Abstract
Cellulose nanofibers (CNFs) are an emerging engineered nanomaterial that are utilized in a variety of applications, including as a replacement for urea-formaldehyde, and other adhesives, as the binding agent in manufactured fiber and particle boards. To ensure the health and well-being of those producing, installing, or otherwise using cellulose nanofiber boards (CNFBs) it is imperative that the particulate matter (PM) produced during CNFB manipulation be evaluated for toxicity. We developed and internally verified a generation system to examine the PM produced by sanding CNFB using aluminum oxide sandpaper. With 80-grit sandpaper our system produced a low dispersity aerosol, as determined by a scanning mobility particle sizer and an optical particle counter, with a geometric mean of 28 nm (GSD = 1.60). ICP-MS evaluation showed little difference in metal concentrations between CNFB PM and nonsanded CNFB stock. We then used the system to simultaneously generate and expose both male and female C57BL/6J mice acutely for 4 hours at a concentration of 7.9 mg/m3. Sham-exposed controls were treated similarly but without sanding the CNFB. Analysis of bronchoalveolar lavage (BAL) fluid biomarkers showed no signs of inflammatory response at either 4- or 24-hours post exposure. Further, BAL cell viability, number of total cells, and pulmonary cellular recruitment were not significantly changed between the sham-exposed controls and CNFB-exposed mice. Histology further confirmed no pulmonary toxicity as a result of CNFB PM inhalation. We conclude that inhalation of a high concentration of the PM from manipulation of a CNFB did not produce acute toxic responses within 24 hours of exposure.
Graphical Abstract

1. Introduction
Within the past several decades, the incorporation of engineered nanomaterials (ENMs) into consumer goods, industrial processes, medical applications, pharmaceutics, and other facets of commerce has increased at an astonishing rate (Khan et al., 2017; Ng et al., 2018; Stark et al., 2015; Yang and Westerhoff, 2014). Commonly, ENMs are defined as having at least one dimension within the range of 1 and 100 nm. The nano-size range of ENMs often grants them distinct characteristics when compared to larger sized materials of the same chemical composition. These characteristics include shape, crystal size, surface functionality, tensile strength, electronic band gap, dissolution, and increased surface-to-volume ratio. It is these augmented physico-chemical properties that often makes ENMs desirable additives for a cornucopia of goods and production techniques.
Along with being characteristically different than their larger counterparts, some ENMs fall within the classification of being environmentally friendly, renewable, sustainable, or “green”. These green ENMs have garnered much attention because they may be able to replace materials that have negative environmental impacts in selected scenarios. One example of a spotlight material for green ENMs is cellulose (Kargarzadeh et al., 2018; Moon et al., 2016). Cellulose, (C6H10O5)n, is a polysaccharide constructed from β(1→4) linked D-glucose units. Classically, cellulose ENMs are manufactured via an extraction process where the cellulose is removed from the cell walls of wood (Osong et al., 2016). However, recent advances in manufacturing have created the possibility for cellulose ENMs to be made from other plants, such as tomatoes, and several non-plant processes, like growth in algae and bacteria (Trache et al., 2017). There are currently two types of cellulose-based ENMs: cellulose nanocrystals (CNCs) and cellulose nanofibers (CNFs). CNCs commonly have a needle-like microscopic appearance with a smaller aspect ratio, and CNFs have a nano-scale diameter and a micro-scale length (Sofla et al., 2016).
Recently, a novel fiber/particle board has been developed that uses CNFs to adhere the bulk wood material together instead of urea-formaldehyde (Amini et al., 2017; Diop et al., 2017a; Diop et al., 2017b). The resulting CNF board (CNFB) has the potential to be integrated into the construction industry as a product with similar properties and use to that of conventional, urea-formaldehyde containing particle and fiberboards. The replacement of the urea-formaldehyde binding agent with CNFs grants the boards one major health benefit over their predecessors: the reduction of occupational and non-occupational exposure to formaldehyde. Formaldehyde is a well-researched toxicant with a host of adverse health consequences associated with its exposure (Ballenger, 1984; Cockcroft et al., 1982; Godish, 1981; Gupta et al., 1982; İnci et al., 2013; Kamata et al., 1997; Ritchie and Lehnen, 1987; Rusch et al., 1983; Songur et al., 2010). It is classified by the International Agency for Research on Cancer as a known human carcinogen (Baan et al., 2009). One study found that workers manipulating classic formaldehyde bound fiber boards were exposed to formaldehyde and coarse particulate matter during their shift. This exposure caused several workers to have both increased eosinophils and cytokines levels in their nasal lavage fluid (Priha et al., 2004). By removing the urea-formaldehyde binder from the new material, occupational exposure to the toxicant is eliminated from both the production and use standpoints. However, a potential new risk is introduced into the exposome of a worker preparing or installing CNFB at a construction site or carpentry workshop that was not present with the use of classical fiber and particle boards: exposure to CNF particulate material (PM) that is aerosolized when a CNFB is cut, sanded, or otherwise manipulated. The possibility for CNF and other fiber board material airborne exposure was exemplified in a study that found between 1.71 and 11.24 mg/m3 of airborne wood dust from fiber board manufacturing in an operating plant (Thetkathuek et al., 2016).
The goal of the research described in this article was to assess the potential for pulmonary health consequences following acute airborne exposure to dust generated via an occupationally relevant manipulation of CNFB using an in vivo model. The progression of the research was twofold. First, a custom apparatus, the Sanding Aerosol Generation and Exposure System (SAGES), was designed, built, and rigorously tested to ensure it produced repeatable airborne dust profiles from CNFB stock. Second, the SAGES, in conjunction with a nose-only inhalation exposure system, was used to acutely expose C57Bl/6J mice to the SAGES-generated CNFB aerosol. After the exposure, biomarkers for pulmonary toxicity were evaluated from the exposed animals to determine potential inhalation risks posed by CNFB use and manipulation.
2. Methods and Materials
2.1. SAGES Design and Operation
2.1.1. SAGES Construction
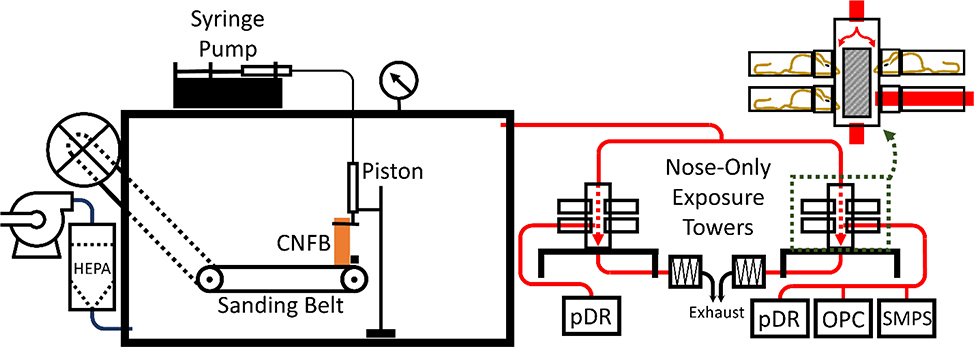
An apparatus was designed and constructed to simultaneously produce and supply aerosols generated from the sanding of CNFB for animal inhalation exposure studies (Fig. 1). The SAGES was inspired by research of Kang, et al. (Kang, 2016; Kang et al., 2016). The SAGES is comprised of a modified belt sander (Central Machinery 97181; Calabasas, CA) confined within a sealable 0.19 m3 aluminum box (Sportsman’s Guide WX2–702056; S. St. Paul, MN) and driven by an external 560 W electric motor. The motor drove the belt at a surface speed of 3.9 m/s. Modifications to the belt sander facilitated external mounting of the drive motor to remove any possibility of particles generated from the running motor contributing to the CNFB dust profile (Szymczak et al., 2007). All factory welds and mounting protrusions on the housing were sealed with silicone caulk. The silicon was given adequate time to dry and off-gas before the SAGES was used for exposure experiments. A rubber gasket and closed-cell PVC foam weather stripping sealed the door to the main body of the chamber. Sealed bearings for the supporting drums of the sanding belt were installed to reduce the possibility of particle contamination from bearing wear.
Fig. 1.
Diagram of the sanding aerosol generation and exposure system (SAGES) in conjunction with a pair of nose-only inhalation towers and auxiliary characterization devices. Design components and operation of the SAGES are discussed in detail in section 2.1. Red tubing indicates particle-laden airflow. Magnification of an exposure tower, outlined by the green dashed box, displays a more detailed representation of the nose-only exposure design. Calibrated pDRs were used to measure real time aerosol concentrations and determine a TWA exposure concentration. The combined use of a SMPS and an OPC allowed for aerosol size characterization for particles ranging from 10 nm to 30 µm.
2.1.2. Stock Feed and Support System
Stock material was fed onto the sanding belt using a system of connected hydraulic pistons. One piston, located externally, was operated using a variable rate syringe pump (Harvard Apparatus 55–2226; Holliston, MA) and in turn actuated the internally mounted piston. Measurements and calculations were conducted to determine a conversion factor between piston pump flow rates to stock feed velocities. Stock material was connected to the internal piston to force the stock against the sanding belt. A fence was installed over the farthest flat edge of the sanding belt to give lateral support to the stock thus keeping the stock perpendicular to the sanding belt during operation. This system allowed for precise control over the stock feed rate and maintained a consistent stock surface area in contact with the sanding belt.
2.1.3. System Airflow
During aerosol exposure studies, HEPA-filtered air was forced by a blower through a port in the left, bottom center of the SAGES. The resulting positive pressure within the system produced a particle laden airflow to aerosol characterization equipment and mouse nose-only exposure towers. While in operation, the system pressure was monitored by a Magnehelic® pressure gauge (Dwyer Instruments Inc. 2030; Michigan City, IN) and was held constant at 0.44 kPa. At this pressure each nose-only inhalation tower was supplied with 4 LPM of exposure air (8 LPM total through the SAGES).
Air was exhausted from the SAGES towards downstream equipment through an exhaust port located 31 cm horizontally and 20 cm above the point of particle generation (Fig. 1). The distance between particle source and exhaust port (37 cm) was chosen to replicate a representative distance between the point of particle generation and breathing zone for a primary exposure in an occupational carpentry or construction setting (Cena et al., 2016). Once through the SAGES exhaust port, the particle-laden air flowed through grounded, conductive exhaust tubing, through a 85Kr charge neutralizer (TSI 3012; Shoreview, MN), and into one of two nose-only inhalation towers (SCIREQ InExpose; Montréal, CA). The SAGES exhaust tubing was kept straight and level with only one 90° turn located at a “T” that split the air stream into the towers with equal length of tubing beyond the “T”.
The CNFB particulate atmosphere entered the top of an exposure tower and was divided into 12 identical exposure airstreams in separate channels. After passing through the tower, the airstreams recombined at the bottom of the tower and were exhausted. Each tower is designed to expose a maximum of 12 mice and each mouse is subject to exposure from one exposure channel. During exposure, mice are held by soft restraints perpendicularly to and with only their snouts protruding into the exposure channels. The use of the nose-only exposure system ensures that the oral and dermal routes of exposure are minimized.
2.1.4. Aerosol Characterization
Aerosol characteristics were determined via direct, isokinetic sampling from one nose-only port on each tower. Three instruments were used to characterize the aerosol: a scanning mobility particle sizer (SMPS) (TSI Inc. 3938; Shoreview, MN) outfitted with an aerosol neutralizer (TSI Inc. 3088; Shoreview, MN) and a standard differential mobility analyzer (DMA) (TSI Inc. 3081L; Shoreview, MN), an optical particle counter (OPC) (Grimm Aerosol Technik 11-C; Ainring, DE), and an aerosol photometer (personal dataRAM [pDR], Thermo Scientific pDR-1500; Waltham, MA). The SMPS – OPC combination allowed for size distribution analysis of particles ranging from 10 nm to 30 μm in 130 size bins. The pDRs were utilized to monitor real-time aerosol concentration during an exposure trial and to collect particulate matter onto a filter to determine the time-weighted average (TWA) concentration. TWA was determined gravimetrically using pre-weighed 37-mm glass microfiber filters (Whatman 1827–037; Middlesex, UK) that were incorporated into the pDRs and a six-place microbalance (Mettler Toledo XP26; Columbus, OH) in a climate-controlled gravimetrics laboratory. We also determined the mass distribution of the aerosol using a Mercer Cascade Impactor.
Samples for transmission electron microscopy (TEM) were collected from the nose-only towers using an electrostatic precipitator (ESPnano Model 100) onto copper grids with carbon support film. Micrographs of CNFB PM were obtained using a JEOL JEM-1230 TEM.
To avoid cross contamination between experiments the SAGES was thoroughly cleaned by vacuuming and dusting with electrostatic wipes between uses. To ensure no residual particulate matter was remaining in the system, a background aerosol measurement was always performed while the SAGES was running but prior to the any stock material making contact with the sanding belt. The background aerosol measurements were then compared to aerosol measurements obtained from the SAGES prior to it ever being used for sanding; i.e. the system was operating but no stock was present.
2.2. Metals Analysis
CNFB stock and collected PM were acid digested in a solution of 9 mL concentrated HNO3 and 1 mL of 30% H2O2 in acid-washed Teflon digestion vessels using a high-performance microwave assisted acid digestion system (ETHOS UP, Milestone Srl.; Sorisole, Italy). The microwave digestion program was set to a ramp for 20 minutes to 210°C followed by a hold for 15 minutes at 210°C. All reagents used for metals analysis were of trace metal grade or better. The digested samples were then diluted with deionized water (>18.2 MΩ cm) and analyzed for metal content using an inductively coupled plasma-mass spectrometer (ICP-MS) (Agilent Technologies 7900; Santa Clara, CA). Quality was assured by analyzing certified reference material purchased from the National Institute of Standards and Technology (NIST No. 1640a; Washington D.C.) and the United States Geological Survey (USGS No. RGM-2; Reston, VA). Recoveries for these samples ranged from 90–105%.
2.3. Animals
C57bl/6 mice (males and females, 4 and 5 weeks old, respectively) were purchased from Jackson Laboratories (Bar Harbor, ME). All animals were housed in polypropylene, fiber covered cages in HEPA-filtered caging units (Thoren; Hazelton, PA) in an AALAC-accredited vivarium provided with food and water ad libitum. Mice were maintained in a 12-hr light/dark cycle. Mice were acclimated for one week prior to study. Animals were selected into CNFB exposure, sham exposed control, and latency groups using a random number generator. Mice were euthanized by an overdose of isoflurane followed by cervical dislocation, thoracotomy, and exsanguination through the heart. All protocols were approved by the University of Iowa’s Institutional Animal Care and Use Committee. Animal handling and exposures conformed to the NIH Guide for the Care and Use of Laboratory Animals.
2.4. BAL Fluid Evaluation
Bronchoalveolar lavage (BAL) was performed on all mice during necropsy. Lungs were washed 3 times with 1 mL of 0.9% sterile sodium chloride solution (Baxter; Deerfield, WI) through an intratracheal catheter inserted just inferior to the cricoid cartilage and collecting the BAL fluid from each wash. BAL fluid was centrifuged at 800 × g for 5 min at 4 °C, supernatants were frozen at −80 °C for future analysis, and pelleted cells were resuspended in Hank’s balanced salt solution (Life-Technologies; Grand Island, NY). An aliquot of homogenous cell suspension was stained with propidium iodide (ORFLO; Ketchum, ID) for cell viability assessment and total cells were counted using a Moxi GO II flow cytometer (ORFLO MXG102; Ketchum, ID). The remainder of the cell suspension was fixed onto microslides with fetal calf serum using a Cytospin 4 (Thermo Scientific; Waltham, MA). Cells were stained using Protocol HEMA 3 stain set (Fischer Diagnostics; Pittsburgh, PA). Cytokines/chemokines were assessed using a multiplex bead assay (Millipore MILLIPLEX MAP; Burlington, MA) with LLODs of 2 pg/mL for all analytes. Total protein was quantified using a Bradford assay. Lactate dehydrogenase (LDH) concentrations were determined via the fluorescent emission produced by the conversion of tetrazolium to formazan in the presence of LDH (Roche Diagnostics US 11644793001; Indianapolis, IN). BAL differential cells were evaluated by light microscopy by two individuals blindly counting 400 cells from each sample.
2.5. Histopathology
After collection of BAL fluid, right lobes of lungs were perfused with 10% Zn formalin (Fisher Scientific; Kalamazoo, MI). Lungs were then embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. Tissues were evaluated for key histopathologic changes including: the presence of inflammatory cell infiltration, evidence of acute lung injury, and lymphoid agglomerates.
2.6. Estimated Deposited Dose
Estimated pulmonary region dose was determined using the computational modeling of regional deposition fraction for mice reported by Asgharian et al. (Asgharian et al., 2014). Assumed parameters of minute volume, and deposition fraction were 25 mL/min and 0.3, respectively.
2.6. Statistical Analysis
Data are expressed as mean ± standard deviation (SD) or geometric mean (GM) with geometric standard deviation (GSD) in parenthesis. Data were analyzed using a one-way analysis of variance (ANOVA) and followed by a Tukey’ Range Test or Dunnett test dependent upon equality of group sizes if significant differences were found between compared groups (Graphpad Software Inc. Prism 8; San Diego, CA) with a p value less than 0.05 being considered significant.
3. Results and Discussion
3.1. SAGES Generated Aerosols
3.1.1. Sanding Belt Grit and Size Distribution
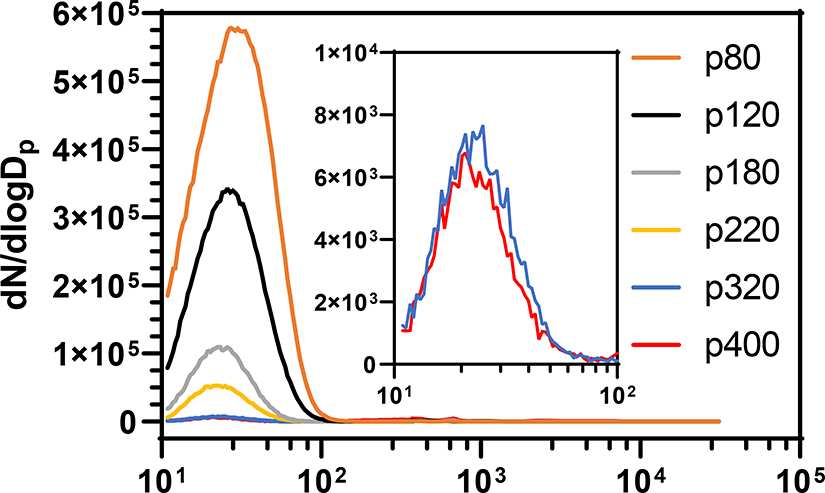
There is debate within the literature about aerosol size distributions that are generated when using different grits of sandpaper (Carlton et al., 2003; Thorpe and Brown, 1994). To guide experimental direction, samples of a consumer grade medium density fiberboard were sanded with one of six aluminum oxide sanding belts (Red Label Abrasives; Belding, MI) of varying grit coarseness using the SAGES at a stock feed rate of 0.5 mm/min (Fig. 2). A new sanding belt was used for each trial and at least two trials were conducted using each grit. Belt grits tested and resulting aerosol size parameters are listed in Table 1. The coarsest grit examined, p80, produced the aerosol with the greatest amount of PM less than 100 nm in diameter. The size profile did not change substantially between sanding belt grits, however, the number of particle counts decreased as the belts decreased in coarseness. The GMs ranged between 24 and 29 nm for all grits less than p400 and the aerosols had GSDs less than or equal to 1.6 for all grits less than p320. All sanding belt grits tested yielded particulate accumulation at the point where the airstream was split for diversion into the two nose-only towers and within the nose-only towers. Most of this accumulation occurred at the split as a result of aerosol impaction. The accumulation within the towers was minor, barely enough to visually identify, and occurred at the point where the exposure airstream met the port for the mouse. Subsequent experiments were conducted using p80 aluminum oxide sanding belts because it produced the greatest amount of ultrafine PM; that is PM with a dimeter less than 100 nm. Recall that all size distribution measurements were taken from the air flowing through the nose-only towers and not within the SAGES chamber. The measured particles represent the smallest of those generated by each sandpaper grade; those that did not settle prior to exiting the chamber. Therefore, a more pronounced difference between the size distribution of the aerosols generated from different sandpaper grades may have been demonstrated if measurements were made within the SAGES chamber.
Fig. 2.
SAGES generated particle size distributions of aerosols produced from medium density fiber board using aluminum oxide sanding belts of various coarseness. Samples of fiber board were sanded at a feed rate of 0.5 mm/min. Size distributions were determined through the combined use of a scanning mobility particle sizer and an aerosol spectrometer/optical particle counter. The resulting data from the instruments were corrected for bin width and dN/dlogDp values were assigned to the bin midpoint before separate instrument data were combined for complete size distribution comparison. The inset shows the ultrafine size distribution of p320 and p400 grits on a different Y-axis scale.
Table 1.
Aerosol GM and GSD by belt grit
Geometric standard deviation
Geometric mean
The findings from the aerosol characterizations showed that a coarser grit sandpaper produced more ultrafine PM than a finer grit. The data in Table 1 show that there is a relatively small difference in GM for grits less than and including p320 and Fig. 2 shows a stark reduction in dN/dlogDp as grits become finer. This evidence indicates that during SAGES sanding more particles are created with coarser grits and finer grits produce a more heterogeneous size distribution.
3.1.2. CNFB Aerosol Concentration, Size Distribution, and Fiber Potential
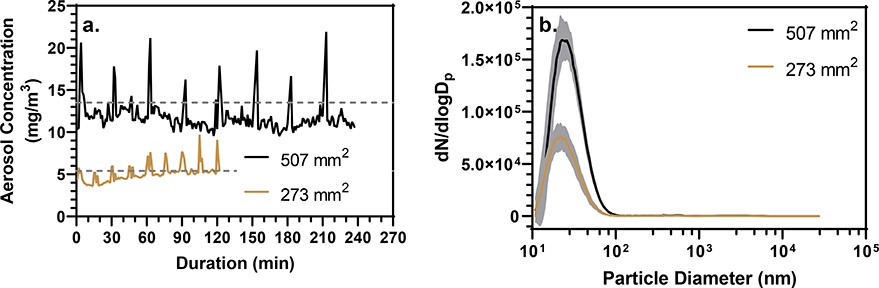
In similar research conducted by our group, an aerosol concentration of 3.5 mg/m3 has been used to model relevant occupational exposures to ENMs (Adamcakova-Dodd et al., 2015; Adamcakova-Dodd et al., 2014; Stebounova et al., 2011). Since the basis of possible CNFB particulate exposure is like that of our previous nanotoxicology studies, it was decided that an inhalable concentration of at least 3.5 mg/m3 would be used for a CNFB exposure. To exercise control over the aerosol concentration the surface area of CNFB in contact with the sanding belt was varied. The stock feed system in conjunction with the support fence were engineered to maintain a perpendicular stock position in relation to the sanding belt and thus a constant sandable surface area. Surface areas of 507 and 273 mm2 at a feed rate of 0.34 mm/min using a p80 aluminum oxide sanding belt produced TWA concentrations, determined gravimetrically, within the exposure towers of 13.5 and 5.4 mg/m3, respectively (Fig. 3a) Continuous pDR monitoring of the aerosol showed a stable concentration over the duration of sanding except for spikes in concentration that occurred when a size distribution sample was taken.
Fig. 3.
(a) Concentration vs. time plot of CNFB aerosol within the exposure towers of two CNFB stocks of different surface areas as monitored by a calibrated pDR. The spikes in concentration occurred during sampling for size distribution analysis by SMPS and OPC. Dashed lines through each concentration profile indicate the TWA concentrations of 13.5 and 5.4 mg/m3 that were determined gravimetrically from the pDR filters. (b) Aerosol size distributions produced from two CNFB stocks of different surface areas as determined by SMPS and OPC. Shaded areas around mean lines display the standard deviation of the eight samples taken during each experiment. The aerosols had GMs (GSDs) of 27 (1.52) and 24 nm (1.49) for the 507 and 273 mm2 CNFB stocks, respectively.
TEM analysis of SAGES generated CNFB samples showed two different particle types (Fig. S1). Most visible particles were less than 100 nm across their largest dimension, thus aligning with the other size distribution data obtained by SMPS/OPC. Larger particles were ranging from 600 to 1000 nm across their longest dimension, however, counts of larger particles were low. Larger particles showed angular protrusions off of the main body, whereas the smaller particles were amorphous.
In a similar fashion to how CNF are incorporated into CNFB, carbon nanotubes have been added to several materials for form new composites. A major concern with all of these ENM composite materials is the potential for the ENM to be released, in any form, to possibly create a health hazard. Our TEM analysis shows no evidence of a “pristine” CNF fiber being released from the CNFB. This finding agrees with the majority of ENM composite materials that have been evaluated to date (Froggett et al., 2014).
CNFB aerosols produced from the 507 and 273 mm2 stocks had GMs of 27 (1.52) and 24 (1.49) nm, respectively (Fig. 3b). Changes in CNFB surface area did not produce a drastic change in GM, however in accordance with the observed decrease in concentration, there were less total particles produced by the 273 mm2 stock compared with the 507 mm2 stock. These data indicate that the concentration of the CNFB aerosol can be controlled through manipulation of the CNFB surface area in contact with the sanding belt, the SAGES can produce a relatively consistent aerosol concentration, and that surface area does not have a large impact of the GM of the produced aerosol.
3.2. Metal Analysis of CNFB Aerosol
Acid digested samples of CNFB stock and collected PM were analyzed for metal content by ICP-MS (Al, Fe, Na, Mg, K, Ca, Cr, Mn, Ni, Cu, and Zn, Table 2). Al was found to be unenriched with only a 1% increase in concentration compared to nonsanded CNFB stock. Al enrichment was of interest because aluminum oxide was the abrasive of the sanding belts. Fe was enriched with a 20% concentration increase in the collected PM compared to the nonsanded stock. Mg was the only other metal to be enriched through the aerosol generation process increasing from 191 ppm in the nonsanded stock to 205 ppm in the particulate, a 7% increase. Ca, Na, K, Mn, Zn, and Ni were found to have deceased concentration in the generated PM compared to the nonsanded stock. Concentrations of Cu and Cr were unchanged between the samples. These data indicate that the SAGES enriches the generated aerosol, when compared with nonsanded stock, with Fe; slightly alters the concentrations of Ca, Na, K, Mg, Mn, and Ni; and, most notably, does not change the aluminum composition.
Table 2.
CNFBa aerosol metal analysis
| Metal | ppm |
||
|---|---|---|---|
| Nonsanded CNFBa Stock Material | SAGESb CNFB PM | PMc % change from stock | |
| Al | 75 | 76 | −1 |
| Fe | 132 | 159 | −20 |
| Ca | 1096 | 1060 | −3 |
| Na | 357 | 327 | −8 |
| K | 272 | 253 | −7 |
| Mg | 191 | 205 | −7 |
| Mn | 57 | 53 | −7 |
| Zn | 39 | 13 | −67 |
| Cu | 3 | 3 | −0 |
| Ni | 4 | 2 | −50 |
| Cr | 2 | 2 | −0 |
cellulose nanofiber board
sanding aerosol generation and exposure system
particulate matter
3.3. CNFB and SAGES Sham Exposures
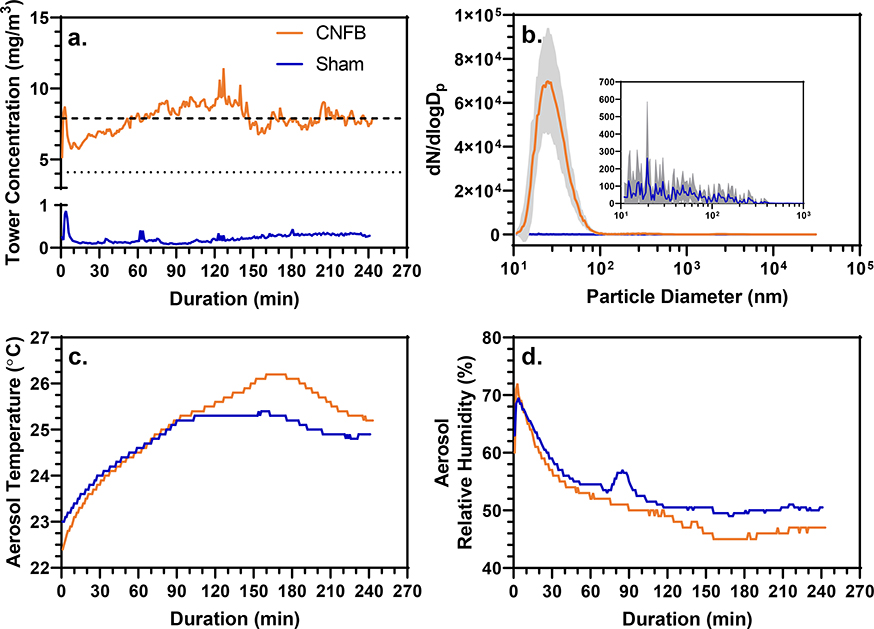
Using the SAGES in conjunction with two mouse nose-only inhalation towers an acute exposure to a CNFB aerosol was conducted with male and female C57Bl/6J mice (11 each). The exposure was 4 hrs in duration with an average concentration of 7.9 mg/m3 produced from a 450 mm2 CNFB stock being sanded by a p80 aluminum oxide sanding belt at 0.34-mm/min stock feed rate (Fig. 4a). Our exposure concentration is slightly higher than the OSHA regulation for soft wood dust (8-hour TWA-TLV is 5 mg/m3), however it is still occupationally relevant because there is evidence of factories operating with occupational exposures near this concentration (Thetkathuek et al., 2016). The CNFB exposure aerosol had a GM of 28 nm (1.48) (Fig. 4b). At the completion of the exposure the mice were removed from the tower and randomly assigned to one of two latency groups with necropsy 4 hours after the beginning of the exposure or 24 hours post exposure.
Fig. 4.
(a) Tower concentration vs. exposure duration of the CNFB and sham exposures as monitored by gravimetrically calibrated pDRs. The dashed line indicates the TWA total particulate concentration over the duration of the CNFB exposure (7.9 mg/m3) determined gravimetrically from pDR filters. The dotted line indicates the TWA CNFB mouse inhalable fraction (4.1 mg/m3. The SAGES control exposure had a TWA concentration of 0.22 mg/m3 determined from pDR filter gravimetric analysis. (b) Aerosol size distributions of the CNFB and sham exposures. The CNFB and sham exposures had a GM(GSD)s of 28 nm (1.48) and 39 nm (2.24), respectively. Gray area surrounding average line shows standard deviation of the size distribution samples from each exposure. (c and d) Aerosol temperature and relative humidity over the exposure duration.
The results from the mass distribution of the CNFB aerosol utilizing a Mercer Cascade impactor showed that 20% if the aerosol mass is less than 3 μm in aerodynamic diameter and thus inhalable by a mouse (Fig. S2) (Asgharian et al., 2014). Applying the 20% inhalable fraction to the exposure concentration of 7.9 mg/m3 results in an inhalable concentration of 1.6 mg/m3. Using the exposure parameters described above, the pulmonary regional deposition of the aerosol was calculated to be 25.3%. Thus, the estimated deposited dose in the pulmonary region was 2.5 μg per mouse. The inhalable fraction of the gross concentration falls within the OSHA regulation for soft wood dust of 5 mg/m3. However, there is concern about whether PM from bulk materials containing ENMs need to be further regulated to include the possibility for ENM release during bulk material manipulation. Currently, there is no regulatory standard within the U.S. for CNF. Being that CNF is a fibrous material, regulation of it, along with carbon nanotubes, has been proposed to follow the regulation standards for asbestos (Zumwalde, 2013). As noted above, the CNFB PM we generated, did not contain and fibers, and thus would not exhibit the same health hazards as an aerosol containing fibers.
Sham exposure was conducted using the same parameters as for the CNFB exposure except the stock feed system held no material. Prior to the sham exposure, the interior of the system was thoroughly cleaned via vacuuming and electrostatic dust wiping and all tubing was replaced. The average airborne particulate concentration within the inhalation towers was 0.22 mg/m3 with a GM of 39 nm (2.24) (Figs. 4a and b). There were no major differences between the sham exposure and CNFB exposure with regard to temperature and relative humidity of the air in the exposure system (Figs. 4c and d). In accordance with the CNFB-exposed mice, the sham-exposed mice were separated into two groups and necropsied at 4- and 24-hours post exposure.
3.4. Toxicological Assessment
3.4.1. BAL Total and Differential Cell Counts and Histology
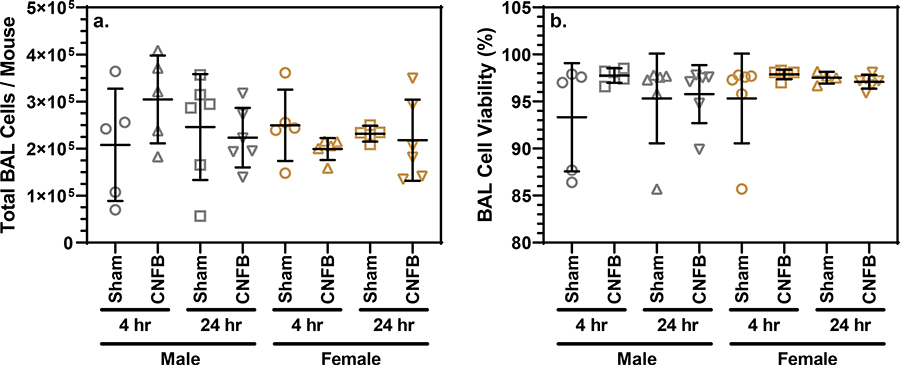
Inhalation of toxic materials may result in recruitment of inflammatory cells into the bronchoalveolar space. We observed no significant differences in total BAL fluid cell populations of CNFB-exposed mice when compared to sex- and latency-matched controls (Fig. 5a). Macrophages dominated the cell population with 0.09%, 0.06%, and 0.02% of cells being neutrophils, lymphocytes, and eosinophils, respectively. Stained sections of right lung lobes were examined for the presence of inflammatory cell infiltration, evidence of acute lung injury, and lymphoid agglomerates. No histological differences were observed between CNFB-exposed and sham-exposed mice (Fig. S3). The lack of granulocytes within the BAL of CNFB-exposed, no cellular recruitment, and the absence of pathology in lung sections provided evidence that there was minimal inflammation within 24 hours of CNFB PM inhalation.
Fig. 5.
(a) Total BAL cells per mouse compared by treatment and latency grouping. (b) BAL cell viability compared by treatment and latency grouping. Bars indicate mean and standard deviation. One-way ANOVA testing revealed lack of statistically significance differences between sex- and latency-matched sham and exposure groups.
3.4.2. BAL Cell Viability, LDH, and Total Protein
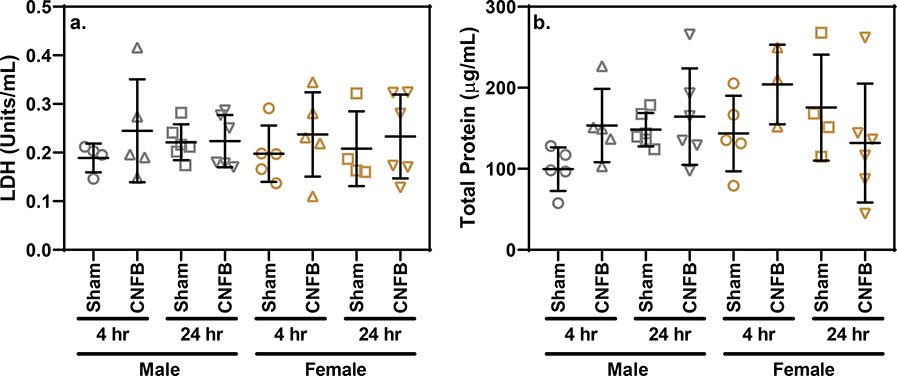
There were no significant differences observed between sex- and latency-matched sham-exposed controls and CNFB-exposed mice with regard the cell viability within BAL (Fig. 5b). All groups had mean BAL cell viability above 93%. The fact that BAL cells are nearly all viable from mice exposed to CNFB shows that CNFB particulate inhalation was not harmful to lung cells at the concentration administered. LDH quantification also showed no significant difference between CNFB-exposed and sham-exposed latency- and sex-matched groups (Fig. 6a). Similar to other biomarkers, one-way ANOVA analysis showed no significant difference between groups with regard to total protein concentration (Fig 6b). The lack of total protein accumulation or LDH release among CNFB-exposed mice indicates a low potential for acute broad spectrum lung toxicity upon CNFB inhalation.
Fig. 6.
(a) LDH concentration within BAL supernatant compared by treatment and latency grouping. (b) Total protein concentration with BAL supernatant compared by treatment and latency grouping. Bars indicate mean and standard deviation. One-way ANOVA testing revealed lack of statistically significance differences between sex- and latency-matched sham and exposure groups.
3.5.3. BAL Cytokines
Previous studies on nanoparticle pulmonary toxicity have shown increases in inflammatory cytokine and chemokine production in response to the inhalation of ENMs (Adamcakova-Dodd et al., 2015; Adamcakova-Dodd et al., 2014; Grassian et al., 2007; Kim et al., 2011). In this study, most of the cytokines determined in BAL fluid: IFN-γ, IL-1β, TNF-α, IL-6, KC, and G-CSF were below LLOD [2 pg/mL]. Samples with quantifiable concentrations of a cytokine were randomly distributed between the cohorts and near the LOD. Since the cytokines examined are maintained in a homeostatic environment at low levels within the lung, these data confirm that there was no inflammation produced by the inhalation of CNFB PM.
3.6. Study Considerations and Limitations
Commercial and industrial applications using fiberboards, like CNFB, include sawing, drilling, and sanding. One limitation of our study is that only sanding was chosen as the method of particle generation. Following are the reasons for this selection: 1) a belt sander provided a compact and safe platform for particle generation. Saws are larger, more hazardous, and more difficult to integrate into a generation system. 2) the use of a belt sander provided enhanced control over PM concentration as established in fig. 4, with little effect on the size distribution of the aerosol. Concentration control with the SAGES was exercised through changing the surface area of the stock in contact with the sanding belt. Particle generation from sawing would be difficult to control. The SAGES feed rate of 0.34 mm/min was chosen because it allowed for the maximum length of stock to be sanded in 4.5 hours and thus didn’t require any other user input during the exposure. 3) research has shown that sanding is the application that generates the most airborne PM in occupational woodworking environments (Brosseau et al., 2001; Scheeper et al., 1995).
Our study employed an acute screening inhalation protocol. Further investigation utilizing a sub-chronic exposure design is warranted. However, our acute inhalation protocol has demonstrated inflammatory responses to particulate exposure that are predictive of longer-term outcomes (Areecheewakul et al., submitted). Others have also reported acute effects of PM exposure at both, 4 and 24 hours after exposure (Uski et al., 2012). These previous studies indicate that inhalation of PM can have an acute impact and if present, the screening methodology described here would have detected it.
4. Conclusions
We created and tested a system for the repeatable production and characterization of CNFB particulate matter. The results from our research show that our PM generation system produced primarily ultrafine PM, by particle count, from the sanding of CNFB. We also noted that our system produces more PM with coarser sanding belts with minor changes to aerosol size characteristics seen between sanding belt coarseness. No evidence of airborne fibers was found in any of the samples. The lack of fibers leads us to believe that CNFB PM resembles that of soft wood dust and not that of fibrous materials. From a regulation standpoint, we conclude that CNFB PM should fall under soft wood dust and not be subject to the stricter regulations associated with fibrous materials. We used our system to expose a cohort of mice to CNFB PM. Evaluation of several inhalation toxicology screening endpoints revealed no evidence of acute toxicity as a result of CNFB PM inhalation. Being that we were only able to perform an acute exposure, further investigation is needed to rule out the possibility of toxic effects associated with chronic exposures and longer latency periods. More research is warranted before the safety profile of CNFB is established. Since CNFB is a new material, still in the research and development phase we are the first study to examine its toxic potential. CNFB PM released during SAGES manipulation does not contain fibers and shows little potential for acute toxic effects at the concentration and dose studied.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number (NIH grant # U01ES027252) as part of the Nanotechnology Health Implications Research (NHIR) Consortium which focuses on comprehensive evaluation of interactions between ENMs and biological systems. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The CNFB described in the research presented in this publication was procured/developed, characterized, and provided by the University of Maine’s Laboratory of Renewable Materials. The CNF used to produce the CNFB was procured/developed, characterized, and provided by the Engineered Nanomaterials Resource and Coordination Core (NIH grant # U24ES026946) as part of the Nanotechnology Health Implications Research Consortium.
The research was conducted in laboratory facilities supported by the Environmental Health Sciences Research Center funded by NIH P30 ES005605.
We would also like to acknowledge the University of Iowa’s Comparative Pathology Laboratory and Central Microscopy Research Facility for their assistance with the research conducted.
Funding
This work was supported by the National Institute of Environmental Health Sciences Grants NIH U01 ES027252 and NIH P30 ES005605.
List of Abbreviations
- BAL
bronchoalveolar lavage
- BALF
bronchoalveolar lavage fluid
- CNC
cellulose nano-crystal
- CNF
cellulose nano-fiber
- CNFB
cellulose nano-fiber board
- EF
enrichment factor
- ENM
engineered nanomaterial
- GM
geometric mean
- GSD
geometric standard deviation
- ICP-MS
inductively coupled plasma-mass spectrometer
- LDH
lactate dehydrogenase
- LLOD
lower limit of detection
- OPC
optical particle counter
- pDR
personal dataRAM
- PM
particulate matter
- SAGES
sanding aerosol generation and exposure system
- SMPS
scanning mobility particle sizer
- TWA
time-weighted average
Footnotes
Declarations
The authors declare they have no conflicts of interest.
Citations
- Adamcakova-Dodd A, Monick MM, Powers LS, Gibson-Corley KN, and Thorne PS (2015). Effects of prenatal inhalation exposure to copper nanoparticles on murine dams and offspring. Part Fibre Toxicol 12, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamcakova-Dodd A, Stebounova LV, Kim JS, Vorrink SU, Ault AP, O’Shaughnessy PT, Grassian VH, and Thorne PS (2014). Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part Fibre Toxicol 11, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini E, Tajvidi M, Gardner DJ, and Bousfield DW (2017). Utilization of Cellulose Nanofibrils as a Binder for Particleboard Manufacture. Bioresources 12. [Google Scholar]
- Asgharian B, Price OT, Oldham M, Chen L-C, Saunders EL, Gordon T, Mikheev VB, Minard KR, and Teeguarden JG (2014). Computational modeling of nanoscale and microscale particle deposition, retention and dosimetry in the mouse respiratory tract. Inhal Toxicol 26, 829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baan R, Grosse Y, Straif K, Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, and Cogliano V (2009). A review of human carcinogens—Part F: Chemical agents and related occupations. Lancet Oncol 10, 1143–1144. [DOI] [PubMed] [Google Scholar]
- Ballenger JJ (1984). Some effects of formaldehyde on the upper respiratory tract. Laryngoscope 94, 1411–1413. [PubMed] [Google Scholar]
- Brosseau L, Parker D, Lazovich D, Dugan S, Milton T, and Pan W (2001). Inhalable dust exposures, tasks, and use of ventilation in small woodworking shops: a pilot study. AIHAJ 62, 322–329. [DOI] [PubMed] [Google Scholar]
- Carlton GN, Patel KB, Johnson DL, and Hall TA (2003). The effectiveness of handheld ventilated sanders in reducing inhalable dust concentrations. Applied occupational and environmental hygiene 18, 51–56. [DOI] [PubMed] [Google Scholar]
- Cena LG, Chen BT, and Keane MJ (2016). Evolution of Welding-Fume Aerosols with Time and Distance from the Source: A study was conducted on the spatiotemporal variability in welding-fume concentrations for the characterization of first- and second-hand exposure to welding fumes. Weld J 95(suppl), 280s–285s. [PMC free article] [PubMed] [Google Scholar]
- Cockcroft DW, Hoeppner VH, and Dolovtch J (1982). Occupational Asthma Caused by Cedar Urea Formaldehyde Particle Board. Chest 82, 49–53. [DOI] [PubMed] [Google Scholar]
- Diop CIK, Tajvidi M, Bilodeau MA, Bousfield DW, and Hunt JF (2017a). Evaluation of the incorporation of lignocellulose nanofibrils as sustainable adhesive replacement in medium density fiberboards. Ind Crops Prod 109, 27–36. [Google Scholar]
- Diop CIK, Tajvidi M, Bilodeau MA, Bousfield DW, and Hunt JFJC (2017b). Isolation of lignocellulose nanofibrils (LCNF) and application as adhesive replacement in wood composites: example of fiberboard. Cellulose 24, 3037–3050. [Google Scholar]
- Froggett SJ, Clancy SF, Boverhof DR, and Canady RA (2014). A review and perspective of existing research on the release of nanomaterials from solid nanocomposites. Part Fibre Toxicol 11, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godish T (1981). Formaldehyde and Building-Related Illness. JEH 44, 116–121. [Google Scholar]
- Grassian VH, O’Shaughnessy PT, Adamcakova-Dodd A, Pettibone JM, and Thorne PS (2007). Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ Health Perspect 115, 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta KC, Ulsamer AG, and Preuss PW (1982). Formaldehyde in indoor air: Sources and toxicity. Environ Int 8, 349–358. [Google Scholar]
- Inci M, Zararsiz I, Davarci M, and Görür S (2013). Toxic effects of formaldehyde on the urinary system. Turk J Urol 39, 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata E, Nakadate M, Uchida O, Ogawa Y, Suzuki S, Kaneko T, Saito M, and Kurokawa Y (1997). Results of a 28-month chronic inhalation study of formaldehyde in male Fisher-344 rats. J Toxicol Sci 22, 239–254. [DOI] [PubMed] [Google Scholar]
- Kang J (2016). A Lab-based Study for the Generation and Characterization of Particle Emission from Composite Materials Containing Carbon Nanotubes. (ProQuest Dissertations Publishing, West Virginia University; ), p. 84. [Google Scholar]
- Kang J, Erdely A, Afshari A, Casuccio G, Bunker K, Lersch T, Dahm M, Farcas D, and Cena L (2016). Generation and characterization of aerosols released from sanding composite nanomaterials containing carbon nanotubes. NanoImpact 5. [Google Scholar]
- Kargarzadeh H, Mariano M, Gopakumar D, Ahmad I, Thomas S, Dufresne A, Huang J, and Lin N (2018). Advances in cellulose nanomaterials. Cellulose 25, 2151–2189. [Google Scholar]
- Khan A, Wen Y, Huq T, and Ni Y (2017). Cellulosic nanomaterials in food and nutraceutical applications: a review. J Agric Food Chem 66, 8–19. [DOI] [PubMed] [Google Scholar]
- Kim JS, Adamcakova-Dodd A, O’Shaughnessy PT, Grassian VH, and Thorne PS (2011). Effects of copper nanoparticle exposure on host defense in a murine pulmonary infection model. Part Fibre Toxicol 8, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RJ, Schueneman GT, and Simonsen JJJ (2016). Overview of Cellulose Nanomaterials, Their Capabilities and Applications. JOM 68, 2383–2394. [Google Scholar]
- Ng C-T, Baeg G-H, Yu LE, Ong C-N, and Bay B-H (2018). Biomedical applications of nanomaterials as therapeutics. Curr Med Chem 25, 1409–1419. [DOI] [PubMed] [Google Scholar]
- Osong SH, Norgren S, and Engstrand P (2016). Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: a review. Cellulose 23, 93–123. [Google Scholar]
- Priha E, Pennanen S, Rantio T, Uitti J, and Liesivuori J (2004). Exposure to and acute effects of medium-density fiber board dust. J Occup Environ Hyg 1, 738–744. [DOI] [PubMed] [Google Scholar]
- Ritchie IM, and Lehnen RG (1987). Formaldehyde-related health complaints of residents living in mobile and conventional homes. Am J Public Health 77, 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch GM, Clary JJ, Rinehart WE, and Bolte HF (1983). A 26-week inhalation toxicity study with formaldehyde in the monkey, rat, and hamster. Toxicol Appl Pharmacol 68, 329–343. [DOI] [PubMed] [Google Scholar]
- Scheeper B, Kromhout H, and Boleij JS (1995). Wood-dust exposure during wood-working processes. Ann Occup Hyg 39, 141–154. [PubMed] [Google Scholar]
- Sofla MRK, Brown RJ, Tsuzuki T, and Rainey TJ (2016). A comparison of cellulose nanocrystals and cellulose nanofibres extracted from bagasse using acid and ball milling methods. Adv Nat Sci-Nanosci 7, 035004–035013. [Google Scholar]
- Songur A, Ozen OA, and Sarsilmaz M (2010). The Toxic Effects of Formaldehyde on the Nervous System In Reviews of Environmental Contamination and Toxicology, Whitacre DM, ed. (New York, NY: Springer; ), pp. 105–118. [DOI] [PubMed] [Google Scholar]
- Stark WJ, Stoessel PR, Wohlleben W, and Hafner A (2015). Industrial applications of nanoparticles. Chem Soc Rev 44, 5793–5805. [DOI] [PubMed] [Google Scholar]
- Stebounova LV, Adamcakova-Dodd A, Kim JS, Park H, O’Shaughnessy PT, Grassian VH, and Thorne PS (2011). Nanosilver induces minimal lung toxicity or inflammation in a subacute murine inhalation model. Part Fibre Toxicol 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymczak W, Menzel N, and Keck L (2007). Emission of ultrafine copper particles by universal motors controlled by phase angle modulation. J Aerosol Sci 38, 520–531. [Google Scholar]
- Thetkathuek A, Yingratanasuk T, and Ekburanawat W (2016). Respiratory Symptoms due to Occupational Exposure to Formaldehyde and MDF Dust in a MDF Furniture Factory in Eastern Thailand. Adv Prev Med 2016, 3705824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe A, and Brown RC (1994). Measurements of the effectiveness of dust extraction systems of hand sanders used on wood. Ann Occup Hyg 38, 279–302. [DOI] [PubMed] [Google Scholar]
- Trache D, Hussin MH, Haafiz MKM, and Thakur VK (2017). Recent progress in cellulose nanocrystals: sources and production. Nanoscale 9, 1763–1786. [DOI] [PubMed] [Google Scholar]
- Uski OJ, Happo MS, Jalava PI, Brunner T, Kelz J, Obernberger I, Jokiniemi J, and Hirvonen MR (2012). Acute systemic and lung inflammation in C57Bl/6J mice after intratracheal aspiration of particulate matter from small-scale biomass combustion appliances based on old and modern technologies. Inhal Toxicol 24, 952–965. [DOI] [PubMed] [Google Scholar]
- Yang Y, and Westerhoff P (2014). Presence in, and release of, nanomaterials from consumer products In Nanomaterial Impacts on Cell Biology and Medicine, Capco DG, and Chen Y, eds. (Dordrecht: Springer; ), pp. 1–17. [DOI] [PubMed] [Google Scholar]
- Zumwalde RD (2013). Occupational exposure to carbon nanotubes and nanofibers. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.