Abstract
Background:
Liquid-based cytology is one of the most useful methods to diagnose a patient with serous effusion, especially when malignancy is suspected. As an alternative to the use of liquid-based cytology only, the serous effusion can be further processed using the technique of DNA image cytometry, which may augment diagnostic utility. The aim of this study was to compare the diagnostic yields of liquid-based cytology, DNA image cytometry, and both in combination, regardless of serous-effusion etiology.
Methods:
We conducted a descriptive study on patients with serous effusions from July 2016 to June 2018. All samples were submitted for liquid-based cytology and DNA image cytometry techniques. We compared the results of cytopathological studies to the final diagnoses.
Results:
For a total of 798 samples, final diagnoses included 412 (51.6%) malignancies, 280 (35.1.%) inflammatory diseases, and 106 (13.3%) transudative serous effusions. Liquid-based cytology had a more sensitive diagnostic yield than DNA image cytometry did (38.8% vs 30.7%; P < .05), but the combination of both had a higher yield (43.7%; P < .05) compared with that of liquid-based cytology alone. For the 412 malignant serous effusions, diagnostic yields of liquid-based cytology and DNA image cytometry were 73.8% and 59.5%, respectively. The difference in sensitivity was significant (P < .05). Combined liquid-based cytology + DNA image cytometry improved diagnostic yield to 83.3% (P < .05). However, both liquid-based cytology and DNA image cytometry had low diagnostic yields for inflammatory diseases and transudative serous effusions.
Conclusion:
In serous effusion, liquid-based cytology’s diagnostic performance is better than that of DNA image cytometry. Application of both techniques can significantly increase diagnostic yield.
Keywords: serous effusion, liquid-based cytology, DNA ploidy, malignant tumor, DNA image cytometry
Introduction
Serous effusion is a common clinical sign of many diseases.1 The appearance of malignant cells in an effusion is a common complication of malignancy in the pleural, pericardial, or peritoneal space. Early detection and treatment can significantly improve patients’ survival rate.2 The most common cause of malignant pleural effusions is lung cancer, followed by breast cancer.3 Ovarian cancer is the most common cause of malignant peritoneal effusion,4 which is detected at diagnosis in two-thirds of cases. By contrast, cancer indicated by pericardial effusion is less common and is detected in only about 2% to 30% of patients with cancer at time of autopsy.5 When malignant effusion is suspected, cytological diagnosis is extremely useful, as its diagnostic rate is 60% (range, 40%-87%).6 Cytological diagnosis is currently considered the first-line diagnostic method for effusions; it is effective not only in diagnosis but also in staging and further guiding treatment for malignancies.
Both direct smear and liquid-based cytology (LBC) are methods that can be used to diagnose serous effusion cytologically.7 Both are widely used to prepare gynecological and nongynecological cytological samples. On the one hand, LBC offers cleaner background smears, better cell distribution, better-preserved cell morphology, less screening time, and longer storage time of well-preserved cells in solution. On the other hand, LBC is a highly morphological examination method, which requires strict training, experienced pathologists, and high-quality production technology. Inadequate diagnostic experience of pathologists and visual fatigue caused by long-term film reading can lead to false positives and false negatives.
DNA image cytometry (DNA-ICM) is a new method of automatic detection based on the gene level. It has been shown to be a sensitive and reliable technique for evaluating DNA content and proliferation of tumors.3 Because background mesothelial cells are usually diploid, the presence of aneuploid cells may indicate malignancy in serous fluid. DNA aneuploidy (content abnormality) determined by image cytometry (ICM) is internationally accepted as a marker of malignant cell transformation, which is the cytometric equivalent of chromosomal aneuploidy.8 DNA content abnormality is thus an important indicator of numerous chromosomal alterations, and its emergence is often a critical early event during carcinogenesis. The aim of this study was to compare the diagnostic yields of LBC, DNA-ICM, and both in combination in detecting malignant cells in serous effusion.
Materials and Methods
Specimen Collection
This preliminary study included 798 patients with serous effusion, which included peritoneal, pericardial, and pleural effusion fluids, as well as intraoperative peritoneal washings, received in the Cytology Division of the Department of Pathology at the Affiliated Hospital of Southwest Medical University. Each specimen was divided into 2 halves for processing by LBC and DNA-ICM techniques. The final diagnoses included 412 (51.6%) malignancies, 280 (35.1%) inflammatory diseases, and 106 (13.3%) transudative serous effusions. The final diagnosis in LBC and DNA-ICM results was reached by thoracoscopic pleural biopsy, biochemical results, microbiological results, clinical manifestations, radiographic findings, and improvement or progression on treatment and follow-up.
Examination of LBC
We collected 100 mL serous-effusion specimens and centrifuged them at room temperature for 15 minutes at 1500 r/min. The sediments were then collected for suspension. We added 15 mL of cell preservation solution (Hologic) to the remaining sediment in each sample and centrifuged the mixture at 600 r/min for 20 minutes. The supernatant was discarded, and the sediment was placed into a vial of ThinPrep PreservCyt solution (Hologic) and left to stand for 15 minutes. The vial was run on an automated ThinPrep 2000 processor (Hologic), yielding 1 liquid-based slide per patient. We then fixed the slide and submitted it for Papanicolaou staining. The liquid-based slides were reviewed, and diagnoses were provided by doctors of intermediate rank and higher in the Pathology Department of the Affiliated Hospital who had rich experience in cytological diagnosis. Criteria for LBC results: no cancer cells were negative, while suspicious cancer cells or cancer cells were positive.
Measurement of DNA Content
DNA content was measured as described previously.9 We spread the postcentrifugation serous-effusion sediments on slides and stained the slide depositions using the Feulgen-thionin method. The nuclei of more than 5000 serous-effusion cells were scanned using a MotiSavant automatic cell image scanning and analysis system (Motic, Inc). The system can simultaneously measure 132 parameters of nuclear DNA, automatically classify and count tested cells, automatically print a DNA ploidy analysis histogram and cell dot matrix distribution, and determine the main content of nuclear DNA by measuring the integrated optical density of chromosome nuclei.
The DNA index (DI) of diploid cells (G1/G0) is 1. When DI = 2, most normal cells are tetraploid (G2/M phase). When DI is 1.1 to 1.9 or 2.1 to 2.9, cells are considered aneuploid. When the DI of aneuploid cells peaks between 1.1 and 1.9, or >3 cells have a DI value ≥2.5, this may be a positive indicator for DNA-ICM in malignant serous effusion. Diagnostic results of DNA-ICM are usually classified into 3 grades: I, normal standard, no obvious aneuploid-cell peak and aneuploid cell; II, possibly malignant standard, a small number of DNA ploidy abnormal cells (1-2 cells; DI ≥ 2.5); III, malignant standard, a large number of DNA ploidy abnormal cells (≥3 cells; DI ≥ 2.5) and aneuploid-cell peak. Slides of specimens with DI ≥2.5 should be viewed under a microscope by experienced cytopathologists, in case the system produces a false-positive result by mistaking deeply stained impurities and crowded overlapping nuclei for malignant cells. In statistical analysis, we classified grade I as negative serous effusion, and grades II and III as positive serous effusion.
Statistical Analysis
We collected data using the Microsoft Excel application. All data are given as mean ± standard deviation. We used SPSS software version 17.0 for Windows (SPSS, Inc) for statistical analyses. Diagnostic yields were compared using McNemar test. A P value <.05 was considered statistically significant.
Results
There were 798 cases of patients hospitalized with serous effusion, comprising 416 males and 382 females with a mean age of 60.4 ± 13.8 years. Demographics of the study population are shown in Table 1. DNA content analysis of the same patient is shown in Figure 1. The diagnostic yields of LBC and DNA-ICM were 38.8% and 30.7%, respectively (P < .001). Further comparisons revealed that the combination of LBC + DNA-ICM had a higher yield (43.7%) than LBC alone (P < .001).
Table 1.
Demographic Data of 798 Patients.
| Variables | N (%) or mean ± SD |
|---|---|
| Age, years | 60.4 ± 13.8 |
| Gender | |
| Male | 416 (52.1%) |
| Female | 382 (47.9%) |
| Final diagnosis | |
| Malignancies | 412 (51.6%) |
| Inflammatory diseases | 280 (35.1%) |
| Transudative serous effusions | 106 (13.3%) |
Abbreviation: SD, standard deviation.
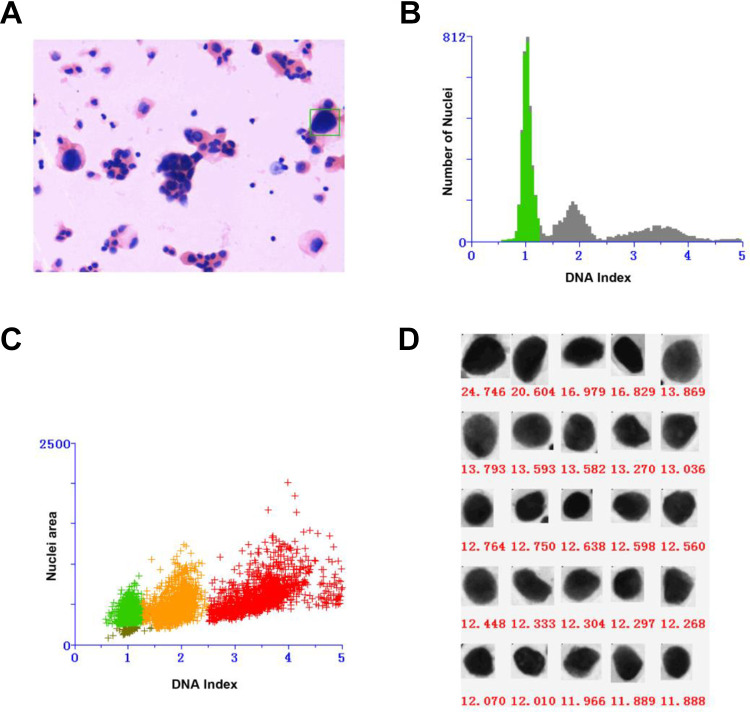
Figure 1.
Cytopathology and DNA content analysis of a 61-year-old male patient presented with suspicious lesions in pleural effusion (A) moderate dysplasia (magnification, ×200). B, Two separate G0 to G1 peaks in DNA content histogram. C, A diploid peak (green) and an aneuploid peak (yellow) in DNA content scatter plot. D, The aneuploid cell nuclei were arranged from large to small according to DNA content (red spots in C) with DNA index value >2.5.
Diagnostic yields of LBC and DNA-ICM in malignant serous effusion were 73.8% and 59.5%, respectively. The difference in sensitivity was significant (P < .05). Combined LBC + DNA-ICM improved the diagnostic yield to 83.3% (P < .05). In 108 patients with LBC results that were negative for malignant serous effusion, DNA-ICM revealed malignant disease in 39 (36.1%), and LBC + DNA-ICM improved the diagnostic yield significantly compared with LBC (P < .05; Table 2). Regarding inflammatory diseases, LBC was able to diagnose 2 cases of empyema thoracis and 4 cases of tuberculous pleuritis. DNA-ICM had no ability to diagnose inflammatory diseases, and neither LBC nor DNA-ICM had any diagnostic role in cases of transudative serous effusion.
Table 2.
Diagnostic Yields of LBC, DNA-ICM, and the Combination of Both Techniques (LBC + DNA-ICM).
| Final diagnosis | n | Diagnostic yield | ||
|---|---|---|---|---|
| DNA-ICM (n, %) | LBC (n, %) | LBC + DNA- ICM (n, %) | ||
| Malignancies | 412 | 245 (59.5) | 304 (73.8)a | 343 (83.3)b |
| Inflammatory diseases | 280 | 0 | 6c | 6d |
| Transudative serous effusions | 106 | 0 | 0 | 0 |
| Total | 798 | 245 (30.7) | 310 (38.8)a | 349 (43.7)b |
Abbreviations: DNA-ICM, DNA image cytometry; LBC, liquid-based cytology.
a P < .05 versus DNA-ICM.
b P < .01 versus LBC.
c P < .01 versus DNA-ICM.
d P < .01 versus LBC.
Liquid-based cytology tended to have a higher diagnostic yield than DNA-ICM in all malignancy subgroups; the difference was significant (P < .01). Liquid-based cytology + DNA-ICM achieved a diagnostic yield similar to that of LBC in all malignancy subgroups (Table 3). However, of 386 patients who were negative benign serous effusion, LBC was false positives for 73, while DNA-ICM produced false positives for 21.
Table 3.
Diagnostic Yields of LBC, DNA-ICM, and the Combination of Both Techniques (LBC + DNA-ICM) in Malignancy Subgroups.
| Final diagnosis | n | Diagnostic yield | ||
|---|---|---|---|---|
| DNA-ICM (n, %) | LBC (n, %) | LBC + DNA- ICM (n, %) | ||
| Solid malignancy | 372 | 0 | 228 (61.3)a | 228 (61.3) |
| Hematological malignancy | 40 | 0 | 17 (42.5)a | 17 (42.5) |
| Total | 412 | 0 | 245 (59.5)a | 245 (59.5) |
Abbreviation: DNA-ICM, DNA image cytometry.
a P < .01 versus DNA-ICM.
Discussion
Clinicians see many cases of serous effusion. In addition to alleviating symptoms, the main hope is to clarify the nature of the effusion from a cytopathological point of view; that is, to judge whether the effusion is caused by malignant tumors or by other non-neoplastic disease. It is a difficult point in cytopathological diagnosis.10 There are many methods of diagnosing malignant serous effusion, including cytological examination, DNA ploidy quantitative analysis, imaging examination, tumor markers, molecular markers of effusion, and endoscopic biopsy. Among them, endoscopic-biopsy histopathology is the most accurate examination method and the gold standard.11,12 Cytopathological examination is the most commonly used classic method for diagnosis of serous effusion, especially for patients who cannot tolerate invasive examinations such as endoscopy. However, the sensitivity of cytological examination is correlated with the experience of the cytopathologist, the quality of the specimens sent for examination, and the classification and differentiation of the tumors.
Quantitative analysis of DNA ploidy is an important method in early detection of tumors. Numerous studies at home and abroad have reported a diagnosis of benign and malignant serous effusion via DNA ploidy analysis; it has become one of the routine clinical detection methods in North America and Europe.13,14 Currently, flow cytometry (FCM) and ICM are mostly used for quantitative analysis of cellular DNA. However, FCM equipment is expensive, necessitating the preparation of a single-cell suspension, which is complex and unsuitable for primary hospitals. The DNA-ICM analysis can automatically detect changes to cell DNA content by scanning the specimen slide from every field of view with a fully automatic digital microscope, thus determining whether the cells have canceration. Malignant cells prior to morphological changes can be found by using the self-determination analysis system and specific data to describe features of tumor biology and morphology.15,16 Therefore, determination of DNA content can be used as an objective basis for the clinical diagnosis of malignant serous effusion.
In this study, we analyzed and compared diagnostic rates of LBC and DNA-ICM in malignant serous effusion. We found that the sensitivity of LBC was higher than that of DNA-ICM: diagnostic yields of LBC and DNA-ICM in malignant serous effusion were 38.8% and 30.7%, respectively, and the difference was significant (P < .05). Although DNA-ICM can provide much more objective diagnostic information than LBC, this study found that DNA-ICM also produced false positives and false negatives. We speculate that false positives in DNA-ICM may occur due to degeneration or nuclear pyknosis of a few cells in inflammatory serous effusion. These cells can appear as alloploid cells with DI ≥ 2.5 when stained using the Feulgen-thionin method. At this time, repeated examinations are needed, and they should be combined with LBC results and immunohistochemistry to identify inflammatory or malignant serous effusion. The main reason for false negatives in DNA-ICM is that dense clusters of cancer cells cannot be identified. Metastatic adenocarcinoma is the most common malignancy indicated by serous effusion, and adenocarcinoma cells often cluster.
Further study and analysis showed that if LBC and DNA-ICM were combined to diagnose malignant serous effusion, diagnostic yields could be increased to 83.3% (P < .05). Our results indicated that combined diagnosis offered significantly higher sensitivity and that the positive-detection rate of hydroceles was significantly increased compared with either LBC or DNA-ICM alone, which was consistent with previous studies reported in the cervix.17 In addition, in the 386 patients with negative benign serous effusion, LBC diagnosed 73 cases of malignant tumors, while DNA-ICM results produced only 21 false positives. Moreover, DNA-ICM detected 39 positive samples in 108 patients who received negative diagnoses of malignant serous effusion via LBC. This is because cytological diagnosis mainly depends on the characteristics of cell morphology and structure. The LBC technology is mature, but serous effusion is complicated in composition; more importantly, normal mesothelial cells can proliferate, even atypically, under various stimuli such as inflammation. Such proliferation and metastasis of mesothelial and metastatic cancer, especially in cases of highly differentiated cancer cells, can occur in microscopic cytopathy, and physicians are challenged to identify these cases morphologically. DNA-ICM can detect changes in DNA content in cells to determine whether the cells have canceration, which is not affected by changes in cell morphology. Therefore, combined diagnosis is conducive to early and accurate diagnosis of malignant serous effusion.
In conclusion, DNA-ICM in the diagnosis of serous effusion effectively compensates for possible missed diagnoses and misdiagnoses caused by human error in LBC diagnosis. Liquid-based cytology combined with DNA-ICM has better sensitivity than either LBC or DNA-ICM alone in such diagnosis. It can compensate for not only diagnostic errors by cytopathologists but also inherent defects in DNA-ICM analysis. Combined diagnosis can thus further control and ensure the quality of serous-effusion diagnosis, yielding great clinical value. Liquid-based cytology + DNA-ICM can therefore be used as a comprehensive method in screening for malignant serous effusion.
Acknowledgments
This work was supported by the Department of Pathology for comprehensive help with this study. In addition, I deeply appreciate the contribution to this thesis made in various ways by my colleagues and friends.
Abbreviations
- DI
DNA index
- DNA-ICM
DNA image cytometry
- FCM
flow cytometry
- LBC
liquid-based cytology.
Authors’ Note: The study protocol was reviewed and approved by the guidelines of the Institutional Ethics Committee of the Affiliated Hospital of Southwest Medical University (approval no. 2019058), Luzhou, China. All patients provided written informed consent prior to enrollment in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Natural Science Foundation of the Sichuan Provincial Education Office (18ZA0529), the Sichuan Provincial Health and Family Planning Commission Research Project (No. 18PJ023 and 18PJ024), the Key Scientific Research Projects of Southwest Medical University (2017-ZRZD-011), and the Doctoral scientific research startup Fund Project of Affiliated hospital of Southwest Medical University.
ORCID iD: Shaohua Wang  https://orcid.org/0000-0003-0594-0264
https://orcid.org/0000-0003-0594-0264
References
- 1. Chen D, Song X, Shi F, et al. Greater efficacy of intracavitary infusion of bevacizumab compared to traditional local treatments for patients with malignant cavity serous effusion. Oncotarget. 2017;8(21):35262–35271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dadhich H, Toi PC, Siddaraju N, Sevvanthi K. A comparative analysis of conventional cytopreparatory and liquid based cytological techniques (Sure Path) in evaluation of serous effusion fluids. Diagn Cytopathol. 2016;44(11):874–879. [DOI] [PubMed] [Google Scholar]
- 3. Chongmei L, Liuyan H, Xuechun Z, Yang J. Combination of DNA ploidy analysis and miR-21 or miR-24 in screening malignant pleural effusion. Interact CardioVasc Thorac Surg. 2018;26(3):376–381. [DOI] [PubMed] [Google Scholar]
- 4. Javorska L, Krcmova L K, Solich P, Kaska M. Simple and rapid quantification of vancomycin in serum, urine and peritoneal/pleural effusion via UHPLC-MS/MS applicable to personalized antibiotic dosing research. J Pharm Biomed Anal. 2017;142:59–65. [DOI] [PubMed] [Google Scholar]
- 5. Razek AAKA, Samir S. Differentiation malignant from benign pericardial effusion with diffusion-weighted MRI. Clin Radiol. 2019;74(4):325.e19–e24. [DOI] [PubMed] [Google Scholar]
- 6. Sen R, Hasija S, Kalra R, et al. Morphometric analysis and immunocytochemical staining on cytospin preparation in effusion cytology: a study. J Cytol Histol. 2015;10:345–351. [Google Scholar]
- 7. Matsuo Y, Yoshida T, Yamashita K, Satoh Y. Reducing DNA damage by formaldehyde in liquid-based cytology preservation solutions to enable the molecular testing of lung cancer specimens. Cancer Cytopathol. 2018;126(12):1011–1021. [DOI] [PubMed] [Google Scholar]
- 8. Zhao M, Yang L, Fu X, et al. Endoscopic ultrasound-guided fine-needle aspiration cytology combined with automated quantitative DNA cytometry can improve the value in the detection of pancreatic malignancy. Pancreas. 2018;47(1):40–52. [DOI] [PubMed] [Google Scholar]
- 9. Xiao X, Shi L, Li H, Song Y, Liu W, Zhou Z. DNA content status using brush biopsy with image cytometry correlated with staging of oral leukoplakia: a preliminary study. Oral Oncol. 2015;51(1):59–63. [DOI] [PubMed] [Google Scholar]
- 10. Kundu R, Handa U, Mohan H. Role of DNA flow cytometry and immunocytochemical analysis in diagnosis of malignant effusions. Diagn Cytopathol. 2012;40(10):887–892. [DOI] [PubMed] [Google Scholar]
- 11. Porcel JM, Palma R, Bielsa S, et al. TTF-1 and napsin A on cell blocks and supernatants of pleural fluids for labeling malignant effusions. Respirology. 2015;20(5):831–833. [DOI] [PubMed] [Google Scholar]
- 12. Liu J, Luo X, Wang W, et al. Clinicopathological study of 9 cases of megakaryocytes in pleural and peritoneal fluids. Medicine (Baltimore). 2018;97(33):e11923–11928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang M, Hao C, Ma Q, et al. DNA image cytometry test for primary screening of esophageal cancer: a population-based multi-center study in high-risk areas in China. Chin J Cancer Res. 2016;28(4):404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fritcher E G B, Brankley S M, Kipp B R, et al. A comparison of conventional cytology, DNA ploidy analysis, and fluorescence in situ hybridization for the detection of dysplasia and adenocarcinoma in patients with Barrett’s esophagus. Hum Pathol. 2008;39(8):1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghizoni J S, Sperandio M, Lock C, et al. Image cytometry DNA ploidy analysis: correlation between two semi-automated methods. Oral Dis. 2018;24(7):1204–1208. [DOI] [PubMed] [Google Scholar]
- 16. Shi A, Min W, Xiang L, et al. Value of automatic DNA image cytometry for diagnosing lung cancer. Oncol Lett. 2018;16(1):915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong O G, Ho M W, Tsun O K, et al. An automated quantitative DNA image cytometry system detects abnormal cells in cervical cytology with high sensitivity. Cytopathology. 2018;29(3):267–274. [DOI] [PubMed] [Google Scholar]