with ovarian cancer following breast cancer, the better the patient's prognosis and it was an independent risk factor (P < .001). Although it was not significant in patients with breast cancer following ovarian cancer (P = .061), a similar trend had been displayed, and it was significantly related to OCPFS (P = .003). The impact of the time intervals between breast cancer and ovarian cancer on prognosis has not been reported in other literature. What causes patients to be more susceptible to a second primary cancer in the short term and worse prognosis requires further study. It is worth mentioning that in our multivariate analysis, the older the diagnosis of breast cancer in patients with ovarian cancer following ovarian cancer, the worse the prognosis, which was not consistent with the generally believed that the younger breast cancer patients had a worse prognosis.[26,27] The reason behind this might be the prognosis of these patients was mainly affected by ovarian cancer, and the sample size needs to be expanded for further research.
This was the first study about the clinical characteristics and survival outcomes of patients with both breast cancer and ovarian cancer, as well as the differences between patients with different sequences of these 2 cancers. The main limitation of our study was the small sample size, especially patients with breast cancer following ovarian cancer. Also the selection bias may have been introduced due to the retrospective nature of the study. And we did not do genetic tests which ended up to no information of BRCA mutation. As a result, we could not confirm the effects of BRCA1/2 on the survival of patients.
5. Conclusions
Our study compared the clinical characteristics and evaluated the survival of a sub-cohort of patients with both primary breast cancer and primary ovarian cancer. The diagnosis time intervals form the first tumor to the second were statistically different in patients with the different order of the two tumors, but most had second primary cancer within the first several years after diagnosed with the first cancer. Patients with ovarian cancer following breast cancer have well-described prognostic factors for improved survival rates including age at diagnosis of first tumor and the time intervals between two tumors.
Acknowledgments
We thank the Department of Obstetrics and Gynecology, Peking Union Medical College Hospital for caring and following up with some of the patients included in this study. The authors declare that they have no conflict of interest.
Author contributions
Chang Chen performed the result analysis and wrote the draft manuscript. Chang Chen and Yali Xu designed the study. Chang Chen and Ying Xu performed the data analysis. Xin Huang, Feng Mao, SongjieShen modified article language. Qiang Sun edited the draft. All authors reviewed the manuscript.
Footnotes
Abbreviations: BCPFS = breast cancer progress free survival, BCSS = breast cancer-specific survival, OCPFS = ovarian cancer progress free survival, OCSS = ovarian cancer-specific survival, OS = overall survival, U.S. = the United States.
How to cite this article: Chen C, Xu Y, Huang X, Mao F, Shen S, Xu Y, Sun Q. Clinical characteristics and survival outcomes of patients with both primary breast cancer and primary ovarian cancer. Medicine. 2020;99:32(e21560).
The authors declare that they have no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- [2].DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin 2019;69:438–51. [DOI] [PubMed] [Google Scholar]
- [3].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [4].Evans HS, Lewis CM, Robinson D, et al. Incidence of multiple primary cancers in a cohort of women diagnosed with breast cancer in southeast England. Br J Cancer 2001;84:435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cluze C, Delafosse P, Seigneurin A, et al. Incidence of second cancer within 5 years of diagnosis of a breast, prostate or colorectal cancer: a population-based study. Eur J Cancer Prev 2009;18:343–8. [DOI] [PubMed] [Google Scholar]
- [6].Soerjomataram I, Louwman WJ, de Vries E, et al. Primary malignancy after primary female breast cancer in the South of the Netherlands, 1972–2001. Breast Cancer Res Treat 2005;93:91–5. [DOI] [PubMed] [Google Scholar]
- [7].Ricceri F, Fasanelli F, Giraudo MT, et al. Risk of second primary malignancies in women with breast cancer: Results from the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer 2015;137:940–8. [DOI] [PubMed] [Google Scholar]
- [8].Molina-Montes E, Pollán M, Payer T, et al. Risk of second primary cancer among women with breast cancer: a population-based study in Granada (Spain). Gynecol Oncol 2013;130:340–5. [DOI] [PubMed] [Google Scholar]
- [9].Bergfeldt K, Rydh B, Granath F, et al. Risk of ovarian cancer in breast-cancer patients with a family history of breast or ovarian cancer: a population-based cohort study. Lancet 2002;360:891–4. [DOI] [PubMed] [Google Scholar]
- [10].McGee J, Giannakeas V, Karlan B, et al. Risk of breast cancer after a diagnosis of ovarian cancer in BRCA mutation carriers: is preventive mastectomy warranted? Gynecol Oncol 2017;145:346–51. [DOI] [PubMed] [Google Scholar]
- [11].Hemminki K, Boffetta P. Multiple primary cancers as clues to environmental and heritable causes of cancer and mechanisms of carcinogenesis. IARC scientific publications. 2004(157):289–297. [PubMed] [Google Scholar]
- [12].Zhang S. Comparisons of gene coexpression network modules in breast cancer and ovarian cancer. BMC Syst Biol 2018;12: Suppl 1: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Longacre M, Snyder N, Housman G, et al. A comparative analysis of genetic and epigenetic events of breast and ovarian cancer related to tumorigenesis. Int J Mol Sci 2016;17: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Boyd J, Sonoda Y, Federici MG, et al. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA 2000;283:2260–5. [DOI] [PubMed] [Google Scholar]
- [15].Rubin SC, Benjamin I, Behbakht K, et al. Clinical and pathological features of ovarian cancer in women with germ-line mutations of BRCA1. N Engl J Med 1996;335:1413–6. [DOI] [PubMed] [Google Scholar]
- [16].Gangi A, Cass I, Paik D, et al. Breast cancer following ovarian cancer in BRCA mutation carriers. JAMA Surg 2014;149:1306–13. [DOI] [PubMed] [Google Scholar]
- [17].Domchek SM, Jhaveri K, Patil S, et al. Risk of metachronous breast cancer after BRCA mutation-associated ovarian cancer. Cancer 2013;119:1344–8. [DOI] [PubMed] [Google Scholar]
- [18].Zhang W, Zhang W, Lin Z, et al. Survival outcomes of patients with primary breast cancer following primary ovarian cancer. Med Sci Monit 2019;25:3869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Metcalfe KA, Lynch HT, Ghadirian P, et al. The risk of ovarian cancer after breast cancer in BRCA1 and BRCA2 carriers. Gynecol Oncol 2005;96:222–6. [DOI] [PubMed] [Google Scholar]
- [20].Vencken PM, Kriege M, Hooning M, et al. The risk of primary and contralateral breast cancer after ovarian cancer in BRCA1/BRCA2 mutation carriers: implications for counseling. Cancer 2013;119:955–62. [DOI] [PubMed] [Google Scholar]
- [21].Peters ML, Garber JE, Tung N. Managing hereditary breast cancer risk in women with and without ovarian cancer. Gynecol Oncol 2017;146:205–14. [DOI] [PubMed] [Google Scholar]
- [22].Li T, Mello-Thoms C, Brennan PC. Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence. Breast Cancer Res Treat 2016;159:395–406. [DOI] [PubMed] [Google Scholar]
- [23].Doherty JA, Peres LC, Wang C, et al. Challenges and opportunities in studying the epidemiology of ovarian cancer subtypes. Curr Epidemiol Rep 2017;4:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mallen A, Soong TR, Townsend MK, et al. Surgical prevention strategies in ovarian cancer. Gynecol Oncol 2018;151:166–75. [DOI] [PubMed] [Google Scholar]
- [25].Eisenhauer EA. Real-world evidence in the treatment of ovarian cancer. Ann Oncol 2017;28: suppl_8: viii61–5. [DOI] [PubMed] [Google Scholar]
- [26].Ferguson NL, Bell J, Heidel R, et al. Prognostic value of breast cancer subtypes, Ki-67 proliferation index, age, and pathologic tumor characteristics on breast cancer survival in Caucasian women. Breast J 2013;19:22–30. [DOI] [PubMed] [Google Scholar]
- [27].Azim HA, Jr, Michiels S, Bedard PL, et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res 2012;18:1341–51. [DOI] [PubMed] [Google Scholar]


