Highlights
-
•
Neutralizing antibodies targeting SARS-CoV-2 S protein correlate with IgGs binding RBD.
-
•
NtAb and RBD IgGs levels do not associate with COVID-19 severity in hospitalized patients.
-
•
NtAb and RBD IgG levels do not correlate with serum levels of inflammatory biomarkers.
Keywords: SARS-CoV-2, COVID-19, Neutralizing antibodies, Inflammatory biomarkers
Abstract
Background
The involvement of SARS-CoV-2 antibodies in mediating immunopathogenetic events in COVID-19 patients has been suggested. By using several experimental approaches, we investigated the potential association between SARS-CoV-2 IgGs recognizing the spike (S) protein receptor-binding domain (RBD), neutralizing antibodies (NtAb) targeting S, and COVID-19 severity.
Patients and methods
This unicenter, retrospective, observational study included 51 hospitalized patients (24 at the intensive care unit; ICU). A total of 93 sera from these patients collected at different time points from the onset of symptoms were analyzed. SARS-CoV-2 RBD IgGs were quantitated by ELISA and NtAb50 titers were measured in a GFP reporterbased pseudotyped virus platform. Demographic and clinical data, complete blood counts, as well as serum levels of ferritin, Dimer-D, C reactive protein (CRP), lactose dehydrogenase (LDH), and interleukin-6 (IL-6) were retrieved from clinical charts.
Results
The overall correlation between levels of both antibody measurements was good (Rho = 0.82; P = 0 < 0.001). SARS-CoV-2 RBD IgG and NtAb50 levels in sera collected up to day 30 after the onset of symptoms were comparable between ICU and non-ICU patients (P=>0.1). Four ICU patients died; two of these achieved NtAb50 titers ≥1/160 while the other two exhibited a 1/80 titer. Very weak (Rho=>0.0–<0.2) or weak (Rho=>0.2–<0.4) correlations were observed between anti-RBD IgGs, NtAb50, and serum levels pro-inflammatory biomarkers.
Conclusions
The data presented herein do not support an association between SARS-CoV-2 RBD IgG or NtAb50 levels and COVID-19 severity.
1. Background
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in late 2019 and has been declared a pandemic [1]. Clinical presentation of COVID-19 varies widely, ranging from asymptomatic to mild or severe forms [2,3]. Worse clinical outcomes are related to an imbalanced immune response skewed toward a Th1 pro-inflammatory profile, which leads to the uncontrolled release of cytokines and chemokines, such as interleukin-6 (IL-6), that mediates progression into acute respiratory distress syndrome, multiorgan failure, and death [4,5].
Adaptive humoral immunity is thought to protect from acquiring SARS-CoV-2 infection, of which neutralizing antibodies (NtAb) seemingly play a major role [6]. Although epitopes mapping within all SARS-CoV-2 structural proteins have been shown to elicit NtAb, the receptor-binding domain (RBD) of the viral spike protein (S) is immunodominant and a highly specific target of most potent NtAbs in COVID-19 patients [[6], [7], [8], [9]]. The involvement of functional antibodies in SARS-CoV-2 clearance and modulation of COVID-19 severity remains to be precisely defined [10]. Data obtained in experimental models indicated that adoptive transfer of neutralizing monoclonal antibodies reduces viral burden in the lung, ameliorates local inflammation and decreases mortality [7,11,12]. Moreover, passive immunization of critically ill COVID-19 patients with plasma from individuals who had recovered from SARS-CoV-2 infection and seroconverted was associated with improved clinical outcomes in uncontrolled case series [13,14]. Yet, the possibility that antibodies could potentially trigger immunopathogenic events in SARS-CoV-2-infected patients or enhance infection is a major concern [6,15,16]. In this context, higher antibody titers, either neutralizing or not, have been reported to be present in patients developing severe forms of COVID-19 when compared to mildly symptomatic individuals who did not require hospitalization [[17], [18], [19], [20], [21], [22], [23]].
2. Objectives
Here, we aimed to explore the potential relationship between the magnitude of SARS-CoV-2 antibodies binding to RBD and NtAb targeting the S protein with the severity of COVID-19 in a cohort of hospitalized patients.
3. Study design
3.1. COVID-19 patients
In this unicenter, retrospective observational study, 51 non-consecutive hospitalized patients with laboratory-confirmed SARS-CoV-2 infection by RT-PCR, admitted to Hospital Clínico Universitario of Valencia between March 5 to April 30, 2020, were included. Patients were hospitalized within 24 h after seeking medical attention at the emergency service. All patients presented with pneumonia and imaging/laboratory findings compatible with COVID-19 [2,3]. Medical history and laboratory data were retrospectively reviewed. The current study was approved by the Research Ethics Committee of Hospital Clínico Universitario INCLIVA (March, 2020).
3.2. Patient samples
A total of 93 sera from 51 patients with COVID-19 were included for the analyses detailed below. Forty-seven sera were obtained within the first two weeks after the onset of symptoms, 32 between the third and the forth weeks and 14 afterwards (between days 31 and 45). Sequential specimens were available from 20 out of the 51 patients (median 3 specimens/patients; range 2–6), 17 of whom were in ICU. Sera from 51 individuals collected prior to the epidemic outbreak (within years 2018 and 2019) served as controls in the SARS-CoV-2 RBD IgG immunoassay and the SARS-CoV-2 neutralizing antibody assays described below. Nine patients had tested positive for Coronavirus 229E by the xTAG Respiratory Viral Panel (Luminex Corporation, Austin, Tx, USA).
3.3. SARS-CoV2-2 RT-PCR
Nasopharyngeal or oropharyngeal specimens were obtained with flocked swabs in universal transport medium (Beckton Dickinson, Sparks, MD, USA, or Copan Diagnostics, Murrieta, CA, USA) and conserved at 4 °C until processed (within 6 h). Undiluted tracheal aspirate samples obtained from mechanically ventilated patients were also processed when available. Commercially-available RT-PCR kits were used for SARS-CoV-2 RNA testing, as previously detailed [24].
3.4. SARS-CoV-2 RBD IgG immunoassay
An enzyme-linked immunosorbent assay (ELISA) was used to quantitate IgG antibodies binding to SARS-CoV-2 RBD [25,26]. A detailed description of the assay can be found in Supplementary Methods.
3.5. SARS-CoV-2 neutralizing antibody assay
A green fluorescent protein (GFP) reporter-based neutralization assay which used a non-replicative vesicular stomatitis virus pseudotyped with the SARS-CoV-2 spike protein (VSV-S) was optimized as previously described (see supplementary methods) [[27], [28], [29]]. We considered high NtAb50 titers those ≥1/160, as this is the minimum NtAb titer of plasma from COVID-19 convalescent individuals recommended by the FDA for therapeutic use [30].
3.6. Laboratory measurements
Clinical laboratory investigation included complete blood count and levels of ferritin, Dimer-D, C reactive protein (CRP), lactose dehydrogenase (LDH) and interleukin-6 (IL-6) quantitated in sera that were later used for SARS-CoV-2 RBD IgGs and NtAb testing.
3.7. Statistical methods
Frequency comparisons for categorical variables were carried out using the Fisher exact test. Differences between medians were compared using the Mann–Whitney U test Spearman’s rank test was used to assess the correlation between continuous variables using the entire dataset. Receiver operating characteristic (ROC) curve analysis was performed to identify the optimal SARS-CoV-2 RBD IgG level predicting NtAb titers above a certain threshold. Two-sided exact P-values are reported. A P-value <0.05 was considered statistically significant. The analyses were performed using SPSS version 20.0 (SPSS, Chicago, IL, USA).
4. Results
4.1. Clinical characteristics of COVID-19 patients
Patients hospitalized in the pneumology ward (n = 27) and ICU (n = 24) were matched for sex and age, the presence of co-morbidities and the time elapsed from the day of onset of symptoms to first serum sample collection (Table 1 ). As expected, ICU patients were hospitalized for longer periods and the median serum levels of several pro-inflammatory biomarkers, (LDH, dimer-D and IL-6) were significantly higher in ICU patients, further confirming their association with COVID-19 severity [[2], [3], [4], [5]]. Four ICU patients died.
Table 1.
Demographic, clinical and laboratory characteristics of patients with COVID-19.
| Parameter | All patients | Patients hospitalized in the pneumology ward | Patients hospitalized in the intensive care unit | P value |
|---|---|---|---|---|
| Sex: Male/Female; no. (%) | 32 (63)/19 (37) | 14 (52)/13 (48) | 18 (75)/(6 (25) | 0.15 |
| Age; median (range) | 53 (21−77) | 58 (42−76) | 65 (29−77) | 0.07 |
| Days of hospitalization; median (range) | 17 (2−67) | 9 (2−22) | 36 (8−67) | <0.001 |
| Days from onset symptoms to first serum sample; median (range) | 12 (5−36) | 11 (5−32) | 13 (7−36) | 0.33 |
| Co-morbidities; no. (%) | 35 (69) | 18 (67) | 17 (71) | 0.75 |
| Number of comorbidities; median (range) | 1 (0−5) | 1 (0−3) | 2 (0−5) | 0.18 |
| Comorbidity; median (range) | ||||
| Arterial hypertension | 23 (45) | 11 (41) | 12 (50) | 0.58 |
| Chronic renal disease | 2 (4) | 0 | 2 (8) | 0.22 |
| Diabetes mellitus | 12 (24) | 5 (19) | 7 (29) | 0.51 |
| Dyslipidemia | 16 (31) | 7 (26) | 9 (38) | 0.37 |
| Ischemic cardiovascular disease | 4 (8) | 2 (7) | 2 (8) | 0.90 |
| Myocardial infarction | 2 (4) | 1 (4) | 1 (4) | 1.00 |
| Pulmonar diseasea | 7 (14) | 2 (7) | 5 (21) | 0.16 |
| Tumor | 3 (6) | 1 (4) | 2 (8) | 0.48 |
| Laboratory findingsb; median (range) | ||||
| CRP (in mg/l) | 44 (0.8−273) | 70 (0.8−242) | 24.80 (1.00−273) | 0.24 |
| Ferritin (ng/mL) | 674 (2.5−2986) | 565 (9.2−2779) | 959 (2.50−2986) | 0.17 |
| Dimer-D (ng/mL) | 903 (91−5445) | 488 (91−1894) | 1328 (489−5445) | <0.001 |
| LDH (U/l) | 666 (357−1328) | 556 (357−825) | 790 (518−1328) | <0.001 |
| IL-6 (pg/mL)c | 1012 (4.6−5000) | 79 (4.6−124) | 1277 (186−5000) | 0.009 |
| Total lymphocyte count (*109/L) | 1.15 (0.17−3.98) | 1.13 (0.17−2.95) | 1.31 (0.38−3.98) | 0.17 |
Including asthma, atelectasis and chronic obstructive pulmonary disease.
The median was calculated in patients with more than one sample. Normal values: 12−300 ng/mL for ferritin, <100 ng/mL for Dimer-D, and <10 mg/L for C-reactive protein (CRP), 140–280 U/L Lactic acid dehydrogenase (LDH), 5–15 pg/ml for IL-6, and 1–4.8 lymphocytes ×109/L.
Data available from 18 patients.
4.2. Correlation between SARS-CoV-2 RBD IgG levels and neutralizing antibody titers
We first aimed to determine whether SARS-CoV-2 RBD IgGs could be used as a proxy for NtAb50 titers. As shown in Fig. 1 , the overall correlation between levels of both antibody assays was strong (Rho = 0.82; P < 0.001). ROC analysis showed that SARS-CoV-2-RBD IgG levels ≥1.15 AU/mL predicted the presence of NtAb50 titers ≥160 with a sensitivity of 90 % and a specificity of 94 % (Supplementary Fig. 1).
Fig. 1.
Correlation between SARS-CoV-2 RBD IgG levels quantitated by ELISA and NtAb50 titers measured by a reporter-based pseudotype (VSV-S) neutralization assay in sera from COVID-19 patients. Rho and P values are shown.
4.3. Kinetics of SARS-CoV-2 RBD IgGs and neutralizing antibodies
Overall, serum levels of both antibody tests were seen to increase significantly in parallel over time (Fig. 2 ), although the median peak NtAb50 titer was reached earlier (between days 11–20) than that of RBD-specific IgGs (between days 20–30). After peaking, NtAb50 levels remained stable through the end of the study period, while RBD-specific IgGs decreased slightly afterwards. Sequential sera were available from 20 patients, most of whom (n = 17) were at ICU. The kinetics profile from both antibody assays was found to vary widely across patients (Fig. 3 ), some of whom exhibited increasing levels while others displayed either constant or fluctuating titers.
Fig. 2.
SARS-CoV-2 RBD IgG levels (A) and NtAb50 titers (B) at different time points after the onset of symptoms in patients with COVID-19.
Fig. 3.
Kinetics patterns of SARS-CoV-2 RBD IgGs (A,B,C) and NtAb (D,E,F) in 20 COVID-19 patients (17 admitted to the intensive care unit).
4.4. SARS-CoV-2 RBD IgG avidity
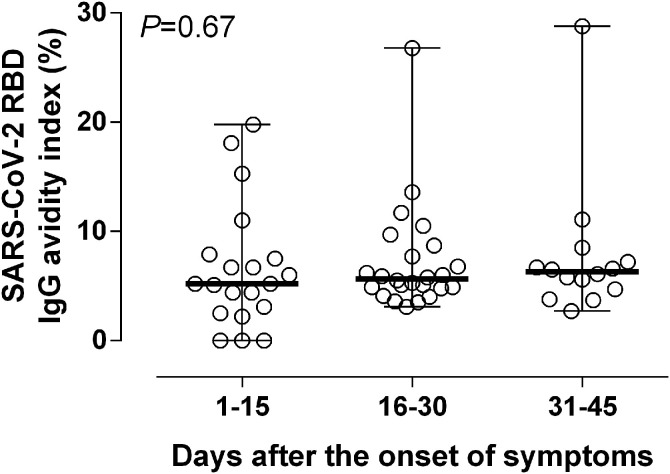
Avidity of SARS-CoV-2 IgGs in sera from COVID-19 patients was assessed by a conventional urea dissociation assay [26]. Overall, AIs were very low (median 5 %; range 2–28 %). Most sera (40 out of 51) displayed AI ≤ 10 %. Analysis of sequential sera from 20 patients revealed that SARS-CoV-2 IgG AI slightly increased over time (Fig. 4 ). SARS-CoV-2 RBD IgG AI did not correlate with NtAb50 titers (Rho = 0.07; P = 0.56)
Fig. 4.
SARS-CoV-2 RBD IgG avidity indices (AIs) of serial sera from COVID-19 patients collected at different times following the onset of symptoms.
4.5. SARS-CoV-2 antibodies and COVID-19 severity
We next compared SARS-CoV-2 RBD IgG and NtAb50 levels in ICU and non-ICU patients in sera collected within the first 30 days after the onset of symptoms. We did not notice a significant difference in the magnitude of either antibody response across groups (Fig. 5 ). Of note, 4 ICU patients died, of which two achieved NtAb50 titers ≥1/160 while the other two exhibited a 1/80 titer.
Fig. 5.
SARS-CoV-2 RBD IgG levels (A) and NtAb50 titers (B) at different time points after the onset of symptoms in patients with COVID-19 either admitted to the intensive care unit or the pneumology ward. P values for comparisons are shown.
4.6. SARS-CoV-2 antibody levels and biomarkers of COVID-19 prognosis
Finally, we sought to determine whether the magnitude of SARS-CoV-2 RBD IgG and NtAb responses was related to an inflammatory state, as inferred from serum levels of CRP, ferritin, Dimer-D, LDH and IL-6. For this, we first performed correlation analyses between these parameters. Very weak (Rho=>0.0–<0.2) or weak (Rho=>0.2–<0.4) correlations (either positive or negative) were found between SARS-CoV-2 RBD IgG levels or NtAb50 titers and all selected biomarkers when considering the entire data set (Fig. 6 ) or when analyses were done separately for specimens collected at different time frames after the onset of symptoms (days 1–15 or days 15–30; not shown). Measurements from both antibody assays weakly correlated with total lymphocyte counts. As a complementary approach, we grouped sera into two categories (high NtAb50 titers: ≥1/160 and low NtAb50 titers: <1/160), and assessed whether median levels of the abovementioned parameters differed across groups. We found this not to be the case (Supplementary Fig. 2).
Fig. 6.
Correlation between SARS-CoV-2 RBD IgG levels and NtAb50 titers with serum levels of C-reactive protein (CRP), Dimer-D, ferritin, lactate dehydrogenase (LDH), interleukin-6 (IL-6) and absolute lymphocyte counts. Rho and P values are shown.
5. Discussion
Here, in addition to further characterizing the antibody response to SARS-CoV-2 in hospitalized COVID-19 patients, we mainly aimed to determine whether a relationship could be established between the magnitude of SARS-CoV-2 RBD IgG and NtAb levels and the “inflammatory state” of patients, which has been shown to directly correlate with COVID-19 severity and prognosis [[2], [3], [4], [5]].
We found that SARS-CoV-2 RBD IgG levels correlated well with NtAb titers, as quantitated by a VSV reporter virus pseudotyped with SARS-CoV-2 S protein (VSV-S), thus lending support to the assumption that the former parameter is a reasonably reliable proxy for the latter [8,9]. Moreover, we could define a SARS-CoV-2 RBD IgG threshold (≥1.15 AU/mL) predicting NtAb titers ≥1/160 with high sensitivity and specificity, this being the lowest titer of plasma recommended by FDA for passive transfer therapy [30].
Previous studies have reported a correlation between RBD IgG levels and NtAb titers in patients with comparable or less severe clinical presentations of COVID-19, using either live native SARS-CoV-2 virus, engineered SARS-CoV-2 pseudotype virus systems or replication-competent SARS-CoV-2 chimeric viruses [18,22,[30], [31], [32], [33], [34], [35], [36]]. Here, the degree of correlation between these two antibody assays was found to be strong (Rho = 0.82), but not absolute (Rho = 1), as previously reported [18,[30], [31], [32], [33], [34], [35], [36]], which is consistent with data showing that highly immunogenic epitopes within the S protein outside the RBD elicit potent NtAb responses [6,37].
The kinetics of SARS-CoV-2 RBD IgGs and NtAb followed a predictable course [18,22,[30], [31], [32], [33], [34], [35], [36]], with antibody levels in both assays showing a consistent increase over time, and reaching a peak within the second and third week after the onset of symptoms for NtAb or slightly later for RBD-specific IgGs.
An interesting observation was that SARS-CoV-2 RBD IgGs avidity was quite low (<10 %) in most sera, which were collected up to 2 months following the onset of symptoms, and showed minimal increase over time. This antibody avidity maturation pattern is reminiscent of that observed during SARS [38]. Remarkably, no correlation was found between SARS-CoV-2 RBD IgG AIs and NtAb50 titers. This finding is in agreement with the idea that limited to no affinity maturation is required from the germline to achieve a potent NtAb response to RBD [39].
The alleged association between high SARS-CoV-2 antibody levels and COVID-19 severity [[17], [18], [19], [20], [21], [22]] is a matter of concern. If found to be the case, a plausible explanation for this observation may be that patients experiencing severe forms of the disease are exposed to higher and more perdurable viral burdens [18]; this, however, would call into question the role of antibodies in contributing to SARS-CoV-2 clearance. Alternatively, it may simply represent an epiphenomenom in the setting of an overall exaggerated immune response driven by “cytokine storms”, or may constitute a relevant pathogenetic mechanism involved in lung tissue damage (antibody-dependent enhancement) [15].
The data presented herein do not support the abovementioned association. In effect, we failed to find differences in SARS-CoV-2 RBD IgGs or SARS-CoV-2 NtAb50 levels within the first 30 days after the onset of symptoms between ICU and non-ICU patients who were matched for age, sex and co-morbidities. Furthermore, 2 out of the 4 ICU patients who died had relatively low NtAb50 titers (1/80). Liu and colleagues [19] showed that oxygen requirement in patients was independently associated with NtAb50 levels, as measured by both a pseudotyped reporter virus or live SARS-CoV-2 neutralization assay. Nevertheless, this finding should be interpreted with caution provided that only 8 ICU patients were recruited and these were much older than those in the non-ICU group. Wang et al. [18] also reported higher NtAb50 titers quantitated by a pseudotyped-virus based neutralization assay in severely ill patients as compared to mild COVID-19 patients. Other studies including relatively small cohorts also pointed to an association of COVID-19 severity with SARS-CoV-2 NtAb [20,22,38]. In our view, comparison between studies addressing the abovementioned issue is rather problematic because of notable differences in clinical characteristics and therapeutic management of patients, categorization of severity, the timing of serum collection, and methods employed for SARS-CoV-2 antibodies detection and quantitation.
Disregulated synthesis and release of pro-inflammatory cytokines is thought to be a pathogenetic hallmark of most severe forms of COVID-19 [4,5]. Although the mechanisms of COVID-19–induced lung injury remain unclear, the so-called “cytokine storm” may likely play a critical role in the process of disease worsening and thus in COVID-19 prognosis [40]. Here, we investigated whether SARS-CoV-2 RBD IgG and NtAb50 levels correlate with serum concentrations of ferritin, Dimer-D, CRP, LDH and IL-6, which have been consistently shown to be markedly increased in patients with progressive disease and poor outcomes [4,5]. At most, we observed weak or very weak correlations between the antibody assays and these inflammatory biomarkers. Moreover, serum levels of the latter overlapped between patients with either high or low NtAb50 titers (≥1/160). Taken together, these data argue against a robust relationship between the magnitude of the antibody responses subjected to analysis herein and the state of inflammation in COVID-19 patients. To our knowledge, only one pre-print study used a similar approach to ours to address this issue [35], reporting a modest correlation (Rho = 0.5) between NtAb50 titers and blood CRP levels. In addition, in contrast to what was observed here, a moderate negative correlation (Rho=-0.45) between NtAb50 titers and absolute lymphocyte counts was observed. As stated above, the comparison between the two studies is not straightforward.
The current study has several limitations. First, its retrospective nature. Second, cohort size is relatively small in our study. Third, IL-6 data was only available from 18 patients (all but one at ICU); in addition, all these patients were treated with tocilizumab. Fourth, SARS-CoV-2 antibodies and inflammatory biomarkers levels were measured in the blood compartment, which may not necessarily mirror those in lung tissue. Fifth, serum levels of other cytokines (i.e. TNF-α, or IL1-β) or chemokines (IFNγ-induced protein 10) that may reflect more accurately the overall state of inflammation were not measured [4,5]. Sixth, epitope specificities of SARS-CoV-2 antibodies other than for the S protein in the case of the neutralization assays or RBD in the case of the IgG tests were not assessed. In this sense, antibodies mediating immunopathogenetic events, especially through ADE, are more likely to behave as sub- or non-neutralizing and target epitopes outside RBD [4].
6. Conclusion
The data presented herein do not support an association between SARS-CoV-2 RBD IgG or NtAb50 levels and COVID-19 severity. Further, well-powered studies overcoming the abovementioned limitations are warranted to solve this question, which is of paramount relevance for vaccine design and for the safety of passive transfer therapies with plasma from convalescent COVID-19 individuals.
Funding
This work was supported by Valencian Government grant DIFEDER/2018/056 to JRD, The Generalitat Valenciana grant Covid_19-SCI to RG, The Spanish National Research Council grant CSIC-COV19-082 and Fondo Supera Covid-19 grant BlockAce to RG and AM, and Instituto de Salud Carlos III-Fondo COVID19 grant COV20_0043 to AM.
Ethical approval
The current study was approved by the Research Ethics Committee of Hospital Clínico Universitario INCLIVA (March, 2020).
Author statement
RG: Methodology, investigation, validation, review & editing; EG: Formal analysis, review and editing; VL: Methodology, investigation; CFG: Methodology, investigation; EA: resources, project administration, review and editing; JB: Supervision; review & editing; AM: Methodology, investigation, funding acquisition; MLB: Patient attendance, review & editing; JSC: Patient attendance, review & editing; JRD: Conceptualization, supervision, funding acquisition, review& editing; RG: Methodology, investigation, validation, funding acquisition, review & editing; DN: Conceptualization, supervision, writing the original draft, review & editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
The members of the Decoy-SARS-COV-2 Study Group from the Institute of Biomedicine of Valencia are the following ones: Vicente Rubio, Alberto Marina, Jeronimo Bravo, José Luis LLacer, Clara Marco, Alonso Felipe, Anmol Adhav, Carla Sanz, Nadine Gougeard, Susana Masiá, Francisca Gallego, Sara Zamora, Lidia Orea, Alicia Forcada, Alba Iglesias, Mónica Escamilla, Laura Villamayor, Borja Sáez, Carolina Espinosa and María Pilar Hernández. They form a group for production of proteins involved in SARS-COV-2 entry into cells and for analysis of their interactions. Their support as a team led by A. Marina was key to production of RBD protein used in the present study. The authors would like to thank Gert Zimmer (Institute of Virology and Immunology, Mittelhäusern/Switzerland), Stefan Pöhlmann and Markus Hoffmann (both German Primate Center, Infection Biology Unit, Goettingen/Germany) for providing the reagents required for the generation of VSV pseudotypes. Estela Giménez holds a Juan Rodés research contract from the Carlos III Health Institute (Ref. JR18/00053). Eliseo Albert holds a Río Hortega research contract from the Carlos III Health Institute (Ref. CM18/00221). Ron Geller holds a Ramón y Cajal fellowship from the Spanish Ministry of Economy and Competitiveness (RYC-2015-17517).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jcv.2020.104611.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.https://www.who.int/dg/speeches/detail/who-director-general-s-opening.
- 2.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.-j, Ni Z.-y, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. New Engl. J. Med. 2020;(382):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allegra A., Di Gioacchino M., Tonacci A., Musolino C., Gangemi S. Immunopathology of SARS-CoV-2 infection: immune cells and mediators, prognostic factors, and immune-therapeutic implications. Int. J. Mol. Sci. 2020;21:E4782. doi: 10.3390/ijms21134782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lega S., Naviglio S., Volpi S., Tommasini A. Recent insight into SARS-CoV2 immunopathology and rationale for potential treatment and preventive strategies in COVID-19. Vaccines (Basel) 2020;8:E224. doi: 10.3390/vaccines8020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore J.P., Klasse P.J. SARS-CoV-2 vaccines: ‘warp speed’ needs mind melds not warped minds. J. Virol. 2020 doi: 10.1128/JVI.01083-20. Jun 26:JVI.01083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers T.F., Zhao F., Huang D. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model science. Science. 2020 doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Premkumar L., Segovia-Chumbez B., Jadi R. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes C.O., West A.P., Jr, Huey-Tubman K.E. Structures of human antibodies bound to SARS-CoV-2 spike reveal common epitopes and recurrent features of antibodies. Cell. 2020 doi: 10.1016/j.cell.2020.06.025. S0092-8674(20)30757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zohar T., Alter G. Dissecting antibody-mediated protection against SARS-CoV-2. Nat. Rev. Immunol. 2020;20:392–394. doi: 10.1038/s41577-020-0359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan A.O., Case J.B., Winkler E.S. A SARS-CoV-2 infection model in mice demonstrates protection by neutralizing antibodies. Cell. 2020 doi: 10.1016/j.cell.2020.06.011. S0092-8674(20)30742-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alsoussi W.B., Turner J.S., Case J.B. A potently neutralizing antibody protects mice against SARS-CoV-2 infection. J. Immunol. 2020 doi: 10.4049/jimmunol.2000583. Jun 26:ji2000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen C., Wang Z., Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan K., Liu B., Li C. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. PNAS. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eroshenko N., Gill T., Keaveney M.K., Church G.M., Trevejo J.M., Rajaniemi H. Implications of antibody-dependent enhancement of infection for SARS-CoV-2 countermeasures. Nat. Biotechnol. 2020;38:789–791. doi: 10.1038/s41587-020-0577-1. [DOI] [PubMed] [Google Scholar]
- 16.Klasse P.J., Moore J.P. Antibodies to SARS-CoV-2 and their potential for therapeutic passive immunization. Elife. 2020;9 doi: 10.7554/eLife.57877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long Q.X., Tang X.J., Shi Q.L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020 doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Zhang L., Sang L. Kinetics of viral load and antibody response in relation to COVID-19 severity. J. Clin. Invest. 2020 doi: 10.1172/JCI138759. Jul 7:138759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L., To K.K., Chan K.H. High neutralizing antibody titer in intensive care unit patients with COVID-19. Emerg. Microbes Infect. 2020 doi: 10.1080/22221751.2020.1791738. Jul 3:1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wölfel R., Corman V.M., Guggemos W. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 21.Okba N.M.A., Müller M.A., Li W. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg. Infect. Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salazar E., Kuchipudi S.V., Christensen P.A. bioRxiv; 2020. Relationship Between Anti-Spike Protein Antibody Titers and SARS-CoV-2 in Vitro Virus Neutralization in Convalescent Plasma. Jun 9:2020.06.08.138990. [Google Scholar]
- 23.Wang X., Guo X., Xin Q. Neutralizing antibodies responses to SARS-CoV-2 in COVID-19 inpatients and convalescent patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa721. Jun 4:ciaa721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giménez E., Albert E., Torres I. SARS-CoV-2-reactive interferon-γ-producing CD8+ T cells in patients hospitalized with coronavirus disease 2019. J. Med. Virol. 2020 doi: 10.1002/jmv.26213. Jun 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lan J., Ge J., Yu J. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 26.Baccard-Longere M., Freymuth F., Cointe D., Seigneurin J.M., Grangeot-Keros L. Multicenter evaluation of a rapid and convenient method for determination of cytomegalovirus immunoglobulin G avidity. Clin. Diagn. Lab. Immunol. 2001;8:429–431. doi: 10.1128/CDLI.8.2.429-431.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger Rentsch M., Zimmer G. A vesicular stomatitis virus replicon-based bioassay for the rapid and sensitive determination of multi-species type I interferon. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025858. Mossman KL, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanika A., Larisch B., Steinmann E., Schwegmann-Weßels C., Herrler G., Zimmer G. Use of influenza C virus glycoprotein HEF for generation of vesicular stomatitis virus pseudotypes. J. Gen. Virol. 2005;86:1455–1465. doi: 10.1099/vir.0.80788-0. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020:1–10. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.2020. Recommendations for Investigational COVID-19 Convalescent Plasma.https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma (Accessed July 5, 2020) [Google Scholar]
- 31.Suthar M.S., Zimmerman M., Kauffman R. medRxiv; 2020. Rapid Generation of Neutralizing Antibody Responses in COVID-19 Patients. May 8:2020.05.03.20084442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L., Zhang W., Hu Y. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020 doi: 10.1001/jama.2020.10044. Jun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harvala H., Robb M., Watkins N. medRxiv; 2020. Convalescent Plasma Therapy for the Treatment of Patients With COVID-19: Assessment of Methods Available for Antibody Detection and Their Correlation with Neutralising Antibody Levels. 05.20.20091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.To K.K., Tsang O.T., Leung W.S. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu F., Wang A., Liu M. medRxiv; 2020. Neutralizing Antibody Responses to SARS-CoV-2 in a COVID-19 Recovered Patient Cohort and Their Implications. [DOI] [Google Scholar]
- 36.Ni L., Ye F., Cheng M.-L. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020 doi: 10.1016/j.immuni.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L., Wang P., Nair M.S. bioRxiv; 2020. Potent Neutralizing Monoclonal Antibodies Directed to Multiple Epitopes on the SARS-CoV-2 Spike. Jun 18;2020.06.17.153486. [DOI] [PubMed] [Google Scholar]
- 38.Chan P.K., Lim P.L., Liu E.Y., Cheung J.L., Leung D.T., Sung J.J. Antibody avidity maturation during severe acute respiratory syndrome-associated coronavirus infection. J. Infect. Dis. 2005;192:166–169. doi: 10.1086/430615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burton D.R., Walker L.M. Rational vaccine design in the time of COVID-19. Cell Host Microbe. 2020;27:695–698. doi: 10.1016/j.chom.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinha P., Matthay M.A., Calfee C.S. Is a "cytokine storm" relevant to COVID-19? JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.3313. Jun 30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.