CONSPECTUS:
Lipid membranes are more than just barriers between cell compartments; they provide molecular environments with a finely tuned balance between hydrophilic and hydrophobic interactions that enable proteins to dynamically fold and self-assemble to regulate biological function. Characterizing dynamics at the lipid–water interface is essential to understanding molecular complexities from the thermodynamics of liquid–liquid phase separation down to picosecond-scale reorganization of interfacial hydrogen-bond networks. Ultrafast vibrational spectroscopy, including two-dimensional infrared (2D IR) and vibrational sum-frequency generation (VSFG) spectroscopies, is a powerful tool to examine picosecond interfacial dynamics. Two-dimensional IR spectroscopy provides a bond-centered view of dynamics with subpicosecond time resolutions, as vibrational frequencies are highly sensitive to the local environment. Recently, 2D IR spectroscopy has been applied to carbonyl and phosphate vibrations intrinsically located at the lipid–water interface. Interface-specific VSFG spectroscopy probes the water vibrational modes directly, accessing H-bond strength and water organization at lipid headgroup positions. Signals in VSFG arise from the interfacial dipole contributions, directly probing headgroup ordering and water orientation to provide a structural view of the interface.
In this Account we discuss novel applications of ultrafast spectroscopy to lipid membranes, a field that has experienced significant growth over the past decade. In particular, ultrafast experiments now offer a molecular perspective on increasingly complex membranes. The powerful combination of ultrafast, interface-selective spectroscopy and simulations opens up new routes to understanding multicomponent membranes and their function. This Account highlights key prevailing views that have emerged from recent experiments: (1) Water dynamics at the lipid–water interface are slow compared to those of bulk water as a result of disrupted H-bond networks near the headgroups. (2) Peptides, ions, osmolytes, and cosolvents perturb interfacial dynamics, indicating that dynamics at the interface are affected by bulk solvent dynamics and vice versa. (3) The interfacial environment is generally dictated by the headgroup structure and orientation, but hydrophobic interactions within the acyl chains also modulate interfacial dynamics. Ultrafast spectroscopy has been essential to characterizing the biophysical chemistry of the lipid–water interface; however, challenges remain in interpreting congested spectra as well as designing appropriate model systems to capture the complexity of a membrane environment.
Graphical Abstract

MOTIVATION
The modern picture of cell membranes as a two-dimensional “fluid mosaic” has been a standard for nearly 50 years.5 Triggered by the lipid raft hypothesis in 1997, which has remained a source of motivation in contemporary studies, we now find ourselves in a new era of lipid membrane research.6–8 Novel developments in imaging, biochemistry, and spectroscopy have begun to access the complexity and dynamism of lipid bilayers, revealing vast diversity in lipid composition and organization with links to cellular function.9,10 With new experimental techniques and modeling capabilities, membrane physical chemistry is in the midst of a rapid expansion toward revisiting, revising, and replacing previous membrane models.
Lipids often drive membrane behavior in addition to providing the environment for integral membrane proteins. Membranes create a 1 nm-thick interface between hydrophobic and hydrophilic regions that support a wide range of biochemical processes. The resulting environments are complex and include partially disrupted hydrogen-bond networks near the polar headgroups.11 Ultrafast vibrational spectroscopy has been an essential tool to characterize interfacial structure and motions in atomistic detail. Infrared measurements probe the intrinsic vibrations of lipids, water, and embedded biomolecules. The combination of structure sensitivity and subpicosecond time resolution provides direct experimental access to rapidly evolving interfacial hydrogen-bond networks. Ultrafast techniques combined with molecular dynamics simulations have provided the most complete picture to date. Naturally, there is a tight interplay between membrane composition and interfacial environments. In this Account we provide an overview of recent developments in ultrafast methods and highlight key findings in membrane research.
APPLICATIONS OF ULTRAFAST SPECTROSCOPY
Why Vibrational Spectroscopy?
Vibrational spectroscopy has been used to study lipid bilayers over the past several decades. These methods include Raman, IR absorption, and vibrational sum-frequency generation (VSFG), among others.12–14 IR absorption spectra reveal several aspects of membrane structure: headgroup conformation and interfacial hydration through phosphate and ester carbonyl stretching modes; and alkyl tail conformation and phase from CH2 and CH3 stretching bands. One advantage of IR spectroscopy is the ability to exploit the intrinsic modes of the lipids as vibrational probes (Figure 1), which are sensitive to the local environment; vibrational spectroscopy does not generally require extrinsic probes, which are common in numerous biophysical techniques.15,16 Isotope incorporation, typically 13C or 13C=18O in IR spectroscopy, effectively isolates the vibrational spectrum of the labeled component by shifting it to a lower frequency.17 Isotope labels are a valuable tool in vibrational spectroscopy of multilipid systems, as they provide access to single species in a multicomponent environment. Of course, challenges remain in the chemical synthesis of lipids, sample preparation, and interpretation of spectra, which are discussed later. In ultrafast experiments, systems are commonly studied in near-native, aqueous environments, including self-assembled vesicles, monolayers, and multibilayers.
Figure 1.

Diagram of functional groups commonly used as vibrational reporters of lipid membranes. Labels indicate the approximate region of the spectrum where each functional group is most often probed.
Static spectra contain ensemble-averaged structural information, but they cannot resolve fast dynamics. Recent implementations of ultrafast vibrational spectroscopy can measure the picosecond dynamics of lipids, including interfacial motions and H-bond network rearrangements. These new techniques have reinvigorated the field of lipid interfaces and present a promising path toward more complete descriptions of structural dynamics at interfaces. Specifically, this Account focuses on two ultrafast spectroscopic methods: two-dimensional infrared (2D IR) and multidimensional VSFG, which reveal information about the solvent structure and dynamics at the interface. These techniques offer advantages over traditional linear vibrational spectroscopy, including background suppression, well-defined lineshapes, stronger signals, and inherent interface selectivity stemming from the vibrational modes accessed by each method.
Probing the Interface with 2D IR Spectroscopy
Ultrafast 2D IR spectroscopy is a frequency-resolved pump–probe technique in which infrared pump pulses excite a specific vibrational mode, and the probe detects subsequent vibrations over a broad frequency range. Pump and probe pulses are separated by a femtosecond to picosecond delay, typically referred to as the “waiting time”. Spectra are represented as two-dimensional plots composed of an excitation frequency axis and a detection frequency axis, as shown in Figure 2. Short waiting times produce elongated 2D IR peaks as a result of the high correlation between the frequencies of excited and detected modes, whereas at longer waiting times, this correlation is lost to frequency fluctuations, resulting in rounder peaks. Ultrafast dynamics can be extracted by quantifying the degree of correlation as a function of the waiting time, which is equivalent to measuring the ensemble-averaged correlation function of the frequency fluctuations. Off-diagonal peak elongation resulting from loss of frequency correlation is referred to as “spectral diffusion”, which represents the rate of “exchange” among environments within the ensemble. The spectral diffusion curve typically follows an exponential decay, and the maximum waiting time is limited by the excited-state lifetime of the vibrational mode. Typically for an ester with a C=O vibration in lipids, the lifetime is 1.0 to 1.7 ps, and therefore, ultrafast measurements are limited to picosecond time scales.18 The loss of frequency correlation that occurs on longer time scales (>10 ps) is typically referred to as “static inhomogeneity”, which represents slow molecular motions, which present as “static” compared to the experimental time scale.19
Figure 2.

Cartoon representation of spectral diffusion. Diagonally elongated 2D peaks (middle panel) become round at long pump–probe waiting times due to population exchange. Analogous steady-state spectra (top) show no line shape change upon spectral diffusion. Points overlaid on the 2D spectra represent the assigned peak maxima at each pump frequency. The white lines indicate the diagonal elongation of each 2D peak. The slope of this line is plotted versus the waiting time (bottom). Time constants from an exponential fit to this data represent the ensemble-averaged frequency–frequency correlation lifetime, from which dynamics such as H-bond lifetimes are extracted. The loss of correlation at longer waiting times is referred to as “spectral diffusion”.
Two-dimensional infrared spectroscopy is not an inherently interface-selective technique; in 2D IR, interface selectivity is achieved by the choice of the vibrational probe. In a lipid bilayer, the lipid ester C=O is located at the ~1 nm interface between the bulk water and the membrane hydrophobic region. Figure 3 shows the distribution of water oxygen atoms and the lipid P, N, and ester C=O oxygen atoms along the membrane normal. Spatial overlap between the C=O oxygen positions and the interfacial region between the hydrophilic (maximum) and hydrophobic (minimum) water populations makes this ester C=O an intrinsic local reporter of the interfacial environment. Since absorption frequencies are sensitive to the local environment around the probe, ultrafast dynamics extracted from spectral diffusion can be used to describe the motions of molecules in its vicinity.
Figure 3.

Distribution of functional group locations along the membrane normal (Z-coordinate). The distributions are extracted from a molecular dynamics (MD) trajectory of a dimyristoylphos-phatidylcholine bilayer. Histograms computed from MD trajectories are published in ref 4.
The molecular origin of the motions driving the frequency fluctuations is often unclear from experiments, illustrating the need for well-developed computational models to accurately describe frequency shifts in these complex membrane environments. Conceptually, the vibrational frequency of the oscillator shifts in response to the intermolecular forces imparted on the oscillator by the neighboring molecules, and while intermolecular interactions contain multiple contributions, electrostatic interactions dominate the frequency fluctuations. Taking advantage of these effects, electrostatic maps can accurately predict infrared spectra and frequency–frequency correlation functions of C=O probes to near-quantitative agreement.20,21 Upon agreement between experiments and simulations, the atomistic information contained in the MD trajectory can then be used to describe in atomistic detail the molecular fluctuations that lead to the observed frequency fluctuations.
Sum-Frequency Generation Probes Interfacial Water Dynamics
Spectral lineshapes and frequency fluctuations measured by 2D IR are often linked to the structure and dynamics of the solvent, yet it is the lipid, not the solvent, that is probed. Experimental access to a solvent-structure-based interpretation requires direct interrogation of the solvent dynamics. The challenge is that bulk molecules vastly outnumber interfacial ones, and therefore, measurements that are only sensitive to interfacial solvent molecules are essential. VSFG, a second-order nonlinear process, is inherently interface-selective, as the VSFG signals arise only from the interface.22,23 Briefly, VSFG experiments use a visible and an infrared pulse that are combined at a sample surface or interface and generate an output signal with a frequency equal to the sum of the input pulses. Scanning the wavelength of the IR beam produces a VSFG spectrum that can be interpreted similarly to an absorption spectrum where VSFG signals are localized to the interface. One important consideration is that multiple interfaces can produce interfering signals, making the spectrum difficult to interpret. Therefore, most VSFG spectra are measured on a single interface such as the one shown in Figure 4; typically consisting of a monolayer at the air–water interface. The lipid packing density can be controlled using a Langmuir–Blodgett trough, which allows for the investigation of multiple phases and packing densities.24
Figure 4.
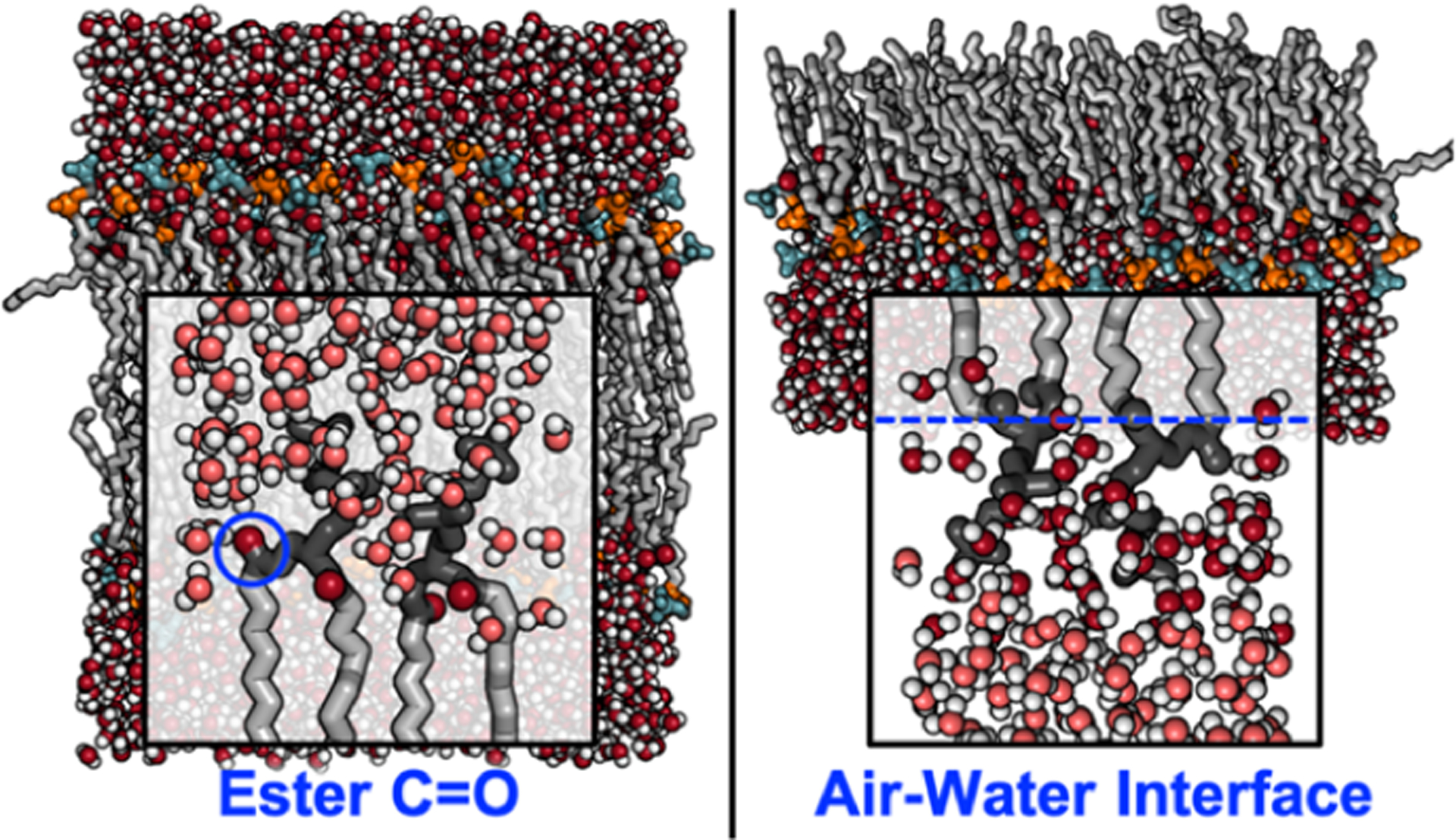
Location and identity of vibrational probes in a lipid bilayer for 2D IR (left) and at the air–water interface for VSFG (right) experiments. The lipids are shown as gray sticks, with the headgroups (dark gray) and the carbonyl groups (red) highlighted for clarity. The configurations were sampled from a MD trajectory of dipalmitoylphosphatidylcholine (DPMC) published in ref 4
Interfacial water spectra collected by VSFG typically capture the O–H stretching modes and contain characteristic peaks around 3200 cm−1, 3400 cm−1, and 3700 cm−1 with the lower two bands associated with bound O–H and the high frequency band with free O–H, respectively.25 The measurements provide a structural picture of the interfacial H-bonding network.26 Similar to 2D IR, VSFG spectral interpretation often involves MD simulations. Where VSFG can directly access water orientation and structure at the interface and the relative populations thereof, simulations that accurately reproduce water configurations can then be used to describe the structure of neighboring functional groups, such as lipid phosphate, choline, and ester groups, to resolve links between the lipid structure, solvent structure, and solvent dynamics.27
Extensions of SFG have been developed recently to distinguish net orientations of water dipole moments by heterodyne-detected VSFG (HD-VSFG), which have enabled a more complete picture of interfacial water structure.28 The major advantage of HD-VSFG over homodyne-detected VSFG is, where VSFG can reveal only the magnitude of the electric field, the use of a local-oscillator in HD-VSFG enables an interferometric measurement of the electric field amplitude and phase, providing direct information about the up/down orientation of surface water molecules.28–30 Extensions toward transient VSFG have also been developed by introducing an additional pump pulse similar to the one used in transient absorption, or “pump-probe” IR experiments, to excite the sample prior to VSFG measurement.31 Two-dimensional VSFG (2D HD-VSFG) was initially developed by Bonn and co-workers by incorporating an IR “pump” within the homodyne SFG pulse sequence which allowed for multidimensional measurements on a molecular monolayer at the air/water interface.32,33 This novel technique is analogous to 2D IR featuring frequency-resolved pump and probe axes.34 These ultrafast methods show promise toward linking local water dynamics to specific structural motifs at the lipid–water interface.24,26,35
MODEL SYSTEMS AND CASE STUDIES
Interfacial Water Dynamics Are Dependent on Headgroup Structure
Monolayers and bilayers composed of single lipid species are popular model systems because of their simplicity. These systems have been useful to characterize the effects of lipids on the structure of water molecules at the interface. For example, using phase-sensitive SFG, Allen and co-workers determined that all lipids strongly orient interfacial waters with hydrogens pointing toward the acyl chains and that the net orientation is stronger for anionic compared to zwitterionic lipids.36
Building on one-dimensional VSFG investigations of water orientations,36,37 the dynamics of interfacial water have been measured with both time-resolved and two-dimensional heterodyne detected VSFG (TR-VSFG and 2D HD-VSFG, respectively), combining interface specificity and ultrafast time resolution. Tahara and co-workers found that the charge of a lipid or surfactant has a significant impact on the water dynamics as observed through spectral diffusion, which was significantly slower with anionic species compared to cationic.38–40 Backus and co-workers determined that the slowdown is independent of the water orientation.41 Interestingly, water dynamics at the zwitterionic lipid interface cannot be described as a combination of cationic and anionic interfaces, suggesting that dipole–dipole interactions,38–40 as well as H-bonding, drive interfacial dynamics.39 The presence of H-bond donors in the lipids impacts water dynamics, as surfactants with primary amines slow down interfacial water significantly more than similar surfactants with tertiary amines.42 H-bonding groups in lipids, membrane-associated proteins, glycolipids, glycoproteins, and sterols are integral to biological membranes. Therefore, disentangling interfacial electrostatics and local H-bonding will remain an important future challenge.
Minimally hydrated samples provide another route to interfacial dynamics using bulk measurements. In these experiments, sufficiently low hydration is maintained such that all waters directly interact with a lipid headgroup. One potential pitfall of this approach is that lipids undergo multiple phase changes with the addition of water before adopting the laminar fluid phase in bulk water, and therefore, lipid packing and self-assembly in minimally hydrated systems must be carefully characterized before comparison with fully hydrated membranes. These studies often allow for direct comparison between interfacial water and bulk water, as water dynamics are measured at different hydration levels. Since the lyotropic phase diagrams of common model phospholipids have been characterized (via FTIR, NMR, and calorimetry), samples with sufficient water to fully hydrate the headgroups are prepared following standard protocols.43 This minimum lipid-to-water ratio varies for different species: from 16 waters per lipid, or about 28% wt/wt in dipalmitoylphosphatidylcholine to >40% wt/wt in membranes containing unsaturated phosphatidylcholine lipids as a result of the increased area per lipid that results from unsaturation.43 Additionally, the presence of salts alters the amount of water needed for complete hydration.44 Microhydrated lipids and surfactants can also be assembled into water-in-oil reverse micelles consisting of a nanoscopic “pool” of water suspended in a bulk nonpolar phase. In pump–probe and 2D IR spectra of the OH stretching mode of water, Elsaesser and co-workers found that vibrational lifetimes and spectral diffusion dynamics were slower in reverse micelles compared to bulk water.45,46 Unsurprisingly, the confined water dynamics approached bulk dynamics as the number of water molecules inside the reverse micelle increased. Similar results have been obtained using pump–probe and anisotropy decays of the OD stretching mode of lipids with minimal amounts of D2O,47–49 and the components of the vibrational relaxation have been assigned to two components: a bulk water component and a phosphate-associated component.50 Interestingly, relative contributions of these two components differed above or below their gel-to-fluid phase transition temperature, as the phosphate-associated component became more significant when the lipids were in the fluid phase. This can be attributed to looser lipid packing, allowing increased water penetration into the headgroup region. Cho and co-workers found that the vibrational relaxation of aqueous HN3 in fully hydrated zwitterionic lipid multilayers was comparable to bulk water, concluding that aqueous anions stay largely solvated, rather than interacting directly with the lipid interface.49
Dynamics in the Hydrophobic Core
In saturated lipids, the only feasible modes used to probe the bilayer core are the CH2 or CH3 stretching modes. Vibrational coupling makes it difficult to obtain an atomistic interpretation of measurements in this frequency region, and for that reason, extrinsic probes such as metal carbonyls have been introduced to measure dynamics within the hydrophobic core.51,52 In past studies, the combination of vibrational probes with ultrafast spectroscopy has provided numerous insights into protein environments and dynamics, and the same methods can be directly translated to membrane studies.53 Probes become useful when the intrinsic vibrational modes are weak, insensitive to their environment, or when spectra are difficult to interpret due to spectral congestion or other factors. Overall, vibrational probes are analogous to fluorescent probes such as FRET dye pairs, which provide high sensitivity and specificity but require characterizing their location and orientation for unambiguous interpretations. Similarly, the characterization of the location and orientation of vibrational probes greatly expands utility in membrane biophysics.54 However, while extrinsic vibrational probes often provide clear spectroscopic signals, their location and effect on surroundings can be difficult to characterize. Consequently, control experiments become important to assess the effect of these probes on the system.
Metal carbonyls have been used to measure dynamics within the hydrophobic membrane region. Interestingly, the dynamics of W(CO)6 are insensitive to the amount of hydration water present, suggesting that dynamics in the hydrophobic region are largely decoupled from the headgroup. Vesicles exhibit faster picosecond-scale dynamics compared to planar multibilayers, indicating that membrane curvature plays a role in lipid-packing and dynamics within the bilayer core.51 Metal carbonyls have also been covalently attached to cholesterol, and the measured dynamics were more than twice as slow for the same probe in bulk water.55 Recently, Rubtsov and co-workers incorporated azide probes within the hydrocarbon tails.52 Azide and nitrile probes are commonly used for probing protein dynamics and may become equally useful for membranes.56
The vibrational modes of the lipids themselves provide a detailed molecular picture; however, their location is restricted compared to extrinsic probes. We have begun investigating the effects of chain unsaturation on H-bond dynamics at the lipid–water interface utilizing lipid ester carbonyls as an intrinsic probe (Figure 5) and found that at subpicosecond time scales, there is little difference between saturated and unsaturated lipids, while from 5 to 10 ps, the measured autocorrelations diverge significantly. We have interpreted this effect as being a result of differences in H-bond exchange rates, as the ester carbonyl band in lipid membranes is split into separate peaks by H-bonding. Picosecond dynamics are the result of fast fluctuations, while slower dynamics are related to H-bond exchange. Structurally, the unsaturated lipids increase exposure at the ester positions, producing faster dynamics.
Figure 5.

Dynamics of lipids with differing tail lengths and unsaturation observed via spectral diffusion. (a) Two-dimensional IR sequence spectra of dipalmitoylphosphatidylcholine (DPPC) in the ester C=O region at various waiting times, showing a loss of diagonal elongation due to spectral diffusion. (b) Spectral diffusion analyzed by a center line slope (CLS) analysis of the saturated lipid DPPC (blood red) and the unsaturated lipid dipalmitoylphosphatidylcholine (DOPC) (teal). Experimental methods are described in the Supporting Information.
Characterization of ultrafast dynamics has been varied, yet common themes have emerged from these measurements. Perhaps the most consistent of these is that water dynamics are slower at lipid membrane interfaces than they are in bulk.33–44 This observation is not surprising, given that the membrane significantly disrupts the tetrahedral H-bond networks of water, but the extent of the disruption appears to be influenced by the headgroup structure and composition of the lipid membrane. The slowdown due to lipids has generally been interpreted as constrained motions of waters interacting with lipid headgroups; furthermore, the headgroup structure itself determines its extent. Specifically, anionic and H-bond donating lipids have been shown to slow the interfacial H-bond motions more than those without these characteristics.42,57
Ultrafast Spectroscopy Probes Heterogeneity and Phase Separation
In contrast to the model systems discussed above and many others used in fundamental biophysical experiments, biological membranes are composed of a diverse host of species, which vary widely in their charge, shape, polarity, and size. Studying lipid diversity is an active area of research as simulation tools become better equipped for modeling heterogeneous, complex membranes58,59 and as advances in lipidomics quantify the diversity of lipids in cell membranes.60–63 Some of the recent ultrafast work has focused on the effects of increasing levels of heterogeneity and molecular diversity on model membranes. For example, cholesterol has been linked to domain formation, and consequently, recent efforts have focused on understanding the effect of cholesterol on bilayer dynamics.51,64
Polar lipids themselves exhibit high molecular diversity, as both the headgroup and the tail composition can vary significantly. Lipid species are not necessarily miscible, leading to domain formation. Using intrinsic membrane probes, Volkov and co-workers observed phase separation between two lipid species, evidenced by the absence of strong cross peaks between the amide groups of a sphingolipid and the ester groups of a phosphocholine lipid.65 This work occurred early in the development of ultrafast vibrational spectroscopy of membranes, and it remains a promising proof of concept. Coupling between nearby transition dipoles is an extremely local interaction, allowing for the detection of phase separation on a nanometer scale. Since lineshapes also provide information about molecular environments, this approach could potentially add an atomistic view to phase separation without the addition of extrinsic probes.
Transmembrane Peptides Modulate H-Bond Structure and Dynamics
One of the most striking differences between model bilayers and biological membranes is that proteins occupy 30–50% of the membrane area,66 while common model bilayers are composed primarily of lipids. The effect of peptides and proteins on membrane interfacial dynamics and self-assembly is an emerging area of inquiry.58,66,67 The peptide backbone serves as a convenient intrinsic probe of structure and dynamics in addition to the intrinsic probes present in lipid membranes. Ultrafast vibrational spectroscopy is relatively established for proteins, and some of the earliest ultrafast spectroscopic investigations of lipid membranes used membrane peptides to probe the local environment,68,69 finding that the electrostatic environment is much more heterogeneous near the headgroup region than in the hydrophobic core.68 Using isotope-labeled peptide probes, we recently found that water formed H-bonds to the peptide even where the peptide was buried in the hydrophobic region of the membrane.3 This study, which used a transmembrane helix with a high percentage of hydrophilic residues, suggested that the presence and composition of transmembrane proteins influences the solvation environment within the bilayer core.3 Both results illustrate the importance of viewing the bilayer as a dynamic environment where structure and dynamics are influenced by nearby components. Studies have also been carried out to assess the effects of proteins on the environment experienced by the lipids. Stevenson and Tokmakoff uncovered a ~100% slowdown in ester carbonyls dynamics when gramicidin was added to a lipid membrane.18 Recently, we used 2D IR spectroscopy and MD simulations to study the effects of transmembrane crowding on the ultrafast dynamics of the membrane by sampling the lipid ester C=O dynamics at varied peptide:lipid ratios approaching those representative of biological membrane compositions.4 We found that, indeed, high peptide:lipid ratios drive a slowdown of up to 50% at the lipid–water interface; however, this effect is nonmonotonic with the peptide concentration (Figure 6). We have linked these dynamics to specific water structures at the interface, which is quite susceptible to the presence and locality of the membrane peptide.4
Figure 6.

Transmembrane crowding effects on ultrafast dynamics. (upper) Top-view of representative “pure lipid” and peptide-crowded membranes with a 1:10 peptide:lipid (P:L) ratio. (lower) Center line slope relaxation time constants (left) and computed frequency autocorrelations of the carbonyls, as a function of the peptide:lipid ratio. Reproduced from ref 4. Copyright 2020 American Chemical Society.
Bulk and Interfacial Water Dynamics Are Interdependent
Aqueous species also impact interfacial H-bond networks. One prominent example is cations, which often accumulate near lipid membranes due to the presence of anionic lipid species. Recently, a 2D IR study on the effects of Ca2+ at near-physiological concentrations showed that interfacial dynamics were slowed down by Ca2+ but only in membranes containing anionic lipids.1 Absorption spectra recorded with isotope-labeled anionic lipids indicated that the spectroscopic changes observed were largely localized to the anionic lipids. Similarly, water SFG spectra revealed that NaCl-dependent changes in H-bonding occurred with anionic lipids.70 This is consistent with previous results obtained using traditional biophysical methods, which showed that zwitterionic membranes are far less sensitive to cations than membranes containing anionic lipids.71,72
Commonly used denaturants and cosolvents can significantly modulate interfacial H-bond populations and dynamics in membranes. One noteworthy example is DMSO, a common cryoprotectant. In a contribution to this emerging field, we have characterized the interfacial H-bond properties through ester carbonyl modes. DMSO is preferentially excluded from the ~1 nm region around the headgroups, and therefore, an osmotic effect dehydrates the interface.73 Interestingly, interfacial H-bond dynamics exhibit nonmonotonic trends with an increasing DMSO concentration, with dynamics becoming increasingly faster up to 10 mol % and slowing down above this DMSO concentration. The two solvation regimes observed are best described as a combination of disrupted H-bond networks within the interface and lipid–lipid repulsion effects, which weaken the interfacial H-bond networks. This remains an emerging area of investigation; the effects of commonly used cosolvents such as glycerol, alcohols, and osmolytes should be the focus of future studies.
FUTURE PROSPECTS
Ultrafast experiments offer a unique window to the structure and dynamics at lipid–water interfaces. Where vibrational probes access different components of the membrane environment, the studies discussed here converge on several key conclusions: (1) Water dynamics near the interface are slow compared to those in bulk water; (2) Interfacial dynamics are sensitive to headgroup structure and membrane composition; (3) Ions, osmolytes, and transmembrane peptides all affect lipid interfacial dynamics; however the nature of the effect is difficult to predict as its origin depends on multiple factors. Ultrafast spectroscopy, interpreted through the lens of MD simulations, allows an atomistic interpretation of the membrane environment that is not accessible by either technique alone.1–4,73
Ongoing Experimental Challenges
There are key challenges in fully understanding the membrane environment by ultrafast methods. First, vibrational spectra are often congested, containing multiple overlapping peaks that are difficult to unambiguously interpret on a structural level. The samples themselves present several limitations, including scatter from turbid vesicle solutions, low oscillator strengths, and low concentration limits, all of which contribute to small signals. Recently, scatter suppression methods such as phase cycling have been a driving forces behind the recent uptick in ultrafast studies of lipid membranes and interfaces.74 Computational maps, which link local electrostatics from MD simulations to infrared frequency shifts, have been developed to deconvolve the molecular origins of observed lineshapes and dynamics.75 Recent improvements in simulation complexity, accuracy, and time scale have augmented this development, as simulations approaching biological accuracy have begun to emerge.
Second, the selection of appropriate model systems remains a challenge. Sample design is ultimately limited by the experiment type: 2D IR experiments typically use ~100 nm vesicles or multilayers in solution, and SFG samples must lack inversion symmetry. Considering these constraints, most model systems studied contain few lipid species and low concentrations of membrane proteins/peptides to make them accessible for analysis, and consequently, samples are not always biologically relevant in complexity; in fact, many studies outlined here use systems with low hydration levels. On a more practical note, often these experiments require long data collection times (up to 24 h), making sample stability an important consideration.
Third, ultrafast spectroscopy is limited to the picosecond time scale, whereas longer dynamics appear as a “static” component in the waiting-time 2D spectra. For example, in DMPC bilayers, only 49% of the relaxation occurs on the picosecond time scale; therefore, the static component is important for the interpretation of dynamics.4 For example, consider a perturbation that slows certain motions beyond their original picosecond relaxation, removing their contributions to the measured spectral relaxation, in which case other fast motions may contribute more significantly to the picosecond relaxation and spectra may exhibit “faster” dynamics. For this reason, MD simulations remain a key interpretation tool, especially since simulations capture dynamics on nanosecond time scales. Nonequilibrium 2D IR techniques incorporate additional laser pulses and can be used to measure processes too slow for traditional 2D IR.76,77
Ultrafast Spectroscopy and Multiscale Membrane Dynamics
Motions in cell membranes span decades in time. Protein and lipid conformational changes range from nanoseconds to milliseconds, while ion and water molecules fluctuations range from femtoseconds to tens of picoseconds. Many biophysical techniques, including microscopy and NMR spectroscopy, have been deployed to study the dynamics of conformational changes in lipid membranes.9,62,66 Picosecond dynamics, in contrast, have only recently become available to experimental biophysicists. Solvents fluctuate on a picosecond time scale, and one of the primary applications of ultrafast techniques has been solvents and solvent interfaces. Within this context, ultrafast infrared spectroscopy of lipid membranes can be viewed as an extension of ultrafast solvent spectroscopy into biomimetic interfaces. Ultrafast methods have revealed that the dynamics of this interface are composition-dependent. Every parameter that has been tested on a picosecond time scale, including hydration, ion concentration, lipid composition, and peptide concentration, has impacted the dynamics. Nonequilibrium 2D IR techniques incorporate additional laser pulses and can be used to measure processes too slow for traditional 2D IR.76
Ultrafast spectroscopy provides a link between ensemble properties and bond-centered information, critical for describing cell membranes. In this current era of membrane research, lipid composition is known to drive key processes such as ATP synthesis and ion channel gating through lipid–protein H-bonds and salt bridges.78,79 Ultrafast infrared spectroscopy provides a unique window into H-bond fluctuations and lifetimes, as well as ion-binding interactions. Ultrafast spectroscopy, which occupies a distinct set of fast time scales while also being applicable to long time scales through nonequilibrium techniques, is poised to play a significant role in describing molecular interactions in lipid membranes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Welch Foundation (F-1891), the National Science Foundation (BIO-1815354), and the National Institutes of Health (NIH) (R35GM133359).
Biographies
Jennifer C. Flanagan recently completed her Ph.D. in the Baiz group at the University of Texas at Austin. Her research focuses on the interplay between transmembrane peptides and lipid dynamics using ultrafast 2D IR spectroscopy.
Mason L. Valentine recently graduated with a Ph.D. from the Baiz group at the University of Texas at Austin. His research is focused on lipid–lipid and lipid–cation interactions in multicomponent membranes.
Carlos R. Baiz is an Assistant Professor at the University of Texas at Austin. His research group investigates ultrafast dynamics in complex environments using ultrafast infrared spectroscopy. Carlos earned his Ph.D. working in the group of Professor Kevin Kubarych at the University of Michigan. Prior to joining the University of Texas at Austin in 2015, Carlos was an NIH National Research Service Award Postdoctoral Fellow at Massachusetts Institute of Technology and the University of Chicago in the group of Professor Andrei Tokmakoff.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.accounts.0c00302.
Detailed descriptions of experimental methods used for measuring the 2D IR dynamics of DPPC/DOPC bilayers shown in Figure 5 (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.accounts.0c00302
The authors declare no competing financial interest.
Contributor Information
Jennifer C. Flanagan, Department of Chemistry, University of Texas at Austin, Austin, Texas 78712-1224, United States;.
Mason L. Valentine, Department of Chemistry, University of Texas at Austin, Austin, Texas 78712-1224, United States;.
Carlos R. Baiz, Department of Chemistry, University of Texas at Austin, Austin, Texas 78712-1224, United States;.
REFERENCES
- (1).Valentine ML; Cardenas AE; Elber R; Baiz CR Physiological Calcium Concentrations Slow Dynamics at the Lipid-Water Interface. Biophys. J 2018, 115, 1541–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]; This investigation used isotope-edited ultrafast two-dimensional infrared spectroscopy to probe the lipid–water interface of lipid bilayers with and without Ca2+ in solution. Results indicated a dependence of interfacial dynamics on the Ca2+ concentration in anionic lipid species.
- (2).Valentine ML; Cardenas AE; Elber R; Baiz CR Calcium-Lipid Interactions Observed with Isotope-Edited Infrared Spectroscopy. Biophys. J 2020, 118, 2694–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work used isotope-edited ultrafast two-dimensional infrared spectroscopy to investigate the head- group-specific effect of Ca2+ ions on the lipid–water interface, revealing water reorganization at the lipid–water interface of anionic lipids in the presence of Ca2+ that was not observed for zwitterionic lipid species.
- (3).Flanagan JC; Baiz CR Site-Specific Peptide Probes Detect Buried Water in a Lipid Membrane. Biophys. J 2019, 116, 1692–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]; Here, isotope-labeled transmembrane peptides were used to probe water penetration within the alkyl tail region of model lipid bilayers with ultrafast two-dimensional infrared spectroscopy. This study found increased hydration ~1 nm into the alkyl region, suggesting the membrane environment is perturbed by the presence of transmembrane peptides.
- (4).Flanagan JC; Cardenas AE; Baiz CR Ultrafast Spectroscopy of Lipid–Water Interfaces: Transmembrane Crowding Drives H-Bond Dynamics. J. Phys. Chem. Lett 2020, 11, 4093–4098. [DOI] [PubMed] [Google Scholar]; The effect of the transmembrane peptide content on dynamics at the lipid–water interface of model bilayers was probed by ultrafast two-dimensional infrared spectroscopy. Interfacial dynamics were found to depend nonmonotonically on peptide insertion and are hypothesized to be driven by observed changes in local water structure at the interface.
- (5).Singer SJ; Nicolson GL The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972, 175, 720–731. [DOI] [PubMed] [Google Scholar]
- (6).Simons K; Ikonen E Functional Rafts in Cell Membranes. Nature 1997, 387, 569–572. [DOI] [PubMed] [Google Scholar]
- (7).Kaiser H-J; Lingwood D; Levental I; Sampaio JL; Kalvodova L; Rajendran L; Simons K Order of Lipid Phases in Model and Plasma Membranes. Proc. Natl. Acad. Sci. U. S. A 2009, 106, 16645–16650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Engelman DM Membranes Are More Mosaic than Fluid. Nature 2005, 438, 578–580. [DOI] [PubMed] [Google Scholar]
- (9).Klymchenko AS; Kreder R Fluorescent Probes for Lipid Rafts: From Model Membranes to Living Cells. Chem. Biol 2014, 21, 97–113. [DOI] [PubMed] [Google Scholar]
- (10).Mouritsen OG Lipidology and Lipidomics–Quo Vadis? A New Era for the Physical Chemistry of Lipids. Phys. Chem. Chem. Phys 2011, 13, 19195. [DOI] [PubMed] [Google Scholar]
- (11).Tros M; Zheng L; Hunger J; Bonn M; Bonn D; Smits GJ; Woutersen S Picosecond Orientational Dynamics of Water in Living Cells. Nat. Commun 2017, 8, 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Muller EA; Pollard B; Raschke MB Infrared Chemical Nano-Imaging: Accessing Structure, Coupling, and Dynamics on Molecular Length Scales. J. Phys. Chem. Lett 2015, 6, 1275–1284. [DOI] [PubMed] [Google Scholar]
- (13).Lewis RNAH; Mcelhaney RN Membrane Lipid Phase Transitions and Phase Organization Studied by Fourier Transform Infrared Spectroscopy ☆. Biochim. Biophys. Acta, Biomembr 2013, 1828, 2347–2358. [DOI] [PubMed] [Google Scholar]
- (14).Czamara K; Majzner K; Pacia MZ; Kochan K; Kaczor A; Baranska M Raman Spectroscopy of Lipids: A Review. J. Raman Spectrosc 2015, 46, 4–20. [Google Scholar]
- (15).Schrader AM; Han S Location of the TEMPO Moiety of TEMPO-PC in Lipid Bilayers. Biophys. J 2017, 113, 966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Stone MB; Shelby SA; Veatch SL Super-Resolution Microscopy: Shedding Light on the Cellular Plasma Membrane. Chem. Rev 2017, 117, 7457–7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Torres J; Kukol A; Goodman JM; Arkin IT Site-Specific Examination of Secondary Structure and Orientation Determination in Membrane Proteins: The Peptidic13C?18O Group as a Novel Infrared Probe. Biopolymers 2001, 59, 396–401. [DOI] [PubMed] [Google Scholar]
- (18).Stevenson P; Tokmakoff A Ultrafast Fluctuations of High Amplitude Electric Fields in Lipid Membranes. J. Am. Chem. Soc 2017, 139, 4743–4752. [DOI] [PubMed] [Google Scholar]
- (19).Guo Q; Pagano P; Li Y-L; Kohen A; Cheatum CM Line Shape Analysis of Two-Dimensional Infrared Spectra. J. Chem. Phys 2015, 142, 212427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Reppert M; Tokmakoff A Computational Amide I 2D IR Spectroscopy as a Probe of Protein Structure and Dynamics. Annu. Rev. Phys. Chem 2016, 67, 359–386. [DOI] [PubMed] [Google Scholar]
- (21).Edington SC; Flanagan JC; Baiz CR An Empirical IR Frequency Map for Ester C-O Stretching Vibrations. J. Phys. Chem. A 2016, 120, 3888–3896. [DOI] [PubMed] [Google Scholar]
- (22).Shen YR The Principles of Nonlinear Optics; Wiley-Interscience, 2003. [Google Scholar]
- (23).Shen YR; Ostroverkhov V Sum-Frequency Vibrational Spectroscopy on Water Interfaces: Polar Orientation of Water Molecules at Interfaces. Chem. Rev 2006, 106, 1140–1154. [DOI] [PubMed] [Google Scholar]
- (24).Ghosh A; Smits M; Bredenbeck J; Bonn M Membrane-Bound Water Is Energetically Decoupled from Nearby Bulk Water: An Ultrafast Surface-Specific Investigation. J. Am. Chem. Soc 2007, 129 (31), 9608–9609. [DOI] [PubMed] [Google Scholar]
- (25).Bonn M; Bakker HJ; Tong Y; Backus EHG No Ice-Like Water at Aqueous Biological Interfaces. Biointerphases 2012, 7 (1), 20. [DOI] [PubMed] [Google Scholar]
- (26).McGuire JA; Shen YR Ultrafast Vibrational Dynamics at Water Interfaces. Science 2006, 313, 1945–1948. [DOI] [PubMed] [Google Scholar]
- (27).Lee E; Kundu A; Jeon J; Cho M Water Hydrogen-Bonding Structure and Dynamics near Lipid Multibilayer Surface: Molecular Dynamics Simulation Study with Direct Experimental Comparison. J. Chem. Phys 2019, 151, 114705. [DOI] [PubMed] [Google Scholar]
- (28).Inoue K; Nihonyanagi S; Tahara T Ultrafast Vibrational Dynamics at Aqueous Interfaces Studied by 2D Heterodyne-Detected Vibrational Sum Frequency Generation Spectroscopy; Springer: Singapore, 2019; pp 215–236. [Google Scholar]
- (29).Nihonyanagi S; Mondal JA; Yamaguchi S; Tahara T Structure and Dynamics of Interfacial Water Studied by Heterodyne-Detected Vibrational Sum-Frequency Generation. Annu. Rev. Phys. Chem 2013, 64, 579–603. [DOI] [PubMed] [Google Scholar]
- (30).Verreault D; Hua W; Allen HC From Conventional to Phase-Sensitive Vibrational Sum Frequency Generation Spectroscopy: Probing Water Organization at Aqueous Interfaces. J. Phys. Chem. Lett 2012, 3 (20), 3012–3028. [DOI] [PubMed] [Google Scholar]
- (31).Laaser JE; Xiong W; Zanni MT Time-Domain SFG Spectroscopy Using Mid-IR Pulse Shaping: Practical and Intrinsic Advantages. J. Phys. Chem. B 2011, 115, 2536–2546. [DOI] [PubMed] [Google Scholar]
- (32).Bredenbeck J; Ghosh A; Nienhuys HK; Bonn M Interface-Specific Ultrafast Two-Dimensional Vibrational Spectroscopy. Acc. Chem. Res 2009, 42 (9), 1332–1342. [DOI] [PubMed] [Google Scholar]
- (33).Bredenbeck J; Ghosh A; Smits M; Bonn M Ultrafast Two Dimensional-Infrared Spectroscopy of a Molecular Monolayer. J. Am. Chem. Soc 2008, 130, 2152–2153. [DOI] [PubMed] [Google Scholar]
- (34).Hsieh C-S; Okuno M; Hunger J; Backus EHG; Nagata Y; Bonn M Aqueous Heterogeneity at the Air/Water Interface Revealed by 2D-HD-SFG Spectroscopy. Angew. Chem., Int. Ed 2014, 53, 8146–8149. [DOI] [PubMed] [Google Scholar]
- (35).Smits M; Ghosh A; Sterrer M; Müller M; Bonn M Ultrafast Vibrational Energy Transfer between Surface and Bulk Water at the Air-Water Interface. Phys. Rev. Lett 2007, 98, 098302. [DOI] [PubMed] [Google Scholar]
- (36).Chen X; Hua W; Huang Z; Allen HC Interfacial Water Structure Associated with Phospholipid Membranes Studied by Phase-Sensitive Vibrational Sum Frequency Generation Spectroscopy. J. Am. Chem. Soc 2010, 132, 11336–11342. [DOI] [PubMed] [Google Scholar]
- (37).Sung W; Seok S; Kim D; Tian CS; Shen YR Sum-Frequency Spectroscopic Study of Langmuir Monolayers of Lipids Having Oppositely Charged Headgroups. Langmuir 2010, 26, 18266–18272. [DOI] [PubMed] [Google Scholar]
- (38).Nihonyanagi S; Yamaguchi S; Tahara T Ultrafast Dynamics at Water Interfaces Studied by Vibrational Sum Frequency Generation Spectroscopy. Chem. Rev 2017, 117, 10665–10693. [DOI] [PubMed] [Google Scholar]
- (39).Inoue K; Singh PC; Nihonyanagi S; Yamaguchi S; Tahara T Cooperative Hydrogen-Bond Dynamics at a Zwitterionic Lipid/Water Interface Revealed by 2D HD-VSFG Spectroscopy. J. Phys. Chem. Lett 2017, 8, 5160–5165. [DOI] [PubMed] [Google Scholar]
- (40).Singh PC; Inoue K; Nihonyanagi S; Yamaguchi S; Tahara T Femtosecond Hydrogen Bond Dynamics of Bulk-like and Bound Water at Positively and Negatively Charged Lipid Interfaces Revealed by 2D HD-VSFG Spectroscopy. Angew. Chem., Int. Ed 2016, 55, 10621–10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Deiseroth M; Bonn M; Backus EHG Orientation Independent Vibrational Dynamics of Lipid-Bound Interfacial Water. Phys. Chem. Chem. Phys 2020, 22 (18), 10142–10148. [DOI] [PubMed] [Google Scholar]
- (42).Inoue K; Ahmed M; Nihonyanagi S; Tahara T Effect of Hydrogen-Bond on Ultrafast Spectral Diffusion Dynamics of Water at Charged Monolayer Interfaces. J. Chem. Phys 2019, 150 (5), 054705. [DOI] [PubMed] [Google Scholar]
- (43).Marsh D Handbook of Lipid Bilayers, 2nd ed.; CRC Press: Boca Raton, FL, 2013. [Google Scholar]
- (44).Binder H; Zschörnig O The Effect of Metal Cations on the Phase Behavior and Hydration Characteristics of Phospholipid Membranes. Chem. Phys. Lipids 2002, 115, 39–61. [DOI] [PubMed] [Google Scholar]
- (45).Costard R; Greve C; Heisler IA; Elsaesser T Ultrafast Energy Redistribution in Local Hydration Shells of Phospholipids: A Two-Dimensional Infrared Study. J. Phys. Chem. Lett 2012, 3, 3646–3651. [DOI] [PubMed] [Google Scholar]
- (46).Costard R; Levinger NE; Nibbering ETJ; Elsaesser T Ultrafast Vibrational Dynamics of Water Confined in Phospholipid Reverse Micelles. J. Phys. Chem. B 2012, 116, 5752–5759. [DOI] [PubMed] [Google Scholar]
- (47).Volkov VV; Palmer DJ; Righini R Heterogeneity of Water at the Phospholipid Membrane Interface. J. Phys. Chem. B 2007, 111 (6), 1377–1383. [DOI] [PubMed] [Google Scholar]
- (48).Volkov VV; Takaoka Y; Righini R What Are the Sites Water Occupies at the Interface of a Phospholipid Membrane? † . J. Phys. Chem. B 2009, 113, 4119–4124. [DOI] [PubMed] [Google Scholar]
- (49).Kundu A; Verma PK; Ha J-H; Cho M Studying Water Hydrogen-Bonding Network near the Lipid Multibilayer with Multiple IR Probes. J. Phys. Chem. A 2017, 121, 1435–1441. [DOI] [PubMed] [Google Scholar]
- (50).Kundu A; Błasiak B; Lim J-H; Kwak K; Cho M Water Hydrogen-Bonding Network Structure and Dynamics at Phospholipid Multibilayer Surface: Femtosecond Mid-IR Pump–Probe Spectroscopy. J. Phys. Chem. Lett 2016, 7, 741–745. [DOI] [PubMed] [Google Scholar]
- (51).Kel O; Tamimi A; Fayer MD The Influence of Cholesterol on Fast Dynamics Inside of Vesicle and Planar Phospholipid Bilayers Measured with 2D IR Spectroscopy. J. Phys. Chem. B 2015, 119, 8852–8862. [DOI] [PubMed] [Google Scholar]
- (52).Varner C; Zhou X; Saxman ZK; Leger JD; Jayawickramarajah J; Rubtsov IV Azido Alkanes as Convenient Reporters for Mobility within Lipid Membranes. Chem. Phys 2018, 512, 20–26. [Google Scholar]
- (53).Ma J; Pazos IM; Zhang W; Culik RM; Gai F Site-Specific Infrared Probes of Proteins. Annu. Rev. Phys. Chem 2015, 66, 357–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Shrestha R; Cardenas AE; Elber R; Webb LJ Measurement of the Membrane Dipole Electric Field in DMPC Vesicles Using Vibrational Shifts of P-Cyanophenylalanine and Molecular Dynamics Simulations. J. Phys. Chem. B 2015, 119, 2869–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Osborne DG; Dunbar JA; Lapping JG; White AM; Kubarych KJ Site-Specific Measurements of Lipid Membrane Interfacial Water Dynamics with Multidimensional Infrared Spectroscopy. J. Phys. Chem. B 2013, 117 (49), 15407–15414. [DOI] [PubMed] [Google Scholar]
- (56).Ramos S; Horness RE; Collins JA; Haak D; Thielges MC Site-Specific 2D IR Spectroscopy: A General Approach for the Characterization of Protein Dynamics with High Spatial and Temporal Resolution. Phys. Chem. Chem. Phys 2019, 21, 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Inoue K; Singh PC; Nihonyanagi S; Yamaguchi S; Tahara T Cooperative Hydrogen-Bond Dynamics at a Zwitterionic Lipid/Water Interface Revealed by 2D HD-VSFG Spectroscopy. J. Phys. Chem. Lett 2017, 8, 5160–5165. [DOI] [PubMed] [Google Scholar]
- (58).Marrink SJ; Corradi V; Souza PCT; Ingólfsson HI; Tieleman DP; Sansom MSP Computational Modeling of Realistic Cell Membranes. Chem. Rev 2019, 119, 6184–6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Ingólfsson HI; Melo MN; van Eerden FJ; Arnarez C; Lopez CA; Wassenaar TA; Periole X; de Vries AH; Tieleman DP; Marrink SJ Lipid Organization of the Plasma Membrane. J. Am. Chem. Soc 2014, 136, 14554–14559. [DOI] [PubMed] [Google Scholar]
- (60).Cajka T; Fiehn O Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics. Anal. Chem 2016, 88, 524–545. [DOI] [PubMed] [Google Scholar]
- (61).Klose C; Surma MA; Simons K Organellar Lipidomics — Background and Perspectives. Curr. Opin. Cell Biol 2013, 25, 406–413. [DOI] [PubMed] [Google Scholar]
- (62).Wijesooriya CS; Nyamekye CKA; Smith EA Optical Imaging of the Nanoscale Structure and Dynamics of Biological Membranes. Anal. Chem 2019, 91, 425–440. [DOI] [PubMed] [Google Scholar]
- (63).Lee SC; Knowles TJ; Postis VLG; Jamshad M; Parslow RA; Lin Y; Goldman A; Sridhar P; Overduin M; Muench SP; Dafforn TR A Method for Detergent-Free Isolation of Membrane Proteins in Their Local Lipid Environment. Nat. Protoc 2016, 11, 1149–1162. [DOI] [PubMed] [Google Scholar]
- (64).Stevenson P; Tokmakoff A Infrared Insights into the Effect of Cholesterol on Lipid Membranes. Chem. Phys 2018, 512, 146–153. [Google Scholar]
- (65).Volkov VV; Chelli R; Righini R Domain Formation in Lipid Bilayers Probed by Two-Dimensional Infrared Spectroscopy. J. Phys. Chem. B 2006, 110 (4), 1499–1501. [DOI] [PubMed] [Google Scholar]
- (66).Guigas G; Weiss M Effects of Protein Crowding on Membrane Systems. Biochim. Biophys. Acta, Biomembr 2016, 1858, 2441–2450. [DOI] [PubMed] [Google Scholar]
- (67).Paterson DJ; Tassieri M; Reboud J; Wilson R; Cooper JM Lipid Topology and Electrostatic Interactions Underpin Lytic Activity of Linear Cationic Antimicrobial Peptides in Membranes. Proc. Natl. Acad. Sci. U. S. A 2017, 114, E8324–E8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Mukherjee P; Krummel AT; Fulmer EC; Kass I; Arkin IT; Zanni MT Site-Specific Vibrational Dynamics of the CD3ζ Membrane Peptide Using Heterodyned Two-Dimensional Infrared Photon Echo Spectroscopy. J. Chem. Phys 2004, 120, 10215–10224. [DOI] [PubMed] [Google Scholar]
- (69).Mukherjee P; Kass I; Arkin IT; Zanni MT Picosecond Dynamics of a Membrane Protein Revealed by 2D IR. Proc. Natl. Acad. Sci. U. S. A 2006, 103, 3528–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Doǧangün M; Ohno PE; Liang D; McGeachy AC; Bé AG; Dalchand N; Li T; Cui Q; Geiger FM Hydrogen-Bond Networks near Supported Lipid Bilayers from Vibrational Sum Frequency Generation Experiments and Atomistic Simulations. J. Phys. Chem. B 2018, 122, 4870–4879. [DOI] [PubMed] [Google Scholar]
- (71).Roux M; Bloom M Calcium, Magnesium, Lithium, Sodium, and Potassium Distributions in the Headgroup Region of Binary Membranes of Phosphatidylcholine and Phosphatidylserine as Seen by Deuterium NMR. Biochemistry 1990, 29, 7077–7089. [DOI] [PubMed] [Google Scholar]
- (72).Pabst G; Hodzic A;Štrancar J; Danner S; Rappolt M; Laggner P Rigidification of Neutral Lipid Bilayers in the Presence of Salts. Biophys. J 2007, 93, 2688–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Venkatraman RK; Baiz CR Ultrafast Dynamics at the Lipid-Water Interface: DMSO Modulates H-Bond Lifetimes. Langmuir 2020, 36, 6502–6511. [DOI] [PubMed] [Google Scholar]
- (74).Shim S-H; Zanni MT How to Turn Your Pump-Probe Instrument into a Multidimensional Spectrometer: 2D IR and Vis Spectroscopies via Pulse Shaping. Phys. Chem. Chem. Phys 2009, 11, 748–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Baiz CR; Błasiak B; Bredenbeck J; Cho M; Choi JH; Corcelli SA; Dijkstra AG; Feng CJ; Garrett-Roe S; Ge NH; Hanson-Heine MWD; Hirst JD; Jansen TLC; Kwac K; Kubarych KJ; Londergan CH; Maekawa H; Reppert M; Saito S; Roy S; Skinner JL; Stock G; Straub JE; Thielges MC; Tominaga K; Tokmakoff A; Torii H; Wang L; Webb LJ; Zanni MT Vibrational Spectroscopic Map, Vibrational Spectroscopy, and Intermolecular Interaction. Chem. Rev 2020, 120 (15), 7152–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Hogle DG; Cunningham AR; Tucker MJ Equilibrium versus Nonequilibrium Peptide Dynamics: Insights into Transient 2D IR Spectroscopy. J. Phys. Chem. B 2018, 122, 8783–8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Flanagan JC; Baiz CR Ultrafast PH-Jump Two-Dimensional Infrared Spectroscopy. Opt. Lett 2019, 44, 4937. [DOI] [PubMed] [Google Scholar]
- (78).Gantzel RH; Mogensen LS; Mikkelsen SA; Vilsen B; Molday RS; Vestergaard AL; Andersen JP Disease Mutations Reveal Residues Critical to the Interaction of P4-ATPases with Lipid Substrates. Sci. Rep 2017, 7 (1), 10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Poveda JA; Giudici AM; Renart ML; Millet O; Morales A; González-Ros JM; Oakes V; Furini S; Domene C Modulation of the Potassium Channel KcsA by Anionic Phospholipids: Role of Arginines at the Non-Annular Lipid Binding Sites. Biochim. Biophys. Acta, Biomembr 2019, 1861, 183029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


