Biofilm formation is an important protection mechanism used by most microorganisms and provides cells with many advantages, like high infectivity, antibiotic resistance, and strong survivability. Since most persistent bacterial infections are believed to be associated with biofilms, biofilm control is an important issue in medicine, environmental engineering, and industry. Biofilm formation is influenced by various environmental factors. Temperature is the most direct environmental cue encountered by microorganisms. Here, we investigated the effect of temperature on the biofilm formation of P. aeruginosa, a notorious pathogen, and found that temperature is an important factor determining the amount and structure of biofilms. Low temperatures greatly increase biofilm formation and give biofilms a highly conspicuous structure. Although thermoregulation of biofilm formation is mainly mediated by c-di-GMP, some c-di-GMP-independent regulations were also observed. This study shows how biofilms are formed at various temperatures and provides new insights to control biofilms using temperature.
KEYWORDS: Pseudomonas aeruginosa, temperature, biofilm, c-di-GMP, alginate, Pel, Psl
ABSTRACT
We investigated the effect of temperature on the biofilm formation of Pseudomonas aeruginosa and revealed that the biofilm formation increased rapidly at temperatures lower than 25°C. P. aeruginosa formed the most robust biofilm of a conspicuous mushroom-like structure at 20°C. However, when the temperature increased to 25°C, the biofilm formation rapidly decreased. Above 25°C, as the temperature rose, the biofilm formation increased again little by little despite its less-structured form, indicating that 25°C is the low point of biofilm formation. The intracellular 3′,5′-cyclic diguanylate (c-di-GMP) levels also decreased rapidly as the temperature rose from 20 to 25°C. The expression levels of pelA, algD, and pslA encoding Pel, alginate, and Psl, respectively, were also dramatically affected by temperature, with pelA being regulated in a pattern similar to that of the intracellular c-di-GMP levels, and the pattern seen for algD regulation was the most similar to the actual biofilm formation pattern. Total exopolysaccharide production was thermoregulated and followed the regulation pattern of c-di-GMP. Interestingly, the thermoregulation patterns in biofilm formation were different depending on the strain of P. aeruginosa. Unlike PAO1, another strain, PA14, showed a gradual decrease in biofilm formation and c-di-GMP in the range of 20 to 37°C, and P. aeruginosa clinical isolates also showed slightly different patterns in biofilm formation in conjunction with temperature change, suggesting that different strains may sense different temperature ranges for biofilm formation. However, it is obvious that P. aeruginosa forms more biofilms at lower temperatures and that temperature is an important factor in determining the biofilm formation.
IMPORTANCE Biofilm formation is an important protection mechanism used by most microorganisms and provides cells with many advantages, like high infectivity, antibiotic resistance, and strong survivability. Since most persistent bacterial infections are believed to be associated with biofilms, biofilm control is an important issue in medicine, environmental engineering, and industry. Biofilm formation is influenced by various environmental factors. Temperature is the most direct environmental cue encountered by microorganisms. Here, we investigated the effect of temperature on the biofilm formation of P. aeruginosa, a notorious pathogen, and found that temperature is an important factor determining the amount and structure of biofilms. Low temperatures greatly increase biofilm formation and give biofilms a highly conspicuous structure. Although thermoregulation of biofilm formation is mainly mediated by c-di-GMP, some c-di-GMP-independent regulations were also observed. This study shows how biofilms are formed at various temperatures and provides new insights to control biofilms using temperature.
INTRODUCTION
Surface-attached biofilm formation is an important protection mechanism used by most microorganisms and provides cells with many biological advantages, such as high infectivity, antibiotic resistance, and strong survivability (1, 2). Since most persistent bacterial infections are believed to be associated with biofilms of pathogens, and since biofilm formation causes great loss in many industrial facilities, the control of biofilms is a very important issue in medicine, public health, and industry (3). Pseudomonas aeruginosa, an opportunistic human pathogen that causes a variety of infections on burn wound sites, corneas, and lungs, often forms biofilms in human bodies, which leads to a chronic state of infection by increasing resistance to immunity and antibiotic medication (4, 5).
Biofilm formation is influenced by a variety of environmental cues. Within a cell, these cues sensed by various sensors are merged to a convergent event controlling the intracellular level of 3′,5′-cyclic diguanylate (c-di-GMP) that regulates protein activity or transcription to determine the bacterial lifestyle (4, 6). High concentrations of c-di-GMP promote biofilm formation and suppress planktonic growth by increasing the exopolysaccharide production and decreasing motility (4). Bacterial biofilms are embedded in self-produced extracellular polymeric substances (EPSs), such as exopolysaccharides, extracellular DNA, and proteins, and exopolysaccharides are usually a major component (7). P. aeruginosa has three major exopolysaccharides in its biofilm matrix: alginate, Psl, and Pel (8). In P. aeruginosa, c-di-GMP controls the productions of alginate, Pel, and Psl in two stages: transcription and synthesis. c-di-GMP binds to FleQ, a transcriptional repressor to induce the transcription of the biosynthetic operons for Pel and Psl (pel and psl operons, respectively) (6, 9, 10). c-di-GMP also binds to Alg44 and PelD proteins and enhances their enzymatic activities in alginate and Pel biosynthesis (11, 12). Therefore, while c-di-GMP regulates the production of these three polysaccharides, the intensity of the regulation can be different.
Pathogens such as P. aeruginosa experience big fluctuations in temperature during their infection and transmission cycles. Therefore, some researchers have studied the relationship between temperature and biofilm formation. An early study has shown that in P. aeruginosa strain PA14, biofilm formation and the expression of pel were greatly increased at room temperature compared to those at 37°C (13). A recent study has also demonstrated that in Vibrio cholerae, low temperatures increased biofilm formation and the intracellular c-di-GMP level (14). Interestingly, the study also reported that temperature modulates c-di-GMP levels in a similar fashion in P. aeruginosa, but not in Listeria monocytogenes (14). All of these studies imply that temperature and bacterial biofilm formation are closely related, but despite these prior studies, P. aeruginosa still lacks a series of systematic studies on how temperature change affects biofilm formation. That is, the relationship between biofilms, c-di-GMP, and various EPSs has been reported only in fragments by different research groups, and the detailed temperature range or the difference between P. aeruginosa strains has not been studied. In this study, we focused on elucidating the effects of temperature on biofilm formation, c-di-GMP, and EPSs together in two different P. aeruginosa strains, PAO1 and PA14.
RESULTS
Biofilm formation increases at low temperatures in P. aeruginosa.
To investigate whether temperature affects biofilm formation of P. aeruginosa or not, biofilms of wild-type P. aeruginosa (PAO1) were formed under different temperatures in a static condition. When the biofilms formed at 20, 25, 30, and 37°C were quantified, the biofilms formation was dramatically enhanced at very low temperatures (20°C) compared to those formed at higher temperatures (25, 30, and 37°C) (Fig. 1). Above 25°C, as the temperature rose, the biofilm formation increased again little by little, showing the lowest peak at 25°C (Fig. 1). For comparison, the biofilms were treated with 5 μM sodium nitroprusside (SNP), a well-known biofilm inhibitor that induces biofilm dispersal by generating nitric oxide (NO) (4). In all temperature ranges, SNP reduced the biofilm formation by 20 to 62% (Fig. 1). However, the reduction of biofilm formation by temperature-upshift from 20 to 25°C was 79%, which is much greater than the inhibition by 5 μM SNP.
FIG 1.

Biofilm formation of P. aeruginosa is temperature dependent. Biofilm formation by P. aeruginosa PAO1 was measured using a static biofilm assay. Biofilm was formed at 20, 25, 30, and 37°C for 24 h without shaking; quantified by crystal violet staining (A600); and normalized with planktonic cell growth (OD600). For comparison, biofilm formation was inhibited by 5 μM SNP. The amounts of biofilms are presented as a relative value to that at 20°C. *, P < 0.05; **, P < 0.01; ***, P < 0.005.
Temperature affects the biofilm structure.
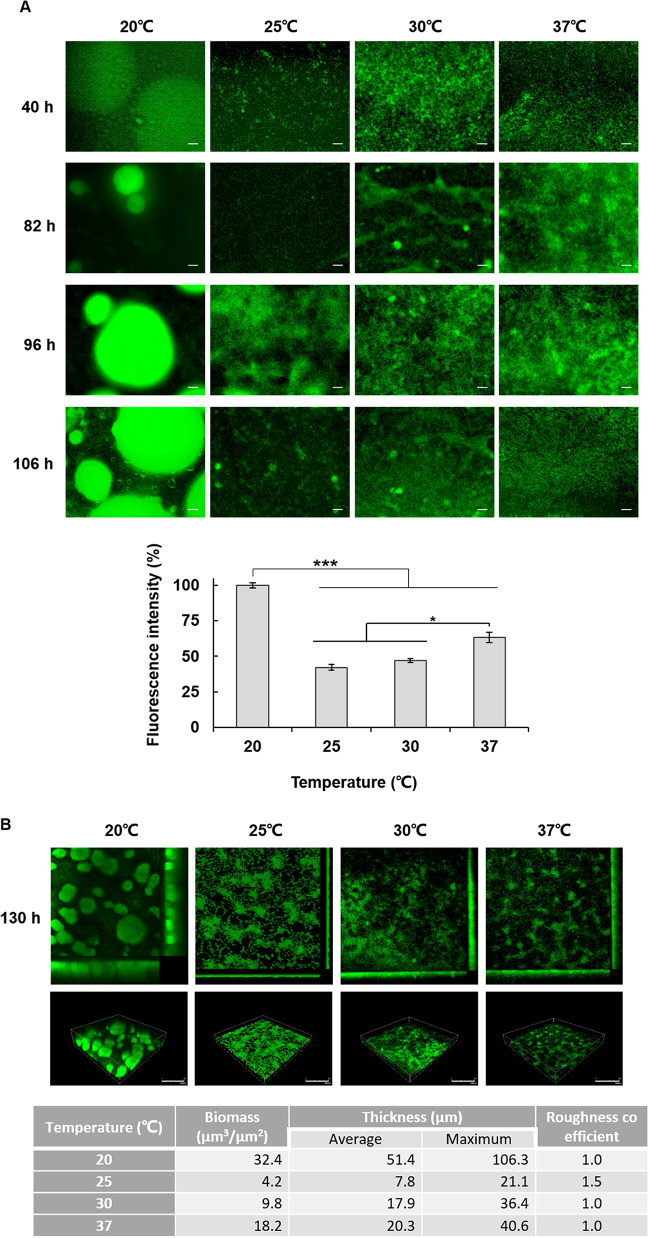
To assess whether temperature influences biofilm structure, biofilms were grown for 130 h in the flow cells at each temperature. The real-time observation of flow-cell biofilms showed that the biofilm grew the largest at 20°C, and as in the case of static biofilm, the smallest biofilm was formed at 25°C and increased slightly again at higher temperatures (Fig. 2A). In addition, biofilms formed at 20°C exhibited a more conspicuous mushroom-like structure (Fig. 2B). COMSTAT analysis revealed that total biofilm biomass and thickness dramatically increased at 20°C compared to those formed at 25, 30, and 37°C (Fig. 2B). Above 25°C, the biofilms were less structured, showing a flat form despite a slight increase in biofilm mass as the temperature rose, and the mushroom-like structure formed only at 20°C (Fig. 2B). These results demonstrate that both the amount and the structure of biofilms are affected by temperature.
FIG 2.
Biofilm structure is also affected by temperature. GFP-expressing P. aeruginosa cells were injected into a flow-cell system, and their biofilms were grown for 6 days at 20, 25, 30, and 37°C in a presence of shear force. (A) Biofilms were observed at the indicated time points by fluorescence microscopy (scale bars, 50 μm). The fluorescence intensity was measured and is presented as a relative value (%). (B) At 130 h, 3D images of the biofilms were taken using CLSM (scale bars, 150 μm) and analyzed by COMSTAT. *, P < 0.05; ***, P < 0.005.
In order to measure the level of planktonic cells in the flow-cell chamber, the optical density (OD) of the drain exiting the flow-cell was measured during biofilm development. Since the level of planktonic cells was very low at 20°C (Fig. 3), it was found that most of the P. aeruginosa cells were growing in biofilm form at 20°C. At higher temperatures, more planktonic cells were detected, demonstrating that high temperature promotes planktonic growth (Fig. 3).
FIG 3.
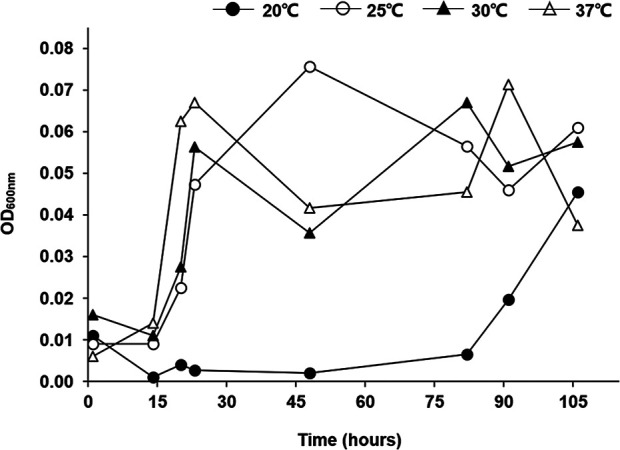
Planktonic growth is reduced at 20°C. At each temperature (20, 25, 30, and 37°C), the density of planktonic bacteria in drains from the flow cells was measured by determining the OD600 for 5 days.
Temperature affects intracellular c-di-GMP level.
c-di-GMP mediates the production of factors required for biofilm formation (4, 6). Because the transcription of cdrA is up- or downregulated according to the c-di-GMP level in P. aeruginosa, it can reflect the intracellular c-di-GMP level. Therefore, we measured the expression of the cdrAp-lacZ reporter with temperature to know the intracellular c-di-GMP levels. Our result showed that it decreased rapidly as the temperature rose from 20 to 25°C, and there was no significant change above 25°C (Fig. 4A). To confirm this result, the intracellular c-di-GMP level was measured again by an independent chemical method, a TO-based fluorescence assay, which showed a consistent result (Fig. 4B). These results clearly show that the rapid decrease in biofilm formation caused by temperature changes between 20 and 25°C is due to a decrease in c-di-GMP. The reason why biofilm formation increased little by little above 25°C without a significant change in c-di-GMP levels is not clear, but we note that Townsley and Yildiz reported that c-di-GMP levels increased slightly but significantly as the temperature rose from 25 to 37°C in P. aeruginosa PAO1 (14).
FIG 4.

Temperature affects c-di-GMP levels in P. aeruginosa. (A) Intracellular c-di-GMP levels were measured by cdrA-lacZ reporter fusion. pSKcdrA (Table 1) was introduced into P. aeruginosa PAO1 by transformation, and the β-galactosidase activity was measured at 20, 25, 30, and 37°C. The c-di-GMP levels are presented as a relative value to the level at 20°C. (B) The c-di-GMP level was measured by using a thiazole orange (TO)-based fluorescence assay. The results are also presented as values relative to the level at 20°C. **, P < 0.01; ***, P < 0.005.
The expression of alg and pel genes is significantly increased at 20°C.
In P. aeruginosa, c-di-GMP controls biofilm formation by modulating the production of alginate, Pel, and Psl, the major EPSs. To assess whether temperature influences the expression of the operons encoding these EPSs, the transcriptions of alg, pel, and psl were measured by using their promoter-lacZ fusion reporters. The results showed that the expression levels of pel, alg, and psl operons were also dramatically affected by temperature, but the patterns were different. The expression of alg rapidly decreased from 20 to 25°C, reaching a low point at 25°C, and then slightly increased again, which was the most similar to the actual biofilm formation pattern (Fig. 5A). The expression of pel was regulated by temperature in a pattern such as a change in intracellular c-di-GMP levels without any increase above 25°C (Fig. 5B). Unexpectedly, the expression of psl gradually increased as the temperature increased from 20 to 37°C (Fig. 5C). Because of this difference in regulation patterns, we directly measured the total amount of exopolysaccharides according to temperature by using a Congo red binding assay. The result showed that exopolysaccharides were produced the most at 20°C; fewer exopolysaccharides were produced at 25, 30, and 37°C, although the levels generated at these three temperatures were similar (Fig. 5D). This result clearly indicates that total exopolysaccharide production is regulated by temperature, and the pattern is similar to the level of intracellular c-di-GMP.
FIG 5.
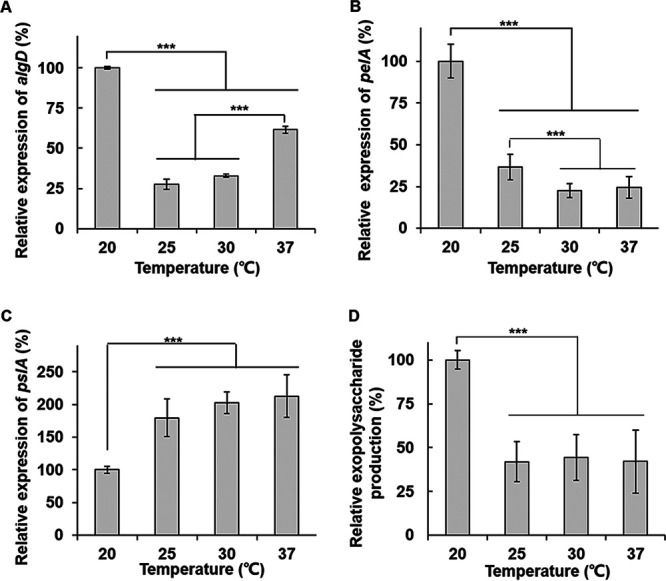
The expressions of alg, pel, and psl operons are thermoregulated in P. aeruginosa PAO1. The expression of the algDp-lacZ (A), pelAp-lacZ (B), and pslAp-lacZ (C) fusions was measured by using a β-galactosidase activity assay in P. aeruginosa PAO1 cells grown at 20, 25, 30, and 37°C. The β-galactosidase activities of each fusion are presented as a relative value to those at 20°C. (D) A Congo red binding assay was carried out with PAO1. The relative amounts of the Congo red-bound exopolysaccharides are presented. ***, P < 0.005.
In conclusion, P. aeruginosa PAO1 biofilms are formed the least at about 25°C. At a temperature lower than 25°C, the level of intracellular c-di-GMP and total exopolysaccharide production rapidly increase, resulting in more and better-structured biofilms. This increase in exopolysaccharides is presumably driven by increases in alginate and Pel. At temperatures higher than 25°C, the c-di-GMP level and the total exopolysaccharide production remain constant, but for some reason, the biofilm still increases little by little as the temperature rises.
Different strains may have different temperature ranges for biofilm formation.
A different P. aeruginosa strain, PA14, was investigated for temperature-dependent biofilm formation. Because strain PA14 does not have functional Psl-biosynthetic operon, the change in biofilm formation with temperature could have been different. When the static biofilm formation of PA14 strain was measured at 20, 25, 30, and 37°C, it was similar to PAO1 in that biofilm formation increased at low temperatures. However, unlike PAO1, the biofilm formation of PA14 gradually increased as the temperature decreased from 37 to 20°C, rather than a dramatic change between 20 and 25°C (Fig. 6A).
FIG 6.
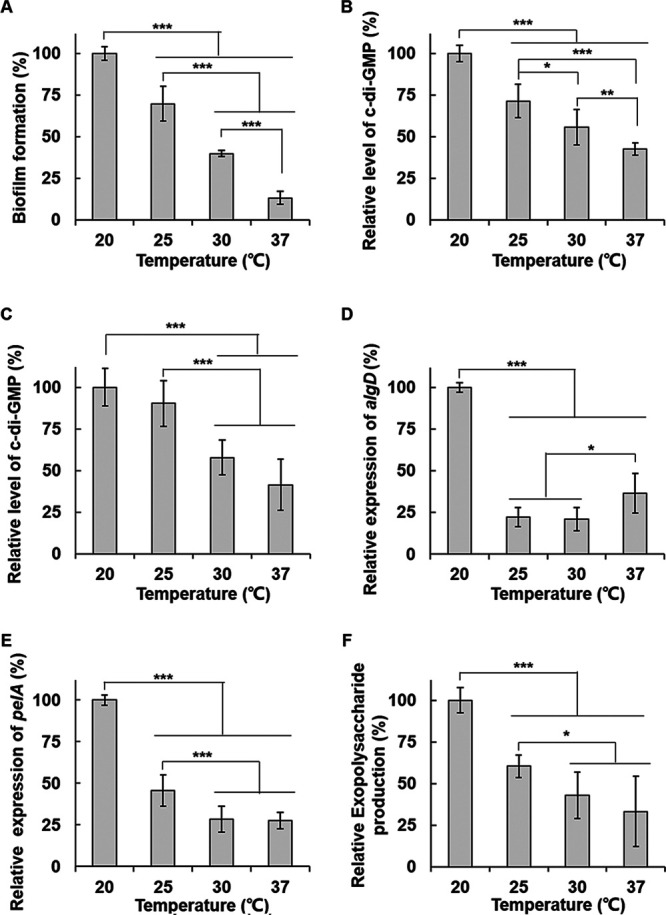
Biofilm formation, intracellular c-di-GMP levels, and the exopolysaccharide production of P. aeruginosa PA14 are thermoregulated in a slightly different pattern. (A) Biofilm formation of PA14 was measured by the static biofilm assay in the same way as in Fig. 1. (B) Intracellular c-di-GMP levels of PA14 were measured by using PA14 cdrAp-lacZ reporter fusion (pQF-cdrA14) in the same way as in Fig. 4A. (C) c-di-GMP levels in PA14 were measured by TO-based fluorescence assay in the same way as in Fig. 4B. (D) The expression of alg operon of PA14 was measured by using PA14 algDp-lacZ fusion (pQF-algD14). (E) The expression of pel operon of PA14 was measured using PA14 pelAp-lacZ fusion (pQF-pelA14). (F) The total exopolysaccharide production in PA14 was measured by using a Congo red binding assay as in Fig. 5D. All data are presented as a relative value to the levels at 20°C. *, P < 0.05; **, P < 0.01; ***, P < 0.005.
In order to find out that this difference is due to intracellular c-di-GMP change, the level of intracellular c-di-GMP was measured in strain PA14 by using PA14 cdrAp-lacZ fusion. The result showed the gradual decrease of c-di-GMP levels at a temperature range between 20 and 37°C (Fig. 6B), which is the same pattern as the biofilm change with temperature. We confirmed this result with the TO-based fluorescence assay, which showed a similar result that the intracellular c-di-GMP levels decreased gradually as the temperature rose from 20 to 37°C (Fig. 6C). These results suggest that the temperature-dependent change of the biofilm formation is also mediated by c-di-GMP in PA14, but the system of PA14 to regulate the c-di-GMP levels recognizes a temperature range different from that of PAO1.
We investigated the expression of exopolysaccharide operons of PA14 using the promoter-lacZ fusions of PA14 algD and pelA. Interestingly, the thermoregulation patterns of alg and pel operons of PA14 were similar to those of PAO1 (Fig. 6D and E). However, when we measured the total exopolysaccharide production, unlike PAO1, it gradually increased as the temperature decreased (Fig. 6F). In PA14, the thermoregulation patterns of the biofilm formation and c-di-GMP levels were similar to that of the total exopolysaccharide production.
We further investigated the temperature-dependent change in biofilm formation with P. aeruginosa clinical strains that were isolated in our previous studies (15). The changes in biofilm formation of the clinical strains with temperature looked like a combination of PAO1 and PA14. Of the five strains tested, four (J108, J153, J161, and J202) showed a gradual increase as the temperature decreased like PA14 but showed a rapid change between 20 and 25°C like PAO1 (Fig. 7). In addition, there were differences between clinical strains. A clinical strain (J16) formed the most biofilm at 25°C and formed less at 20°C (Fig. 7). These results suggest that the temperature range recognized for biofilm formation may vary from strain to strain, whereas it is apparent that P. aeruginosa forms more biofilms at lower temperatures.
FIG 7.
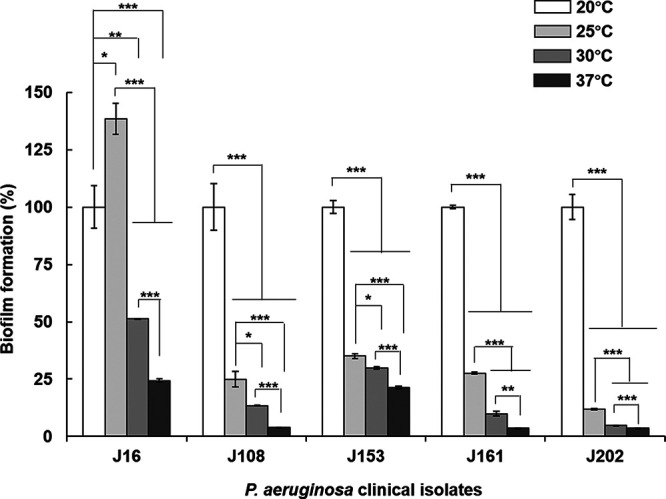
Biofilm formation of P. aeruginosa clinical isolates according to temperature. Biofilm formation of P. aeruginosa clinical isolates (J16, J108, J153, J161, and J202) was investigated using static biofilm analysis in the same way as in Fig. 1. *, P < 0.05; **, P < 0.01; ***, P < 0.005.
DISCUSSION
P. aeruginosa survives in a broad range of temperatures as a ubiquitous bacterium and multihost pathogen. In this study, the effect of temperature on P. aeruginosa biofilm formation was investigated, and the following observations were made. (i) Temperature has a very significant effect on the biofilm formation of P. aeruginosa, which is greater than the effect of biofilm inhibitors known to date. (ii) Low temperatures not only greatly increase the biofilm formation of P. aeruginosa but also give the biofilms a highly conspicuous structure. (iii) The thermoregulation pattern that changes biofilm formation is different depending on the strain of P. aeruginosa, but the most biofilm formation was observed with most strains when the temperature was lowered to 20°C. (iv) Because the biofilm formation and intracellular c-di-GMP level changed with temperature in a similar pattern, the thermoregulation of biofilm formation is mainly mediated by c-di-GMP.
Temperature affects not only biofilm biomass, but also the structure and shape of the biofilm. The results of this study show that this is because the exopolysaccharide biosynthesis is controlled by temperature. In the temperature range between 20 and 37°C, the lower the temperature, the more Pel and alginate are synthesized and more and better-structured biofilms are formed by P. aeruginosa. One peculiarity is the increase of the psl expression with temperature (Fig. 5C), which was unexpected because the transcription of psl has been known to be regulated by FleQ (6). However, the total exopolysaccharide production was highest at 20°C, and there was no significant change above 25°C (PAO1), or it further decreased as the temperature rose (PA14). We think that the decreases in alginate and Pel overwhelm the increase in Psl.
In P. aeruginosa, the temperature-dependent change of biofilm formation is similar to that of c-di-GMP, so it can be said that the biofilm change with temperature is mostly mediated by c-di-GMP. However, the slightly different thermoregulation pattern of c-di-GMP in PAO1 and PA14 suggests that the system of each strain to regulate the c-di-GMP levels may sense temperature changes with different sensitivity. P. aeruginosa clinical isolates also showed slightly different patterns in biofilm formation with temperature changes, supporting the idea that different strains may sense different temperature ranges for biofilm formation. The exact mechanism for this remains to be revealed. Anyway, it is obvious that P. aeruginosa forms more biofilms at lower temperatures.
Finally, the results of this study are consistent with previous studies about the relation between temperature and bacterial biofilm formation. An early study showed that biofilm formation of P. aeruginosa PA14 was affected by temperature and that the expression of pel is dramatically reduced at a high temperature (37°C) (13). A recent study on V. cholerae by Townsley and Yildiz showed that biofilm formation is induced at low temperatures through increased levels of c-di-GMP (14). The temperature range that caused a rapid change in intracellular c-di-GMP levels was between 15 and 25°C in V. cholerae and P. aeruginosa (14). Similarly, most P. aeruginosa strains tested in this study showed a rapid change in biofilm formation between 20 and 25°C. The only difference is seen in a comparison of c-di-GMP levels between 25 and 37°C, which in their experiment was measured as a small but significant difference, and in our experiment, it was determined that there was no significant difference. We do not know why this difference occurred, and it may be because the method of quantifying c-di-GMP is different. However, we think that their results and ours do not contradict much, because the main conclusion—that a dramatic increase in biofilm formation and intracellular c-di-GMP levels occurs below 25°C—is the same.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Bacterial cells were generally grown at 37°C in Luria-Bertani medium (LB; 0.5% yeast extract, 1% Bacto tryptone, 0.5% NaCl) with vigorous shaking. Bacterial growth was measured by optical density at 600 nm (OD600). A green fluorescence protein (GFP)-expressing plasmid, pAB1, was transformed into PAO1 to visualize biofilm formation in a flow cell system. The promoter-lacZ fusion plasmids (Table 1) were also transformed into strain PAO1 or PA14 to measure the gene expression. The transformed cells were selected on carbenicillin-containing media (150 μg/ml). To induce GFP, isopropyl-1-thio-β-d-galactopyranoside (IPTG) was added at 1 mM.
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Description or sequence (5′–3′)a | Source, reference, or purpose |
|---|---|---|
| Strains | ||
| P. aeruginosa | ||
| PAO1 | Wild-type P. aeruginosa | 19 |
| PA14 | Clinical strain of P. aeruginosa | 20 |
| J16 | P. aeruginosa clinical isolate | 15 |
| J108 | P. aeruginosa clinical isolate | 15 |
| J153 | P. aeruginosa clinical isolate | 15 |
| J161 | P. aeruginosa clinical isolate | 15 |
| J202 | P. aeruginosa clinical isolate | 15 |
| E. coli | ||
| DH5α | supE44 ΔlacU169 (ϕ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi‐1 relA1 | 21 |
| Plasmids | ||
| pAB1 | gfp-mut2 gene in pMF54 | 16 |
| pQF50 | Broad-host-range promoterless lacZ fusion plasmid; Apr | 22 |
| pSKcdrA | PAO1 cdrAp-lacZ fusion in pQF50; Apr | 16 |
| pQF-algD | PAO1 algDp-lacZ fusion in pQF50; Apr | This study |
| pQF-pelA | PAO1 pelp-lacZ fusion in pQF50; Apr | This study |
| pQF-pslA | PAO1 pslp-lacZ fusion in pQF50; Apr | This study |
| pQF-cdrA14 | PA14 cdrAp-lacZ fusion in pQF50; Apr | This study |
| pQF-algD14 | PA14 algDp-lacZ fusion in pQF50; Apr | This study |
| pQF-pelA14 | PA14 pelp-lacZ fusion in pQF50; Apr | This study |
| Primers | ||
| AlgDp-F | ACTAGGATCCATTGGCAGGCATTTAA | algDp amplification |
| AlgDp-R | TGATAAGCTTCGCATTCACCTCGATT | algDp amplification |
| PQF50-Up | GCAGCCCAGTAGTAGGTTGAG | Sequence confirmation |
| PQF50-Down | GTTGTAAAACGACGGCCAGTG | Sequence confirmation |
| F-pslAp BamHI | GCCCGGATCCCTTCCGCCTTCGA | pslAp amplification |
| R-pslAp HindIII | AATGAAGCTTGTTTGCTCTGCCG | pslAp amplification |
| F-pelAp BamHI | CGATGGATCCAGACGAAGTGACTT | pelAp amplification |
| R-pelAp HindIII | TGAAAAGCTTGCCCAGCCTA | pelAp amplification |
| F-cdrAp14 BamHI | GATCGGATCCTTGTTGCTGATCGCGA | PA14 cdrAp amplification |
| R-cdrAp14 HindIII | GACGAAGCTTGAAAATCTCCCTATCT | PA14 cdrAp amplification |
| F-algDp14 BamHI | ACTAGGATCCATTGGCAGGCATTTAA | PA14 algDp amplification |
| R-algDp14 HindIII | TGATAAGCTTCGCATTCACCTCGATT | PA14 algDp amplification |
| F-pelAp14 SalI | CTGGGTCGACGAAAACCTGCGGTGT | PA14 pelAp amplification |
| R-pelAp14 HindIII | GCTGAAGCTTATGCCCAGCCTACGCG | PA14 pelAp amplification |
Apr, ampicillin and carbenicillin resistance. Restriction sites are underlined in sequences.
Static biofilm assay.
P. aeruginosa cells were grown overnight in LB broth with vigorous shaking and then inoculated at 2% into a fresh M63 medium (M63 salt [KH2PO4, 12 g/liter; K2HPO4, 28 g/liter; NH4SO4, 8 g/liter], 1 mM MgSO4, 0.5% Casamino Acids, 0.2% citrate) in a 96-well polystyrene plate and incubated at 37°C for 24 h without shaking. Cell growth was then measured at OD600, the planktonic cells were removed, and the plate was washed with water and dried for 10 min. Next, 180 μl of 0.1% crystal violet solution (wt/vol) was added to each well, followed by incubation for 7 min to stain biofilms on well surfaces. After a brief wash, the crystal violet was dissolved by adding 200 μl of absolute ethanol and measured by the absorbance at 600 nm (A600) to quantify biofilm mass. The absorbance was normalized by planktonic cell growth (OD600). All experiments were carried out at least three times.
Flow-cell biofilm assay.
P. aeruginosa cells harboring the GFP-expressing plasmid pAB1 (Table 1) were cultured overnight and diluted to an OD600 of 0.03 in fresh LB broth. Then, 200 μl was injected into a flow-cell chamber (2 mm × 2 mm × 50 mm). After incubation at room temperature for 1 h to allow attachment, the flow cells were placed in 20, 25, 30, and 37°C incubators, and 1% TSB (Bacto tryptic soy broth; BD) containing 150 μg/ml carbenicillin and 1 mM IPTG flowed at 50 μl/min through the flow-cell chambers for 130 h. Temperature was accurately controlled within ±1°C. All experiments were carried out at least three times.
Biofilm imaging and quantification.
Biofilms in flow cells were imaged by confocal laser scanning microscopy (CLSM; Olympus, FV10i) or by fluorescence microscopy (Zeiss, Axioskop FL). Three-dimensional (3D) images of biofilms were reconstructed from plane images using Bitplane Imaris 6.3.1 image analysis software. Fluorescence intensities of flow cell biofilms were quantified using ImageJ software or a COMSTAT plugin in ImageJ.
β-Galactosidase activity assay.
The β-galactosidase activity was measured using a Galacto-Light Plus kit (Applied Biosystems). P. aeruginosa cells were grown overnight in LB broth with vigorous shaking, inoculated into 5 ml of fresh LB medium, and then cultivated for 16 h at the indicated temperature. After measuring the OD600, 100-μl aliquots of cell cultures were mixed with 10 μl of chloroform, vigorously vortexed, and left to stand for 15 min at room temperature. Then, 10-μl portions of supernatants were transferred to a 96-well plate, and a substrate solution of the Galacto-Light Plus kit was added. After a 50-min incubation at room temperature in a dark place, 150 μl of light emission solution (Accelerator II) of the kit was added, and the luminescence was promptly measured using a multiwell plate reader (Tristar LB941; Berthold). The luminescence was normalized using the OD600 of the culture, and the β-galactosidase activities were expressed as luminescence/OD600.
Construction of promoter-lacZ fusions.
The DNA fragments upstream of algD, pelA, and pslA were amplified from P. aeruginosa PAO1 genomic DNA by PCR with specific primers in Table 1 (AlgDp-F/AlgDp-R, F-pelAp BamHI/R-pelAp HindIII, and F-pslAp BamHI/R-pslAp HindIII, respectively). The resulting 493-, 364-, and 419-bp fragments were digested by BamHI-HindIII and cloned into the BamHI-HindIII-digested pQF50, a promoterless lacZ reporter plasmid (Table 1). The PA14 counterparts of these promoter-lacZ reporters were constructed with specific primers in Table 1 (F-cdrAp14 BamHI/R-cdrAp14 HindIII, F-algDp14 BamHI/R-algDp14 HindIII, and F-pelAp14 SalI/R-pelAp14 HindIII, respectively). PA14 does not have the functional psl operon. The correct fusion and no mutation were confirmed by enzyme digestion and sequencing with polycloning site-flanking primers (PQF50-Up and PQF50-Down in Table 1).
Measurement of intracellular c-di-GMP.
The intracellular c-di-GMP levels were measured in the cell cultures by two different methods: (i) measuring β-galactosidase activity of cdrA-lacZ fusion reporter (16) or (ii) measuring fluorescence of ci-di-GMP-bound thiazole orange (TO) (17). For the former method, pSKcdrA-harboring P. aeruginosa strain PAO1 or PA14 was grown overnight, 1% diluted into fresh LB medium, further cultivated for 16 h at the indicated temperature, and then measured for β-galactosidase activity. For the latter method, strain PAO1 or PA14 was cultivated in the same way, harvested, and lysed by sonication in a buffer (10 mM Tris-HCl, 100 mM NaCl [pH 8.0]). Protein concentrations of lysates were determined using Bio-Rad protein assay dye reagent (Bio-Rad). Cellular macromolecules were precipitated by adding perchloric acid (final concentration, 12%) on ice for 10 min and centrifugation. After adding 500 μl of neutralizing buffer (3 M KOH, 0.4 M Tris, 2 M NaCl), the soluble fractions were passed through 0.2-μm filter and a 3-kDa size exclusion column. TO was then added at 30 μM, followed by incubation at 4°C for 12 h. Since TO produces fluorescence when bound to c-di-GMP, the fluorescence intensity reflects the amount of c-di-GMP. Fluorescence was measured using a Tristar LB941 unit (Berthold; excitation, 510 nm; emission, 580 nm).
Measurement of exopolysaccharide production.
To quantify the production of exopolysaccharides, a Congo red binding assay was carried out as described elsewhere (18). Briefly, P. aeruginosa cells were grown in 5 ml of LB medium overnight and diluted 1:100 into 5 ml of fresh LB broth for the main culture. After cultivation to an OD600 of 2, 2 ml of the cultures was harvested by centrifugation at 14,000 × g for 10 min and resuspended in 1 ml of 1% tryptone with 40 μg/ml of Congo red. After incubation for 2 h at 37°C with vigorous shaking, the bacterial surface-associated exopolysaccharide-bound Congo red was removed by centrifugation for 10 min at 14,000 × g, and unbound free Congo red in supernatant was measured by the absorbance at 490 nm (A490). The exopolysaccharide-bound Congo red that reflects the level of exopolysaccharides was quantified by subtracting the A490 value of the sample from the A490 value of the control without cells. All experiments were carried out at least three times.
Statistical analysis.
The Student t test (two-sample assuming equal variances) was used to determine the significance of differences. The analysis was conducted using Excel (Microsoft), and a P value lower than 0.05 was considered a significant difference.
ACKNOWLEDGMENT
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2019R1A2C1010087).
REFERENCES
- 1.Bassler BL, Losick R. 2006. Bacterially speaking. Cell 125:237–246. doi: 10.1016/j.cell.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Parsek MR, Greenberg EP. 2005. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol 13:27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Li XH, Lee JH. 2017. Antibiofilm agents: a new perspective for antimicrobial strategy. J Microbiol 55:753–766. doi: 10.1007/s12275-017-7274-x. [DOI] [PubMed] [Google Scholar]
- 4.Kim SK, Lee JH. 2016. Biofilm dispersion in Pseudomonas aeruginosa. J Microbiol 54:71–85. doi: 10.1007/s12275-016-5528-7. [DOI] [PubMed] [Google Scholar]
- 5.Hancock RE, Speert DP. 2000. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment. Drug Resist Updat 3:247–255. doi: 10.1054/drup.2000.0152. [DOI] [PubMed] [Google Scholar]
- 6.Valentini M, Filloux A. 2016. Biofilms and cyclic di-GMP (c-di-GMP) signaling: lessons from Pseudomonas aeruginosa and other bacteria. J Biol Chem 291:12547–12555. doi: 10.1074/jbc.R115.711507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maunders E, Welch M. 2017. Matrix exopolysaccharides: the sticky side of biofilm formation. FEMS Microbiology Lett 364:fnx120. doi: 10.1093/femsle/fnx120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franklin MJ, Nivens DE, Weadge JT, Howell PL. 2011. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front Microbiol 2:167. doi: 10.3389/fmicb.2011.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baraquet C, Murakami K, Parsek MR, Harwood CS. 2012. The FleQ protein from Pseudomonas aeruginosa functions as both a repressor and an activator to control gene expression from the pel operon promoter in response to c-di-GMP. Nucleic Acids Res 40:7207–7218. doi: 10.1093/nar/gks384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hickman JW, Harwood CS. 2008. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol Microbiol 69:376–389. doi: 10.1111/j.1365-2958.2008.06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitney JC, Whitfield GB, Marmont LS, Yip P, Neculai AM, Lobsanov YD, Robinson H, Ohman DE, Howell PL. 2015. Dimeric c-di-GMP is required for posttranslational regulation of alginate production in Pseudomonas aeruginosa. J Biol Chem 290:12451–12462. doi: 10.1074/jbc.M115.645051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee VT, Matewish JM, Kessler JL, Hyodo M, Hayakawa Y, Lory S. 2007. A cyclic-di-GMP receptor required for bacterial exopolysaccharide production. Mol Microbiol 65:1474–1484. doi: 10.1111/j.1365-2958.2007.05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakuragi Y, Kolter R. 2007. Quorum-sensing regulation of the biofilm matrix genes (pel) of Pseudomonas aeruginosa. J Bacteriol 189:5383–5386. doi: 10.1128/JB.00137-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsley L, Yildiz FH. 2015. Temperature affects c-di-GMP signaling and biofilm formation in Vibrio cholerae. Environ Microbiol 17:4290–4305. doi: 10.1111/1462-2920.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SK, Li XH, Hwang HJ, Lee JH. 2018. Antibiofilm effect of biofilm-dispersing agents on clinical isolates of Pseudomonas aeruginosa with various biofilm structures. J Microbiol 56:902–909. doi: 10.1007/s12275-018-8336-4. [DOI] [PubMed] [Google Scholar]
- 16.Kim SK, Park HY, Lee JH. 2015. Anthranilate deteriorates the structure of Pseudomonas aeruginosa biofilms and antagonizes the biofilm-enhancing indole effect. Appl Environ Microbiol 81:2328–2338. doi: 10.1128/AEM.03551-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XH, Kim SK, Lee JH. 2017. Anti-biofilm effects of anthranilate on a broad range of bacteria. Sci Rep 7:8604. doi: 10.1038/s41598-017-06540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Q, Wood TK. 2009. OmpA influences Escherichia coli biofilm formation by repressing cellulose production through the CpxRA two-component system. Environ Microbiol 11:2735–2746. doi: 10.1111/j.1462-2920.2009.02000.x. [DOI] [PubMed] [Google Scholar]
- 19.Pearson JP, Pesci EC, Iglewski BH. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol 179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung IY, Choi KB, Heo YJ, Cho YH. 2008. Effect of PEL exopolysaccharide on the wspF mutant phenotypes in Pseudomonas aeruginosa PA14. J Microbiol Biotechnol 18:1227–1234. [PubMed] [Google Scholar]
- 21.Sambrook JE, Fritsch F, Maniatis T. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 22.Farinha MA, Kropinski AM. 1990. Construction of broad-host-range plasmid vectors for easy visible selection and analysis of promoters. J Bacteriol 172:3496–3499. doi: 10.1128/jb.172.6.3496-3499.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]