Abstract
Quercetin is a yellow pigment that is found in many common dietary plants, and that protects against oxidative stress, inflammation, and arteriosclerosis. It has also been suggested to prolong the lifespan of, and enhance heat-stress tolerance in nematodes; thus, the present study investigated its effects on both the nematode life- and health span by assessing its capacity to promote nematode motility after aging and/or heat stress, as well as the mechanisms underlying these effects. The results of the conducted analyses showed that quercetin feeding prolonged lifespan, suppressed age-related motility retardation, improved motility recovery after heat stress, and decreased the production of both intercellular and mitochondrial reactive oxygen species in the analysed Caenorhabditis elegans strains, likely by modulating the insulin-like signalling (ILS) pathway and p38-mitogen-activated protein kinase (MAPK) pathway. In particular, the transcription factors DAF-16 and SKN-1 were found to mediate the observed quercetin-induced effects, consistent with their previously demonstrated roles as regulators of aging. Furthermore, we demonstrated, for the first time, that quercetin induced heat-stress tolerance in C. elegans by modulating HSF-1 expression and/or activity. Thus, the present study provides valuable insights into the mechanisms by which quercetin inhibit aging and enhance heat-stress tolerance via ILS and MAPK pathway in C. elegans.
Introduction
Recent studies have identified a range of biologically active substances in foods; for example, polyphenols such as resveratrol and anthocyanin have been detected in the grape pericarp, and shown to exert strong anti-oxidative effects, and to reduce the risk of arteriosclerosis and stroke [1–3]. Quercetin used in this study is contained in many different of dietary plants and classified as a flavonoid, one kind of polyphenol. So far, various flavonoids such as catechin and anthocyanin have been demonstrated to incur physiological effects in many kinds of model organism [4,5]. The yellow flavonoid quercetin and its glycoside have been reported to inhibit oxidative stress, inflammation, and cerebrovascular disease, tumour, and cancer development [6–12]. To date, the effects of quercetin on lifespan and protection against stress have been researched in some model organisms [13–17]. The physiological effects of quercetin should be different depending on the kinds of model organisms and concentrations [13]. The effects on health span have not reported; thus, the present study aimed to analyse the physiological effects of quercetin on ‘health span’ in C. elegans. Health span is the period for which an individual remains healthy and independent; for example, the period for which no extensive medical care or nursing is required in humans, whereas we defined health span as the period maintaining the normal locomotion in C. elegans.
C. elegans is a nematode with a transparent body that makes thrashing movements. It can be easily cultured on an agar medium at room temperature (20°C), and is widely used as a model organism in a wide range of biological studies due to the fact that it shares orthologous genes and various basic organs (e.g. digestive and nervous systems) with higher order animals. In addition, C. elegans has a short lifespan that allows many assays to be conducted in a limited period of time. Many studies have already been conducted to evaluate the effect of functional dietary components on aging and stress tolerance in C. elegans [18–20]; thus, it is a very suitable model to use to conduct preliminary assessments of physiological active compounds, and thereby provide valuable and relevant data to support subsequent studies in higher order animals.
Previous studies have suggested that quercetin may prolong the lifespan and increase stress tolerance in C. elegans [4,16,17,20]; however, its effects on motility are poorly understood in general, and have not yet been assessed in aged or stressed nematodes [21]. Therefore, the present study aimed to elucidate the physiological effects of, and mechanisms by which quercetin impacts motility in aged and heat-stressed C. elegans nematodes. Furthermore, while previous studies have suggested that heat stress tolerance can be induced in nematodes by modulating ILS and p38-mitogen-activated protein kinase (MAPK) pathway [4,15–17], the present study aimed to further clarify the signalling pathways by which quercetin modulates C. elegans aging and heat-stress tolerance.
Materials and methods
Nematodes and quercetin
The C. elegans N2 Bristol (wild type), daf-2(e1370), age-1 (hx546), daf-16 (mgDf50), sek-1 (ag1), hsf-1 (sy441) (Caenorhabditis Genetics Center (CGC), University of Minnesota, Minneapolis, MN, USA), nsy-1 (tm850), pmk-1 (tm676), and skn-1 (tm4241) (National BioResource Project (NBRP), Tokyo Women’s Medical University, Japan) nematode strains were cultured (20°C) on nematode growth medium (NGM) plates that were spread with Escherichia coli (OP50) (Table 1) [22,23]. Quercetin (Cayman chemical company, Michigan, USA) was dissolved in dimethyl sulfoxide (DMSO; Kanto Chemical Co., Inc., Tokyo, Japan) to a concentration of 0.01 M, before being diluted to 50- and 500 μM in OP50, and spread onto NGM plates for each quercetin-feeding assay. We defined the plates spread onto NGM plates with OP50 as OP plate and with quercetin, containing OP50 as Q plate. 200 μl of OP50 and quercetin were spread onto NGM plates and dried.
Table 1. Genes shown in this study.
| Genes | Properties |
|---|---|
| daf-2 | Encodes receptor for insulin-like ligands. Gene deficient mutant shows extended lifespan. |
| age-1 | Encodes an ILS pathway protein homologous to PI3K. Gene deficient mutant shows extended lifespan. |
| daf-16 | Encodes a FOXO-homologue transcription factor that is regulated by ILS, and that mediates aging and stress tolerance. |
| nsy-1 | Encodes a p38-mitogen-activated protein kinase kinase kinase (MAPKKK) protein that acts in the MAPK cascade. |
| sek-1 | Encodes a MAPKK protein that acts in the MAPK cascade. |
| pmk-1 | Encodes a MAPK protein that acts in the MAPK cascade. |
| skn-1 | Encodes a transcription factor homologous to Nrf2 that is regulated by MAPK, and that mediates aging and stress tolerance. |
| hsf-1 | Encodes a transcription factor that regulates heat-stress tolerance. |
| sod-1 | SKN-1 target gene that encodes the reactive oxygen species (ROS) scavenger, SOD-1. |
| sod-2 | SKN-1 target gene that encodes the ROS scavenger SOD-2. |
| sod-3 | DAF-16 target gene that encodes the ROS scavenger SOD-3. |
| ctl-1 | DAF-16 target gene that encodes CTL-1 that metabolizes H2O2 to H2O. |
| ctl-2 | DAF-16 target gene that encodes CTL-2 that metabolizes H2O2 to H2O. |
| hsp-12.6 | Gene regulated by both DAF-16 and HSF-1 that encodes a heat shock protein (HSP). |
| hsp-16.1 | Gene regulated by both DAF-16 and HSF-1 that encodes an HSP. |
| hsp-16.2 | HSF-1 target gene that encodes an HSP. |
| hsp-70 | HSF-1 target gene that encodes an HSP. |
| sip-1 | Gene that is regulated by both DAF-16 and HSF-1, and that mediates heat-stress tolerance. |
| mtl-1 | DAF-16 target gene that mediates heat-stress tolerance. |
Nematode synchronization
To collect synchronized eggs, adult nematodes were crushed in NaClO solution comprising (1:10) 10 N NaOH (Wako Pure Chemical Industries, Ltd., Osaka, Japan) in NaClO (Haiter, KAO, Tokyo, Japan).
Evaluation of lifespan
Synchronized worms were cultured on OP plates at 20°C for 96 h, and then transferred to OP or Q plates (50 μM and 500 μM, day 0) for further culture (20°C). Offspring generation was prevented via the addition of 0.5 mg/ml of 2'-deoxy-5-fluorouridine (FUdR; Wako Pure Chemical Industries, Ltd.) to the plates on days -1, 0, and 2. The worms were transferred to new plates every 2 days, and the survival rate of the wild type worms were counted on each transfer day, where the survival rate at day 0 was set as 100%. Worms that displayed no movement upon gentle probing with a platinum picker were judged as dead. The survival rate of CT (dDW), containing double distilled water (dDW) as solvent instead of DMSO was also counted. Assay was conducted at least three times independently.
Evaluation of motility in ageing nematodes
Synchronized worms were cultured on OP plates at 20°C for 96 h, and then transferred to OP or Q plates (50 μM and 500 μM, day 0) for further culture (20°C). Offspring generation was prevented via the addition of 0.5 mg/ml of 2'-deoxy-5-fluorouridine (FUdR; Wako Pure Chemical Industries, Ltd.) to the plates on days -1, 0, and 3. The worms were transferred to new plates every 3 days, and the thrashing movements in 15 seconds of the wild type, daf-2(e1370), age-1(hx546), daf-16(mgDf50), nsy-1(tm850), sek-1(ag1), pmk-1(tm676), skn-1(tm4241), and hsf-1(sy441) worms were counted on each transfer day in S-basal medium, where the movement at day 0 was set as 100%. The thrashing movements of CT (dDW), containing dDW as solvent instead of DMSO was also counted. The movement of the daf-2(e1370) and age-1(hx546) worms was counted until day 12 due to their extended lifespan [22]. All assays were conducted at least three times independently.
Evaluation of nematode motility recovery after heat stress
Generally, heat stress decreases movement of worms. To measure the motility-recovery rate after heat stress, synchronized wild type, daf-2(e1370), age-1(hx546), daf-16(mgDf50), nsy-1(tm850), sek-1(ag1), pmk-1(tm676), skn-1(tm4241), and hsf-1(sy441) worms were cultured on OP or Q plates (50 μM and 500 μM) at 20°C for 96 h. In order to prevent the growth of E. coli from affecting worms, worms were transferred to NGM plates without spread of OP50, and then incubated at 35°C for 4 h. Heat-stress initiation was designated as 0 h, and the thrashing movements in 15 seconds of both heat-stressed and control (maintained at 20°C) worms were counted every 6 or 12 h in S-basal medium. The ratio of the movement counts generated for the heat-stressed and control worms (n = 10 worms/group) was calculated [24]. The motility-recovery rate of CT (dDW), containing dDW as solvent instead of DMSO was also counted in wild type. The movement of the daf-16(mgDf50) and hsf-1(sy441) worms did not recover sufficiently at 12 h; therefore, they were evaluated for 24 h. All assays were conducted at least three times independently.
Evaluation of mitochondrial ROS
Synchronized worms were cultured on OP or Q plates (50 μM and 500 μM) for 72 h, before 400 μL of 0.5 mg/mL MitoTracker® Orange CM-H2TMRos (Thermo Fisher Scientific, Inc., Waltham, MA, USA) was added to the plates. After 24 h, the worms were washed, and fixed in 10% ethanol (Kanto Chemical Co.). Fluorescence (n > 30 worms/group) was measured using a BZ8000 microscope, and analysed using ImageJ software (NIH). The fluorescence level exhibited by control worms was set as 100%. Assays were conducted at least three times independently.
Evaluation of cellular ROS
Synchronized worms were cultured on OP or Q plates (50 μM and 500 μM) for 96 h, before DCF-DA (Wako) was diluted in dimethyl sulfoxide (Kanto Chemical Co.) to produce a 50 μM DCF-DA solution that was added (400 μL) to each plate. After 1 h, worms were washed, and fixed in 10% ethanol. Again, fluorescence (n > 35 worms/group) was measured using a BZ8000 microscope, and analysed using ImageJ software. The fluorescence level exhibited by control worms was set as 100%. Assays were conducted at least three times independently.
Gene expression
Synchronized worms were cultured on OP or Q plates (50 μM and 500 μM) for 96 h, before mRNA for genes of interest (Table 1) [25–30] was extracted via crushing using the Power Masher® and Bio Masher® (Nippi Inc., Tokyo, Japan), and reverse-transcribed to cDNA using the PrimeScript™ RT Reagent Kit and the gDNA Eraser (Takara, Shiga, Japan). A real-time quantitative PCR (qPCR) was conducted using the Thermal Cycler Dice® Real Time System Lite (Takara) with Thunderbird® SYBR® qPCR Mix (TOYOBO, Osaka, Japan), and appropriate primers (Table 2). Actin was selected as a reference gene and confirmed the suitability by comparing with tba-1 and pmp-3, used as alternative reference genes [31]. Each qPCR reaction was performed in triplicate. Assays were conducted at least three times independently.
Table 2. Primer sequences used in the conducted gene-expression analysis.
| Gene | Sense primer (5’–3’) | Antisense primer (5’–3’) |
|---|---|---|
| actin* | TCGGTATGGGACAGAAGGAC | CATCCCAGTTGGTGACGATA |
| daf-16 | ATCATCTTTCCGTCCCCG | TTGGAATTGCTGGAACCG |
| skn-1 | AGTGTCGGCGTTCCAGATTTC | GTCGACGAATCTTGCGAATCA |
| hsf-1 | ATGTACGGCTTCCGAAAGATGA | TCTTGCCGATTGCTTTCTCTTAA |
| sod-1 | CGTAGGCGATCTAGGAAATGTG | TGACGAGCGTGTCGGTGAG |
| sod-2 | GATACTGTCCAAAGGGAAAGAT | GTAGTAAGCGTGCTCCCAGA |
| sod-3 | ATCTACTGCTCGCACTGCTT | TTTCATGGCTGATTACAGGTT |
| ctl-1 | CGATACCGTACTCGTGATGAT | CCAAACAGCCACCCAAATCA |
| ctl-2 | CTGGGAGAAGGTGTTGGAT | GGATGAACCTTTGAAAAGTGAT |
| hsp-12.6 | TGGAGTTGTCAATGTCCTCG | GACTTCAATCTCTTTTGGGAGG |
| hsp-16.1 | CCACTATTTCCGTCCAGCTCA | GGCGCTTGCTGAATTGGAAT |
| hsp-16.2 | TGTTGGTGCAGTTGCTTCGAATC | TTCTCTTCGACGATTGCCTGTTG |
| hsp-70 | ACCCTTCGTTGGATGGAACG | GCATCCGGAACCTGATTGGGC |
| sip-1 | CGAGCACGGGTTCAGCAAGAG | CAGCGTGTCCAGCAGAAGTGTG |
| mtl-1 | TGTGAGGAGGCCAGTGAGA | TTAATGAGCCGCAGCAGTT |
*Reference gene.
Statistical analysis
Data were presented as the mean ± SEM, and statistical analysis was performed using one way ANOVA followed by Tukey’s HSD post hoc test. The survival rate was analysed using the log-rank test. Graphs were generated using Microsoft Excel and Microsoft PowerPoint software (Microsoft Corp., Redmond, WA, USA). A P value < 0.05 was considered to indicate statistical significance.
Results
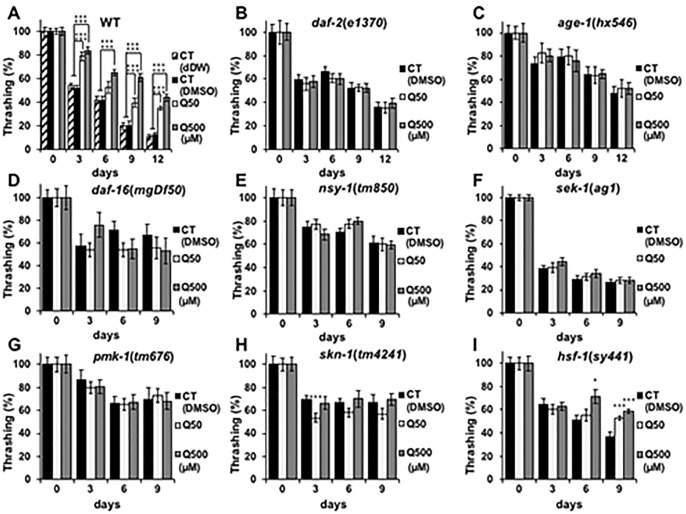
Previous studies have reported that quercetin can prolong the nematode lifespan [4,15–17,20]. At first, we investigated whether DMSO used as solvent has toxicity to the lifespan of nematode and whether 50- and 500μM quercetin used in present study can induce longevity effect to nematode, thereby we designed two controls. CT (dDW) contains the same amount of double distilled water (dDW) with DMSO contained in CT (DMSO), 50- and 500 μM quercetin samples. The survival rate of CT (dDW) and CT(DMSO) didn’t change significantly and 50- or 500 μM quercetin was increased in wild type (Fig 1). These results indicate the concentration of DMSO used in this study don’t affect the longevity of nematode, and 50- and 500 μM quercetin can prolong the lifespan similarly to the previous study [4,15–17,20]. Present study focused on analysis of its effects on the nematode health span, rather than longevity. Specifically, we analysed the effects of quercetin on nematode motility through ageing process by feeding nematodes 50- or 500 μM quercetin and evaluating their movement every 3 days. Motility deterioration was suppressed on days 3,9 and 12 in wild-type worms that were fed 50 μM quercetin, and on days 3, 6, 9 and 12 in wild-type worms that were fed 500 μM quercetin (Fig 2A). In order to find differences with other flavonoids, we used epigallocatechin gallate (EGCG), a species of catechin [5]. EGCG showed similar effect with 500 μM of quercetin (S1 Fig). Suppression of motility deterioration was observed in the hsf-1(sy441) worms similarly (Fig 2I); however, in contrast, quercetin feeding did not suppress motility deterioration in the daf-2(e1370), age-1(hx546), daf-16(mgDf50), nsy-1(tm850), sek-1(ag1), pmk-1(tm676), nor the skn-1(tm4241) nematodes (Fig 2B–2H), suggesting that it may promote motility by modulating daf-2, age-1, daf-16, nsy-1, sek-1, pmk-1 and skn-1, but not hsf-1 expression and/or activity. Similar to lifespan analysis, the motility rate of CT (dDW) and CT(DMSO) didn’t change significantly (Fig 2A).
Fig 1. Quercetin induced life span extension.
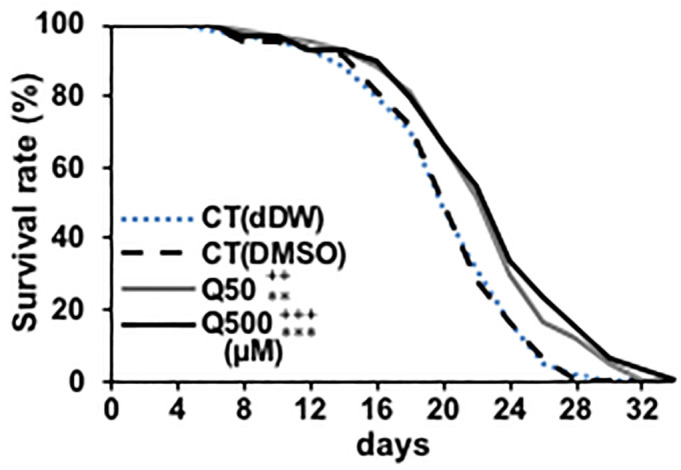
Survival rate analysis was generated for N2 wild-type Caenorhabditis elegans nematodes every 2 days and presented relative to that calculated on day 0. n = 60/group. ++P < 0.01, +++P < 0.005 vs CT (dDW) and **P < 0.01, ***P < 0.005 vs CT (DMSO) according to the conducted log-rank test. All assays were conducted at least three times independently.
Fig 2. Quercetin suppressed age-related retardation.
Movement counts were generated for (A) N2, (B) e1370, (C) hx546, (D) mgDf50, (E) tm850, (F) ag1, (G) tm676, (H) tm4241, and (I) sy441 Caenorhabditis elegans nematodes every 3 days from day 0 until (A, B, E–I) day 9 or (C, D) day 12, and presented relative to that calculated on day 0. Data are presented as the mean ± SEM, n = 10/group. +++P < 0.005 vs CT (dDW) and *P < 0.05, ***P < 0.005 vs CT (DMSO) according to the conducted Tukey’s HSD. All assays were conducted at least three times independently.
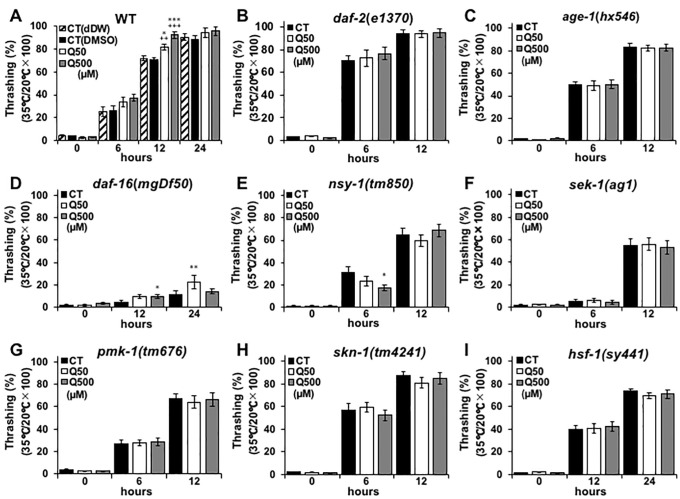
Upregulated stress tolerance has been shown to be closely related to longevity and anti-aging effects in nematodes [19], and previous studies have revealed that quercetin improves nematode heat-stress tolerance [16,20]. Thus, the present study analysed the motility of nematodes after heat-stress induction, with and without quercetin feeding. The motility-recovery rate of the N2 wild-type worms that were fed with 50- and 500 μM quercetin was resultantly shown to be non-significantly and significantly increased compared to controls, respectively (Fig 3A). The thrashing recovery at hours 24 was didn’t change significantly, due to the full recovery from heat damages (Fig 3A). The recovery of motility was shown in the worms fed EGCG similarly to the worms fed 500 μM of quercetin (S2 Fig). In contrast, the recovery rate of the daf-2(e1370), age-1(hx546), nsy-1(tm850), sek-1(ag1), pmk-1(tm676), skn-1(tm4241) and hsf-1(sy441) nematodes after heat-stress induction was unchanged by quercetin feeding (Fig 3B, 3C and 3E–3I). This suggests that quercetin may promote motility-recovery by modulating daf-2, age-1, daf-16, nsy-1, sek-1, pmk-1, skn-1 and hsf-1 expression and/or activity. Notably, the motility of the daf-16(mgDf50) worms that were fed with quercetin after heat stress was partially recovered (Fig 3D), suggesting that motility-recovery after heat stress may occur via incurred effects on daf-16 activity.
Fig 3. Quercetin increased heat stress tolerance.
Movement counts were generated to assess the motility-recovery rate of (A) N2, (B) e1370, (C) hx546, (D) mgDf50, (E) tm850, (F) ag1, (G) tm676, (H) tm4241, and (I) sy441 Caenorhabditis elegans nematodes at (A–C, E–H) 6 h and (D, I) 12 h intervals after heat stress. Data are presented as the mean ± SEM, n = 10/group. +P < 0.01, +++P < 0.005 vs CT (dDW) and *P < 0.05, **P < 0.01 vs CT (DMSO) according to the conducted Tukey’s HSD. All assays were conducted at least three times independently.
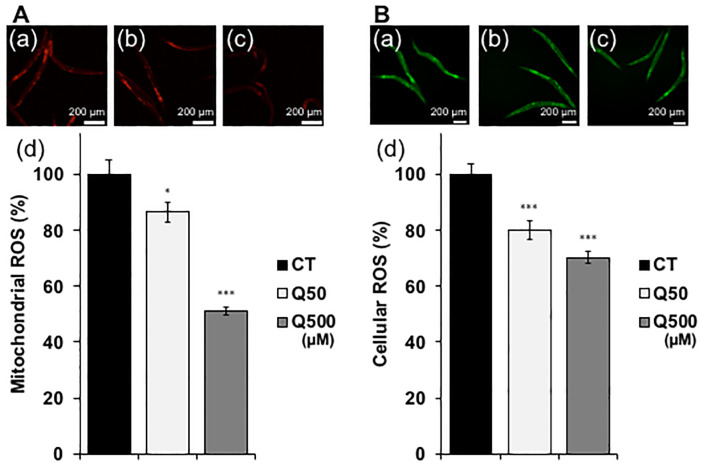
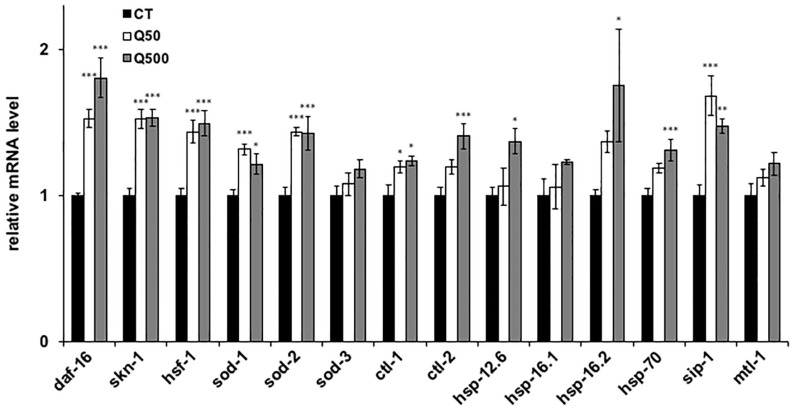
Heat stress has been shown to cause nematodes to produce reactive oxygen species (ROS), which are associated with a range of biological effects, including cell damage, and disease [32]. The mitochondria are a major source of ROS, particularly during ATP generation. Since quercetin improved the motility of the ageing nematodes, we expected that the mitochondria activated during this process would produce increased ROS levels (Fig 2A). Surprisingly, however, mitochondrial and cellular ROS levels were significantly decreased after quercetin feeding in the analysed nematodes (Fig 4A and 4B). In order to clarify the mechanism and to identify genes involved in the observed quercetin-induced physiological effects, qPCR analysis was conducted. We selected actin as a reference gene in this study and it could be appropriate (S3 Fig), because the differences of quantities of actin and other alternative reference genes, tba-1 and pmp-3, in each condition are not significant [31]. We chose some genes mainly related to three transcriptions factors, DAF-16, SKN-1 and HSF-1. Genes chosen in present study were specifically related to stress tolerance (Table 1). Feeding with either 50- or 500 μM quercetin was resultantly shown to significantly upregulate daf-16, skn-1, hsf-1, sod-1, sod-2, ctl-1, and sip-1 mRNA levels compared to controls. Moreover, feeding with 500 μM quercetin significantly increased ctl-2, hsp-12.6, hsp-16.2, and hsp-70 expression compared to controls (Fig 5).
Fig 4. Quercetin decreased mitochondrial- and intercellular ROS.
(A) Mitochondrial and (B) cellular ROS levels in (a) control (CT), and Caenorhabditis elegans nematodes treated with (b) Q50 mM, and (c) Q500 mM quercetin. (d) Analysis of data presented in (a–c). Scale bars, 200 μm. Data are presented as the mean ± SEM. *P < 0.05, ***P < 0.005 according to the conducted Tukey’s HSD. All assays were conducted at least three times independently.
Fig 5. Quercetin changed expression of genes.
The mRNA expression levels of the analysed genes are shown relative to that of the selected reference gene (actin). Data are presented as the mean ± SEM. *P < 0.05, ***P < 0.005 according to the conducted Tukey’s HSD. Assays were conducted at least three times independently.
Discussion
Recent studies have discussed the concepts of a ‘health span’ and ‘lifespan’ to differentiate between the period for which an individual remains healthy, versus the period between their birth and death. The C. elegans health span is represented by the time until worms begin to exhibit decreased motility in aged worms; therefore, the present study mainly examined the effects of quercetin on motility. No studies have previously evaluated quercetin in this context, and no previous reports have investigated the protective effects of quercetin on the motility of aged and stressed nematodes. Since severe heat stress disrupts nematode proteostasis (by causing aberrant protein folding and aggregation) in a manner similar to aging, the motility of heat-stressed worms is considered to be equivalent to that of aged worms, and to be representative of the nematode health span [33,34]. Thus, the present study investigated the physiological effects of quercetin on the motility of both aged and heat-stressed C. elegans nematodes.
In order to search similarity and differences between quercetin and other flavonoids, we choose epigallocatechin gallate (EGCG). EGCG didn’t prolong the life span but enhance the heat stress tolerance of C. elegans [5]. In this study EGCG increased the motility of heat stressed- and aged nematodes similarly to quercetin (S1 and S2 Figs). These results suggested there are some similarity and differences of their physiological actions among flavonoids.
Consistent with previous studies, many genes were herein shown to adjust the nematode lifespan, including daf-2, age-1, daf-16, and skn-1 [25,35,36]. Previous studies have also revealed that quercetin promotes longevity in nematodes by modulating the expression of daf-2, age-1, unc-43, and sek-1, but not daf-16 nor skn-1 [16]. Notably, DAF-16 and SKN-1 are transcription factors that are controlled by the ILS and MAPK pathways, respectively [37,38], which themselves regulate the transcription of several genes related to stress tolerance [39]. In fact, DAF-16 is a FOXO homologue that has been shown to mediate both longevity and stress tolerance [36]. Similarly, SKN-1 is a homologue of Nrf2 that has been shown to contribute to phase-2 detoxification, and prolong lifespan [38]. The present study used daf-2, age-1, daf-16, nsy-1, sek-1, pmk-1, skn-1, and hsf-1-deficient mutants to assess whether the effects of quercetin on motility occur via the ILS and/or MAPK pathways. The transcription factor HSF-1 was also analysed, because it is known to mediate heat-stress tolerance, to interact with the ILS pathway, and to act with DAF-16 to drive the transcription of several heat-shock proteins [26,37,40]. Thus, the study analysed the interactions between quercetin, the ILS pathway, and HSF-1.
We analysed whether the concentration of DMSO used in this study affected to the lifespan, health span and heat stress tolerance of nematodes, since previous study showed DMSO shortened the lifespan of C. elegans [41]. Consequently, however, DMSO didn’t have no influence on their lifespan, health span and heat stress tolerance (Figs 1, 2A and 3A). That’s probably because in previous study the amount of DMSO was possibly much higher than that used in this study, since the authors dissolved DMSO in agar [41].
Quercetin feeding was found to prolong the lifespan of C. elegans (Fig 1), and suppress age-related motility retardation in the wild-type compared to the control nematodes (Fig 2A); however, this effect was not observed in the daf-2(e1370), age-1(hx546), daf-16(mgDf50), nsy-1(tm850), sek-1(ag1), pmk-1(tm676) nor the skn-1(tm4241) worms (Fig 2B–2H). Present studies have suggested that quercetin promotes motility in ageing nematodes via incurred effects on the ILS and MAPK pathway. Interestingly, previous studies have reported that neither daf-16 nor skn-1 mediate the quercetin-induced enhancement of nematode longevity [16]; however, in contrast, the results of the present suggest that quercetin increased the expression of both of these genes to enhance motility in aged worms, and thus increase their health span. Given that the phenotype of the aged hsf-1(sy441) worms was similar to that of the aged wild-type worms after quercetin feeding, it is unlikely that HSF-1 contributed to this phenotype (Fig 2I).
The improved recovery of the quercetin-fed nematodes from heat stress was suggested to occur via the upregulation of daf-2, age-1, nsy-1, sek-1, pmk-1, skn-1, hsf-1, and to a lesser extent, daf-16 (Fig 3B–3I). Notably, the mRNA expression of mtl-1 was not increased, despite the fact that this gene was previously shown to be associated with heat-stress tolerance, and to be a target of DAF-16 (Fig 5) [26]. In contrast, quercetin feeding induced increased transcription of the HSF-1 targets hsp-16.2 and hsp-70 (Fig 5) [25–27]. Together, these findings indicate that the effect of quercetin on heat-stress recovery was mediated predominantly and partially via the activation of HSF-1 and DAF-16, respectively, consistent with previous studies that showed that coactivating DAF-16 and HSF-1 significantly upregulates hsp-12.6 and sip-1 (but not hsp-16.1) mRNA expression (Fig 4) [26].
The present study further suggests that quercetin enhanced nematode motility after aging and heat stress via SKN-1 and the MAPK pathway. Interestingly, both have been shown to control the transcription of antioxidant enzymes, and thereby promote ROS scavenging [28]; thus, the observed improved nematode motility may have occurred because quercetin feeding induced the scavenging of ROS generated by senescence and heat stress. This hypothesis is supported by the fact that quercetin feeding upregulated transcription of the known ROS-scavenging SKN-1 target genes sod-1 and sod-2 (Fig 5). SOD-1 and SOD-2 have been shown to scavenge ROS in both the cytoplasm and mitochondria during nematode development [29], consistent with the fact that quercetin feeding herein decreased both cytoplasmic and mitochondrial ROS levels (Fig 4). We suggest that quercetin herein activated SOD-1 and SOD-2, and thereby caused ROS to be metabolized to H2O2 by SOD, and subsequently to H2O by catalase [40]. Furthermore, ctl-1 and ctl-2 are known to be regulated by DAF-16 [42]; thus, the herein observed upregulation of these genes suggests that DAF-16 indirectly contributed to ROS metabolism (Fig 5). Thus, we suggest that quercetin feeding induces ROS scavenging to both inhibit aging, and prolong the nematode health span.
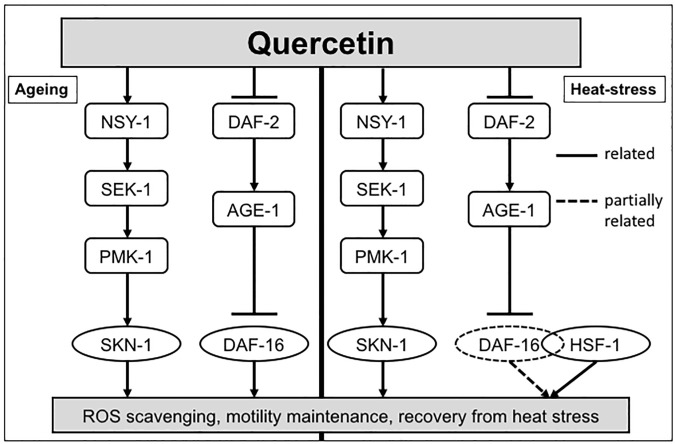
The ILS pathway has been previously shown to regulate both DAF-16 and SKN-1 [35], consistent with the findings of the present study, which suggest that quercetin enhanced nematode motility after aging and heat stress by coactivating DAF-16 and SKN-1. Importantly, the present study revealed a novel quercetin-induced interaction between DAF-16 and HSF-1 (Fig 6); thus, it provides novel insights into the complex combination of signalling changes by which quercetin induces anti-aging effects [15]. Since these transcription factors are conserved in higher order animals, quercetin is a promising potential therapeutic target to improve and prolong the human health span.
Fig 6. Effects of quercetin on signalling pathways in Caenorhabditis elegans.
Signalling pathways shown to be modulated by quercetin in this study. Solid lines indicate relatedness, hashed lines indicate partial relatedness.
Supporting information
(DOCX)
Movement counts were generated for N2 Caenorhabditis elegans nematodes every 3 days from day 0 until day 12, and presented relative to that calculated on day 0. Data are presented as the mean ± SEM, n = 10/group. *P < 0.05, ***P < 0.005 vs CT according to the conducted Tukey’s HSD. All assays were conducted at least three times independently.
(TIFF)
Movement counts were generated to assess the motility-recovery rate of N2 Caenorhabditis elegans nematodes at 6 h intervals after heat stress. Data are presented as the mean ± SEM, n = 10/group. *P < 0.05, ***P < 0.005 vs CT (DMSO) according to the conducted Tukey’s HSD. All assays were conducted at least three times independently.
(TIFF)
The mRNA expression levels of the analysed genes are shown relative to that of the quantity of actin in CT. Data are presented as the mean ± SEM. The quantities of genes in each condition are not significantly. Assays were conducted at least three times independently.
(TIFF)
Acknowledgments
We thank CGC and NBRP for providing us nematodes. We appreciate to Suntory Global Innovation Center Limited for their help.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
We clarify that this work was supported in part by Suntory Global Innovation Center Limited and Grants-in-Aid for Scientific Research and Education from the University of Tsukuba, Japan. We declare as follows; “The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.” There was no additional external funding received for this study.
References
- 1.Riccioni G. et al. Resveratrol and anti-atherogenic effects. Int. J. Food Sci. Nutr. 66, 603–610 (2015). 10.3109/09637486.2015.1077796 [DOI] [PubMed] [Google Scholar]
- 2.Lopez M. S., Dempsey R. J. & Vemuganti R. Resveratrol neuroprotection in stroke and traumatic CNS injury. Neurochem. Int. 89, 75–82 (2015). 10.1016/j.neuint.2015.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitterman J. L. & Chung J. H. Metabolic effects of resveratrol: addressing the controversies. Cell Mol. Life Sci. 72, 1473–1488 (2015). 10.1007/s00018-014-1808-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pallauf K., Duckstein N. & Rimbach G. A literature review of flavonoids and lifespan in model organisms. Proc. Nutr. Soc. 76, 145–162 (2017). 10.1017/S0029665116000720 [DOI] [PubMed] [Google Scholar]
- 5.Zhang L. et al. Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Radical Biology and Medicine, 46(3), 414–421 (2009). 10.1016/j.freeradbiomed.2008.10.041 [DOI] [PubMed] [Google Scholar]
- 6.Men K. et al. Nanoparticle-delivered quercetin for cancer therapy. Anticancer Agents Med. Chem. 14, 826–832 (2014). 10.2174/1871520614666140521122932 [DOI] [PubMed] [Google Scholar]
- 7.Duenas M. et al. Deglycosylation is a key step in biotransformation and lifespan effects of quercetin-3-O-glucoside in Caenorhabditis elegans. Pharmacol. Res. 76, 41–48 (2013). 10.1016/j.phrs.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 8.Yilmaz M. Z. et al. The therapeutic effects of anti-oxidant and anti-inflammatory drug quercetin on aspiration-induced lung injury in rats. J. Mol. Histol. 45, 195–203 (2014). 10.1007/s10735-013-9542-3 [DOI] [PubMed] [Google Scholar]
- 9.Ishizawa K. et al. Pharmacology in health food: metabolism of quercetin in vivo and its protective effect against arteriosclerosis. J. Pharmacol. Sci. 115, 466–470 (2011). 10.1254/jphs.10r38fm [DOI] [PubMed] [Google Scholar]
- 10.Marunaka Y. et al. Quercetin is a Useful Medicinal Compound Showing Various Actions Including Control of Blood Pressure, Neurite Elongation and Epithelial Ion Transport. Curr. Med. Chem. 25, 4876–4887 (2018). 10.2174/0929867323666160919095043 [DOI] [PubMed] [Google Scholar]
- 11.Brull V. et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: a randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 114, 1263–1277 (2015). 10.1017/S0007114515002950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan F. et al. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update. Nutrients 8, pii: E529 (2016). 10.3390/nu8090529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proshkina E. et al. Geroprotective and Radioprotective Activity of Quercetin, (-)-Epicatechin, and Ibuprofen in Drosophila melanogaster. Front. Pharmacol. 7, 505 (2016). 10.3389/fphar.2016.00505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kampkotter A. et al. Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 149, 314–323 (2008). 10.1016/j.cbpb.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 15.Pietsch K. et al. Meta-Analysis of Global Transcriptomics Suggests that Conserved Genetic Pathways are Responsible for Quercetin and Tannic Acid Mediated Longevity in C. elegans. Front. Genet. 3, 48 (2012). 10.3389/fgene.2012.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietsch K., Saul N., Menzel R., Sturzenbaum S. R. & Steinberg C. E. Quercetin mediated lifespan extension in Caenorhabditis elegans is modulated by age-1, daf-2, sek-1 and unc-43. Biogerontology 10, 565–578 (2009). 10.1007/s10522-008-9199-6 [DOI] [PubMed] [Google Scholar]
- 17.Saul N., Pietsch K., Menzel R. & Steinberg C. E. Quercetin-mediated longevity in Caenorhabditis elegans: is DAF-16 involved? Mech. Ageing Dev. 129, 611–613 (2008). 10.1016/j.mad.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 18.Sugawara T. & Sakamoto K. Killed Bifidobacterium longum enhanced stress tolerance and prolonged life span of Caenorhabditis elegans via DAF-16. Br. J. Nutr. 120, 872–880 (2018). 10.1017/S0007114518001563 [DOI] [PubMed] [Google Scholar]
- 19.Feng S. et al. Thermal stress resistance and aging effects of Panax notoginseng polysaccharides on Caenorhabditis elegans. Int. J. Biol. Macromol. 81, 188–194 (2015). 10.1016/j.ijbiomac.2015.07.057 [DOI] [PubMed] [Google Scholar]
- 20.Kampkotter A. et al. Investigations of protective effects of the flavonoids quercetin and rutin on stress resistance in the model organism Caenorhabditis elegans. Toxicology 234, 113–123 (2007). 10.1016/j.tox.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 21.O’Fallon K. S. et al. Effects of quercetin supplementation on markers of muscle damage and inflammation after eccentric exercise. Int. J. Sport Nutr. Exerc. Metab. 22, 430–437 (2012). 10.1123/ijsnem.22.6.430 [DOI] [PubMed] [Google Scholar]
- 22.Dorman J. B., Albinder B., Shroyer T. & Kenyon C. The age-1 and daf-2 genes function in a common pathway to control the lifespan of Caenorhabditis elegans. Genetics 141, 1399–1406 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheesman H. K. et al. Aberrant Activation of p38 MAP Kinase-Dependent Innate Immune Responses Is Toxic to Caenorhabditis elegans. G3 (Bethesda) 6, 541–549 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furuhashi T. & Sakamoto K. FoxO/Daf-16 restored thrashing movement reduced by heat stress in Caenorhabditis elegans. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 170, 26–32 (2014). 10.1016/j.cbpb.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 25.Seo H. W. et al. Catalpol Modulates Lifespan via DAF-16/FOXO and SKN-1/Nrf2 Activation in Caenorhabditis elegans. Evid Based. Complement. Alternat Med. 2015, 524878 (2015). 10.1155/2015/524878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu A. L., Murphy C. T. & Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300, 1142–1145 (2003). 10.1126/science.1083701 [DOI] [PubMed] [Google Scholar]
- 27.Morton E. A. & Lamitina T. Caenorhabditis elegans HSF-1 is an essential nuclear protein that forms stress granule-like structures following heat shock. Aging Cell. 12, 112–120 (2013). 10.1111/acel.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.An J. H. & Blackwell T. K. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 17, 1882–1893 (2003). 10.1101/gad.1107803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaar C. E. et al. Mitochondrial and cytoplasmic ROS have opposing effects on lifespan. PLoS Genet. 11, e1004972 (2015). 10.1371/journal.pgen.1004972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy C. T. et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424, 277–283 (2003). 10.1038/nature01789 [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y et al. Selection of reliable reference genes in Caenorhabditis elegans for analysis of nanotoxicity. PloS one 73 e31849 (2012). 10.1371/journal.pone.0031849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schieber M. & Chandel N. S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–62 (2014). 10.1016/j.cub.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonani Ravi Raghav, et al. Phycoerythrin extends life span and health span of Caenorhabditis elegans. Age 365: 9717 (2014). 10.1007/s11357-014-9717-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verbeke Philippe, et al. Heat shock response and ageing: mechanisms and applications. Cell biology international 259: 845–857 (2001). 10.1006/cbir.2001.0789 [DOI] [PubMed] [Google Scholar]
- 35.Lithgow G. J., White T. M., Melov S. & Johnson T. E. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc. Natl. Acad. Sci. U. S. A. 92, 7540–7544 (1995). 10.1073/pnas.92.16.7540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tullet J. M. et al. DAF-16/FoxO directly regulates an atypical AMP-activated protein kinase gamma isoform to mediate the effects of insulin/IGF-1 signaling on aging in Caenorhabditis elegans. PLoS Genet. 10, e1004109 (2014). 10.1371/journal.pgen.1004109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tullet J. M. et al. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 132, 1025–1038 (2008). 10.1016/j.cell.2008.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inoue H. et al. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 19, 2278–2283 (2005). 10.1101/gad.1324805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papp D., Csermely P. & Soti C. A role for SKN-1/Nrf in pathogen resistance and immunosenescence in Caenorhabditis elegans. PLoS Pathog. 8, e1002673 (2012). 10.1371/journal.ppat.1002673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y., Branicky R., Noe A. & Hekimi S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 217, 1915–1928 (2018). 10.1083/jcb.201708007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X., Wang X., Li L. & Wang D. Lifespan extension in Caenorhabditis elegans by DMSO is dependent on sir-2.1 and daf-16. Biochem. Biophys. Res. Commun. 400, 613–618 (2010). 10.1016/j.bbrc.2010.08.113 [DOI] [PubMed] [Google Scholar]
- 42.Govindan S. et al. Phytochemicals-induced hormesis protects Caenorhabditis elegans against alpha-synuclein protein aggregation and stress through modulating HSF-1 and SKN-1/Nrf2 signaling pathways. Biomed. Pharmacother. 102, 812–822 (2018). 10.1016/j.biopha.2018.03.128 [DOI] [PubMed] [Google Scholar]