Abstract
The SARS-Cov-2 pandemic that currently affects the entire world has been shown to be especially dangerous in the elderly (≥65 years) and in smokers, with notably strong comorbidity in patients already suffering from chronic diseases, such as Type 2 diabetes, cancers, chronic respiratory diseases, obesity, and hypertension. Inflammation of the lungs is the main factor leading to respiratory distress in patients with chronic respiratory disease and in patients with severe COVID-19. Several studies have shown that inflammation of the lungs in general and Type 2 diabetes are accompanied by the degradation of glycosaminoglycans (GAGs), especially heparan sulfate (HS). Several studies have also shown the importance of countering the degradation of HS in lung infections and Type 2 diabetes. D-xylose, which is the initiating element for different sulfate GAG chains (especially HS), has shown regeneration properties for GAGs. D-xylose and xylitol have demonstrated anti-inflammatory, antiglycemic, antiviral, and antibacterial properties in lung infections, alone or in combination with antibiotics. Considering the existing research on COVID-19 and related to D-xylose/xylitol, this review offers a perspective on why the association between D-xylose and antibiotics may contribute to significantly reducing the duration of treatment of COVID-19 patients and why some anti-inflammatory drugs may increase the severity of COVID-19. A strong correlation with scurvy, based on gender, age, ethnicity, smoking status, and obesity status, is also reviewed. Related to this, the effects of treatment with plants such as Artemisia are also addressed.
Chemical compounds
D-xylose; xylitol; l-ascorbic Acid; D-glucuronic acid; N-acetylglucosamine; D-N-acetylglucosamine; N-acetylgalactosamine; galactose.
Keywords: Xylose; Xylitol, SARS-Cov-2; Inflammatory; Lung; Acute kidney injury; Diabetes; Scurvy
Graphical abstract
1. Introduction
COVID-19 caused by the SARS-Cov-2 virus remains at pandemic level without any established treatment for patients developing the severe forms of the disease that involve respiratory distress (with resuscitation). The mortality rate in the three countries most affected in terms of number of deaths, as of June 14, 2020, is 5.56% for the United States, 5.02% for Brazil, and 14.11% for the United Kingdom [1]. In patients developing a severe form of COVID-19 (about 5% [2]), the organs most affected are the lungs, followed by the kidney and heart; about 65% to 75% of these cases require invasive ventilation with intensive care management [3,4]. This high percentage of patients requiring admission to an intensive care unit (ICU) quickly saturates the resuscitation capacity in areas with outbreaks of the disease [[5], [6], [7]]. This in turn increases mortality [8].
The mechanism for fatality due to COVID-19 is beginning to be understood, based on practitioners' observations and several studies carried out on COVID-19 patients. Faced with a “cytokines storm” that causes acute inflammation of the lungs, leading to respiratory distress, practitioners have administered different anti-inflammatory drugs to patients and have also issued warnings about the harmful consequences of prolonged use of some of these anti-inflammatory drugs, including some nonsteroidal anti-inflammatory drugs (NSAIDs), the use of which leads to cardiovascular problems [[9], [10], [11], [12]]. It is therefore essential to find alternative anti-inflammatory treatments that significantly reduce the duration of ICU management of patients with severe COVID-19.
Glycosaminoglycans (GAGs) found on the surface of different cells and in the extracellular matrix (ECM) play a primary role in inflammation of the lungs and more generally during viral infections and in diseases with high comorbidity with COVID-19: Type 2 diabetes, cancer, hypertension, kidney diseases, coronary artery disease (CAD), and so on.
This review considers a therapeutic pathway based on a molecule related to GAGs, D-xylose, in combination with antibiotics. A narrative review of the literature is provided, including all relevant high-quality information that addresses the points presented in Table 1 for the use of D-xylose.
Table 1.
COVID-19-Associated Factors Correlated with D-Xylose.
| No. | Points | SARS-Cov-2 (COVID-19) |
|---|---|---|
| 1 | Lungs | SARS-Cov-2 (COVID-19) induces lung inflammation [13]. |
| 2 | Anti-inflammatory drugs | Some anti-inflammatory drugs might contribute to severe manifestations of COVID-19 [[9], [10], [11], [12]]. |
| 3 | Antibiotics | Doxycycline (tetracycline) has been proposed by doctors in the proposed protocol for the management of COVID-19 patients [14]. |
| 4 | Type 2 Diabetes (T2D) | Comorbidity with SARS-Cov-2 [[15], [16], [17], [18]]. |
| 5 | Hypertension | Comorbidity with SARS-Cov-2 [[15], [16], [17], [18]]. |
| 6 | Coronary artery disease (CAD) | Comorbidity with SARS-Cov-2 [15,16,18]. |
| 7 | Cancers | Comorbidity with SARS-Cov-2 [15,18]. |
| 8 | Chronic obstructive pulmonary disease (COPD) | Comorbidity with SARS-Cov-2 [15,16,18]. |
| 9 | Acute kidney injury (AKI) | Comorbidity with SARS-Cov-2 [2,15,16] |
| 10 | Vitamin C | Some studies have reported that high dose intravenous vitamin C may helps fight the virus [19,20] |
| 11 | Elderly (≥65 years old) | About 21% of elderly (≥65 years old) infected with COVID-19 develop a severe form (admission to intensive care or death) [21] |
| 12 | Smokers | Smokers are around 2.4 times more likely to be admitted to an ICU, need mechanical ventilation or die or die compared to non-smokers [22] |
| 13 | Gender | Men are 1.7 to 2.1 times more likely to be admitted to an ICU, need mechanical ventilation or die compared to women [23] |
| 14 | Ethnicity | Blacks in New York City are 2 times more likely to dying of COVID-19 compared to Whites and Asians [24] |
| 15 | Obesity | A study of 3615 individuals who tested positive for COVID-19 show that patients under age 60 with a BMI of 30-34 were 1.8 times more likely to be admitted to ICU compare to those at same age with BMIs <30 [25] |
| 16 | Glycosaminoglycans (GAGs) |
Glycosaminoglycan may facilitate SARS-CoV-2 host cell entry [26] |
| 17 | Biomarker: Hyaluronan |
Hyaluronic acid and type III procollagen, can be used as early warning indicators of poor prognosis for critical patients with COVID-19 [[27], [28], [29]] |
| 18 | Idiopathic Pulmonary fibrosis (IPF) |
IPF is a major risk factor for severe COVID-19 [28,30]. The use of anti-fibrotic therapy has been hypothesized for COVID-19 [30] |
| 19 | Peripheral. Lymphocyte subset |
The significantly reduced numbers of CD3+CD8+T cells, but not CD3+CD4+T cells, can be used as early warning indicators of poor prognosis for critical patients with COVID-19 [31] |
| 20 | Thrombosis | Thrombosis is a major risk factor for severe COVID-19 [32] |
| 21 | Medicinal plants (Artemisia) | Artemisia: the “COVID organics,” a decoction based on Artemisia set up by Malagasy researchers and presented by their president is currently used by several African countries [33] |
Symptoms, biomarkers, therapeutic pathways, and risk factors associated with severe COVID-19.
Point by point, the involvement or relationship with D-xylose or xylitol is considered in the following for each of the 21 aspects listed in Table 1.
2. D-xylose/xylitol and glycosaminoglycans
D-xylose is a monosaccharide found in various plant components (some fruits, birch tree bark, some other plants). It can be evaluated in terms of malabsorption using the “D-xylose test” because it is directly used in the body in its natural form and therefore does not undergo metabolism [34].
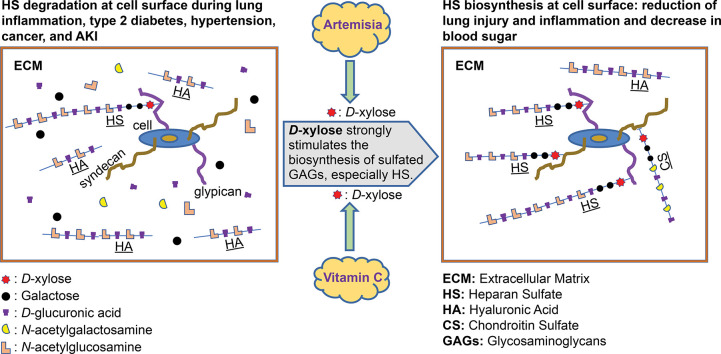
In an organism, D-xylose is used at the level of sulfated glycosaminoglycans (GAGs), which are long linear chains that consist of disaccharide repetitive units found in the extracellular matrix (ECM), at the surface of the endothelial cells, fibroblasts, macrophages and hepatocytes [35]. There are five types of sulfated GAGs: heparan sulfates (HS), heparins (Hep), chondroitin sulfates (CS), dermatan sulfates (DS), and keratan sulfates (KS). Sulfated GAGs are bound to a core protein (syndecans, glypicans, decorins, etc.) through a covalent link, forming proteoglycans (PGs).
The main core proteins of PGs in the extracellular matrix are perlecan (with HS type chains), agrin (heparan sulfate proteoglycan [HSPG]), aggrecan (chondroitin sulfate proteoglycan [CSPG]/dermatan sulfate proteoglycan [DSPG]), and decorin.
The membranous core proteins of PGs are categorized as either syndecans or glypicans. Syndecans are transmembrane proteins, unlike glypicans, which are entirely extracellular proteins (fixed on the cellular membrane). The membranous PGs are HSPGs, CSPGs, and DSPGs, but are predominantly HSPGs. HSPGs are found on the surfaces of several types of cells, such as endothelial cells, epithelial cells, fibroblasts, and neuronal tissues [35,36].
Except for KS, all the other sulfated GAGs are connected to the core proteins by an identical linkage region, consisting of the trisaccharide sequence xylose–galactose–galactose [35,36]. The biosynthesis of the HS chain (and also CS, DS, and Hep chains) begins with the fixation of a D-xylose molecule to specific serine residues placed on the core protein (syndecan, glypican, decorin, etc.). This initiation of HS biosynthesis is carried out through xylosyltransferase 1 and xylosyltransferase 2 enzymes [35,36]. After the D-xylose molecule is in place, binding of two galactose molecules follows. The chain thus formed constitutes the linkage region. This is then completed with a repetition of the same basic unit specific to each type of sulfated GAGs (HS/CS/DS/Hep).
The base unit for HS is a D-glucuronic acid linked to N-acetylglucosamine. The base unit of CS is an N-acetylgalactosamine linked to a D-glucuronic acid. Hyaluronic acid is the only nonsulfated GAG; this type of glycosaminoglycan is not linked to a core protein. The disaccharides that compose it are themselves comprised of D-glucuronic acid and D-N-acetylglucosamine [35].
HSPGs are quickly recycled and renewed on the surface of the cell; their half-life is approximately 2–3 h [37]. The degradation of HSPGs on the cell surface depends on the action of several extracellular and lysosomal enzymes, and in particular on heparanase, which cleaves HS chains [35,36].
Xylose is therefore the element binding these GAGs to the core proteins and only has one position. This role of D-xylose in the biosynthesis of HS/CS/DS/Hep indicates that the amount of D-xylose in the organism directly influences the amount of these GAGs present in that organism.
A large French company has already demonstrated this: Patent number WO1999024009A1, filed in 1999, records the surprising discovery that D-xylose significantly stimulated the synthesis and secretion of PGs and GAGs on the surface of epidermis cells (fibroblasts, keratinocytes) [38].
Apart from the GAGs, the only other pathway where xylose is used is called Notch signaling. The rest of this review focuses mainly on the use of xylose at the level of the GAGs.
D-xylose is a direct metabolite of xylitol (known as wood sugar) via the enzyme xylose reductase and coenzyme NAD(P)+ [39]. The following sections review the evidence for use of xylose and xylitol that supports this line of approach.
3. GAGs and viral infection: SARS-Cov-2
Many studies have demonstrated the central role played by sulfated GAGs, particularly HS, during viral infections. Various viruses interact with GAGs on the cell surface This is the case, for example, for filovirus [40], human respiratory syncytial virus [41], and human adenovirus type 37, where researchers proposed the use of sulfated GAGs as viral decoy receptors as a therapeutic pathway by the after an in vitro study [42]; this also applied to the rabies virus, where acute respiratory distress syndrome (ARDS) is one of the first lethal complications of infection [43], and for dozens of other viruses and bacteria [44,45].
A recent in vitro study in the United States on SARS-Cov-2 allowed Kim et al. to demonstrate that GAGs can facilitate the entry of SARS-Cov-2 and suggested that, following that study, consideration be given to implementing GAGs-based therapeutic approaches [26]. Hao et al., through an in vitro study conducted in China, also generated support for these results, demonstrating that the glycoprotein Sipke S of SARS-Cov-2 attaches to HS on the cell surface. This study also suggests exploring the use of HS to fight the virus [46].
These therapeutic avenues based on HS are also supported by the observation that just like SARS-Cov-2, coronavirus Hcov-NL63 and SARS-Cov use ACE2 as a receptor [47], but also HS. In addition, Buijsers and her colleagues recently demonstrated an involvement of the heparanase enzyme and HS chains that relates to the severity of COVID-19 [48].
With these elements demonstrating the possible involvement of GAGs, especially HS, in SARS-Cov-2 infection, then, contrary to the HS inhibition strategy that has been proposed by some researchers, it appears that GAGs should be regenerated.
4. D-xylose/xylitol, lung inflammation, and respiratory infections
Viral and bacterial infections affecting the lungs are usually accompanied by the degradation of GAGs.
In 2017, Rajas et al. showed in an in vitro study that GAGs in general and more specifically HS play an essential role in the attachment and adhesion of infectious agents to the surface of lung cells [49].
Schmidt et al. demonstrated in 2012, reporting a study in mice, that acute pulmonary lesions and lung diseases were triggered by the degradation of endothelial pulmonary glycocalyx (consisting of glycoproteins and PGs) and that this degradation specifically concerned HS. They noted that such degradation resulted in an increased availability of adhesion molecules (CAMs) on the endothelial surface. Inhibition of the enzyme responsible for HS degradation (heparanase) has been observed to be followed by reduced lesions, inflammation, and mortality [50].
Indeed, the inhibition of the heparanase enzyme stops HS chain degradation (see Section 2). Thus, stopping the destruction of HS causes a reduction in lesions, inflammation, and mortality. On the other hand, the degradation of HS causes an increase in CAMs, and it is known that an increase in CAMs promotes infection [51], and promotes or testifies to the inflammatory state [52].
Buijsers and her colleagues recently demonstrated in a study in vivo that heparanase activity in patients developing a severe form of COVID-19 is significantly more important than it is in noninfected individuals. They reported that the level of severity was directly related to the level of such activity. The same applies to the level of HS circulating in the plasma of infected patients, which is significantly higher than in noninfected persons [48]. The high level of circulating HS reflects a degradation of GAGs on the cell surface, which induces, as the researchers point out, the deregulation of the integrity of the endothelial glycocalyx.
From a literature review on the subject in 2016, Haeger et al. also concluded that HS plays a central role in severe lung diseases and that one of the avenues of treatment for severe lung diseases could be treatment to counteract HS degradation [53]. However, as described in Section 2, patent WO1999024009A1, filed by a large French company in 1999, presented the surprising discovery that D-xylose significantly stimulated the synthesis and secretion of PGs and GAGs on the surface of epidermis cells (fibroblasts, keratinocytes) [38]. The lungs have abundant cells that are fibroblast types; thus, in view of the preceding, D-xylose prevents the degradation of HS by stimulating the biosynthesis of HS in a significant way at the lungs and thereby prevents injury and inflammation. The use of antifibrotic therapy has been proposed for treatment of COVID-19 (see Table 1). Other studies have also demonstrated the importance of xylitol in the prevention or treatment of respiratory infections.
In vitro studies from the University of Iowa (patent number US20040192786A1) have found that xylitol sugar lowers the salt concentration of the airway surface liquid lining the interior of the lungs, improving the antibody activity in the lungs. For researchers, this xylitol-based method has promise for the prevention or treatment of respiratory infections, respiratory pneumonia, and chronic bronchitis [54]. Taking into account the foregoing and the fact that xylitol is the alcoholic form of D-xylose, the results obtained seem logical.
Moreover, in 2011, Ferreira et al. demonstrated in an in vitro study that xylitol plays an important role in cell adhesion on macrophages. The researchers reported that there were 10 times more cells glued to the macrophage controls compared to the xylitol-treated macrophages, and concluded that the presence of xylitol could be adapted to control inflammation [55], since cell adhesion is a crucial step in lung inflammation [56]. The already-mentioned study by Schmidt et al. reported that the increase in CAMs correlated with the degradation of HS. This study by Aline et al. can be viewed as indicating that xylitol-treated cells had 10 times fewer CAMs than untreated cells. This is consistent with the conclusion reached by researchers on the potential for use of xylitol to control inflammation in the lungs.
In vivo studies conducted at Chung-Ang University in 2016 by Xu et al. reported that mice that received dietetic xylitol for 14 days before and for 3 days after human respiratory syncytial virus (hRSV) inoculation had a significantly higher reduction of viral load [57]. These results corroborate the results of the studies reported above. Note that, like SARS-Cov-2, hRSV interacts with HS on the cell surface (see Section 3). The study also reported significant reduction in CD3+ and CD3 + CD8+ lymphocytes in mice receiving 3.3 mg of xylitol per kilogram per day, and no decrease in CD3 + CD4+ lymphocytes in all groups. This significant decrease in CD3 + CD8+ lymphocytes was not observed in mice receiving 33 mg xylitol per kilogram per day [57]. The same researchers previously reported positive effects of xylitol on mice with influenza A virus infection (H1N1) [58]. However, a decrease in CD3 + CD8+ lymphocytes is a predictor of mortality for COVID-19 patients (see Table 1). These anti-inflammatory and antiviral properties of D-xylose/xylitol in respiratory conditions are the subject of another patent application (number WO1999048361A1) filed in 1998 in the United States by Alonzo H. Jones [59].
5. D-xylose/xylitol, type 2 diabetes (T2D), and hyaluronan (HA)
Between 1932 and 1940, early studies on the metabolism of D-xylose and L-xylose in rats demonstrated a significant decrease in glycogen in the blood, liver (up to 50%), and muscles after absorption of xylose [60]. Similar results have been found in recent studies with groups of rats fed diets supplemented with xylose or xylitol [61,62]. Similar results were obtained in humans in a randomized, double-blind study of 75 Koreans [63].
This can be explained as follows: If there is insufficient xylose in the body (or in the case of some viral infections), one of the first consequences could be an increase in blood sugar. Indeed, some serine locations that should be occupied by xylose on core proteins (syndecans, glypicans, etc.) to initiate the production of GAGs (HS/Hep/CS/DS) are free due to a lack of xylose (or are occupied due to viruses glycosylation in case of some viral infections), thus preventing the initiation of GAG biosynthesis that should occur in these locations. The sugars that should be used in the production of these GAGs (D-glucuronic acid, galactose, N-acetylglucosamine, N-acetylgalactosamine) are found in the bloodstream, and some studies seem to support this explanation.
A 2011 study showed that sulfated GAGs were altered during Type 2 diabetes, with levels of HS and chondroitin and dermatan sulfates (CS/DS) decreased by about 14% [64]. Several other studies reported the degradation of HS during diabetes [65,66]. Also, in view of the half-life of the GAGs on the surface of the cell, which is 2–3 h, this degradation contributes to the origin of the problems of accumulation of HS/CS/DS chains, sources of certain cardiovascular diseases (see Section 9). This in turn may explain the strong association between diabetes and these diseases. Other studies have already shown that N-acetylglucosamine levels increase during Type 2 diabetes and that N-acetylglucosamine can be used as a biomarker for Type 2 diabetes [67,68].
Another consequence is the increase of other types of GAGs, such as hyaluronic acid. Indeed, hyaluronic acid not bound to a core protein can act to fix the excess unused sugar for the synthesis of HS/CS/Hep/DS. One study showed that hyaluronic acid levels for Type 2 diabetes were higher and that such levels could be used as a biomarker [69]. This accumulation of HA has already been reported in the lung in adult respiratory distress syndrome, where it is about six times higher than in control patients [70].
A 2010 study also showed that there is an increase of about 66% in D-glucuronic acid in the blood of diabetic compared to nondiabetic persons [71]. Another consequence is a decrease in the activity of xylosyltransferase enzymes (XYLT1, XYLT2) due to the decrease in attachment locations of xyloses (caused by lack of D-xylose or due to glycosylation of viruses at these positions). Indeed, Götting et al., in a study on 100 diabetic patients (Type 1 and Type 2) and 100 blood donations from people without diabetes, demonstrated that xylosyltranferase serum in diabetic patients was significantly lower compared to that in nondiabetic patients. These researchers concluded that the serum activity of xylosyltransferase could be used as a biomarker for the reduction of biosynthesis of GAGs in diabetics [72]. Further, a study using rats found that such variation in the activity of the enzyme xylosyltransferase 2 induced lung injury [73]. This again confirms the anti-inflammatory properties of D-xylose and provides an explanation for the process leading to inflammation during diabetes [74].
Thus, D-xylose has antiglycemic effects, demonstrated during studies in rats and in humans and also by a theoretical explanation, which supports an understanding that D-xylose allows the secretion of HS/CS/DS/Hep and decreases the accumulation of HA, which thus appears to be a cause of COVID-19 fatality (see Table 1).
6. D-xylose/xylitol and some cancers
As with diabetes, from the beginning of the COVID-19 pandemic, the first data showed that patients with cancer had high comorbidity with COVID-19, and this was later confirmed by several studies (see Table 1). Several studies have shown a strong association between Type 2 diabetes and cancers [75,76]. Other studies have explored the association between viruses and cancers [[77], [78], [79]].
As with viruses and diabetes, GAGs—in particular, sulfated GAGs, HS and CS—play a central role in the development of cancers [16,80]. This link, illustrated across years of research, has enabled researchers to consider the use of GAGs as a therapeutic pathway for cancer treatment [81,82].
The accumulated knowledge on cancers indicates that sulfated GAGs, HS/CS/DS/Hep, modulate transduction signaling, and act as receptors of epidermal growth factor, and this knowledge can be used to control the proliferation of cancer cells. The deregulation in cancers of these sulfated GAGs, particularly HS/CS, due to changes in their biosynthesis has been reported in several studies [[81], [82], [83]]. As explained in Section 5 for diabetes, the deregulation of HS/CS/DS/Hep leads to an increase in the blood of the sugars that make up these chains. Several studies carried out on cancers report that cancers in general are accompanied by an increase in serum D-glucuronic acid [84], and an increase of serum N-acetylglucosamine, resulting in hyperactivity of the enzyme process of O-GlcNacylation [85]. O-GlcNac transferase (E.C. 2.4.1.255) that catalyzes the addition of N-acetylglucosamine from UDP-GlcNac to a serine or threonine residue of the target protein [86]. This enzyme plays a crucial regulatory role in cell proliferation, in the development of metastases, and so on. An increase of the level of this enzyme is now considered to be characteristic of cancer cells [[85], [86], [87]].
As a consequence of an increase in these sugars due to the deregulation of HS/CS/DS/Hep, there is an increase of HA in the ECM. This has been widely reported in cancers and is correlated with cancer cell proliferation and inflammation [[88], [89], [90], [91]]. The descriptions in the preceding sections considering the link between HS degradation and inflammation provide an explanation of the inflammatory process associated with tumor progression [92,93]. This also supports an understanding of why people already suffering from cancer are among the people most at risk of COVID-19. This is also true for the increase of CAMs in cancers, an effect that actively contributes to the metastatic spread of tumor cells [[94], [95], [96]]. As reported in the preceding sections, this increase in CAMs is also correlated with the degradation of HS. These points support the deduction that D-xylose/xylitol via the initiation of biosynthesis of HS/CS/DS/Hep would produce anticancer effects, in addition to its anti-inflammatory, antiviral, and anti-glycemic properties already demonstrated. The words of Professor Katrin Mani from Lund University, noted in April 2010, are very important in this regard: “We have previously discovered that a carbohydrate, xylose, linked to a naphthalene preferentially inhibits growth of tumor cells but not normal cells. This is something the researchers have been aware of for around 10 years. Since then, they have been working on the mechanism of action of the drug to try to understand why the xylose compound works so well against tumor cells” [97]. The proportion of 97% treated cancer cells reported in the article is remarkable.
Studies have shown that xylitol inhibits cancer cells, including lung cancer. These results indicate that xylitol has potential for use in lung cancer therapy, acting by inhibiting cell proliferation [98,99].
7. Interaction between D-xylose and anti-inflammatory drugs
Since the beginning of the COVID-19 pandemic, practitioners have warned about the dangers of prolonged use of certain nonsteroidal anti-inflammatory drugs (NSAIDs) in countering the “cytokines storm” observed in severe cases of COVID-19 [[9], [10], [11], [12]]. Given the properties of D-xylose already described in this report, it is reasonable to wonder whether the NSAIDs react with D-xylose. The answer seems to be affirmative; indeed, the effects of NSAIDs in the “D-xylose test” have been the subject in the past of several studies that have shown that patients on NSAIDs over the long term exhibited malabsorption of D-xylose [[100], [101], [102], [103]].
The interaction between D-xylose and some anti-inflammatory drugs has been the subject of a number of studies that have found that in the presence of some NSAIDs (aspirin, indomethacin, paracetamol, etc.), the amount of D-xylose excreted in urine is significantly lower [103,104]. There is sufficient reason to believe that these anti-inflammatory medications react with D-xylose to form a new molecule. Taking into account the already described concern over the role of D-xylose in lung inflammation and its antiglycemic and anticancer effects, if the amount of xylose in the body is reduced by interaction with anti-inflammatory drugs, this leads to a reduction in HS, which in turn results in inflammation of the lungs, thus aggravating the symptoms of COVID-19 (see Table 1).
Dexamethasone, a corticosteroid that has been randomized in the management of severe cases of COVID-19, appears to have positive effects according to the World Health Organization (WHO), with which the authors of a study shared their preliminary results. Dexamethasone may reduce mortality by one-fifth to one-third, depending on the type of respiratory assistance being received by patients [105]. One study showed that dexamethasone did not affect the absorption of D-xylose [106].
One of the NSAIDs that has been the subject of more alerts by practitioners for its prolonged use in severe cases of COVID-19 is ibuprofen. This molecule reacts by esterification with xylan (xylose polymer) to yield an ester, xylan Ibuprofen ester [107,108].
Note: The “D-xylose test” measures the level of D-xylose in the blood or urine and can be used to diagnose intestinal malabsorption. The test is conducted by oral administration of xylose dissolved in water, followed by urine collection after 1 h or 2 h to measure the amount of D-xylose excreted. The “D-xylose test” studies considered in this section are not very complete (not all NSAIDs) and they only give some idea of a possible interaction or not between the D-xylose molecule and anti-inflammatory drugs. Also, most of these studies were conducted with a small number of patients.
8. D-xylose/xylitol and antibiotics
Infectious agents that infect cells adhere to these cells [109,110]; however, as discussed in Section 4, studies have shown that xylitol can be used to achieve a reduction by at least 10-fold of the number of adhesion molecules favoring adhesion to the surface of macrophages, when compared to control macrophages [55]. Generally, D-xylose reduced adhesion molecules by reducing HS degradation. This in turn suggests possible use of D-xylose to improve antibiotic activity. This was suggested in the case of xylitol in the patent claims filed by the University of Iowa [54]. Additionally, in 2017, Hidalgo et al. observed in a study of mice infected with Actinetobacter baumannii and Klebsiella pneumonia that mixtures of xylose with tetracycline and xylose with chloramphenicol produced a decrease of these bacteria by at least 10-fold, compared to mice treated with antibiotic only [111]. In 2012, with an in vitro study, researchers concluded that “this work could serve as starting point for the development of a new treatment against infections, for instance combining antibiotics with xylose when used in topical compositions” [112].
Under the arguments just described, it is very likely that similar results may be obtained for the lungs.
9. Xylose/xylitol, hypertension, acute kidney injury, coronary artery disease, obesity
Like Type 2 diabetes and cancers, many other pathologies that display comorbidity with COVID-19 are accompanied by inflammation. The “cytokines storm” induced by SARS-Cov-2 adds to the inflammatory state associated with these pathologies, so that patients who suffer from this become particularly at risk.
This is the case for forms of hypertension, 90% of which remain of unknown origin and for which inflammation is a major characteristic. Some researchers recommend the use of anti-inflammatory drugs primarily for the treatment of hypertension [113,114].
Given the anti-inflammatory properties of D-xylose described in the preceding sections, D-xylose (intravenously) is a seriously considered candidate for the management of patients with severe COVID-19 already suffering from hypertension.
Indeed, Guo et al. demonstrated, in an in vivo study in rats, that pulmonary hypertension can be regulated by protecting the integrity of the glycocalyx [115]. As already discussed, a majority of the glycocalyx is made up of PGs and GAGs, and D-xylose promotes the biosynthesis of GAGs, thus promoting the protection of the integrity of the glycocalyx.
Acute kidney injury (AKI) is a severe manifestation of COVID-19 (see Table 1). As with other pathologies, inflammation plays a central role here as well and is the main subject of the various research studies on AKI [116,117].
Several studies have demonstrated that heparanase has a central role in the process leading to AKI. Almost all research on the role of heparanase in this pathology has led researchers to conclude that inhibition of the heparanase enzyme is a very promising therapeutic approach for AKI [118,119]. Recall that the inhibition of heparanase can halt the degradation of HS (see Section 2) and thus reduce inflammation (see Section 4).
Moreover, other studies have found that preventing the deregulation of the glycocalyx on endothelial cells can repair and prevent AKI, sepsis shock, and ARDS [120,121]. Another study showed that the production matrix of GAGs was suppressed in AKI with poor nutrition [122]. This again supports the proposal in this review of using D-xylose/xylitol in the management of severe cases of COVID-19.
The preceding descriptions regarding inflammation and therapeutic pathways using GAGs are equally applicable to CAD, also known as coronary heart disease, and to other cardiovascular diseases. Studies have found that countering the degradation of GAGs, in particular the accumulation of CS/DS in myocardial cells, is a major therapeutic pathway for several cardiovascular diseases [48,123,124].
In terms of obesity, in addition to the direct link between obesity and inflammation that has already been demonstrated, the effects of D-xylose and xylitol on body weight have been studied in several in vivo studies. In 2011, Amo et al. found, in a study of the effects of xylitol in dietetics (for 8 weeks) in mice with a high-fat diet, that visceral fat and the concentrations of plasma insulin and lipids were significantly lower in xylitol-fed rats compared to the control group [125]. In 2015, Lim et al. showed in a similar study, in mice with a fat-rich diet supplemented with D-xylose (for 12 weeks), that D-xylose helps to prevent and mitigate the progression of metabolic disorders related to obesity [126]. Other similar studies have led to the same conclusion: that D-xylose/xylitol can significantly reduce body weight and lower blood glucose levels [127].
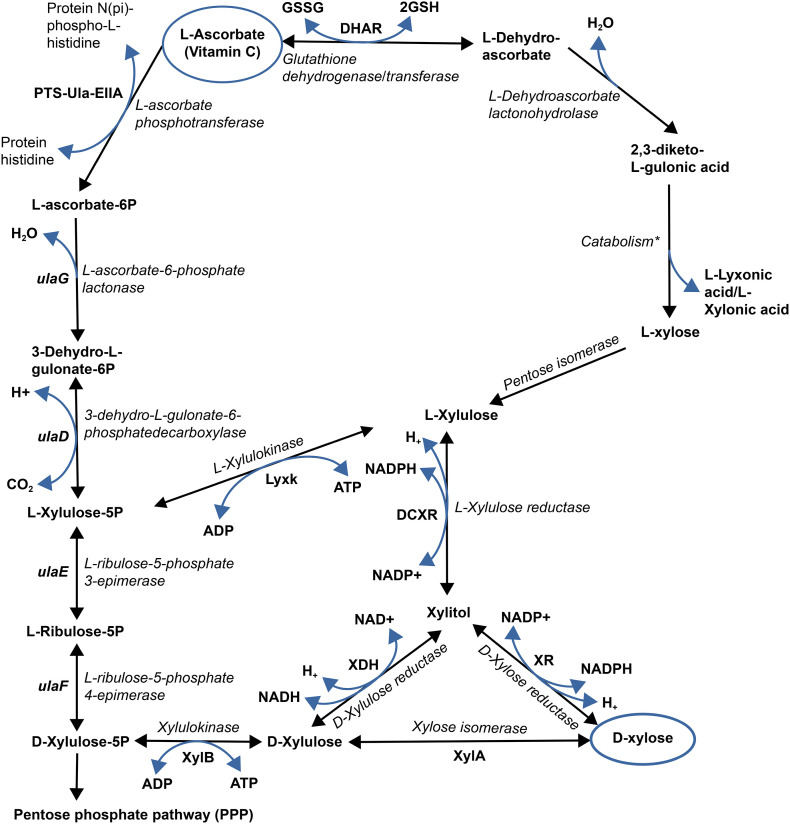
The fact that D-xylose and xylitol are metabolites of ascorbic acid (see Fig. 1 ) suggests that vitamin C has the same effects on obesity. This has been confirmed in dozens of studies [128].
Fig. 1.
Vitamin C, D-xylose, and xylitol. l-ascorbic acid (Vitamin C) pathway showing that D-xylose and xylitol are part of its metabolites.
10. D-xylose, gender, the elderly, smokers, ethnicity, obesity
The metabolic pathway for vitamin C (ascorbic acid) proposed in Fig. 1 is drawn mainly from the Kyoto Encyclopedia of Genes and Genomes (KEGG) and some literature reports. It demonstrates step by step that D-xylose and xylitol are the metabolites of vitamin C.
Several authors have already reported that D-xylose is a metabolite of vitamin C, in particular, Richard Colombo in his thesis published in 2001 in France, where he gave the proportions of some metabolites of ascorbic acid [129]. Professor Alain Raisonnier from the Pierre & Marie Curie Faculty of Medicine demonstrated in his Metabolic Biochemistry course that xylitol is a metabolite of ascorbic acid [130]. This is also presented in a book by Hollmann that describes L-xylose as a metabolite of vitamin C [131].
Indeed, in 1957, Chan, Becker, and King, reporting an in vivo study on guinea pigs involving products of the metabolism of l-ascorbic acid, conducted at Columbia University, showed that dehydroascorbic acid, L-xylose, xylitol, D-xylulose, and L-xylulose are the metabolites of vitamin C [132].
Based on the antiviral, anti-inflammatory, and anticancer properties and antiglycemic effects of D-xylose/xylitol reported in the previous sections, the fact that D-xylose and xylitol are metabolites of vitamin C may explain these different properties that are often attributed to vitamin C, sometimes controversially.
Based on these anti-inflammatory and antiviral properties, vitamin C infusion was administered to SARS-CoV-2-infected pneumonia patients, and numerous clinical trials (NCT04323514, NCT04264533, NCT03680274, and NCT04326725) are ongoing for its therapeutic usage.
Thus, one of the main sources of D-xylose in humans is vitamin C, so this review considers some results from studies already done on vitamin C, which have sometimes been supported by studies conducted on the “D-xylose test.” Depending on how the “D-xylose test” is performed (see Section 8), a potential deficiency of D-xylose in a human would logically be characterized by a small amount of D-xylose excreted in the urine, especially if xylose were administered intravenously. Ingesting D-xylose could compensate for the deficit and result in a surplus being excreted.
The already-mentioned thesis by Colombo reported that 18.3% of patients (ages 67 to 84 years) had an acute vitamin C deficiency of <11.4 μmol/L [129]. The High Health Authority, commenting on the determination of vitamin C in France, reported after literature review that 5% of women and 12% of men, and after age 65 years, 15% of women and 20% of men had an acute vitamin C deficiency [133]. The same study reported that about 6% of the population ages 6 years and older had an acute vitamin C deficiency [133]. This percentage is also close to the percentage of the total population developing severe forms of COVID-19.
This information includes the point that about 20% of people over 65 years of age have a severe vitamin C deficiency (<11.4 μmol/L). Recall here that this is about the same percentage of infected people in the same age group developing a severe form of COVID-19 (see Table 1).
A review by Meyer of the literature reports on respiratory infections in the elderly revealed that national statistics and studies indicated that this segment of the population (≥65 years) is most at risk for various respiratory infections [134]. Moreover, a report from the European Union in 2018 revealed that this tranche alone includes 90% of the total deaths due to respiratory diseases [135], and the same is true for COVID-19. Studies of the effect of age on results of the “D-xylose test” when administering D-xylose intravenously and orally have shown that the amount of D-xylose excreted in urine decreases significantly with age [136,137]. These studies attribute this to kidney problems occurring with age, and according to the hypothesis of the present review, D-xylose deficiency could be the main cause. A patent filed by a large French company (patent number WO1999024009A1), cited in Section 2, mentions a strong decline in PG and GAG with age [38]. These points also support the hypothesis proposed in this review.
From information presented by the High Health Authority of France, the risk for men to show severe vitamin C deficiency is 1.3 (20%/15%) to 2.4 (12%/5%) times that of women. This is about the same ratio for the risk of infected people of the same age group developing a severe form of COVID-19 (see Table 1). The risk for a man has to develop a severe form of COVID-19, compared to a woman, is 1.7 to 2.1 times as great (see Table 1).
The already-mentioned European Union (EU) report of 2018 declares that men die of respiratory diseases 1.85 times more often than women, and this is found across all the countries of the EU [135]. Studies on the effect of sex in results of the “D-xylose test” when administering D-xylose intravenously and orally have shown that the amount of D-xylose excreted in the urine is significantly higher in women than in men [138]. For correlations with age, there is an attribution of the cause to kidney problems.
From the National Health and Nutrition Examination Survey (NHANES) II study of 11,592 people it was found that the risk for a person who smokes to have a severe deficiency of vitamin C is 3.0 times that for a nonsmoker [139]. Smokers are around 2.4 times more likely to develop severe form of COVID-19 (see Table 1). A smoker is 2.6 to 3 times more likely to develop any severe lung infection than is a nonsmoker [140,141].
A study conducted in the United States between 1976 and 1980 on 9252 adults between the ages of 30 and 74 years (another stage of NHANES II) showed that African Americans were 1.4 times (for women) to 1.8 times (for men) more likely to have severe vitamin C deficiency compared to white women and white men, respectively [142]. Taking into account work-related exposure of African Americans to COVID-19, these ratios should be multiplied by these coefficients of exposures (for independent events) in order to compare them to the odds ratios of 2 given in the reports, which calculated ratios for the overall population (see Table 2 ).
Table 2.
Risk factors: COVID-19 severity, vitamin C deficiency, lung diseases, and type 2 diabetes.
| Elderly (≥ 65 years) | Gender | Smokers | Ethnicity | Obesity | |
|---|---|---|---|---|---|
| COVID-19 severity | around 21% of elderly infected develop severe COVID-19 (§1) 90% of total deaths | Men are 1.7 to 2.1 times more likely to develop severe COVID-19 compared to women (§1) | Smokers are around 2.4 times more likely to develop severe COVID-19, compared to non-smokers(§1) | Blacks in New York City are 2 times more likely to dying of COVID-19 compared to Whites and Asians (§1) | A person obese is 1.8 times more likely to develop severe COVID-19 compared to non-obese person (§1) |
| Vitamin C deficiency | Around 20% of elderly have vitamin C deficiency (§10) | Men are 1.3 to 2.4 times more likely to have acute vitamin C deficiency compared to women. (§10) | Smokers are around 3.0 times more likely to have acute vitamin C deficiency compared to non-smokers (§10) | African Americans are 1.4 to 1.8 times more likely to have severe vitamin C deficiency compared to whites Americans (§10). | A person obese is 1.3 to 1.4 times more likely to have a severe deficiency in vitamin C compared to a non-obese person.(§10) |
| Lung diseases /Respiratory diseases severity | Around 20% of infected elderly develop severe lung or respiratory disease [147], 90% of total deaths [135] | Men die of respiratory diseases around 1.85 times more than women [135] | Smokers are 2.6 to 3 times more likely to develop a severe form of lung infections or respiratory diseases than non-smokers [140,141]. | Black Americans are 2.4 times more likely to develop bacterial pneumonia than white Americans [143]. | Obese people were 2.9 times more likely to develop a severe form of the H1N1 disease compared to non-obese [146]. |
| Type 2 Diabetes | around 22% to 25% of prevalence [148,149] | Men are around 1.95 times more likely to develop Type 2 diabetes compared to women [150] | Smokers are around 2.6 times more likely to develop Type 2 diabetes compared to non-smokers [151] | African Americans are 1.4 to 2.3 times more likely to have Type 2 diabetes compared to white Americans [152]. | Obese people are 1.5 to 5 times more likely to have Type 2 diabetes compared to people with normal BMI [153] |
Type 2 Diabetes Risk factors: age, gender, smoking, obesity, and ethnicity associated with COVID-19 severity are the same risk factors for Vitamin C deficiency, lung diseases/respiratory diseases severity, and Type 2 Diabetes.
In 2010, Burton et al. reported an analysis of data on 4780 adults with bacterial pneumonia, identified in nine states in the United States, indicating that black Americans were 2.4 times more likely to develop bacterial pneumonia than were white Americans [143].
A study conducted between 2003 and 2004 of 7277 noninstitutionalized civilians aged 6 years or older (another stage of NHANES-III) supports an assessment that an obese person is 1.3 (for women) to 1.4 (for men) times more likely to have a severe deficiency in vitamin C compared to a non-obese person [144]. Jedrychowski and colleagues showed in 1998, through a study of 1129 preadolescents, that children with a body mass index (BMI) ≥ 20 were twice as likely to be susceptible to acute respiratory infections compared to children with low BMI [145]. A 2009 data analysis of the H1N1 outbreak, conducted by the WHO, showed that obese people were 2.9 times more likely to develop a severe form of the disease compared to non-obese individuals [146].
Risk ratios for the elderly, for smokers, by sex, for obesity, and certain ethnic groups have shown similar values for COVID-19 and for acute vitamin C deficiency. When these data are added to the fact that D-xylose and xylitol are vitamin C metabolites, there is reason to believe that severe cases of COVID-19 may have a direct link to vitamin C deficiency and therefore a link to D-xylose deficiency. This is a generic hypothesis and can have many other variables associated with it in addition to vitamin C deficiency for COVID-19. Thus, it is advisable to consider also a set of specific studies where groups with the same age or other risk factor (smokers, sex, obese, ethnic) have been demonstrated to be susceptible to other viral infections/lung diseases or associated disease, in order to provide supporting evidence.
In addition, venous thromboembolism (VTE), another reported cause of lethality of COVID-19 (see Table 1), is one of the main symptoms of scurvy [154], and the correlations of this with severe cases of COVID-19 seem very strong (see Table 2). Studies of 184 patients in intensive care units have drawn attention to complications related to venous and arterial thrombosis [155]. Other related studies have shown that obesity and cigarettes are the two most important risk factors for VTE [156].
11. D-xylose, Artemisia, other plants, and COVID-19
Several plants that have been reported as useful in treatment for COVID-19 in the first countries affected by the virus contain high levels of D-xylose (Japanese honeysuckle, which is Lonicera japonica, green chiretta, other honeysuckle, red algae, water spinach, etc.). Some of these plants have become the subjects of clinical trials on COVID-19 in China [157]. The antiviral, antibacterial, and especially anti-inflammatory properties of Japanese honeysuckle for the lungs, for example, have already been demonstrated in several studies [158,159]. This also applies to bark or tree sap (from birch, African birch) containing D-xylose, which is already used in alternative medicine to treat pulmonary or respiratory diseases (asthma, bronchitis, cough, pneumonia, etc.).
The same is true for Artemisia, as an Artemisia decoction (COVID Organics) utilized by Malagasy researchers was described as having therapeutic and preventive properties against COVID-19. Xylose is the main monosaccharide found in Artemisia annua [160]. Its use in treatment for COVID-19 and more generally as treatment for respiratory infections is currently being explored by researchers [161,162].
12. Discussion and conclusions
This review is the first to explore the use of xylose in a novel therapeutic regimen for COVID-19. It is the first pharmacological report exploring a hypothesis explaining such action.
The review demonstrated that D-xylose/xylitol has shown anti-inflammatory properties (Section 4), antiviral properties (Section 4), antiglycemic properties (Section 5), and anticancer properties for lung cancer (Section 6).
These different properties, as discussed in this review, are consequences of the ability of D-xylose/xylitol to stimulate the biosynthesis of chains of sulfated GAGs (except KS), especially HS. This GAGs regeneration property of D-xylose was the subject of a patent filed in 1999 (described in Section 2). The use of D-xylose and or xylitol for the prevention and treatment of respiratory infections and to endow anti-inflammatory effects has been the subject of other invention patents as well (Section 4). This study pointed out that the efficacy of D-xylose in combination with antibiotics (tetracycline) has already been demonstrated in vivo (Section 8). There is also a central role played by GAGs, especially HSPGs, in various pathologies, including SARS-COV-2 and pathologies with a high comorbidity with COVID-19, such as hypertension, diabetes, cancer, AKI, and obesity [163]. Therapeutic avenues specified using GAGs have also been proposed for the treatment of most of these pathologies, mirroring those for COVID-19. In addition to the use of D-xylose, xylan-based medicines (xylose polymer) or related esters have been the subject of other patents, in terms of their use for the treatment of respiratory diseases and in the treatment of other diseases associated with inflammation, particularly cancer [164,165].
The review included the correlation of D-xylose/xylitol with vitamin C and with certain medicinal plants (such as Artemisia), some of which are already being explored in clinical research for COVID-19 treatment. Findings indicate that the risk factors associated with generic parameters such as age, gender, smoking status, obesity, and ethnicity for COVID-19 (see Table 1) seem not to be specific to COVID-19, but apply to several other pathologies, such as scurvy, lung diseases/infections in general, and Type 2 diabetes (see Table 2, which is not exhaustive).
The management of patients critically ill with COVID-19 addresses patients who undergo a “cytokine storm” that has been treated with anti-inflammatory drugs. This review arrives at an understanding of how long term use of some NSAIDs can influence the D-xylose level in the body. This applies to ibuprofen, paracetamol, indomethacin, and aspirin, which are NSAIDs where studies in the literature have demonstrated links to the presence of D-xylose (see Section 7).
In addition to these facets, results of an earlier study conducted by Cook and published in January 1975 strongly reinforce the idea of using D-xylose in severe cases of COVID-19. Cook found that the serum concentration of gamma-globulin was significantly inversely proportional to the amount of D-xylose excreted in urine in healthy Africans [166]. This implies that when there is an increase of D-xylose in the bloodstream (i.e., less passing into the urine), the concentration of gamma-globulin serum increases significantly, thus strengthening the immune defense; thus would especially be the case in infections like that from SARS-Cov-2.
Xylitol in use generally as a table sugar has been approved by the Food and Drug Administration [167]. Xylitol has no known major side effects when used in reasonable amounts. One study showed that it is well tolerated even in parents with kidney failure [168], although minor side effects may be observed (diarrhea).
On the other hand, an abnormally high dose of xylitol can be fatal. It should not be used as a glucose substitute [169].
D-xylose is already used in medicine for the “D-xylose test” described in Section 7. It can be administered orally or given directly by intravenous administration. It is generally without known side effects at the levels used for the “D-xylose test” [136,137]. These “safe” doses may be selected as a starting point for preclinical trials in COVID-19 patients.
Taking into account the average half-life of the HSPGs at the cell surface, which is 2 to 3 h, various regimens of D-xylose administration and antibiotics can be considered in preclinical trials: both at the same time, or with D-xylose first and antibiotic at 1 h, 2 h, 3 h, or 5 h later, or with antibiotic first and D-xylose at the subsequent time points, with different adjustments by practitioners according to the parameters noted.
If preclinical trials for D-xylose are encouraging, clinical trials may be considered, starting with a xylosemia assay in some severe cases of COVID-19, grouped by age group and level of severity of the disease, and including dosage in some people infected with SARS-COV-2 but who have a benign form, also grouped by age group. This should provide reliable comparison levels, which can be used by clinicians as a reference.
13. Limitations
The number of patients in the “D-xylose test” studies was generally quite low. Several related studies and concerning several pathologies (in particular, studies on GAGs, on vitamin C, and the concurring studies on medicinal plants) were not included in this review, given the already wide span of this literature review.
This presentation has focused on the sugars forming the structure of the chain of GAGs, in particular with HS, without discussing the importance of their sulfated forms, which, according to several studies, play an equally important role in the various pathologies addressed in this review.
Another limitation is represented by the difficulty in finding studies on D-xylose/xylitol associated with some of the pathologies discussed in this review.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
Acknowledgements
Great thanks to the two experts of ENAGO who made a unquantifiable expertise, having made it possible to improve very significantly this work. Thanks also to the editors of the same company (www.enago.com) for the English language review.
Ethical considerations
This is a review article. Hence, informed consent and ethics committee approvals as well as compliance with the Declaration of Helsinki are not applicable.
Declaration of competing interest
The author declares no conflict of interest.
References
- 1.JHU COVID-19 Resource Center 2020. https://coronavirus.jhu.edu/map.html [PubMed]
- 2.Ronco C., Reis T., Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir. Med. 2020;8:738–742. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., Greninger A.L., Pipavath S., Wurfel M.M., Evans L., Kritek P.A., West T.E., Luks A., Gerbino A., Dale C.R., Goldman J.D., O’Mahony S., Mikacenic C. Covid-19 in critically ill patients in the Seattle region – case series. N. Engl. J. Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannucci E., Silverii G.A., Monami M. Saturation of critical care capacity and mortality in patients with the novel coronavirus (COVID-19) in Italy. Trends in Anaesthesia and Critical Care. 2020 doi: 10.1016/j.tacc.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooke M.W., Wilson S., Halsall J., Roalfe A. Total time in English accident and emergency departments is related to bed occupancy. Emerg. Med. J. 2004;21:575–576. doi: 10.1136/emj.2004.015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fatovich D.M., Hirsch R.L. Entry overload, emergency department overcrowding, and ambulance bypass. Emerg. Med. J. 2003;20:406–409. doi: 10.1136/emj.20.5.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stowell A., Claret P.G., Sebbane M., Bobbia X., Boyard C., Genre Grandpierre R., Moreau A., de La Coussaye J.E. Hospital out-lying through lack of beds and its impact on care and patient outcome. Scand. J. Trauma Resusc. Emerg. Med. 2013;21:17. doi: 10.1186/1757-7241-21-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Little P. Non-steroidal anti-inflammatory drugs and covid-19. BMJ. 2020;368 doi: 10.1136/bmj.m1185. [DOI] [PubMed] [Google Scholar]
- 10.Rothuizen L.E., Livio F., Buclin T. Traitements aggravant Une infection par le COVID-19 : vraiment? [drugs that aggravate the course of COVID-19 : really?] Rev. Med. Suisse. 2020;691–2:852–854. [PubMed] [Google Scholar]
- 11.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., Zeng X., Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell B., Moss C., Rigg A., Van Hemelrijck M. COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? Ecancermedicalscience. 2020;14:1023. doi: 10.3332/ecancer.2020.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conti P., Gallenga C.E., Tetè G., Caraffa Al., Ronconi G., Younes A., Toniato E., Ross R., Kritas S.K. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1. J. Biol. Regul. Homeost. Agents. 2020;34 doi: 10.23812/Editorial-Conti-2. [DOI] [PubMed] [Google Scholar]
- 14.Raybaud P.-J. COVID-19: proposition d'un nouveau traitement et protocole par trithérapie. https://blogs.mediapart.fr/pierre-jacques-raybaud/blog/310320/covid-19-proposition-d-un-nouveau-traitement-et-protocole-par-tritherapie
- 15.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Boretti A., Banik B.K. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition. 2020;12 doi: 10.1016/j.phanu.2020.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S., China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. published online 28 February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vardavas C.I., Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob. Induced Dis. 2020;18:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R.E. Irwin, Misinformation and de-contextualization: international media reporting on Sweden and COVID-19, Glob. Health 50/50. https://globalhealth5050.org/covid19/. (Accessed 12 April 2020) 16 (2020) 62. doi: 10.1186/s12992-020-00588-x. [DOI] [PMC free article] [PubMed]
- 24.NYC blacks and hispanics DYING OF COVID-19 AT TWICE THE RATE OF WHITES, ASIANS by Yoav Gonen, Ann. Choi Josefa Velasquez. https://thecity.nyc/2020/04/nyc-blacks-and-hispanics-dying-of-covid-19-at-twice-the-rate.html
- 25.J. Lighter, MD, M. Phillips, MD, S. Hochman, MD, S. Sterling, MD, D. Johnson, MD, F. Francois, MD, A. Stachel, Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission, Clin. Infect. Dis. (2020) 896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed]
- 26.Kim S.Y., Jin W., Sood A., Montgomery D.W., Grant O.C., Fuster M.M., Fu L., Dordick J.S., Woods R.J., Zhang F., Linhardt R.J. 2020. Glycosaminoglycan Binding Motif at S1/S2 Proteolytic Cleavage Site on Spike Glycoprotein May Facilitate Novel Coronavirus (SARS-CoV-2) Host Cell Entry, bioRxiv. [DOI] [Google Scholar]
- 27.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding M., Zhang Q., Li Q., Wu T., Huang Y.Z. Correlation analysis of the severity and clinical prognosis of 32 cases of patients with COVID-19. Respir. Med. 2020;167 doi: 10.1016/j.rmed.2020.105981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mong M.A., Awkal J.A., Paul E. 2020. Marik Accelerated Hyaluronan Concentration as the Primary Driver of Morbidity and Mortality in High-Risk COVID-19 Patients: With Therapeutic Introduction of an Oral Hyaluronan Inhibitor in the Prevention of “Induced Hyaluronan Storm” Syndrome medRxiv. [DOI] [Google Scholar]
- 30.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M., Guo G.Y., Du J., Zheng C.L., Zhu Q., Hu M., Li X.Y., Peng P., Shi H.Z. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.COVID-19: Reflections on VTE, patient risk assessment, and therapeutic challenges. https://venousnews.com/covid-19-reflections-venous-thromboembolism/ (Accessed 25 June 2020).
- 33.C. Herrmann, COVID-19: tests for 'miracle cure' herb Artemisia begin. https://p.dw.com/p/3cEne. Accessed 04 June, 2020, 2020.
- 34.Craig R.M., Atkinson A.J. d-xylose testing: a review. Gastroenterology. 1988;95:223–231. doi: 10.1016/0016-5085(88)90318-6. [DOI] [PubMed] [Google Scholar]
- 35.Vivès R.R. Héparanes sulfate: structure, fonctions, régulation. 2011. https://tel.archives-ouvertes.fr/tel-01063291
- 36.Sarrazin S., Lamanna W.C., Esko J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egeberg M., Kjeken R., Kolset S.O., Berg T., Prydz K. Internalization and stepwise degradation of heparan sulfate proteoglycans in rat hepatocytes. Biochim. Biophys. Acta. 2001;1541:135–149. doi: 10.1016/s0167-4889(01)00132-x. [DOI] [PubMed] [Google Scholar]
- 38.Dumas M., Bonte F. Utilisations du d-xylose, de ses esters et des oligosaccharides contenant du xylose pour ameliorer la fonctionnalite des cellules de l'epiderme. 1999. https://patents.google.com/patent/WO1999024009A1/fr
- 39.KEGG Orthology https://www.genome.jp/dbget-bin/www_bget?K17743+1.1.1.307+R01431+R09477 K17743.
- 40.O’Hearn A., Wang M., Cheng H., Lear-Rooney C.M., Koning K., Rumschlag-Booms E., Varhegyi E., Olinger G., Rong L. Role of EXT1 and glycosaminoglycans in the early stage of filovirus entry. J. Virol. 2015;89:5441–5449. doi: 10.1128/JVI.03689-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feldman S.A., Audet S., Beeler J.A. The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate. J. Virol. 2000;74:6442–6447. doi: 10.1128/jvi.74.14.6442-6447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foo C.H., Lou H., Whitbeck J.C., Ponce-de-León M., Atanasiu D., Eisenberg R.J., Cohen G.H. Vaccinia virus L1 binds to cell surfaces and blocks virus entry independently of glycosaminoglycans. Virology. 2009;385:368–382. doi: 10.1016/j.virol.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sasaki M., Anindita P.D., Ito N., Sugiyama M., Carr M., Fukuhara H., Ose T., Maenaka K., Takada A., Hall W.W., Orba Y., Sawa H. The role of heparan sulfate proteoglycans as an attachment factor for rabies virus entry and infection. J. Infect. Dis. 2018;217:1740–1749. doi: 10.1093/infdis/jiy081. [DOI] [PubMed] [Google Scholar]
- 44.Aquino R.S., Park P.W. Glycosaminoglycans and infection. Front. Biosci. (Landmark Ed.) 2016;21:1260–1277. doi: 10.2741/4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cagno V., Tseligka E.D., Jones S.T., Tapparel C. Heparan sulfate proteoglycans and viral attachment: true receptors or adaptation bias? Viruses. 2019;11:596. doi: 10.3390/v11070596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hao W., Ma B., Li Z., Wang X., Gao X., Li Y., Qin B., Shang S., Cui S., Tan Z. Binding of the SARS-CoV-2 spike protein to glycans. bioRxiv. 2020 doi: 10.1101/2020.05.17.100537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brielle E.S., Schneidman-Duhovny D., Linial M. The SARS-CoV-2 exerts a distinctive strategy for interacting with the ACE2 human receptor. Viruses. 2020;12:497. doi: 10.3390/v12050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.B. Buijsers, C. Yanginlar, I. Grondman, A. de Nooijer, M.L. Maciej-Hulme, I. Jonkman, N. Janssen, N. Rother, M. de Graaf, P.r Pickkers, M. Kox, L. Joosten, T. Nijenhuis, M.G. Netea, L. Hillbrands, F. van de Veerdonk, R. Duivenvoorden, Q. de Mast, J. van der Vlag., Increased plasma heparanase activity in COVID-19 patients, medRxiv (2020). doi: 10.1101/2020.06.12.20129304. [DOI] [PMC free article] [PubMed]
- 49.Rajas O., Quirós L.M., Ortega M., Vazquez-Espinosa E., Merayo-Lloves J., Vazquez F., García B. Glycosaminoglycans are involved in bacterial adherence to lung cells. BMC Infect. Dis. 2017;17:319. doi: 10.1186/s12879-017-2418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt E.P., Yang Y., Janssen W.J., Gandjeva A., Perez M.J., Barthel L., Zemans R.L., Bowman J.C., Koyanagi D.E., Yunt Z.X., Smith L.P., Cheng S.S., Overdier K.H., Thompson K.R., Geraci M.W., Douglas I.S., Pearse D.B., Tuder R.M. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bevilacqua M.P., Nelson R.M., Mannori G., Cecconi O. Endothelial-leukocyte adhesion molecules in human disease. Annu. Rev. Med. 1994;45:361–378. doi: 10.1146/annurev.med.45.1.361. [DOI] [PubMed] [Google Scholar]
- 52.Albelda S.M., Smith C.W., Ward P.A. Adhesion molecules and inflammatory injury. FASEB J. 1994;8:504–512. doi: 10.1096/fasebj.8.8.8181668. [DOI] [PubMed] [Google Scholar]
- 53.Haeger S.M., Yang Y., Schmidt E.P. Heparan sulfate in the developing, healthy, and injured lung. Am. J. Respir. Cell Mol. Biol. 2016;55:5–11. doi: 10.1165/rcmb.2016-0043TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.M.J. Welsh, J. Zabner, Use of xylitol to reduce ionic strength and activate endogenous antimicrobials for prevention and treatment of infections. US Patent 6.716.819: B2, University of Iowa Research Foundation, 2004.
- 55.Ferreira A.S., Ad Souza M., Raposo N.R.B., Ferreira A.P., Silva S.Sd. Xylitol inhibits J774A.1 macrophage adhesion in vitro. Braz. Arch. Biol. Technol. 2011;54:1211–1216. doi: 10.1590/S1516-89132011000600017. [DOI] [Google Scholar]
- 56.Wegner C.D., Gundel R.H., Rothlein R., Letts L.G. Expression and probable roles of cell adhesion molecules in lung inflammation. Chest. 1992;101 Suppl:34S–39S. doi: 10.1378/chest.101.3_supplement.34s. [DOI] [PubMed] [Google Scholar]
- 57.Xu M.L., Wi G.R., Kim H.J., Kim H.J. Ameliorating effect of dietary xylitol on human respiratory syncytial virus (hRSV) infection. Biol. Pharm. Bull. 2016;39:540–546. doi: 10.1248/bpb.b15-00773. [DOI] [PubMed] [Google Scholar]
- 58.Yin S.Y., Kim H.J., Kim H.J. Protective effect of dietary xylitol on influenza A virus infection. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.A.H. Jones, Xylitol compositions for treating upper respiratory conditions. https://patents.google.com/patent/WO1999048361A1/en, 1999 (Accessed 13 April 2020).
- 60.Miller M.M., Lewis H.B. Pentose metabolism. I. the rate of absorption of d-xylose and the formation of glycogen in the organism of the white rat after oral administration of d-xylose. J. Biol. Chem. 1932;98:133–140. [Google Scholar]
- 61.Islam M.S., Indrajit M. Effects of xylitol on blood glucose, glucose tolerance, serum insulin and lipid profile in a type 2 diabetes model of rats. Ann. Nutr. Metab. 2012;61:57–64. doi: 10.1159/000338440. [DOI] [PubMed] [Google Scholar]
- 62.Rahman M.A., Islam M.S. Xylitol improves pancreatic islets morphology to ameliorate type 2 diabetes in rats: a dose response study. J. Food Sci. 2014;79:H1436–H1442. doi: 10.1111/1750-3841.12520. [DOI] [PubMed] [Google Scholar]
- 63.Jun Y.J., Lee J., Hwang S., Kwak J.H., Ahn H.Y., Bak Y.K., Koh J., Lee J.H. Beneficial effect of xylose consumption on postprandial hyperglycemia in Korean: a randomized double-blind, crossover design. Trials. 2016;17:139. doi: 10.1186/s13063-016-1261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Joladarashi D., Salimath P.V., Chilkunda N.D. Diabetes results in structural alteration of chondroitin sulfate/dermatan sulfate in the rat kidney: effects on the binding to extracellular matrix components. Glycobiology. 2011;21:960–972. doi: 10.1093/glycob/cwr029. [DOI] [PubMed] [Google Scholar]
- 65.Hiebert L.M., Han J., Mandal A.K. Glycosaminoglycans, hyperglycemia, and disease. Antioxid. Redox Signal. 2014;21:1032–1043. doi: 10.1089/ars.2013.5695. [DOI] [PubMed] [Google Scholar]
- 66.Hiebert L.M. Proteoglycans and diabetes. Curr. Pharm. Des. 2017;23:1500–1509. doi: 10.2174/1381612823666170125154915. [DOI] [PubMed] [Google Scholar]
- 67.Wang Z., Park K., Comer F., Hsieh-Wilson L.C., Saudek C.D., Hart G.W. Site-specific GlcNAcylation of human erythrocyte proteins: potential biomarker(s) for diabetes. Diabetes. 2009;58:309–317. doi: 10.2337/db08-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wells L., Vosseller K., Hart G.W. A role for N-acetylglucosamine as a nutrient sensor and mediator of insulin resistance. Cell. Mol. Life Sci. 2003;60:222–228. doi: 10.1007/s000180300017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mine S., Okada Y., Kawahara C., Tabata T., Tanaka Y. Serum hyaluronan concentration as a marker of angiopathy in patients with diabetes mellitus. Endocr. J. 2006;53:761–766. doi: 10.1507/endocrj.k05-119. [DOI] [PubMed] [Google Scholar]
- 70.Hällgren R., Samuelsson T., Laurent T.C., Modig J. Accumulation of hyaluronan (hyaluronic acid) in the lung in adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1989;139:682–687. doi: 10.1164/ajrccm/139.3.682. [DOI] [PubMed] [Google Scholar]
- 71.Fiehn O., Garvey W.T., Newman J.W., Lok K.H., Hoppel C.L., Adams S.H. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Götting C., Kuhn J., Kleesiek K. Serum xylosyltransferase activity in diabetic patients as a possible marker of reduced proteoglycan biosynthesis. Diabetes Care. 2008;31:2018–2019. doi: 10.2337/dc08-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koslowski R., Pfeil U., Fehrenbach H., Kasper M., Skutelsky E., Wenzel K.W. Changes in xylosyltransferase activity and in proteoglycan deposition in bleomycin-induced lung injury in rat. Eur. Respir. J. 2001;18:347–356. doi: 10.1183/09031936.01.00085601. [DOI] [PubMed] [Google Scholar]
- 74.Tsalamandris S., Antonopoulos A.S., Oikonomou E., Papamikroulis G.A., Vogiatzi G., Papaioannou S., Deftereos S., Tousoulis D. The role of inflammation in diabetes: current concepts and future perspectives. Eur. Cardiol. 2019;14:50–59. doi: 10.15420/ecr.2018.33.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giovannucci E., Harlan D.M., Archer M.C., Bergenstal R.M., Gapstur S.M., Habel L.A., Pollak M., Regensteiner J.G., Yee D. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gariani K., Tran C., Philippe J. Diabète et cancer : une association pernicieuse. Internet. Rev. Med. Suisse. 2010;6:1193–1198. [PubMed] [Google Scholar]
- 77.Schiller J.T., Lowy D.R. Virus infection and human cancer: an overview. Recent Results Cancer Res. 2014;193:1–10. doi: 10.1007/978-3-642-38965-8_1. [DOI] [PubMed] [Google Scholar]
- 78.Luo G.G., Ou J.H. Oncogenic viruses and cancer. Virol. Sin. 2015;30:83–84. doi: 10.1007/s12250-015-3599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang Y., Moore P.S., Weiss R.A. Human oncogenic viruses: nature and discovery. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2017;372 doi: 10.1098/rstb.2016.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morla S. Glycosaminoglycans and glycosaminoglycan mimetics in cancer and inflammation. Int. J. Mol. Sci. 2019;20:1963. doi: 10.3390/ijms20081963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Afratis N., Gialeli C., Nikitovic D., Tsegenidis T., Karousou E., Theocharis A.D., Pavão M.S., Tzanakakis G.N., Karamanos N.K. Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 2012;279:1177–1197. doi: 10.1111/j.1742-4658.2012.08529.x. [DOI] [PubMed] [Google Scholar]
- 82.Yip G.W., Smollich M., Götte M. Therapeutic value of glycosaminoglycans in cancer. Mol. Cancer Ther. 2006;5:2139–2148. doi: 10.1158/1535-7163.MCT-06-0082. [DOI] [PubMed] [Google Scholar]
- 83.Nagarajan A., Malvi P., Wajapeyee N. Heparan sulfate and heparan sulfate proteoglycans in cancer initiation and progression. Front. Endocrinol. 2018;9:483. doi: 10.3389/fendo.2018.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fishman W.H., Smith M., Thompson D.B., Bonner C.D., Kasdon S.C., Homburger F. Investigation of glucuronic acid metabolism in human subjects 12. J. Clin. Invest. 1951;30:685–696. doi: 10.1172/JCI102481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma Z., Vosseller K. Cancer metabolism and elevated O-GlcNAc in oncogenic signaling. J. Biol. Chem. 2014;289:34457–34465. doi: 10.1074/jbc.R114.577718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hart G.W., Akimoto Y. In: Essentials of Glycobiology. second ed. Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Darvill A.G., Kinoshita T., Packer N.H., Prestegard J.H., Schnaar R.L., Seeberger P.H., editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 2009. The O-GlcNAc modification. [Google Scholar]
- 87.Ferrer C.M., Sodi V.L., Reginato M.J. O-GlcNAcylation in cancer biology: linking metabolism and signaling. J. Mol. Biol. 2016;428:3282–3294. doi: 10.1016/j.jmb.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stern R. Hyaluronan in cancer biology. Semin. Cancer Biol. 2008;18:237. doi: 10.1016/j.semcancer.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 89.Li X., Shepard H.M., Cowell J.A., Zhao C., Osgood R.J., Rosengren S., Blouw B., Garrovillo S.A., Pagel M.D., Whatcott C.J., Han H., Von Hoff D.D., Taverna D.M., LaBarre M.J., Maneval D.C., Thompson C.B. Parallel accumulation of tumor hyaluronan, collagen, and other drivers of tumor progression. Clin. Cancer Res. 2018;24:4798–4807. doi: 10.1158/1078-0432.CCR-17-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu M., Tolg C., Turley E. Dissecting the dual nature of hyaluronan in the tumor microenvironment. Front. Immunol. 2019;10:947. doi: 10.3389/fimmu.2019.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Price Z.K., Lokman N.A., Ricciardelli C. Differing roles of hyaluronan molecular weight on cancer cell behavior and chemotherapy resistance. Cancers (Basel) 2018;10:482. doi: 10.3390/cancers10120482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singh N., Baby D., Rajguru J.P., Patil P.B., Thakkannavar S.S., Pujari V.B. Inflammation and cancer. Ann. Afr. Med. 2019;18:121–126. doi: 10.4103/aam.aam_56_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bendas G., Borsig L. Cancer cell adhesion and metastasis: selectins, integrins, and the inhibitory potential of heparins. Int. J. Cell Biol. 2012;2012:676731. doi: 10.1155/2012/676731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Okegawa T., Pong R.C., Li Y., Hsieh J.T. The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta Biochim. Pol. 2004;51:445–457. (doi:035001445) [PubMed] [Google Scholar]
- 96.Moh M.C., Shen S. The roles of cell adhesion molecules in tumor suppression and cell migration: a new paradox. Cell Adhes. Migr. 2009;3:334–336. doi: 10.4161/cam.3.4.9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lunds Universitet, Cancer tumours that fight themselves, ScienceDaily, Science www.sciencedaily.com/releases/2010/04/100429093603.htm, 2010 (Accessed 12 April 2020).
- 98.Trachootham D., Chingsuwanrote P., Yoosadiang P., Mekkriangkrai D., Ratchawong T., Buraphacheep N., Kijanukul S., Saekhow S., Pongpitchayadej O., Vongvachvasin K., Sittikornpaiboon P., Tuntipopipat S. Partial substitution of glucose with xylitol suppressed the glycolysis and selectively inhibited the proliferation of oral cancer cells. Nutr. Cancer. 2017;69:862–872. doi: 10.1080/01635581.2017.1339097. [DOI] [PubMed] [Google Scholar]
- 99.Park E., Park M.H., Na H.S., Chung J. Xylitol induces cell death in lung cancer A549 cells by autophagy. Biotechnol. Lett. 2015;37:983–990. doi: 10.1007/s10529-014-1757-1. [DOI] [PubMed] [Google Scholar]
- 100.Sigthorsson G., Tibble J., Hayllar J., Menzies I., Macpherson A., Moots R., Scott D., Gumpel M.J., Bjarnason I. Intestinal permeability and inflammation in patients on NSAIDs. Gut. 1998;43:506–511. doi: 10.1136/gut.43.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bjarnason I., Fehilly B., Smethurst P., Menzies I.S., Levi A.J. Importance of local versus systemic effects of non-steroidal anti-inflammatory drugs in increasing small intestinal permeability in man. Gut. 1991;32:275–277. doi: 10.1136/gut.32.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dyer N.H., Kendall M.J., Hawkins C.F. Malabsorption in rheumatoid disease. Ann. Rheum. Dis. 1971;30:626–630. doi: 10.1136/ard.30.6.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kendall M.J., Nutter S., Hawkins C.F. Xylose test: effect of aspirin and indomethacin. Br. Med. J. 1971;1:533–536. doi: 10.1136/bmj.1.5748.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Markiewicz A. The influence of anti-inflammatory drugs on the values of xylose test in man. Pol. J. Pharmacol. Pharm. 1975;27:265–272. [PubMed] [Google Scholar]
- 105.WHO welcomes preliminary results about dexamethasone use in treating critically ill COVID-19 patients. https://www.who.int/news-room/detail/16-06-2020-who-welcomes-preliminary-results-about-dexamethasone-use-in-treating-critically-ill-covid-19-patients
- 106.Malagon I., Onkenhout W., Klok M., Linthorst L., van der Poel P.F.H., Bovill J.G., Hazekamp M.G. Dexamethasone improves gut permeability in paediatric cardiac surgery. J. Thorac. Cardiovasc. Surg. 2005;130:265–271. doi: 10.1016/j.jtcvs.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 107.Peesa J.P., Yalavarthi P.R., Rasheed A., Mandava V.B.R. A perspective review on role of novel NSAID prodrugs in the management of acute inflammation. J. Acute Dis. 2016;5:364–381. doi: 10.1016/j.joad.2016.08.002. [DOI] [Google Scholar]
- 108.Daus S., Heinze T. Xylan-based nanoparticles: prodrugs for ibuprofen release. Macromol. Biosci. 2010;10:211–220. doi: 10.1002/mabi.200900201. [DOI] [PubMed] [Google Scholar]
- 109.Pizarro-Cerdá J., Cossart P. Bacterial adhesion and entry into host cells. Cell. 2006;124:715–727. doi: 10.1016/j.cell.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 110.Bonazzi M., Cossart P. Impenetrable barriers or entry portals? The role of cell–cell adhesion during infection. J. Cell Biol. 2011;195:349–358. doi: 10.1083/jcb.201106011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hidalgo A.A., Arias Á.J., Fuentes J.A., García P., Mora G.C., Villagra N.A. Xylose improves antibiotic activity of chloramphenicol and tetracycline against K pneumoniae and A. baumannii in a murine model of skin infection. Can. J. Infect. Dis. Med. Microbiol. 2018;2018:1–6. doi: 10.1155/2018/3467219. (PubMed: 3467219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Villagra N.A., Fuentes J.A., Jofré M.R., Hidalgo A.A., García P., Mora G.C. The carbon source influences the efflux pump-mediated antimicrobial resistance in clinically important gram-negative bacteria. J. Antimicrob. Chemother. 2012;67:921–927. doi: 10.1093/jac/dkr573. [DOI] [PubMed] [Google Scholar]
- 113.Dinh Q.N., Drummond G.R., Sobey C.G., Chrissobolis S. In: Biomed Res Int. Guzik T., editor. 2014. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension; p. 406960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.De Miguel C., Rudemiller N.P., Abais J.M., Mattson D.L. Inflammation and hypertension: new understandings and potential therapeutic targets. Curr. Hypertens. Rep. 2015;17:507. doi: 10.1007/s11906-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guo J., Yang Z.C., Liu Y. Attenuating pulmonary hypertension by protecting the integrity of glycocalyx in rats model of pulmonary artery hypertension. Inflammation. 2019;42:1951–1956. doi: 10.1007/s10753-019-01055-5. [DOI] [PubMed] [Google Scholar]
- 116.Rabb H., Griffin M.D., McKay D.B., Swaminathan S., Pickkers P., Rosner M.H., Kellum J.A., Ronco C. Acute Dialysis quality initiative, inflammation in AKI: current understanding, key questions, and knowledge gaps. J. Am. Soc. Nephrol. 2016;27:371–379. doi: 10.1681/ASN.2015030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Murashima M., Nishimoto M., Kokubu M., Hamano T., Matsui M., Eriguchi M., Samejima K.I., Akai Y., Tsuruya K. Inflammation as a predictor of acute kidney injury and mediator of higher mortality after acute kidney injury in non-cardiac surgery. Sci. Rep. 2019;9:20260. doi: 10.1038/s41598-019-56615-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abassi Z., Goligorsky M.S. In: Heparanase. Adv. Exp. Med. Biol. 1221. Vlodavsky I., Sanderson R., Ilan N., editors. Springer; Cham: 2020. Heparanase in acute kidney injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abassi Z., Hamoud S., Hassan A., Khamaysi I., Nativ O., Heyman S.N., Muhammad R.S., Ilan N., Singh P., Hammond E., Zaza G., Lupo A., Onisto M., Bellin G., Masola V., Vlodavsky I., Gambaro G. Involvement of heparanase in the pathogenesis of acute kidney injury: nephroprotective effect of PG545. Oncotarget. 2017;8:34191–34204. doi: 10.18632/oncotarget.16573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Song J.W., Zullo J., Lipphardt M., Dragovich M., Zhang F.X., Fu B., Goligorsky M.S. Endothelial glycocalyx-the battleground for complications of sepsis and kidney injury. Nephrol. Dial. Transplant. 2018;33:203–211. doi: 10.1093/ndt/gfx076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schmidt E.P., Overdier K.H., Sun X., Lin L., Liu X., Yang Y., Ammons L.A., Hiller T.D., Suflita M.A., Yu Y., Chen Y., Zhang F., Cothren Burlew C., Edelstein C.L., Douglas I.S., Linhardt R.J. Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2016;194:439–449. doi: 10.1164/rccm.201511-2281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mitsuhashi M., Mitsuhashi H., Yano S., Tsukada Y., Nojima Y., Naruse T. Levels of serum glycosaminoglycans in renal failure. Res. Commun. Mol. Pathol. Pharmacol. 1998;99:225–232. [PubMed] [Google Scholar]
- 123.Grande-Allen K.J., Osman N., Ballinger M.L., Dadlani H., Marasco S., Little P.J. Glycosaminoglycan synthesis and structure as targets for the prevention of calcific aortic valve disease. Cardiovasc. Res. 2007;76:19–28. doi: 10.1016/j.cardiores.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 124.Zhao R.R., Ackers-Johnson M., Stenzig J., Chen C., Ding T., Zhou Y., Wang P., Ng S.L., Li P.Y., Teo G., Rudd P.M., Fawcett J.W., Foo R.S.Y. Targeting chondroitin sulfate glycosaminoglycans to treat cardiac fibrosis in pathological remodeling. Circulation. 2018;137:2497–2513. doi: 10.1161/CIRCULATIONAHA.117.030353. [DOI] [PubMed] [Google Scholar]
- 125.Amo K., Arai H., Uebanso T., Fukaya M., Koganei M., Sasaki H., Yamamoto H., Taketani Y., Takeda E. Effects of xylitol on metabolic parameters and visceral fat accumulation. J. Clin. Biochem. Nutr. 2011;49:1–7. doi: 10.3164/jcbn.10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lim E., Lim J.Y., Shin J.H., Seok P.R., Jung S., Yoo S.H., Kim Y. d-xylose suppresses adipogenesis and regulates lipid metabolism genes in high-fat diet-induced obese mice. Nutr. Res. 2015;35:626–636. doi: 10.1016/j.nutres.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 127.Ahmad N.E.Q., Yusoff N.M. The effects of xylitol on body weight loss management and lipid profile on diet-induced obesity mice. J. Biosci. Med. 2015;03:54–58. doi: 10.4236/jbm.2015.310007. [DOI] [Google Scholar]
- 128.Garcia-Diaz D.F., Lopez-Legarrea P., Quintero P., Martinez J.A. Vitamin C in the treatment and/or prevention of obesity. J. Nutr. Sci. Vitaminol. 2014;60:367–379. doi: 10.3177/jnsv.60.367. [DOI] [PubMed] [Google Scholar]
- 129.R. Colombo, Etude de la carence en vitamine C dans une population gériatrique hospitalisée (Study of vitamin C deficiency in a geriatric hospitalized population). Sciences du Vivant [q-bio]. 2001, ffhal-01731911f https://hal.univ-lorraine.fr/hal-01731911/document.
- 130.A. Raisonnier, FMPMC-PS- Réserves Energétiques - Objectifs au cours de Biochimie PCEM2 Biochimie métabolique et Régulations (Energy Reserves - Objectives of the Biochemistry Course PCEM2 Metabolic Biochemistry and Regulation). http://www.chups.jussieu.fr/polys/biochimie/REbioch/POLY.Chp.5.42.&Ext.2.html. (Accessed 12 April 2020).
- 131.Hollmann S. Elsevier; Oxford, UK: 2012. Non-Glycolytic Pathways of Metabolism of Glucose. (287p) [Google Scholar]
- 132.Chan P.C., Becker R.R., King C.G. Metabolic products of L-ascorbic acid. J. Biol. Chem. 1958;231:231–240. [PubMed] [Google Scholar]
- 133.Dosage de la vitamine C dans le sang (Determination of vitamin C in the blood). Haute Autorité de Santé. https://www.has-sante.fr/upload/docs/application/pdf/2018-04/argumentaire_vitamine_c_vd.pdf
- 134.Meyer K.C. Lung infections and aging. Ageing Res. Rev. 2004;3:55–67. doi: 10.1016/j.arr.2003.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Organization for economic co-operation and development/European Union, Mortality from Respiratory Diseases, Health, OECD Publishing, Paris/European Union, Brussels State of health in the European Union. Cycle. 2018:94–95. [Google Scholar]
- 136.Kendall M.J. The influence of age on the xylose absorption test. Gut. 1970;11:498–501. doi: 10.1136/gut.11.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Johnson S.L., Mayersohn M., Conrad K.A. Xylose disposition in humans as a function of age. Clin. Pharmacol. Ther. 1986;39:697–702. doi: 10.1038/clpt.1986.121. [DOI] [PubMed] [Google Scholar]
- 138.Kendall M.J., Nutter S. The influence of sex, body weight, and renal function on the xylose test. Gut. 1970;11:1020–1023. doi: 10.1136/gut.11.12.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.J. Lykkesfeldt. Smoking depletes vitamin C: Should smokers be recommended to take supplements? http://eknygos.lsmuni.lt/springer/580/237-260.pdf (Accessed 12 April 2020).
- 140.Winnall W.R., Bellew B., Greenhalgh E.M. In: Tobacco in Australia: Facts and Issues. Greenhalgh E.M., Scollo M.M., Winstanley M.H., editors. Cancer Council Victoria; Melbourne: 2020. 3.9 increased susceptibility to infection in smokers.http://www.tobaccoinaustralia.org.au/3-9-increased-susceptibility-to-infection-in-smoke [Google Scholar]
- 141.Office of the Surgeon General, (US); Office on Smoking and Health (US). The Health Consequences of Smoking: A Report of the Surgeon GeneralAtlanta (GA): Centers for Disease Control and Prevention (US); (2004). https://www.ncbi.nlm.nih.gov/books/NBK44694/. [PubMed]
- 142.Loria C.M., Whelton P.K., Caulfield L.E., Szklo M., Klag M.J. Agreement among indicators of vitamin C status. Am. J. Epidemiol. 1998;147:587–596. doi: 10.1093/oxfordjournals.aje.a009491. [DOI] [PubMed] [Google Scholar]
- 143.Burton D.C., Flannery B., Bennett N.M., Farley M.M., Gershman K., Harrison L.H., Lynfield R., Petit S., Reingold A.L., Schaffner W., Thomas A., Plikaytis B.D., Rose C.E., Whitney C.G., Schuchat A. Active bacterial core surveillance/emerging infections program network, socioeconomic and racial/ethnic disparities in the incidence of bacteremic pneumonia among US adults. Am. J. Public Health. 2010;100:1904–1911. doi: 10.2105/AJPH.2009.181313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schleicher R.L., Carroll M.D., Ford E.S., Lacher D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES) Am. J. Clin. Nutr. 2009;90:1252–1263. doi: 10.3945/ajcn.2008.27016. [DOI] [PubMed] [Google Scholar]
- 145.Jedrychowski W., Maugeri U., Flak E., Mroz E., Bianchi I. Predisposition to acute respiratory infections among overweight preadolescent children: an epidemiologic study in Poland. Public Health. 1998;112:189–195. doi: 10.1038/sj.ph.1900438. [DOI] [PubMed] [Google Scholar]
- 146.Van Kerkhove M.D., Vandemaele K.A., Shinde V., Jaramillo-Gutierrez G., Koukounari A., Donnelly C.A., Carlino L.O., Owen R., Paterson B., Pelletier L., Vachon J., Gonzalez C., Hongjie Y., Zijian F., Chuang S.K., Au A., Buda S., Krause G., Haas W., Bonmarin I., Taniguichi K., Nakajima K., Shobayashi T., Takayama Y., Sunagawa T., Heraud J.M., Orelle A., Palacios E., van der Sande M.A., Wielders C.C., Hunt D., Cutter J., Lee V.J., Thomas J., Santa-Olalla P., Sierra-Moros M.J., Hanshaoworakul W., Ungchusak K., Pebody R., Jain S., Mounts A.W., WHO Working Group for Risk Factors for Severe H1N1pdm Infection, WHO Working Group for Risk Factors for Severe H1N1pdm Infection Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li W., Ding C., Yin S. Severe pneumonia in the elderly: a multivariate analysis of risk factors. Int. J. Clin. Exp. Med. 2015;8:12463–12475. [PMC free article] [PubMed] [Google Scholar]
- 148.Kirkman M.S., Briscoe V.J., Clark N., Florez H., Haas L.B., Halter J.B., Huang E.S., Korytkowski M.T., Munshi M.N., Odegard P.S., Pratley R.E., Swift C.S. Diabetes in older adults. Diabetes Care. 2012;35:2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jain A., Paranjape S. Prevalence of type 2 diabetes mellitus in elderly in a primary care facility: an ideal facility. Indian J. Endocrinol. Metab. 2013;17(Suppl. 1):S318–S322. doi: 10.4103/2230-8210.119647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nordström A., Hadrévi J., Olsson T., Franks P.W., Nordström P. Higher prevalence of type 2 diabetes in men than in women is associated with differences in visceral fat mass. J. Clin. Endocrinol. Metab. 2016;101:3740–3746. doi: 10.1210/jc.2016-1915. [DOI] [PubMed] [Google Scholar]
- 151.Persson P.G., Carlsson S., Svanström L., Ostenson C.G., Efendic S., Grill V. Cigarette smoking, oral moist snuff use and glucose intolerance. J. Intern. Med. 2000;248:103–110. doi: 10.1046/j.1365-2796.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 152.M.C. Marshall Jr, Diabetes in African Americans, Postgrad. Med. J. 81 (2005) 734–740. doi: 10.1136/pgmj.2004.028274. [DOI] [PMC free article] [PubMed]
- 153.Ganz M.L., Wintfeld N., Li Q., Alas V., Langer J., Hammer M. The association of body mass index with the risk of type 2 diabetes: a case-control study nested in an electronic health records system in the United States. Diabetol. Metab. Syndr. 2014;6:50. doi: 10.1186/1758-5996-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Reuler J.B., Broudy V.C., Cooney T.G. Adult scurvy. JAMA. 1985;253:805–807. [PubMed] [Google Scholar]
- 155.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Holst A.G., Jensen G., Prescott E. Risk factors for venous thromboembolism: results from the Copenhagen City Heart Study. Circulation. 2010;121:1896–1903. doi: 10.1161/CIRCULATIONAHA.109.921460. [DOI] [PubMed] [Google Scholar]
- 157.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guo Y.P., Lin L.G., Wang Y.T. Chemistry and pharmacology of the herb pair Flos Lonicerae japonicae-Forsythiae fructus. Chinese Med. 2015;10:16. doi: 10.1186/s13020-015-0044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee H., Lee D., Kim Y., Lee G., Kim S., Jung S., Jung H., Bae H. Lipopolysaccharide induced lung inflammation is inhibited by Lonicera japonica. Mol. Cell. Toxicol. 2011;7:87–93. doi: 10.1007/s13273-011-0012-2. [DOI] [Google Scholar]
- 160.Xie G., Schepetkin I.A., Siemsen D.W., Kirpotina L.N., Wiley J.A., Quinn M.T. Fractionation and characterization of biologically-active polysaccharides from Artemisia tripartita. Phytochemistry. 2008;69:1359–1371. doi: 10.1016/j.phytochem.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cheong D.H.J., Tan D.W.S., Wong F.W.S., Tran T. Anti-malarial drug, artemisinin and its derivatives for the treatment of respiratory diseases. Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Haq F.U., Roman M., Ahmad K., Rahman S.U., Shah S.M.A., Suleman N., Ullah S., Ahmad I., Ullah W. Artemisia annua: trials are needed for COVID-19. Phytother. Res. 2020 doi: 10.1002/ptr.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rops A.L., van der Vlag J., Lensen J.F., Wijnhoven T.J., van den Heuvel L.P., van Kuppevelt T.H., Berden J.H. Heparan sulfate proteoglycans in glomerular inflammation. Kidney Int. 2004;65:768–785. doi: 10.1111/j.1523-1755.2004.00451.x. [DOI] [PubMed] [Google Scholar]
- 164.Coombe D.R., Kett W. Sulphated xylans for treatment or prophylaxis of respiratory diseases. 2020. https://patents.google.com/patent/US20200054668A1/en?oq=US20200054668A1
- 165.Hongping Y., Yang L., Zhengqi Y., Ying W., Su-yin W. Application of the xylan esterification products in prevention or treatment diseases associated with inflammation and cancer drug is prepared. 2018. https://patents.google.com/patent/CN108079001A/en?oq=CN108079001A
- 166.Cook G.C. Relation between malaria serum gamma-globulin concentration and the d-xylose absorption test. Trans. R. Soc. Trop. Med. Hyg. 1975;69:143–145. doi: 10.1016/0035-9203(75)90024-3. [DOI] [PubMed] [Google Scholar]
- 167.Council on foreign relations, Code of Federal Regulations Title 21 https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.395. (Accessed 14 June 2020).
- 168.Spitz I.M., Rubenstein A.H., Bersohn I., Bässler K.H. Metabolism of xylitol in healthy subjects and patients with renal disease. Metabolism. 1970;19:24–34. doi: 10.1016/0026-0495(70)90114-9. [DOI] [PubMed] [Google Scholar]
- 169.Leidig P., Gerding W., Arns W., Ortmann M. Renal oxalosis with renal failure after infusion of xylitol. Dtsch. Med. Wochenschr. 2001;126:1357–1360. doi: 10.1055/s-2001-18650. [DOI] [PubMed] [Google Scholar]