Abstract
Background
For people with HIV (PWH) and alcohol use disorder (AUD) who initiated behavioral treatment (BAUD) we: 1) describe BAUD intensity and medication (MAUD); and 2) examine whether BAUD and MAUD were associated with changes in HIV-related outcomes (CD4 cell count, HIV-1 viral load [VL], VACS Index score 2.0, and antiretroviral [ARV] adherence) from before to one year after treatment initiation.
Methods
We used Veterans Aging Cohort Study (VACS) data to describe BAUD intensity and MAUD (acamprosate, disulfiram, and naltrexone, gabapentin or topiramate). Linear regression models estimated changes in outcomes and included BAUD, MAUD, age and race/ethnicity.
Results
We identified 7,830 PWH who initiated BAUD from 01/2008–09/2017. Median age was 53, 53% were African-American and 35% white. BAUD intensity groups were: 1) Single Visit - 35%; 2) Minimal - 44% recieved ~2 visits during first month; 3) Sustained Moderate - 17% recieved ~8 visits/month initially; and 4) Intensive - 4% started out receiving ~14–16 visits/month. Only 9% recieved MAUD, the majority of which was gabapentin. Among those with detectable VL: all HIV-related outcomes improved more among those with more intensive BAUD. Among those with undetectable VL: adherence improved more among those with greater BAUD intensity. MAUD was associated with increased CD4 among those with detectable VL and with improved adherence among both groups.
Conclusion
Of those with >1 BAUD visit, only 21% received at least moderate BAUD and 9% received at least 6 months of MAUD. Increasing AUD treatment intensity may improve HIV-related outcomes, especially among those with detectable VL.
Keywords: HIV, alcohol use disorder, behavioral treatment, medication treatment, HIV outcomes
1. Introduction
Unhealthy alcohol use is common among people with HIV (PWH) and is associated with increased HIV severity and decreased antiretroviral (ARV) medication adherence, quality of HIV care, viral suppression, and survival (Baum et al., 2010; Bensley et al., 2019; Deiss et al., 2016; Justice et al., 2016; Korthuis et al., 2012; Marshall et al, 2017; Samet et al., 2007; Williams et al., 2019, 2018, 2017a, 2016). DSM-5 defined alcohol use disorder (AUD) is estimated to be prevalent in 8% to 42% of PWH in the U.S. (Galvan et al., 2002; Justice et al., 2016; Samet et al., 2004; Williams et al., 2017b) and is manifest by criteria that include loss of control over drinking, social and legal consequences, alcohol tolerance, continued drinking despite physical and mental health consequences, and physiological withdrawal (O’Connor et al., 2018).
Behavioral and/or medication treatment for AUD (BAUD and MAUD, respectively) is indicated for all individuals with AUD, regardless of HIV status (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2019). BAUD includes psychosocial therapy such as behavioral couples, cognitive behavioral and motivational enhancement therapy. Recommended MAUD includes prescription of a first line US Food and Drug Administration (FDA) approved medication (acamprosate, disulfiram, naltrexone); gabapentin and topiramate have also been shown to be effective treatments for AUD (Guglielmo et al., 2015; Manhapra et al., 2019; Rentsch et al., 2019). However, most individuals with AUD do not receive BAUD or MAUD. In a recent study, 93% of patients with AUD did not utilize any alcohol treatment services over a timeframe of more than a year (Gilbert et al, 2019) and another study reported that only 9% who may benefit from MAUD received it (Kranzler and Soyka, 2018). Likewise, other studies have found that only a small minority of PWH with AUD received treatment (Kraemer et al., 2019; Oldfield et al., 2020, Williams et al., 2017b). Although non-receipt of BAUD and MAUD may have adverse consequences for PWH with AUD, such outcomes are uncertain as prior research on the impact of changes in alcohol intake and receipt of AUD treatment on HIV outcomes were inconclusive (Parsons et al., 2007; Samet et al., 2005), and clinical trials addressing this problem have been challenged by enrollment (Edelman et al., 2019a). While it is imperative to improve access to BAUD and MAUD for all patients with AUD, it is crucial also to determine if the receipt and intensity of BAUD and MAUD improves measurable HIV outcomes. Such knowledge could further guide efforts and increase motivation for initiation and retention in treatment for PWH with AUD. We used data from a national cohort of PWH with AUD to: 1) describe patterns of and intensity of BAUD and MAUD over the 12 months after an initial BAUD encounter; 2) identify which treatment facility and regional factors, patient demographic and clinical characteristics are associated with treatment type and intensity received; and 3) examine whether there are measurable differences in changes in CD4 cells/mm3, HIVRNA-1 viral load copies/mL (VL), VACS Index score 2.0 (an indicator of HIV disease severity), and ARV adherence from pre-AUD treatment to one year after start (post-treatment) by intensity and type of treatment. We hypothesized that higher levels of BAUD intensity and MAUD receipt for at least 6 months would be associated with improved HIV biomarkers (CD4, VL, VACS Index 2.0) and adherence from pre to post treatment compared to receipt of lower levels of treatment. Assessing changes in both HIV biomarkers and ARV adherence is useful even though changes in HIV biomarkers would be theoretically due to (mediated through) changes in adherence. First, change in adherence is limited to those who are on ARVs whereas change in HIV biomarkers includes all PWH. Second, adherence is based on fill/refill data and although these data have been shown to be accurate, we must assume that filled doses are consumed doses. Third, finding an association between changes in alcohol use and adherence does not mean we will also find an association between changes in alcohol use and HIV biomarkers due to the above mentioned assumption for adherence data and/or due to the time it may take for sustained change in adherence to confer to changes in HIV biomarkers.
2. Methods
2.1. Data Source and Sample
We used data from the Veterans Aging Cohort Study (VACS), a national cohort study of 55,986 PWH and 116,705 matched 2:1 by age, race/ethnicity, gender, and site to uninfected patients who received care at the Veterans Health Administration (VHA) between October 1996 and September 2017. The cohort was identified using the VHA’s national Corporate Data warehouse (Fultz et al., 2006). Of the PWH in VACS, we identified 7,830 with a “new” AUD-related behavioral health treatment encounter from January 2008 to September 2017. We required a 12 month period free of BAUD or MAUD treatment prior so that we could consider this a “new” treatment course.
2.2. Data and definitions
2.2.1. Behavioral Treatment for Alcohol Use Disorder (BAUD)
BAUD intensity was defined using methodology adapted from Harris and colleagues, which reported that SUD inpatient and outpatient codes had positive predictive value of 90% and 96%, respectively, using chart review as the gold standard (Harris et al., 2015). Their method identifies substance use disorder (SUD) treatment received in specialty in and outpatient settings using a combination of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes, treatment specialty codes, including outpatient clinic stop codes (a unit of healthcare utilization location and type of care), inpatient bed section codes, and mental health procedure codes including outpatient CPT codes and inpatient ICD-9 procedure codes. Types of BAUD include group psychotherapy, behavioral health counseling and therapy, individual psychotherapy, admission to residential treatment programs, inpatient and outpatient medically managed withdrawal and/or detoxification, and treatment consisting of “interaction between provider and patient” (Harris et al., 2015). Our study extends and adapts this methodology by adding ICD-10 codes which replaced ICD-9 codes in the VA on October 1, 2015 and including only AUD codes (rather than all SUD codes). Inpatient and outpatient treatment was identified if there was an ICD diagnostic code and either a SUD treatment specialty code (inpatient bed section code or outpatient clinic stop code) or mental health procedure code (inpatient ICD-9 and ICD-10) or outpatient CPT code. The ICD diagnostic codes used for AUD were 303.01–303.03, 303.90–303.93 and 305.01–305.03 for ICD-9, and F10.1x and F10.2x for ICD-10. See Appendix A and B for more specific details.
To define BAUD intensity, we determined number of behavioral clinic or inpatient treatment days for each month during the 12 months following the first treatment day identified. The number of BAUD days following the first treatment date was summed in two ways: 1) for each month over a 12 month period; and 2) for the overall 12 month period. We used a semi-parametric group-based trajectory model to fit treatment trajectories (Jones et al., 2001) based on number of treatments per month over 12 months. The trajectory procedure sorts each individual’s set of month treatments into “clusters”, and estimates a single model consisting of distinct trajectories. The trajectory with the highest probability of membership is then assigned to each individual. We used a zero-inflated Poisson outcome distribution for all trajectory-based models based on the distribution of the data. Model selection requires determining the number of groups and the trajectory shapes which best describe the data (Jones and Nagin, 2012). We examined model fit statistics (Bayesian information criterion [BIC]), significance of polynomial terms, and the values of average posterior group membership probability. For sensitivity analyses, we created a simpler variable, categorizing BAUD intensity by number of days over the one year timeframe into 5 groups (BAUD days: 1, 2, 3–9, 10–44, 45+).
2.2.2. Medication for AUD (MAUD)
We calculated the number of days over the 12-month period that were covered by a prescription medication filled/refilled for the following medications: the three FDA-approved medications (acamprosate, disulfiram, naltrexone) and gabapentin and topiramate that have strong evidence for effectiveness. Gabapentin is approved by FDA for postherpetic neuralgic, adjunctive therapy for partial seizures, and restless leg syndrome; although we cannot be certain that prescribed gabapentin was for MAUD, specifically, in this dataset, we do know it was prescribed around the same time that BAUD was received. Current VA and Department of Defense clinical practice guidelines support the use of these medications for AUD treatment (Management of Substance Use Disorders Working Group, 2009). We identified those with a prescription for >6 months (Kranzler et al., 2018) at any point during the 12 months after start of treatment.
2.2.3. Outcome Measures
Primary outcomes were changes from pre-treatment to post-treatment for: 1) CD4; 2) log VL; 3) VACS Index Score 2.0; and 4) adherence. Changes in CD4, log VL, and VACS Index Score 2.0 are based on differences between pre-treatment (value closest to and up to one year prior to start of treatment) and post-treatment (value closest to 12 months following start of treatment ranging from 3 to 18 months following start of treatment). The VACS Index 2.0 uses age, CD4, VL, hemoglobin, renal and hepatic function (FIB4, eGFR, platelets, aspartate and alanine transaminase, and creatinine), hepatitis C virus (HCV) infection, white blood count, albumin and body mass index (BMI) to estimate HIV disease severity (Tate et al., 2019). VACS Index 2.0 scores range from 0 to 164; higher scores reflect higher disease severity and increasing risk of all-cause mortality (Tate et al., 2019). An advantage of the VACS Index over individual laboratory values is that it provides a mechanism to correlate health status change with mortality benefits. For instance, a 5 point increase in the VACS Index 2.0 is associated with a 30% increased risk of 5 year mortality (Hazard Ratio 1.31; 95% CI 1.30–1.31) (Tate et al., 2019). ARV adherence was based on percent of days with medication coverage over the one year timeframe prior (pre) to and one year after (post) start of AUD treatment using fill/refill pharmacy data.
2.2.4. Other Measures
Demographic characteristics include age at start of treatment, gender (male/female), and race/ethnicity. ICD-9 codes documented in the one year prior to six months after enrollment in VACS were used to measure presence of severe mental illness (severe mental illness (SMI); major depression, schizophrenia, schizoaffective disorder, bipolar disease, and posttraumatic stress disorder) and drug use diagnoses (amphetamines, cocaine, cannabis, hallucinogens, or opioids). Smoking status was based on the most common value (never, past, current) in the electronic medical record (EMR) Health Factors dataset which captures smoking status that is routinely collected at patient visits via clinical reminders (McGinnis et al., 2011).
Rural versus urban location was based on rural-urban commuting area codes for each 3 digit station at which first treatment occurred. The VHA’s Drug and Alcohol Program Survey (DAPS) was used to identify the availability of weekend/evening hours for outpatient SUD services, presence of methadone clinic, and number of providers specializing in addiction or twelve step programs at each site. The DAPS is administered biannually by the VHA Program Evaluation and Resource Center and is usually completed by an on-site program manager. In 2010, the DAPS had a 100% response rate. Homelessness is based on having ICD-9 code V60.0 or ICD-10 code Z59.0. We ascertained HCV status using the electronic health record; those having a positive HCV viral load, positive antibody test, or clinical diagnosis as based on ICD-9 codes were defined as having HCV. Alcohol use severity was based on the 3-item Alcohol Use Disorders Identification Test Consumption (AUDIT-C) which is administered annually to over 90% of outpatients in the VA and documented in the EMR (Bradley et al., 2006). We generated a mean of all available AUDIT-C scores documented during the 12 months prior to initial AUD treatment date and during the 12–24 month window after start of treatment.
2.3. Statistical Analyses
To understand and portray BAUD treatment patterns, we graphed the average number of treatment days per month by the 12 month treatment intensity trajectory groups. Patient characteristics were described overall and by BAUD treatment intensity trajectory groups using ANOVA, Kruskall-Wallis, and chi-square tests as appropriate. For variables associated with BAUD in univariate analyses, we also examined their association with BAUD in a multivariate linear regression model in which BAUD treatment intensity trajectory group was the outcome variable. Patient characteristics were also compared between those who did and did not receive >6 months of MAUD. For variables associated with MAUD receipt in univariate analysis, we also examined their association with MAUD receipt in a multivariate logistic regression model.
We used Kruskall-Wallis tests to compare the unadjusted association between BAUD intensity and changes in biomarker outcomes (log VL, CD4, VACS Index Score 2.0) and adherence among those with pre and post measures. Wilcoxon rank-sum tests were used to compare the unadjusted association between MAUD receipt and changes in biomarker outcomes (log VL, CD4, VACS Index Score 2.0) and adherence among those with pre and post measures. Linear regression models were used to assess the adjusted association of BAUD intensity and MAUD receipt with change in biomarker outcomes (log VL, CD4, VACS Index Score 2.0) and adherence among those with pre and post measures. Because there is more room for ARV adherence and HIV biomarker improvement among those with detectable VL, outcome models were stratified by VL (<50 copies/mL / > 50 copies/mL). Models were adjusted for: 1) demographic characteristics (age, race/ethnicity, and gender); and also for 2) smoking, mental health related diagnoses, HCV, drug use diagnoses, baseline AUDIT-C, and baseline biomarker or adherence corresponding to outcomes for each model, time between outcome measures (for all outcomes except adherence). The significance of the association between treatment intensity and change in biomarker outcomes and adherence was tested using an overall Wald test.
We ran several secondary analyses. Because only a small percent of the sample received >6 months of MAUD and that was disproportionate by treatment intensity groups, we ran the models excluding those who received >6 months of MAUD. Additionally, we tested for the effect of BAUD intensity combined with >6 months of MAUD interaction by running a model including an interaction term.
3. Results
3.1. Demographics and characteristics
Of the 7,830 PWH who received AUD treatment from January 2008 to September 2017, the mean age was 53 ranging from 23 to 84, 60% were African American, 28% white, and 8% other, 3% female, 41% had HCV, 75% were current smokers, and 77% were on ARVs. (Table 1)
Table 1.
Characteristics of PWH by BAUD Intensity and Receipt of MAUD Among those who Received at Least One Day of Treatment (n=7,830)
| Treatment Intensity | >6 Months of MAUD | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All | 1 Only | Minimal | Moderate | Intensive | P-Value | No | Yes | P-Value | |
| N | 7,830 | 2,757 | 3,410 | 1,365 | 298 | - | 7112 | 718 | |
| Mean Age (sd) | 53 (8.9) | 54 (9) | 54 (9) | 53 (8) | 53 (7) | <.001 | 53 | 55 | <.001 |
| Race (%) | .001 | <.001 | |||||||
| Black | 60 | 58 | 58 | 68 | 77 | 62 | 52 | ||
| White | 28 | 31 | 30 | 24 | 16 | 28 | 37 | ||
| Hispanic | 8 | 8 | 9 | 7 | 5 | 8 | 8 | ||
| Other | 3 | 3 | 3 | 2 | 2 | 3 | 3 | ||
| Female Gender (%) | 3 | 3 | 3 | 3 | 2 | .9 | 3 | 3 | .7 |
| Hepatitis C (%) | 41 | 37 | 40 | 47 | 50 | <.001 | 40 | 45 | .021 |
| Smoking Status (%) | <.001 | .8 | |||||||
| Never | 15 | 16 | 16 | 12 | 9 | 15 | 15 | ||
| Past | 10 | 11 | 11 | 7 | 6 | 10 | 9 | ||
| Current | 75 | 73 | 74 | 81 | 85 | 75 | 75 | ||
| Drug Related Diagnosis (%) | 35 | 30 | 34 | 46 | 56 | <.001 | 35 | 37 | .4 |
| Mental Health Diagnosis (%) | 26 | 24 | 26 | 30 | 27 | .002 | 26 | 34 | <.001 |
| Homelessness (%) | 52 | 44 | 59 | 66 | 77 | <.001 | 52 | 53 | .4 |
| Mean Pre AUDIT-C* (SD) | 3.0 | 3.0 | 3.0 | 3.1 | 3.4 | <.001 | 3.2 | 2.7 | <.001 |
| CD4>200 (%) | 65 | 68 | 66 | 62 | 50 | <.001 | 12 | 8 | <.001 |
| CD4≤200 | 11 | 19 | 12 | 13 | 13 | 65 | 74 | ||
| Missing | 23 | 22 | 22 | 25 | 37 | 23 | 18 | ||
| VL≤50 (%) | 36 | 45 | 42 | 36 | 27 | <.001 | 36 | 31 | <.001 |
| VL>50 | 41 | 34 | 36 | 38 | 36 | 41 | 51 | ||
| Missing | 23 | 22 | 21 | 26 | 37 | 23 | 18 | ||
| On ARV | 71 | 73 | 72 | 67 | 59 | <.001 | 77 | 84 | <.001 |
| Facility/Location | |||||||||
| SUD Weekend Hours* (%) | 52 | 51 | 52 | 52 | 57 | .2 | 52 | 52 | .8 |
| Rural (%) | 11 | 10 | 19 | 7 | 8 | .006 | 9 | 12 | .024 |
| Brief Intervention Prior to Treatment (%) | 18 | 17 | 18 | 19 | 26 | .001 | 18 | 17 | .7 |
| Brief Intervention After Start of Treatment | 24 | 16 | 24 | 36 | 55 | <.001 | 24 | 24 | .7 |
| Treatment Types | |||||||||
| Inpatient (%) | 13 | 0.4 | 7 | 41 | 84 | <.001 | |||
| >30 Days of Medication (%) | 21.9 | 17.3 | 21.1 | 30.6 | 32.6 | <.001 | |||
| Acamprosate (%) | .4 | 0 | .4 | 1.0 | 1.0 | <.001 | |||
| Disulfiram (%) | .3 | 0.1 | 0.2 | 0.7 | 1.0 | .002 | |||
| Naltrexone (%) | 1.9 | 0.3 | 1.6 | 5.7 | 3.7 | <.001 | |||
| Gabapentin (%) | 17.5 | 15.3 | 17.1 | 21.8 | 22.8 | <.001 | |||
| Topiramate (%) | 1.5 | 1.1 | 1.4 | 2.1 | 3.0 | .01 | |||
| >6 Months Medication (%) | 9.2 | 8.1 | 9.0 | 11.3 | 12.1 | .002 | |||
| Acamprosate (%) | 0.1 | 0 | 0.1 | 0.2 | 0.0 | .3 | |||
| Disulfiram (%) | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | .7 | |||
| Naltrexone (%) | 0.8 | 0.1 | 0.7 | 2.4 | 1.7 | <.001 | |||
| Gabapentin (%) | 7.8 | 7.7 | 7.7 | 8.1 | 9.1 | .8 | |||
| Topiramate (%) | 0.4 | 0.4 | 0.5 | 0.3 | 0.3 | .6 | |||
| Median Time to Medication (IQR, n=1474) | 49 (11–144) | 45.5 (13–136) | 51 (12–127) | 42 (8–159) | 60 (15–193) | .5 | |||
| Of those not on ARV Pre (n=2126), on ARV Post (%) | 27 | 21 | 27 | 34 | 39 | <.001 | 27 | 25 | .7 |
| Mean Change in Mean AUDIT-C (n=4963) | −0.4 | −0.3 | −0.3 | −0.4 | −1.4 | <.001 | −.5 | −.4 | .5 |
| Mean Change in Adherence % (n=5205) | .9 | −.3 | .3 | 3 | 13 | <.001 | 0.01 | .02 | .19 |
| Mean Change in HIV Disease Severity | |||||||||
| Change in CD4 (n=5677) | 30 | 22 | 33 | 39 | 38 | .02 | 31 | 23 | .43 |
| Change in log VL (n=5731) | −0.3 | −0.1 | −0.3 | −0.3 | −0.6 | .008 | −.3 | −.2 | .9 |
| Change in VACS Index 2.0 (n=5517) | −1.2 | −0.8 | −1.2 | −1.5 | −3.6 | .03 | −1.2 | −1.0 | .7 |
Pre AUDIT-C available for 6,790 and availability of weekend hours available for 7,469
Models should adjust for age, race/ethnicity, HCV, smoking, other drug related dx, SMI, pre AUDIT-C, baseline biomarker, time between biomarkers
3.2. Patterns of BAUD
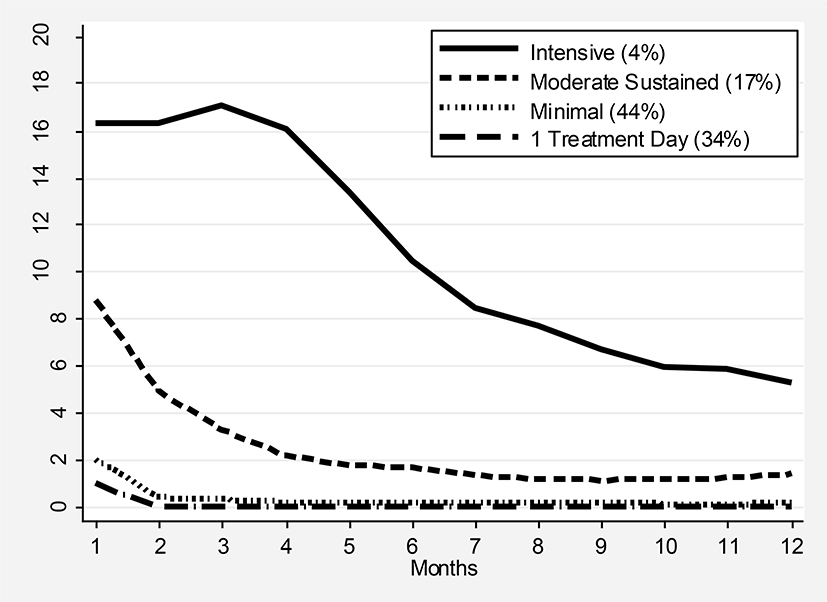
Using number of treatment days per month over a 12 month period after start of treatment as described above, we identified four groups representing levels of AUD treatment intensity over time (three trajectory groups identified, but we split the lowest level trajectory group into two groups – having only one treatment day vs. more than one treatment day). Model fit, as measured by BIC, improved substantially when increased from 2 to 3 groups and the smallest group in the 3-group trajectory contains 4% of the sample. The groups reflect the following pattern of BAUD intensity: 1) Single Visit - 35% recieved just one visit over the 12 months; 2) Minimal - 44% recieved a mean of 2 visits - typically approximately two visits during the first month and an average of 0 visits for each month after that; 3) Sustained Moderate - 17% started at a mean of approximately 8 visits per month and gradually declined to recieving 1 to 2 visits per month from months 5 to 12; and 4) Intensive - 4% started out receiving an average of 14 to 16 visits per month over the first 3 months and then gradually declined to an average of 7 visits per month by the 12th month. Median (interquartile range) number of treatment days over the one 12 month time frame that corresponds to each of these groups is 1 (1–1), 3 (2–5), 27 (18–38), and 116 (85–153). Figure 1 shows the pattern of mean number of treatment days per month by treatment trajectories, and for all trajectory groups more treatment is received in the earlier months of treatment. We also created a simpler variable representing total number of treatment visits over twelve months with the following categories: 35% received 1 day of treatment, 16% 2 days, 25% 3–9 days, 17% 10–44 days, and 7% 45+ days of treatment.
Figure 1.
Number of BAUD Treatment Days Per Month by Intensity of Treatment Trajectory Groups
3.3. Medication for AUD (MAUD)
Of these 7,830 who received BAUD for at least one day, 1,602 (20%) received at least 30 days of MAUD within the following year. Median time to MAUD was 48 days (interquartile range = 12–135) after the first day of BAUD. The most commonly received medication was gabapentin (1,370) followed by naltrexone (152), topiramate (117), acamprosate (31), and disulfiram (23), and only 88 received two or more medications for at least 30 days. 718 (9%) received medication for >6 months with 609 receiving >6 months of gabapentin, 63 naltrexone, 33 topiramate, 6 disulfiram, and 4 acamprosate. Only 52 received 2 or more medications for 6 months or more.
3.4. Variables Associated with BAUD Intensity
Those who received more intensive BAUD were more likely to be African-American, have HCV, report currently smoking, have a drug use diagnosis, have higher mean AUDIT-C, have a history of homelessness, to be living in an urban area, to have missing values for VL and CD4, and to have received at least one brief intervention during the one year before and the one year after start of BAUD. Those who received more intensive BAUD were less likely to be on ARV treatment at start of BAUD. However, of those not on an ARV at the start of BAUD, those in the more intensive treatment were more likely to start on ARV during the year after start of BAUD. Additionally, AUDIT-C scores decreased (improved) the most for those in the most intensive treatment group. (Table 1) In multivariate linear regression models, being African-American, younger in age, infected with HCV, having a drug use diagnosis, homelessness, unhealthy alcohol use (AUDIT-C 4+), and detectable VL were associated with higher levels of BAUD intensity. (Table 2)
Table 2.
Variables Associated with BAUD Intensity and >6 Months of MAUD in Multivariate Regression Models
| BAUD Intensity | >6 Months MAUD | |||
|---|---|---|---|---|
| Coefficient | p | Odds Ratio | p | |
| Age | −0.00 | <0.001 | 1.03 | <0.001 |
| Race/Ethnicity | ||||
| White (referent group) | ||||
| African-American | 0.06 | 0.006 | 0.59 | <0.001 |
| Hispanic | 0.00 | 0.923 | 0.67 | 0.036 |
| Other | −0.05 | 0.399 | 0.88 | 0.598 |
| HCV | 0.07 | 0.001 | 1.08 | 0.408 |
| Smoking | ||||
| Never (referent group) | ||||
| Current | 0.02 | 0.493 | 1.04 | 0.762 |
| Past | −0.03 | 0.403 | 0.91 | 0.645 |
| Drug Dx | 0.09 | <0.001 | 1.02 | 0.885 |
| SMI Dx | 0.02 | 0.474 | 1.48 | <0.001 |
| Homeless Dx | 0.23 | <0.001 | 1.17 | 0.115 |
| Unhealthy Alcohol Use | 0.02 | <0.001 | 0.98 | 0.080 |
| Rural | −0.02 | 0.538 | 1.19 | 0.188 |
| VL | ||||
| Detectable (referent group) | ||||
| Undetectable | −0.09 | <0.010 | 1.37 | 0.001 |
| Not Available | −0.04 | 0.117 | 0.81 | 0.119 |
| Constant | 0.86 | <0.001 | 0.02 | <0.001 |
Abbreviations: HCV, hepatitis C virus; SMI, severe mental illness; VL, viral load
3.5. Variables Associated with MAUD
Compared to those who received less than 6 months of MAUD, those who received at least 6 months of MAUD were slightly older (mean = 55 vs. 53, p=.004), more likely to be current smokers (79% vs. 74%, p<.001), have HCV (48% vs. 39%, p<.001), be on ARV (80% vs. 77%, p=.008), have a drug use diagnosis (39% vs. 34%, p<.001), live in a rural area (12% vs. 9%, p=.024), or have SMI (33% vs. 25% p<.001). MAUD receipt was similar by race/ethnicity, gender, history of homelessness, VL, and CD4. Receiving a brief intervention before or after start of BAUD and change in AUDIT-C did not vary by receipt of MAUD (Table 1). In multivariate logistic regression models, being older, white, having a SMI diagnosis, and undetectable VL was associated with a higher likelihood of receiving >6 months of MAUD (Table 2).
3.6. Association of BAUD Intensity and MAUD with Changes in Biomarkers and Adherence
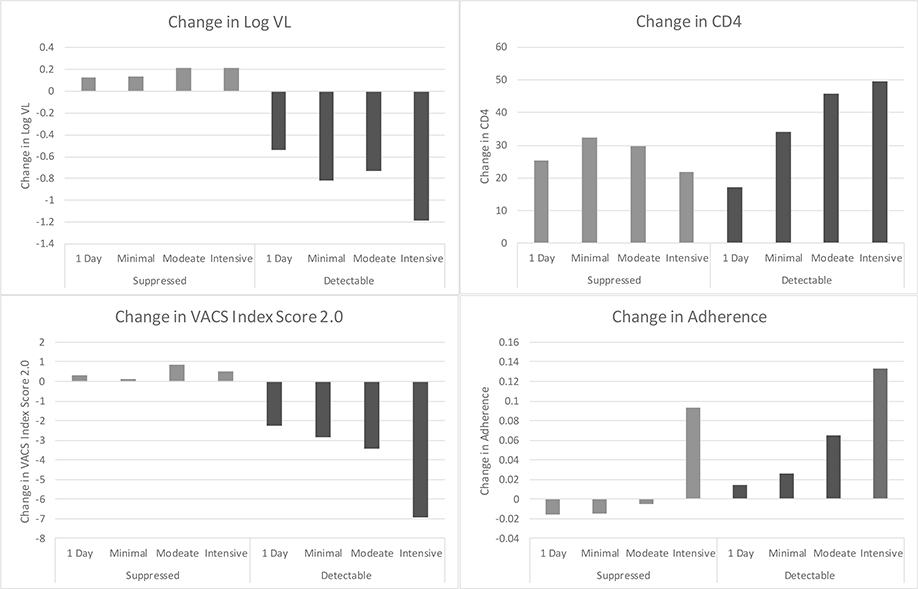
Among those with baseline detectable VL, CD4, log VL, VACS Index 2.0, and adherence improved significantly more among those with greater BAUD intensity compared to those with lower levels of BAUD intensity (p<.01, Figure 2), with improvements in highest level of intensity versus lowest level of intensity as follows: 49 vs. 18 cells/mm3 for CD4; −1.2 vs. − 0.6 copies/mL for log VL; − 6 vs. − 2 for VACS Index 2.0; and 0.13 vs. 0.02 for adherence. Among those with baseline undetectable VL, only adherence improved statistically significantly more among those with greater BAUD intensity compared those with lower intensity (Figure 2).
Figure 2.
Change in Log VL, Change in CD4 cell count, VACS Index Score 2.0, and ARV Adherence by Treatment Intensity and VL Suppression
Differences are statistically significant for:
-change in log VL and adherence for those with both undetectable and detectable VL (all p<.01)
-change in CD4, and change in VACS Index Score 2.0 for those with detectable VL (all p<.01)
In linear regression models predicting changes in biomarkers and adherence, including BAUD intensity and MAUD as the main predictors, adjusted for age, race/ethnicity, gender, the associations of BAUD intensity with changes in biomarkers and adherence are similar to the associations in the univariate analyses (Figure 2 & Table 3). MAUD was associated with improvement in CD4 among those with detectable VL and with improved adherence among those with both detectable and undetectable VL (Table 3).
Table 3.
Adjusted Linear Regression Models: Association of BAUD Intensity and >6 Months of MAUD with Pre- to Post-Treatment Change in CD4, Log VL, VACS Index Score 2.0, and ARV Adherence*
| Detectable VL | Undetectable VL | |||
|---|---|---|---|---|
| Treatment Intensity | Coef | 95% CI | Coef | 95% CI |
| CD4 change | n=2139 | n=2802 | ||
| >6 Months of MAUD | 27.09 | 1.40–52.79 | −10.5 | −31.3–10.3 |
| 1 Day Only (referent group) | ||||
| Minimal | 15.77 | −1.06–32.61 | 2.05 | −12.76–16.86 |
| Moderate | 30.11 | 9.20–51.01 | −2.75 | −23.30–17.80 |
| Intensive | 27.60 | −10.20–65.41 | −10.4 | −53.53–32.64 |
| P=.03 | P=.9 | |||
| Log HIV-1 RNA change | n=2176 | n=2869 | ||
| >6 Months of MAUD | −0.16 | −0.32–0.01 | −0.08 | −0.23–0.06 |
| 1 Day Only (referent group) | ||||
| Minimal | −0.07 | −0.18–0.04 | −0.02 | −0.13–0.08 |
| Moderate | 0.01 | −0.13–0.15 | 0.06 | −0.09–0.20 |
| Intensive | −0.40 | −0.66–(−0.14) | 0.03 | −0.27–0.33 |
| P=.01 | P=.7 | |||
| VACS Index Score 2.0 change | n=2053 | n=2460 | ||
| >6 Months of MAUD | −0.94 | −2.69–0.81 | −.30 | −1.42–0.83 |
| 1 Day Only (referent group) | ||||
| Minimal | −0.55 | −1.67–0.57 | −0.18 | −0.97–0.60 |
| Moderate | −0.71 | −2.11–0.69 | 0.62 | −0.48–1.71 |
| Intensive | −3.79 | −6.45–(−1.13) | −0.03 | −2.25–2.20 |
| P=.047 | P=.5 | |||
| ARV Adherence change** | n=1649 | n=2827 | ||
| >6 Months of MAUD | 0.05 | 0.02–0.08 | 0.04 | 0.02–0.05 |
| 1 Day Only (referent group) | ||||
| Minimal | 0.01 | −0.01–0.03 | −0.00 | −0.01–0.01 |
| Moderate | 0.03 | 0.00–0.05 | −0.01 | −0.02–0.01 |
| Intensive | 0.09 | 0.04–0.15 | 0.08 | 0.05–0.12 |
| P=.004 | P<.001 | |||
Models are adjusted for age and race/ethnicity, gender, smoking, HCV, drug diagnosis, severe mental illness (SMI), AUDIT-C, and for corresponding models, number of days between pre and post values for log VL, CD4, and VACS Index change.
3.7. Secondary Analyses
Our results were similar when the analysis was limited to those who received BAUD alone (no MAUD). There was a statistically significant interaction between BAUD intensity and MAUD among those with undetectable VL for change in VACS Index 2.0 and adherence; and among those with detectable VL for adherence. Among those with undetectable VL, BAUD intensity was associated with change in VACS Index 2.0 only among those who received >6 months of MAUD (lower intensity associated with decreased VACS Index 2.0, coefficient = −3.75, 95% CI = −6.24 – −1.34). Among those with and without detectable VL, BAUD intensity was associated with increased adherence only among those not receiving MAUD (coefficient = 0.10, 95% CI = 0.04–0.16 and coefficient = 0.09, 95% CI = 0.05–0.13, respectively). (Appendix C)
4. Discussion
In this large national VA sample, we found that of PWH who received at least one BAUD treatment, 34% received only 1 day of treatment, 44% received minimal, 17% received moderate and only 4% received high intensity BAUD. Less than 10% received >6 months of MAUD. Age, African-American race/ethnicity, HCV, drug related diagnoses, homelessness, alcohol use severity, and detectable VL were associated with higher intensity of BAUD. Age, white race/ethnicity, SMI, and undetectable VL were associated with receiving MAUD. Among those with detectable VL, CD4, VL, VACS Index 2.0, and adherence improved more among those with more intensive BAUD compared to those with lower intensity BAUD. Among those with undetectable VL, only adherence improved more among those with greater BAUD intensity compared to those with lower intensity BAUD. MAUD was associated with increased CD4 among those with detectable VL and with improved adherence among those with detectable and undetectable VL.
The low rate of at least moderate BAUD and MAUD among those with at least one BAUD visit represents a missed opportunity for substantial AUD treatment, especially for specific treatments among some subgroups (e.g., MAUD for African American PLWH). Differences in predictors of receiving BAUD and MAUD are likely complex and relate to clinic system’s culture, provider practices, and patient preferences and lived experiences. Findings regarding race/ethnicity differences in MAUD, in particular have been previously identified (Williams et al, 2017) and are worthy of further exploration at all levels. Recent calls to consider racism as a fundamental cause of health and healthcare disparities, as outlined on the Health Affairs Blog (https://www.healthaffairs.org/blog), cannot be dismissed and racism needs to be explored as a potential source of differences in AUD treatment. . In addition to research focused on understanding gaps in receipt of BAUD and MAUD overall, and for specific subpopulations, findings from this study suggest a strong need to focus on efforts to increase access to AUD treatment and recovery resources.
Our finding that the association of intensity of AUD treatment with HIV outcomes differed by detectable versus undetectable VL was not expected. All of the pre-post change outcomes improved for those with greater intensity of treatment among those with detectable VL. It is possible that physicians are more likely to recommend and/or PWH are more motivated to engage in a higher level of AUD treatment when virologic control targets are not being met, or, alternatively, engagement in frequent AUD treatment increases access to more frequent HIV care, potentially at the same VA clinical facility. Although adherence improved with more intense AUD treatment among those with undetectable VL, CD4 and VACS Index 2.0 did not. This finding may be because there is less room for the biomarkers to improve or there was not enough time to observe changes among those who already have undetectable VL. While we measured changes in HIV outcomes in addition to changes in ARV adherence, change in adherence is an important mediating variable between the association of AUD treatment with changes in HIV outcomes. In contrast to our findings, two prior intervention studies that aimed to reduce alcohol use found no improvements in adherence, CD4 cell count, VL that were sustained beyond 3 months (Parsons et al., 2007; Samet et al., 2005). Another study found evidence of a delayed effect on viral load (12 months for those who received 6 months of a stepped intervention) (Edelman et al., 2019b).
As in prior studies (Kraemer et al., 2019; Oldfield et al., 2020; Williams et al., 2017b), the proportion of PWH that were prescribed MAUD was very small. The most common medication identified was gabapentin (8% prescribed gabapentin vs. less than 1% for each of the other medications) which is commonly used for other indications; therefore, we may even be overestimating the percent who received MAUD. Current VA guidelines deem the use of naltrexone, acamprosate, disulfiram, topiramate, or gabapentin as appropriate for Veterans with AUD. The low rate of MAUD mirrors what is observed nationally among other patient populations with MAUD. Unfortunately, the low rate of MAUD makes it difficult to render conclusions about its impact on HIV outcomes.
This study has several other limitations. First, non-mental health prescribers (e.g., primary care providers) may prescribe MAUD and so it is possible that MAUD is prescribed in the absence of BAUD. However, it is likely that the majority of our sample on MAUD initiated the medication within a specialty substance use disorder treatment setting as addiction care in the VA is often primarily obtained in these settings. Second, non-medication counseling treatment in office-based settings, which is being emphasized now in the VA, might not be captured in our dataset because our method for detecting BAUD focused on specialty mental health and substance use disorder outpatient and inpatient treatment settings. Third, the majority of our sample on MAUD received gabapentin, which is often prescribed for other indications (e.g., neuropathic pain). Nonetheless, whether gabapentin was prescribed for AUD or a different issue, it could potentially impact alcohol use (Goodman and Brett, 2019); a prior study has shown that gabapentin is effective for AUD even when prescribed for different reasons (Rentsch et al., 2019). Short of medical record review, there is not a simple way to determine the reason for a gabapentin prescription and so all gabapentin prescriptions were included in our analyses (Johansen, 2018). We did not differentiate between oral and injection naltrexone use, so we may have underestimated naltrexone use of at least 30 days or 6 months because injection naltrexone would cover 28 days. However, such a small percent received any naltrexone (only 2.2%/172 received any and only 1.9%/152 received 30 days or more), so this possible underestimate would not impact our main findings. Fourth, while results are only generalizable to those who initiated BAUD; identifying the effects of BAUD and MAUD on those willing to engage in treatment is nonetheless quite important. Fifth, the ICD and CPT codes used to identify BAUD reflected a range of behavioral interventions but we are unable to know precisely which BAUD was delivered nor how well it was delivered. Lastly, there are few women in this sample so results may not be generalizable to women.
In conclusion, among a national sample of PWH and AUD who had an initial AUD treatment encounter, we found low rates of subsequent BAUD beyond minimal intensity, low rates of MAUD, and an association of higher intensity treatment with improved HIV outcomes, especially among PWH with detectable VL. Although all individuals with active AUD should receive treatment, our results indicate that engagement in more frequent AUD treatment may have particular benefit for a subset of PWH. System interventions are needed to increase engagement and retention in behavioral and medication treatments for PWH and AUD. Future work should also assess the benefit of more intensive AUD treatment among those with other comorbidities that require adherence to medication and other interventions to maintain good health.
Highlights.
Among PWH receiving behavioral treatment for AUD, only 9% recieved medication.
Only 21% received at least sustained moderate AUD behavioral treatment.
ARV adherence improved more among those with more intensive behavioral treatment.
Medication for AUD was associated with increased CD4 and adherence.
Increasing AUD treatment intensity may improve HIV-related outcomes.
Acknowledgements
We thank John O’Leary for comparing AUD ICD-9 and ICD-10 codes over time.
Funding Sources and Acknowledgments: This work was supported by the National Institutes of Health: NIAAA (R01 AA022886, U10-AA13566, U24-AA020794, U01-AA020790), the Veterans Health Administration, and the National Clinician Scholars Program. The views expressed are not those of the Department of Veterans Affairs or the United States Government. We thank John O’Leary for comparing AUD ICD-9 and ICD-10 codes over time. The authors have no conflicts of interest.
Appendix A.
Method for identifying AUD ICD-10 Codes to use
| AUD ICD-9 and ICD-10 codes were identified using the following methods: |
| 1) using our original VACS ICD-9 codes for AUD (www.vacohort.org), we applied the Agency for Healthcare Research and Quality (AHRQ) forwards and backwards mapping tool (add reference) to identify potential ICD-10 codes to include; |
| 2) we reviewed published and unpublished AUD ICD-10 codes identified by other research groups; |
| 3) multiple clinical and health services experts reviewed the original ICD-9 codes and list of potential ICD-10 codes and provided extensive input on which codes to include; |
| 4) AUD prevalence was compared pre and post October 1, 2015 using several iterations of ICD-10 code combinations. |
| Note that outpatient AUD prevalence decreased by about 2.5% after the start of ICD-10 codes (outpatient ICD codes are entered by health professionals); inpatient AUD prevalence remained stable (inpatient ICD codes are entered by professional coders). |
Appendix B.
Definitions of AUD Diagnoses, Procedures, and VHA SUD Specialty Locations
| Code Type | Codes |
|---|---|
| ICD-9 AUD Diagnoses | 303.01–303.03, 303.90–303.93, 305.01–305.03 |
| ICD-10 AUD Diagnoses | F10.1x, F10.2x |
| ICD-9 Outpatient Mental Health Related Procedures (CPT) | 90801, 90802, 90804, 90805, 90806, 90807, 90808, 90809, 90810, 90811, 90812, 90813, 90814, 90815, 90816, 90817, 90818, 90819, 90821, 90822, 90823, 90824, 90826, 90827, 90828, 90829, 90845, 90847, 90849, 90853, 90857, 90862, 90875, 90876, 98960, 98961, 98962, 99078, 99201, 99202, 99203, 99204, 99205, 99211, 99212, 99213, 99214, 99215, 99217, 99218, 99219, 99220, 99221, 99222, 99223, 99231, 99232, 99233, 99238, 99239, 99241, 99242, 99243, 99244, 99245, 99251, 99252, 99253, 99254, 99255, 99281, 99282, 99283, 99284, 99285, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, 99384, 99385, 99386, 99387, 99394, 99395, 99396, 99397, 99401, 99402, 99403, 99404, 99408, 99409, 99411, 99412, 99510 |
| ICD-10 Outpatient Mental Health Related Procedures (CPT) | G0155, G0176, G0177, G0396, G0397, H0001, H0002, H0004, H0005, H0007, H0012, H0013, H0015, H0016, H0020, H0022, H0031, H0034, H0035, H0036, H0037, H0039, H0040, H2000, H2001, H2010, H2011, H2012, H2013, H2014, H2015, H2016, H2017, H2018, H2019, H2020, H2035, H2036, M0064, S0201, S9480, S9480, S9484, S9485, T1006, T1012 |
| ICD-9 Inpatient Mental Health Related Procedures (CPT) | 94.61, 94.62, 94.63, 94.64, 94.65, 94.66, 94.67, 94.68, 94.69 |
| ICD-10 Inpatient Mental Health Related Procedures (CPT) | HZ2ZZZZ, HZ30ZZZ, HZ31ZZZ, HZ32ZZZ, HZ33ZZZ, HZ34ZZZ, HZ35ZZZ, HZ36ZZZ, HZ37ZZZ, HZ38ZZZ, HZ39ZZZ, HZ3BZZZ, HZ40ZZZ, HZ41ZZZ, HZ42ZZZ, HZ43ZZZ, HZ44ZZZ, HZ45ZZZ, HZ46ZZZ, HZ47ZZZ, HZ48ZZZ, HZ49ZZZ, HZ4BZZZ, HZ50ZZZ, HZ51ZZZ, HZ52ZZZ, HZ53ZZZ, HZ54ZZZ, HZ55ZZZ, HZ56ZZZ, HZ57ZZZ, HZ58ZZZ, HZ59ZZZ, HZ5BZZZ, HZ5CZZZ, HZ5DZZZ, HZ63ZZZ, HZ80ZZZ, HZ81ZZZ, HZ82ZZZ, HZ83ZZZ, HZ84ZZZ, HZ85ZZZ, HZ86ZZZ, HZ87ZZZ, HZ88ZZZ, HZ89ZZZ, HZ90ZZZ, HZ91ZZZ, HZ92ZZZ, HZ93ZZZ, HZ94ZZZ, HZ95ZZZ |
| Clinic Stops (outpatient) | SUD Individual Session (513) SUD Home Visit (514) SUD/PTSD (519) Intensive SUD Treatment (547) SUD Group Session (560) SUD Telephone (545) |
| Bed Sections (Inpatient) | SUD Residential Rehabilitation (27) High Intensity SUD Treatment Program (74) SUD Domiciliary (86) |
Appendix C.
Adjusted Linear Regression Models to Provide More Information on Interaction Findings
| <6 Months of MAUD | >6 Months of MAUD | |||||||
|---|---|---|---|---|---|---|---|---|
| Detectable VL | Undetectable VL | Detectable VL | Undetectable VL | |||||
| Treatment Intensity | Coef | 95% CI | Coef | 95% CI | Coef | 95% CI | Coef | 95% CI |
| VACS Index Score 2.0 change | NA | n=2186 | NA | n=274 | ||||
| 1 Day Only (referent group) | ||||||||
| Minimal | NA | 0.21 | −0.62–1.04 | NA | −3.75 | −6.24–1.33 | ||
| Moderate | 0.98 | −0.19–2.16 | −1.87 | −5.05–1.10 | ||||
| Intensive | −0.19 | −2.62–2.24 | −0.33 | −6.16–5.51 | ||||
| p=.4 | p=.02 | |||||||
| ARV Adherence change** | n=1488 | n=2494 | n=161 | n=333 | ||||
| 1 Day Only (referent group) | ||||||||
| Minimal | 0.01 | −0.02–0.03 | −0.00 | −0.01–0.01 | 0.04 | −0.02–0.10 | −0.00 | −0.04–0.03 |
| Moderate | 0.02 | −0.01–0.05 | −0.00 | −0.02–0.01 | 0.06 | −0.00–0.13 | −0.02 | −0.06–0.02 |
| Intensive | 0.10 | 0.04–0.16 | 0.09 | 0.05–0.13 | 0.04 | −0.06–0.15 | 0.03 | −0.05–0.11 |
| p=.007 | p<.001 | p=.2 | p=.6 | |||||
Models are adjusted for age and race/ethnicity, gender, smoking, HCV drug diagnosis, severe mental illness (SMI), and AUDIT-C. VACS Index change model is also adjusted for number of days between pre and post values for VACS Index change.
Footnotes
Conflict of Interest No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baum MK, Rafie C, Lai S, Sales S, Page JB, Campa A, 2010. Alcohol use accelerates HIV disease progression. AIDS Res. Hum. Retroviruses. 26, 5, 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensley KM, Fortney J, Chan G, Dombrowski JC, Ornelas I, Rubinsky AD, Lapham GT, Glass JE, Williams EC, 2019. Differences in receipt of alcohol-related care across rurality among VA patients living with HIV with unhealthy alcohol use. The Journal of Rural Health. 35, 3, 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd RW, Lindo EG, Weeks LD, McLemore MR (2020, July 2). On racism: A new standard for publishing on racial health inequalities. Retrieved from https://www.healthaffairs.org/do/10.1377/hblog20200630.939347/full/ [Google Scholar]
- Bradley KA, Williams EC, Achtmeyer CE, Volpp B, Collins BJ, Kivlahan DR, 2006. Implementation of evidence-based alcohol screening in the Veterans Health Administration. Am. J. Manag. Care. 12, 10, 597–606. [PubMed] [Google Scholar]
- Deiss RG, Mesner O, Agan BK, Ganesan A, Okulicz JF, Bavaro M, Lalani T, O’Bryan TA, Bebu I, Macalino GE, 2016. Characterizing the association between alcohol and HIV virologic failure in a military cohort on antiretroviral therapy. Alcoholism: Clinical and Experimental Research. 40, 3, 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman EJ, Moore BA, Holt SR, Hansen N, Kyriakides TC, Virata M, Brown ST, Justice AC, Bryant KJ, Fiellin DA, 2019a. Efficacy of extended-release naltrexone on HIV-related and drinking outcomes among HIV-positive patients: a randomized-controlled trial. AIDS and Behavior. 23, 1, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman EJ, Maisto SA, Hansen NB, Cutter CJ, Dziura J, Deng Y, Fiellin LE, O’Connor PG, Bedimo R, Gibert CL, 2019b. Integrated stepped alcohol treatment for patients with HIV and alcohol use disorder: a randomised controlled trial. The Lancet HIV. 6, 8, e509–e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, Justice AC, 2006. Development and verification of a virtual cohort using the National VA Health Information System. Med. Care, S25–S30. [DOI] [PubMed] [Google Scholar]
- Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, Longshore D, Morton SC, Orlando M, Shapiro M, 2002. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J. Stud. Alcohol, 63, 2, 179–186. [DOI] [PubMed] [Google Scholar]
- Gilbert PA, Pro G, Zemore SE, Mulia N, Brown G, 2019. Gender Differences in Use of Alcohol Treatment Services and Reasons for Nonuse in a National Sample. Alcoholism: Clinical and Experimental Research. 43, 4, 722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CW, Brett AS, 2019. A clinical overview of off-label use of gabapentinoid drugs. JAMA internal medicine. 179, 5, 695–701. [DOI] [PubMed] [Google Scholar]
- Guglielmo R, Martinotti G, Quatrale M, Ioime L, Kadilli I, Di Nicola M, Janiri L, 2015. Topiramate in alcohol use disorders: review and update. CNS drugs. 29, 5, 383–395. [DOI] [PubMed] [Google Scholar]
- Harris AH, Ellerbe L, Phelps TE, Finney JW, Bowe T, Gupta S, Asch SM, Humphreys K, Trafton J, 2015. Examining the specification validity of the HEDIS quality measures for substance use disorders. J. Subst. Abuse Treat. 53, 16–21. [DOI] [PubMed] [Google Scholar]
- Johansen ME, 2018. Gabapentinoid use in the United States 2002 through 2015. JAMA internal medicine. 178, 2, 292–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BL, Nagin DS, Roeder K, 2001. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological methods, research. 29, 3, 374–393. [Google Scholar]
- Jones BL, Nagin DS, 2012. A Stata plugin for estimating group-based trajectory models Research Showcase@ CMU.Carnegie Mellon University; 2015. [Google Scholar]
- Justice AC, McGinnis KA, Tate JP, Braithwaite RS, Bryant KJ, Cook RL, Edelman EJ, Fiellin LE, Freiberg MS, Gordon AJ, 2016. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend. 161, 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthuis PT, Fiellin DA, McGinnis KA, Skanderson M, Justice AC, Gordon AJ, Doebler DA, Asch SM, Fiellin LE, Bryant K, Gibert CL, Crystal S, Goetz MB, Rimland D, Rodriguez-Barradas MC, Kraemer KL, 2012. Unhealthy alcohol and illicit drug use are associated with decreased quality of HIV care. J. Acquir. Immune Defic. Syndr. 61, 2, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer KL, McGinnis KA, Fiellin DA, Skanderson M, Gordon AJ, Robbins J, Zickmund S, Bryant K, Korthuis PT, 2019. Low levels of initiation, engagement, and retention in substance use disorder treatment including pharmacotherapy among HIV-infected and uninfected veterans. J. Subst. Abuse Treat. 103, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Soyka M, 2018. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA. 320, 8, 815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Management of Substance Use Disorders Working Group, 2009. VA/DoD clinical practice guideline for management of substance use disorders (SUD). Washington, DC: Department of Defense, Department of Veterans Affairs. [Google Scholar]
- Manhapra A, Chakraborty A, Arias AJ, 2019. Topiramate Pharmacotherapy for Alcohol Use Disorder and Other Addictions: A Narrative Review . J. Addict. Med. 13, 1, 7–22. [DOI] [PubMed] [Google Scholar]
- Marshall BDL, Tate JP, McGinnis KA, Bryant KJ, Cook RL, Edelman EJ, Gaither JR, Kahler CW, Operario D, Fiellin DA, Justice AC, 2017. Long-term alcohol use patterns and HIV disease severity. AIDS (London, England). 31, 9, 1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, Brown ST, Freiberg MS, Gibert CL, Goetz MB, 2011. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tobacco Res. 13, 12, 1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor EA, Perdue LA, Senger CA, Rushkin M, Patnode CD, Bean SI, Jonas DE, 2018. Screening and Behavioral Counseling Interventions to Reduce Unhealthy Alcohol Use in Adolescents and Adults: An Updated Systematic Review for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality (US) (Evidence Synthesis, 171.) Table 1, Unhealthy Alcohol Use: Terms and Definitions. https://www.ncbi.nlm.nih.gov/books/NBK534919/table/ch1.tab1/. [PubMed] [Google Scholar]
- Oldfield BJ, McGinnis KA, Edelman EJ, Williams EC, Gordon AJ, Akgün K, Crystal S, Fiellin LE, Gaither JR, Goulet JL, 2020. Predictors of initiation of and retention on medications for alcohol use disorder among people living with and without HIV. J. Subst. Abuse Treat. 109, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents, 2019. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Washington, D.C.: US Department of Health and Human Services; https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/0. [Google Scholar]
- Parsons JT, Golub SA, Rosof E, Holder C, 2007. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: a randomized controlled trial. J. Acquir. Immune Defic Syndr. 46, 4, 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentsch CT, Fiellin DA, Bryant KJ, Justice AC, Tate JP, 2019. Association Between Gabapentin Receipt for Any Indication and Alcohol Use Disorders Identification Test—Consumption Scores Among Clinical Subpopulations With and Without Alcohol Use Disorder. Alcoholism: Clinical and Experimental Research. 43, 3, 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JH, Phillips SJ, Horton NJ, Traphagen ET, Freedberg KA, 2004. Detecting alcohol problems in HIV-infected patients: use of the CAGE questionnaire. AIDS Res. Hum. Retroviruses. 20, 2, 151–155. [DOI] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Meli S, Dukes K, Tripps T, Sullivan L, Freedberg KA, 2005. A randomized controlled trial to enhance antiretroviral therapy adherence in patients with a history of alcohol problems. Antivir. Ther. 10, 1, 83–93. [DOI] [PubMed] [Google Scholar]
- Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R, 2007. Alcohol consumption and HIV disease progression. J. Acquir. Immune Defic. Syndr. 46, 2, 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate JP, Sterne JAC, Justice AC, Veterans Aging Cohort Study (VACS) and the Antiretroviral Therapy Cohort Collaboration (ART-CC), 2019. Albumin, white blood cell count, and body mass index improve discrimination of mortality in HIV-positive individuals. AIDS. 33, 5, 903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Gupta S, Rubinsky AD, Glass JE, Jones-Webb R, Bensley KM, Harris AHS 2017. Variation in receipt of pharmacotherapy for alcohol use disorders across racial/ethnic groups: A national study in the U.S. Veteran Health Administration. Drug and Alcohol Dependence. 178, 527–533. [DOI] [PubMed] [Google Scholar]
- Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH, 2016. Alcohol use and human immunodeficiency virus (HIV) infection: current knowledge, implications, and future directions. Alcoholism: Clinical and Experimental Research. 40, 10, 2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Joo YS, Lipira L, Glass JE, 2017a. Psychosocial stressors and alcohol use, severity, and treatment receipt across human immunodeficiency virus (HIV) status in a nationally representative sample of US residents. Substance abuse. 38, 3, 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Lapham GT, Shortreed SM, Rubinsky AD, Bobb JF, Bensley KM, Catz SL, Richards JE, Bradley KA, 2017b. Among patients with unhealthy alcohol use, those with HIV are less likely than those without to receive evidence-based alcohol-related care: a national VA study. Drug Alcohol Depend. 174, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, McGinnis KA, Bobb JF, Rubinsky AD, Lapham GT, Skanderson M, Catz SL, Bensley KM, Richards JE, Bryant KJ, 2018. Changes in alcohol use associated with changes in HIV disease severity over time: A national longitudinal study in the Veterans Aging Cohort. Drug Alcohol Depend. 189, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, McGinnis KA, Edelman EJ, Matson TE, Gordon AJ, Marshall BD, Bryant KJ, Rubinsky AD, Lapham GT, Satre DD, 2019. Level of alcohol use associated with HIV care continuum targets in a national US sample of persons living with HIV receiving healthcare. AIDS and Behavior. 23, 1, 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]