Abstract
The present study explored the modulating apoptosis effect of hydrogen sulfide (H2S) in subarachnoid hemorrhage (SAH) rats and its exact mechanism. A rat SAH model established by intravascular puncturing was used for the present study. After giving NaHS (donor of H2S), an L-type calcium channel opener (Bay K8644), or a calcium channel agonist (nifedipine), the neurological function of the rats, associated pathological changes, and expression of apoptosis-related proteins (Bcl-2, Bax, and caspase-3) and microtubule-associated protein (MAP-2) were examined. The concentration of H2S and expression of cystathionine beta synthase in the hippocampus changed upon early brain injury (EBI) after SAH. Compared with the SAH group, the neurological function of the rats and microstructure observed by electron microscopy were better in the SAH + NaHS group and SAH + Bay K8644 group. It was observed that apoptosis was more obvious in the SAH group than in the control group and was alleviated in the SAH + NaHS group. Furthermore, the alleviating effect of NaHS was partially weakened by nifedipine, indicating that the effect of anti-apoptosis in H2S might be correlated with the calcium channel. The expression of Bax and caspase-3 was elevated, while the expression of Bcl-2 decreased in the SAH group but improved in the SAH + NaHS and SAH + Bay K8644 group. Compared with the SAH + NaHS group, the expression of pro-apoptotic proteins was higher in the SAH + NaHS + nifedipine group. Therefore, upon EBI following SAH, the H2S system plays an important neurological protective effect by modulating the function of the L-type calcium channel and inhibiting apoptosis.
Keywords: Subarachnoid hemorrhage, Early brain injury, Apoptosis, Hydrogen sulfide, Calcium channel
Introduction
Subarachnoid hemorrhage (SAH) is a common acute severe illness in the field of neurosurgery, and ruptured intracranial aneurysm is the most common reason for spontaneous SAH (Macdonald and Schweizer 2017). Previous studies have revealed that post-SAH delayed cerebral vasospasm is the primary reason for poor prognosis. However, several recent studies have found that post-SAH early brain injury (EBI) is the main deciding factor of SAH prognosis (Al-Mufti et al. 2017). In EBI, intracranial pressure increases, and cerebral perfusion pressure and cerebral blood flow decrease, causing cerebral ischemia and cerebral tissue necrosis (Topkoru et al. 2017). At present, the post-SAH EBI pathogenesis has not yet been completely illustrated, and studies have suggested that whole cerebral ischemia, blood–cerebral barrier damage, ion homeostasis imbalance, cerebral edema, excitatory toxicity, thrombin activation, oxidative stress, and immune inflammation may be involved. However, for these mechanisms, the treatment measures cannot produce a good SAH prognosis, prompting that the most important mechanism has yet to be explained (Chowdhury et al. 2013; de Oliveira Manoel and Macdonald 2018; Fujii et al. 2013; Pawlowska et al. 2018; Sun et al. 2018). Recent studies have shown that cell apoptosis occurs in the post-SAH EBI pathophysiological process and plays an important role in EBI (Liu et al. 2018).
Hydrogen sulfide (H2S) is an important gas signal molecule that regulates vascular function. It is produced by catalyzing homocysteine through cystathionine beta synthase (CBS) in the nervous system. Recently, several studies have shown that the H2S system participates in the regulation of cell apoptosis and plays an important role in neural protection (Wang et al. 2014). However, its specific mechanism remains unclear. Other studies have shown that calcium channels are involved in the pathophysiological process of EBI after SAH and that activation of the L-type calcium channel can inhibit nerve cell apoptosis, which plays an important role in the survival of nerve cells (Shi et al. 2014). In addition, H2S plays an important role in regulating the L-type calcium channel, which can be regulated differently in different cells. In cardiac myocytes, H2S inhibits the opening of the L-type calcium channel, while in neurons, H2S promotes the opening of the L-type calcium channel (Avanzato et al. 2014; Tang et al. 2010; Wang et al. 2014). The purpose of this study was to investigate whether the H2S system affects the apoptosis of EBI cells after SAH and whether its specific mechanism is related to its L-type calcium channel regulation.
Materials and Methods
Animal Groups
A total of 50 healthy adult male Sprague–Dawley rats weighting 280–300 g were purchased from Beijing Weitong Lihua Laboratory Animal Technology Co., Ltd. These rats were fed by the Animal Experimental Center of Peking University First Hospital. The test rats were randomly divided into five groups, and each group included 10 rats. The details were as follows: (1) control (normal) group: sham-operated group; (2) SAH group: the operation established the SAH model; (3) SAH + NaHS group: at 1 week before the modeling, the rats were given an intraperitoneal injection of NaHS at a dose of 14 μmol/kg, qd; (4) SAH + Bay K8644 group: at 2 h before the modeling, the rats were given an intraperitoneal injection of Bay K8644 at 2 mg/kg; (5) SAH + NaHS + nifedipine group: at 1 week before the modeling, nifedipine was administered through the oral cavity and stomach at a dose of 1 mg/kg, qd, with the NaHS administration method the same as above. All rats were sacrificed at 24 h after the modeling. All animals were treated in compliance with the National Research Council’s Guide for the Care and Use of Laboratory Animals (1996). The study was approved by the Institutional Review Board of the Ethics Committee of Affiliated Hospital of Peking University First Hospital.
Main Reagents
The tunnel kit was purchased from Roche Company (Basel, Switzerland), and the apoptosis-associated protein antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, U.S.A.). The related buffer solution and ECL chemiluminescence kit were purchased from Millipore (Merck KGaA, Darmstadt, Germany). Additionally, NaHS (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), Bay K8644 (Meilun Biotechnology Co., Dalian, China), nifedipine (Tianjin Pacific Chemical & Pharmaceutical Co., Tianjin, China), microtubule-associated protein (MAP-2) antibody (Zhongsan jinqiao Biotechnology Co., Beijing, China), and CBS antibody (Zhongsanjinqiao Biotechnology Co., Beijing, China) were used in this experiment.
Animal Model Preparation and Evaluation
The intravascular puncture method was applied to the rats to establish the post-SAH EBI model (Sehba 2015). After the rats were given abdominal cavity anesthesia with chloral hydrate, a neck incision was performed, and the right external carotid artery was ligatured and cut off. Then, a small V-shaped incision was made in the proximal part of the right external carotid artery to lead a 5-cm fishing line from the incision and insert it into the distal end along the internal carotid artery. Upon producing the resistive, the insertion was conducted within approximately 2–3 mm to puncture the furcation part of the internal carotid artery, establishing the SAH model. After the modeling, Sugewara et al.’s (2008) evaluation method was applied to evaluate the neurological function of the rats after the SAH for 24 h.
Neurological Scoring
Neurological evaluations of the rats in each group were carried out at 24 h after surgery by a colleague who was blinded to the rats’ treatments. To assess neurological deficits, an established neurological scoring system (Garcia et al. 1995) was used that consisted of six test items: spontaneous activity (in cage for 5 min) (0–3), symmetry of movements (four limbs) (0–3), symmetry of forelimbs (outstretched while held by the tail) (0–3), climbing the wall of the wire cage (1–3), reaction to touch on either side of the trunk (1–3), and response to vibrissae touch (1–3). The total scores ranged from 3 to 18; a lower score indicated worse neurological function.
Determination of H2S Content in Cerebral Tissue
For the cerebral tissue perfusion sampling, the hippocampal CA1 neuron region (six samples from each group, approximately 50 mg each) was sampled, and the weight-to-volume ratio was 1:3 after preparation with the homogenate buffer solution to prepare the homogenate. Then, the homogenate was moved into the reaction flask, and NaOH was added into the central hole of the flask. After incubation in water, trichloroacetic acid was added to incubate again, and the liquid was moved to the central hole to determine the H2S content using the sensitive sulfur electrode method.
Detection of the Hippocampal Apoptotic Cell Quantity of the Rats using the Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Method
The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) kit was applied for the operation. The green cells in the merged figure were the positive cells, i.e., the apoptotic cells. The Leica Q550CW Qwin software was adopted to measure the apoptotic nerve cell quantity. The TUNEL method was completed in eight, seven, seven, and nine rats in the SAH group, SAH + NaHS group, SAH + Bay K8644 group, and SAH + NaHS + nifedipine group, respectively.
Detection of Brain Parenchyma in the Hippocampus by Electron Microscopy
After the hippocampus (approximately 50 mg) was taken out, formalin and glutaraldehyde were used for fixation. After the slices were embedded, hematoxylin and eosin staining and electron microscopy were performed (six samples from each group). A transmission electron microscope was used to observe the morphology and soma of the neurons as well as the organelle structure of the axons and dendrites.
Detection of CBS and Apoptosis-Associated Protein Expression in Cerebral Tissues
The immunohistochemical method was adopted to detect the expression of MAP-2 and apoptosis-associated factor caspase-3 (each sample was approximately 150 mg), while western blotting was adopted to detect the expression of CBS, apoptosis-promoting factor Bax, and apoptosis inhibitor Bcl-2 (each sample was approximately 50 mg). The immunohistochemical method and western blot were completed in eight, seven, seven, and nine rats in the SAH group, SAH + NaHS group, SAH + Bay K8644 group, and SAH + NaHS + nifedipine group, respectively.
The MAP-2 antibody, Bcl-2 antibody, Bax antibody, caspase-3 antibody, and CBS antibody were rabbit anti-rat polyclonal antibodies. The primary antibody concentrations were as follows: MAP-2 (1:800), Bax (1:300), Bcl-2 (1:300), caspase-3 (1:300), and CBS (1:50). The immunohistochemistry was performed according to the kit’s instructions. Western blotting was used to extract the protein sample through lysis buffer, and SDS-PAGE gel electrophoresis was performed. The PVDF membrane was applied to transfer the membrane, followed by the use of the western detergent for rinsing and the addition of the western confining liquid for closing. Then, the primary antibody and secondary antibody were added for incubation, and the BeyoECL Plus (P0018) reagent was used to detect the protein. The positive expression of caspase-3 manifested as brownish-yellow granules in the cytoplasm, while MAP-2 manifested as stained neuronal axons and dendrites.
Statistical Methods
All data were analyzed using the SPSS 16.0 software. For continuous variables with a normal distribution, the single-factor ANOVA was used. The comparisons between the groups were analyzed by t test, and the data were expressed as the mean ± standard deviation. A P value < 0.05 was considered statistically significant.
Results
Among the 50 test rats, 9 died during or shortly after the surgery (2, 3, 3, and 1 in the SAH group, SAH + NaHS group, SAH + Bay K8644 group, and SAH + NaHS + nifedipine group, respectively).
Neurological Functioning Results
The neurological scores of the rats at 24 h after surgery were significantly lower in the SAH group than in the control group, suggesting pronounced neurological impairment was induced by the SAH. This impairment presented mainly as reduced spontaneous activity and limb weakness. The NaHS and Bay K8644 slightly increased the neurological scores of the rats with SAH, but the differences were not significant. The neurological scores in the SAH + NaHS + nifedipine group were slightly lower than in the SAH + NaHS group, but there was no statistical significance (Fig. 1).
Fig. 1.
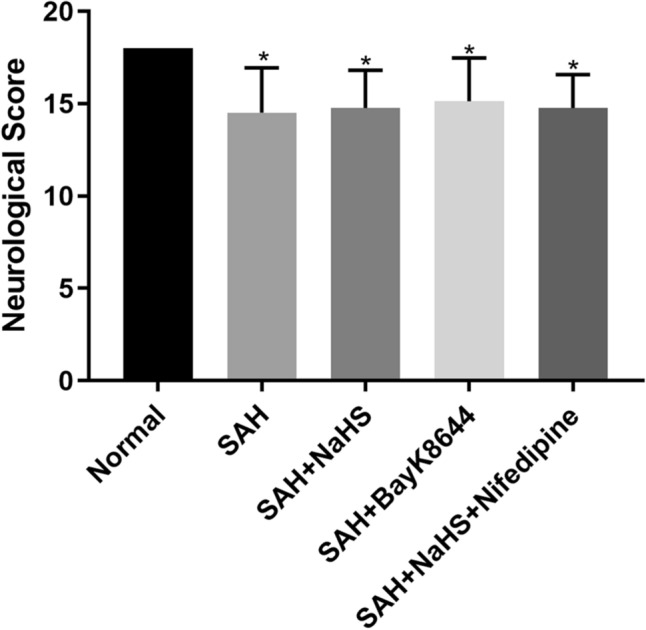
The neurological scores of the rats in each group at 24 h after surgery. The scores were lower in the four SAH model groups compared with the control group. However, there was no statistical significance among the four SAH groups. *Means compared with the control group (normal), P < 0.05
Pathological Changes of Hippocampal Neurons in the SAH Rats
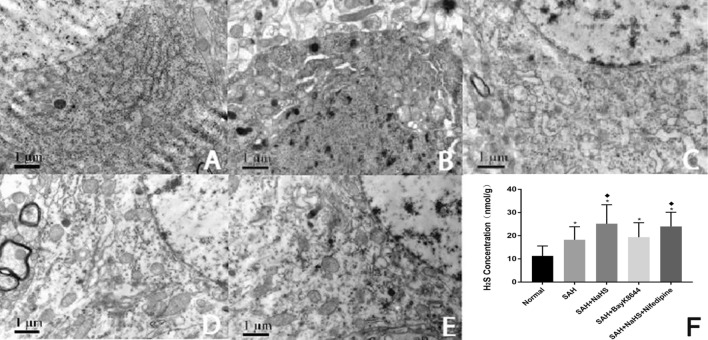
The electron microscopy results revealed that the hippocampal neuron Golgi complex of the SAH group greatly expanded compared to the control group. Meanwhile, rough endoplasmic reticulum degranulation occurred, the nuclear membrane structure was fuzzy, and the mitochondria severely swelled and expanded. Moreover, the crest was broken and dissolved, and the lysosomes significantly increased. The expansion of the Golgi complex and rough endoplasmic reticulum was also inhibited compared to the SAH + Bay K8644 group. Meanwhile, the lysosome increased, and the mitochondrial swelling was improved, though the improving effect of NaHS was reduced by calcium ion agonist nifedipine. The Golgi complex and rough endoplasmic reticulum expansion were aggravated in the SAH + NaHS + nifedipine group. In addition, the mitochondrial swelling was relatively significant in the SAH + NaHS + nifedipine group compared with the SAH + NaHS group (Figs. 2a–e).
Fig. 2.
a–e structure of the hippocampal neurons of the rats in each group under an electron microscope. a control group; b SAH group; c SAH + NaHS group; d SAH + Bay K8644 group; e SAH + NaHS + nifedipine group. f H2S concentration in the hippocampus of the rats in each group. *Means compared with the control group (normal), P < 0.05; (filled diamond) means compared with the SAH group, P < 0.05
Elevated H2S Concentration in Hippocampus of the SAH Rats
The H2S concentration was detected in the hippocampus of rats from each group, showing that the H2S in the SAH group increased compared to the control group. After NaHS was given, the concentration continued to increase, while after Bay K8644 and nifedipine were given, the H2S concentration did not significantly change, indicating that the opener and agonist of the calcium channel cannot influence H2S generation (Fig. 2f).
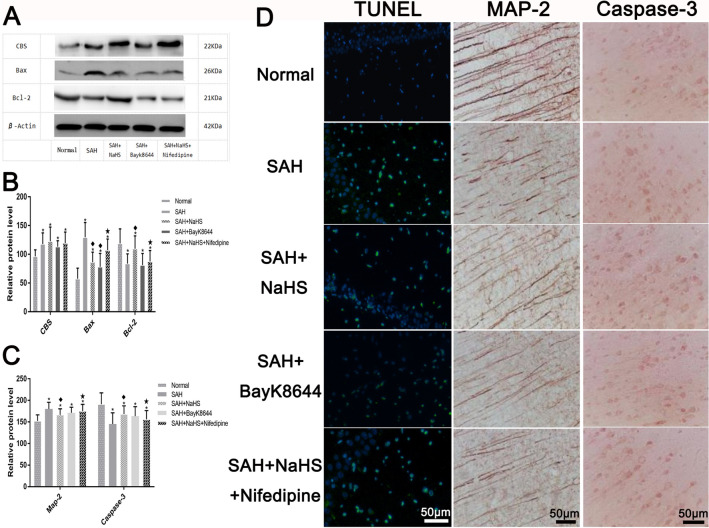
Elevated CBS Expression Upregulation of H2s in Cerebral Tissues
The CBS expression was detected in the hippocampus of rats from each group, showing that the CBS expression of the SAH group significantly increased compared to the control group. However, there were no significant changes when compared among all groups. This indicated that the post-SAH H2S system had started and that the increase in CBS expression caused the increase in H2S concentration, while the calcium channel opener and calcium channel agonist had no significant influence on the CBS expression (Figs. 3a, b).
Fig. 3.
The expression of CBS, Bax, and Bcl-2 via the western blotting in each group (a) and the statistical results (b). *Means compared with the control group (normal), P < 0.05; (filled diamond) means compared with the SAH group, P < 0.05; (filled star) means compared with the SAH + NaHS group, P < 0.05. c The average optical density of the immunohistochemical staining results of MAP-2 and Caspase-3 in each group. *Means compared with the control group (normal), P < 0.05; (filled diamond) means compared with the SAH group, P < 0.05; (filled star) means compared with the SAH + NaHS group, P < 0.05. d: The detection of hippocampal neuron apoptosis in each group; the sections were labeled with TUNEL (green) to detect apoptotic cells and counterstained with DAPI (blue) to detect the nuclei. Immunohistochemical staining of MAP-2 and Caspase-3 in each group; scale bar = 50 μm
Inhibitory Effect of H2S on Apoptosis of Hippocampal Neurons and the Effect of the Calcium Channel
The TUNEL method was applied to detect the hippocampal neuron apoptosis conditions of rats from each group. The following details were revealed: The cell apoptosis was significant in the SAH group compared to the control group, and after the NaHS pretreatment was given, the apoptosis decreased. The number of apoptotic cells decreased in the SAH + Bay K8644 group compared to the SAH group, while the apoptosis increased in the SAH + NaHS + nifedipine group compared to the SAH + NaHS group (Fig. 3d).
Inhibitory Effect of H2S on Apoptosis-Associated Protein Expression and the Effect of the Calcium Channel in Cerebral Tissues
MAP-2 Expression
The immunohistochemical assay revealed the following: The MAP-2-positive staining was mainly distributed in the neuron body, axon, and dendrite. Compared to the control group, the axon staining in the SAH group was shallow, broken, and sparsely and disorderly distributed, suggesting that the microtubule system was damaged. Compared to the SAH group, the axon staining in the SAH + NaHS and SAH + Bay K8644 groups darkened, and the axon distribution was dense and regular under whole microscopic vision, indicating that the H2S and calcium channel opener may have relieved the damage of the microtubule system. Compared to the NaHS group, the axon staining of the SAH + NaHS + nifedipine group was shallow and broken, and the distribution was relatively scattered. However, compared to the SAH group, the axon staining darkened, showing that the protective effect of NaHS was partially antagonized by the nifedipine (Fig. 3c, d).
Bax and Bcl-2 Expression
Western blotting was adopted to detect apoptosis-associated proteins Bax and Bcl-2, and it was found that the Bax expression significantly increased in the SAH group compared to the control group. Compared to the SAH group, the Bax protein expression decreased in the SAH + NaHS and SAH + Bay K8644 groups, while, compared to the SAH + NaHS group, it increased in the SAH + NaHS + nifedipine group (Fig. 3a, b). These results suggested that post-SAH cell apoptosis increased and that the exogenous application of H2S donor NaHS and calcium channel opener Bay K8644 could relieve the cell apoptosis. Meanwhile, the apoptosis-inhibiting function of NaHS may have been partially weakened through the calcium ion agonist.
The anti-apoptotic protein Bcl-2 expression decreased in the SAH group compared to the control group but increased in the SAH + NaHS group compared to the SAH group. However, the Bcl-2 expression did not significantly change in the SAH + Bay K8644 group compared to the SAH group. After nifedipine was given, the Bcl-2 expression decreased compared to the SAH + NaHS group (Fig. 3a, b). This indicated that NaHS could induce the anti-apoptotic protein Bcl-2 expression to increase and that this function was weakened through the calcium ion agonist.
Caspase-3 Expression
Compared to the control group, the cell staining of caspase-3 in the SAH group was significantly deeper. Meanwhile, the positive quantity increased, the arrangement was dense, and the caspase-3 expression increased. The caspase-3 expression decreased in the SAH + NaHS and SAH + Bay K8644 groups compared to the SAH group. Furthermore, it decreased in the SAH + NaHS + nifedipine group compared to the SAH group but increased compared to the SAH + NaHS group, demonstrating that the nifedipine weakened the effect of NaHS to relieve the apoptosis (Fig. 3c, d).
Discussion
Post-SAH EBI is the main factor determining the SAH prognosis (Chowdhury et al. 2013). Recent studies have shown that cell apoptosis participates in the post-SAH EBI pathophysiological process and plays an important role in EBI (Liu et al. 2018). In the early stage of post-SAH, cell apoptosis occurs in the hippocampus, blood–brain barrier, and cerebrovascular system and significantly increases in local cerebral tissues (Sabri et al. 2013). For protein kinase B (Akt), which is a key apoptosis-associated signal molecule, in post-SAH EBI, the Akt/GSK-3β signaling pathway is activated, Akt and GSK-3β phosphorylation significantly increases, and the relevant downstream products, such as caspase-3, also significantly increase (Duris et al. 2011). In addition, studies have shown that inhibiting the activation of the p38-MAPK pathway or caspase-3 can reduce the incidence of neuronal apoptosis, relieving EBI (Hasegawa et al. 2011; Zhang et al. 2011). The present study found that in post-SAH EBI, hippocampal cell apoptosis in the rats significantly increased, Bcl-2 expression in the cerebral tissue decreased, and Bax and caspase-3 expression increased, further proving that cell apoptosis participates in the pathophysiological process of post-SAH EBI formation.
At the end of the last century, H2S was the third gas signaling molecule discovered (after NO and CO), and present studies have shown that the H2S system participates in the regulation of cell apoptosis and plays an important role in neural protection (Wang et al. 2014). In rats with cerebral ischemia reperfusion, the H2S and CBS expression in the cortical and hippocampal regions varies with the time of reperfusion, and giving a low dose of NaHS can reduce nerve injury after reperfusion. Further research has shown that H2S plays a role in protecting against cerebral ischemia reperfusion injury by inhibiting cell apoptosis (Ren et al. 2010). Many studies have shown that H2S participates in the pathophysiological process of multiple diseases in the body by inhibiting cell apoptosis and plays a role in protecting nervous tissues and reducing the incidence of EBI (Grobelny et al. 2011; Lei et al. 2016; Minamishima et al. 2009). Yu et al. (2014) summarized and compared similar research on H2S, NO, and CO in neural protection within the past 10 years, speculating that H2S plays a role in protecting against post-SAH cerebral injury. The present study also found that after the SAH rats were given H2S donor NaHS, the apoptosis-promoting associated protein expression of the rats in the NaHS + SAH group decreased compared to the SAH group. Meanwhile, under electron microscopy, it was found that nerve injury was relieved, indicating that the endogenous H2S system participates in the post-SAH EBI process and that exogenous application of the H2S donor can relieve cell apoptosis in EBI, realizing neural protection. However, the mechanism of H2S in regulating cell apoptosis in post-SAH EBI remains unclear.
Studies have shown that the voltage-gated calcium channel plays an important role in regulating nerve cell apoptosis, dendritic extension, and synaptic plasticity. The central neurons can express nine kinds of voltage-gated calcium channels with at least different functions, but in the hippocampus and other cerebral tissues, the L-type calcium channel is the most common, accounting for 30–50% of the calcium ion flow. The activated L-type calcium channel can effectively stimulate the expression of transcription factors, such as cAMP response element binding protein and myocyte-enhancer factor 2, and inhibit cell apoptosis (Dolphin 2018). Additionally, in the early stage of post-SAH, changes in the calcium ion current in cells can be observed, and the L-type calcium channel opening significantly shrinks, demonstrating that the L-type calcium channel participates in the pathophysiological process of cerebral injury in this early stage (Shi et al. 2014). It has been reported that H2S plays a significant role in regulating the L-type calcium channel. In myocardial cells, H2S inhibits the opening of the L-type calcium channel, while in neurons, it promotes the opening of the L-type calcium channel—which mediates the signal transmission between the neurons and neuroglia—and further serves as an anti-apoptotic, anti-inflammatory, and antioxidative neurotransmitter protecting neurons to reduce secondary nerve injury (Avanzato et al. 2014; Tang et al. 2010; Wang et al. 2014). Therefore, we hypothesized that the mechanism of H2S regulating apoptosis in EBI after SAH is related to the regulation of the L-type calcium channel.
In the present study, the investigators provided L-type calcium channel opener Bay K8644, by which the cell apoptosis significantly reduced and the apoptosis-promoting associated protein expression significantly decreased. This further indicated that opening of the calcium channel can inhibit post-SAH cell apoptosis. Furthermore, after L-type calcium channel agonist nifedipine was given, the ability of H2S in improving post-SAH cell apoptosis significantly declined, and the cell apoptosis increased compared to the NaHS group. Moreover, the apoptosis-associated protein expression significantly increased. Therefore, the role of H2S in improving post-SAH cell apoptosis is theorized to be realized by opening the calcium channel. Additionally, it is hypothesized that an activation mechanism of the channels might result from the formation of a disulfide bridge between the cysteine residues of the pore and that H2S might have an accommodating gate on the channels mentioned above, with “Cys-SH” as the critical target (Zhang et al. 2012).
The limitations of the present study are as follows: In the experiment, the detection of various proteins was performed through the detection of semi-quantitative protein levels, and there was a lack of further detection at the DNA or RNA level. The participation of the calcium channel in the regulation of H2S on cell apoptosis was based on the use of the corresponding agonist drug and indirect detection results. In the future, when the calcium channel patch clamp has been further detected, it will be beneficial to further verify these results.
In conclusion, the regulatory mechanism of H2S on cell apoptosis in post-SAH EBI may be correlated with the calcium channel, which is induced by increasing the activation of the L-type calcium channel to promote the internal flow of calcium ions and reduce cell apoptosis, ultimately weakening the post-SAH EBI and thereby realizing neural protection.
Acknowledgements
This work was supported by Chinese National Nature Science Foundation (Grant No. 81541119), Youth Clinical Research Project of Peking University First Hospital (2019CR02), and Peking University First Hospital Cross Clinical Study Foundation.
Author Contributions
HZD and CWW were responsible for the study design and manuscript writing. SLS and JYZ were responsible for the study execution and data collection. LL was responsible for data management, data analysis, and interpretation. All the authors read and approved the final manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
All animals were treated in compliance with the National Research Council’s Guide for the Care and Use of Laboratory Animals (1996). The study was approved by the Institutional Review Board of the Ethics Committee of Affiliated Hospital of Peking University First Hospital. This article does not contain any studies with human participants performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hong-Zhou Duan and Chong-Wei Wu have contributed equally to this study.
References
- Al-Mufti F, Amuluru K, Smith B, Damodara N, El-Ghanem M, Singh IP, Dangayach N, Gandhi CD (2017) Emerging markers of early brain injury and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. World Neurosurg 107:148–159 [DOI] [PubMed] [Google Scholar]
- Avanzato D, Merlino A, Porrera S, Wang R, Munaron L, Mancardi D (2014) Role of calcium channels in the protective effect of hydrogen sulfide in rat cardiomyoblasts. Cell Physiol Biochem 33(4):1205–1214 [DOI] [PubMed] [Google Scholar]
- Chowdhury T, Dash HH, Cappellani RB, Daya J (2013) Early brain injury and subarachnoid hemorrhage: Where are we at present? Saudi J Anaesth 7(2):187–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Manoel AL, Macdonald RL (2018) Neuroinflammation as a target for intervention in subarachnoid hemorrhage. Front Neurol 9:292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC (2018) Voltage-gated calcium channels: their discovery, function and importance as drug targets. Brain Neurosci Adv 2:2398212818794805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duris K, Manaenko A, Suzuki H, Rolland WB, Krafft PR, Zhang JH (2011) α7 nicotinic acetylcholine receptor agonist PNU-282987 attenuates early brain Injury in a perforation model of subarachnoid hemorrhage in rats. Stroke 42(12):3530–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH (2013) Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res 4(4):432–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ (1995) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 26(4):627–634 discussion 635 [DOI] [PubMed] [Google Scholar]
- Grobelny BT, Ducruet AF, DeRosa PA, Kotchetkov IS, Zacharia BE, Hickman ZL, Fernandez L, Narula R, Claassen J, Lee K, Badjatia N, Mayer SA, Connolly ES Jr (2011) Gain-of-function polymorphisms of cystathionine β-synthase and delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. J Neurosurg 115(1):101–107 [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Suzuki H, Altay O, Zhang JH (2011) Preservation of tropomyosin-related kinase B (TrkB) signaling by sodium orthovanadate attenuates early brain injury after subarachnoid hemorrhage in rats. Stroke 42(2):477–483 [DOI] [PubMed] [Google Scholar]
- Lei Y, Zhen Y, Zhang W, Sun X, Lin X, Feng J, Luo H, Chen Z, Su C, Zeng B, Chen J (2016) Exogenous hydrogen sulfide exerts proliferation, anti-apoptosis, angiopoiesis and migration effects via activating HSP90 pathway in EC109 cells. Oncol Rep 35(6):3714–3720 [DOI] [PubMed] [Google Scholar]
- Liu J, Zhou S, Zhang Y, Li X, Qian X, Tao W, Jin L, Zhao J (2018) Bax inhibitor-1 suppresses early brain injury following experimental subarachnoid hemorrhage in rats. Int J Mol Med 42(5):2891–2902 [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Schweizer TA (2017) Spontaneous subarachnoid haemorrhage. Lancet 389(10069):655–666 [DOI] [PubMed] [Google Scholar]
- Minamishima S, Bougaki M, Sips PY, Yu JD, Minamishima YA, Elrod JW, Lefer DJ, Bloch KD, Ichinose F (2009) Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation 120(10):888–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowska E, Szczepanska J, Wisniewski K, Tokarz P, Jaskólski DJ, Blasiak J (2018) NF-κB-mediated inflammation in the pathogenesis of intracranial aneurysm and subarachnoid haemorrhage. Does autophagy play a role? Int J Mol Sci 19(4):E1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Du A, Li D, Sui J, Mayhan WG, Zhao H (2010) Dynamic change of hydrogen sulfide during global cerebral ischemia-reperfusion and its effect in rats. Brain Res 1345:197–205 [DOI] [PubMed] [Google Scholar]
- Sabri M, Ai J, Lakovic K, Dabbondanza J, Ilodigwe D, Macdonald RL (2013) Mechanisms of microthrombosis and microcirculatory constriction after experimental subarachnoid hemorrhage. Acta Neurochir Suppl 115:185–192 [DOI] [PubMed] [Google Scholar]
- Sehba FA (2015) The rat endovascular perforation model of subarachnoid hemorrhage. Acta Neurochir Suppl 120:321–324 [DOI] [PubMed] [Google Scholar]
- Shi X, Fu Y, Liao D, Chen Y, Liu J (2014) Alterations of voltage-dependent calcium channel currents in basilar artery smooth muscle cells at early stage of subarachnoid hemorrhage in a rabbit model. PLoS ONE 9(1):e84129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T, Ayer R, Jadhav V, Zhang JH (2008) A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods 167(2):327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Yang S, Li S, Hang C (2018) Melatonin upregulates nuclear factor erythroid-2 related factor 2 (Nrf2) and mediates mitophagy to protect against early brain injury after subarachnoid hemorrhage. Med Sci Monit 24:6422–6430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Wu L, Wang R (2010) Interaction of hydrogen sulfide with ion channels. Clin Exp Pharmacol Physiol 37(7):753–763 [DOI] [PubMed] [Google Scholar]
- Topkoru B, Egemen E, Solaroglu I, Zhang JH (2017) Early brain injury or vasospasm? An overview of common mechanisms. Curr Drug Targets 18(12):1424–1429 [DOI] [PubMed] [Google Scholar]
- Wang JF, Li Y, Song JN, Pang HG (2014) Role of hydrogen sulfide in secondary neuronal injury. Neurochem Int 64:37–47 [DOI] [PubMed] [Google Scholar]
- Yu YP, Chi XL, Liu LJ (2014) A hypothesis: hydrogen sulfide might be neuroprotective against subarachnoid hemorrhage induced brain injury. Sci World J 2014:432318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhao XD, Shi JX, Yin HX (2011) Inhibition of the p38 mitogen-activated protein kinase (MAPK) pathway attenuates cerebral vasospasm following experimental subarachnoid hemorrhage in rabbits. Ann Clin Lab Sci 41(3):244–250 [PubMed] [Google Scholar]
- Zhang RY, Sun Y, Tsai HJ, Tang CS, Jin HF, Du JB (2012) Hydrogen sulfide inhibits L-type calcium currents depending upon the protein sulfhydryl state in rat cardiomyocytes. PLoS ONE 33(4):1205–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]