Abstract
Objectives:
Tenofovir alafenamide (TAF) is a preferred nucleotide reverse transcriptase inhibitor used in the treatment of HIV. Co-administration of TAF with rifabutin (RFB) is not recommended due to concerns that RFB decreases TAF gastrointestinal absorption. The objective of this study was to determine the efficacy of antiretroviral therapy regimens that include the co-administration of TAF and RFB.
Methods:
Persons with HIV (PWH) who received TAF–RFB co-administration for ≥1 month were identified retrospectively. The primary outcome was the maintenance of HIV viral load <200 copies/mL (cpm) for those already on HIV therapy at RFB initiation, or suppression of viral load to <200 cpm for those with unsuppressed HIV viral load prior to TAF–RFB co-administration.
Results:
Twenty-two PWH met the inclusion criteria. Four out of five patients (80%) maintained a viral load <200 cpm and 15/17 (88%) achieved a viral load <200 cpm during TAF–RFB co-administration. After the exclusion of patients who self-discontinued therapy or were lost to follow-up, 19/19 (100%) met the combined primary endpoint of HIV viral load <200 cpm.
Conclusions:
This study suggests that TAF–RFB co-administration may be effective despite concerns that RFB could reduce TAF absorption.
Keywords: Tenofovir alafenamide, HIV, Rifabutin, Co-administration
Introduction
Tenofovir alafenamide (TAF), a prodrug of tenofovir, is a well-tolerated and efficacious nucleotide reverse transcriptase inhibitor approved for the treatment of HIV (Eron et al., 2018; Sax et al., 2014; Wang et al., 2016). Due to lower plasma but higher intracellular concentrations of tenofovir diphosphate (TFV-DP), the active drug metabolite, TAF is associated with reduced renal and bone toxicities compared to tenofovir disoproxil fumarate (TDF) (Gupta et al., 2019; Wang et al., 2016). Due to its favorable safety profile, TAF is a component of several recommended initial antiretroviral therapy (ART) regimens (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2020).
Rifabutin (RFB) is a rifamycin antibiotic used in the treatment of mycobacterial disease, in particular among persons with HIV (PWH). The co-administration of RFB with TAF is not recommended due to concerns that RFB may reduce TAF absorption from the gut, potentially through P-glycoprotein induction (Panel on Antiretroviral Guidelines for Adults and Adolescents). However, one prior study in healthy volunteers found that RFB did not induce P-glycoprotein (Lutz et al., 2018). In addition, when TAF was administered with rifampicin, a known P-glycoprotein inducer, intracellular TFV-DP levels remained higher that those achieved with conventional doses of TDF (Cerrone et al., 2019). It was therefore hypothesized that any TAF–RFB interaction would not be clinically significant.
Methods
A retrospective observational study of PWH who received TAF–RFB co-administration for ≥1 month between April 2016 and July 2019 at a single center in San Diego, California, was performed. The decision to administer TAF and RFB was taken by a multidisciplinary team, including an HIV physician and a pharmacist, and was based on HIV resistance profiles, patient adherence, drug interactions, and available evidence. Participants were categorized into two groups: (1) PWH with HIV viral load >200 copies/mL (cpm) initiating both TAF-containing ART and RFB, and (2) PWH with a baseline HIV viral load <200 cpm established on TAF-containing ART and initiating RFB. For group 1, the primary outcome was achieving a viral load <200 cpm, with a secondary outcome of viral load <50 cpm. For group 2, the primary outcome was maintenance of the HIV viral load <200 cpm, with a secondary outcome of viral load <50 cpm after a minimum of 1 month of co-administration. A chart review was performed for individuals who did not achieve the primary outcome to evaluate whether the suspected cause was treatment failure.
Results
Twenty-two PWH received TAF–RFB co-administration for a median duration of 34.2 weeks (interquartile range 11.5–46.0 weeks). All participants received TAF 25 mg daily. Demographics, HIV infection characteristics, and dosing for RFB administration are shown in Table 1. The proportions of participants in group 1 and group 2 who achieved or maintained an HIV viral load <200 cpm or <50 cpm while receiving TAF–RFB co-administration are shown in Fig. 1. From 17 PWH with a viral load >200 cpm at the start of TAF–RFB co-administration, 15 (88.2%) achieved a viral load <200 cpm (64.7% with a viral load ≤50 cpm); the mean time to viral suppression was 53 days (95% confidence interval 29.8–76.2 days). Of the five patients with a viral load <200 cpm at the start of TAF–RFB co-administration, four (80%) maintained a viral load <200 cpm (80% with a viral load <50 cpm) for at least 1 month after the start of co-administration. Of the 18 PWH for whom follow-up was available through to the completion of RFB therapy, 83.3% had an HIV viral load <200 cpm and 72% had an HIV viral load <50 cpm at the end of therapy.
Table 1.
Baseline characteristics.
| Characteristic | Number |
|---|---|
| Total number of participants | 22 |
| Age (years), median (IQR) | 37 (30–49) |
| Female, n (%) | 6 (27.3) |
| White, n (%) | 8 (36.4) |
| Hispanic, n (%) | 12 (54.5) |
| CD4 count (cells/μl), median (IQR) | 36 (22–112) |
| On ART, n (%) | 5 (22.7) |
| Resistance to ≥1 ART class, n (%) NRTI resistance | 9 (40.9) 4 (18.2) |
| Additional ART, n (%) | |
| Integrase inhibitor | 18 (81.8) |
| Protease inhibitor | 2 (9.1) |
| Integrase + protease inhibitor | 2 (9.1) |
| Rifabutin dose, n (%) | |
| 150 mga | 4 (18.2) |
| 300 mg | 18 (81.8) |
| Organism, n (%) | |
| Mycobacterium tuberculosis | 12 (54.5) |
| Mycobacterium avium–intracellulare | 5 (22.7) |
| Other | 5 (22.7) |
ART, antiretroviral therapy; IQR, interquartile range; NRTI, nucleotide reverse transcriptase inhibitor.
Dose adjusted for drug interaction with protease inhibitor use.
Fig. 1.
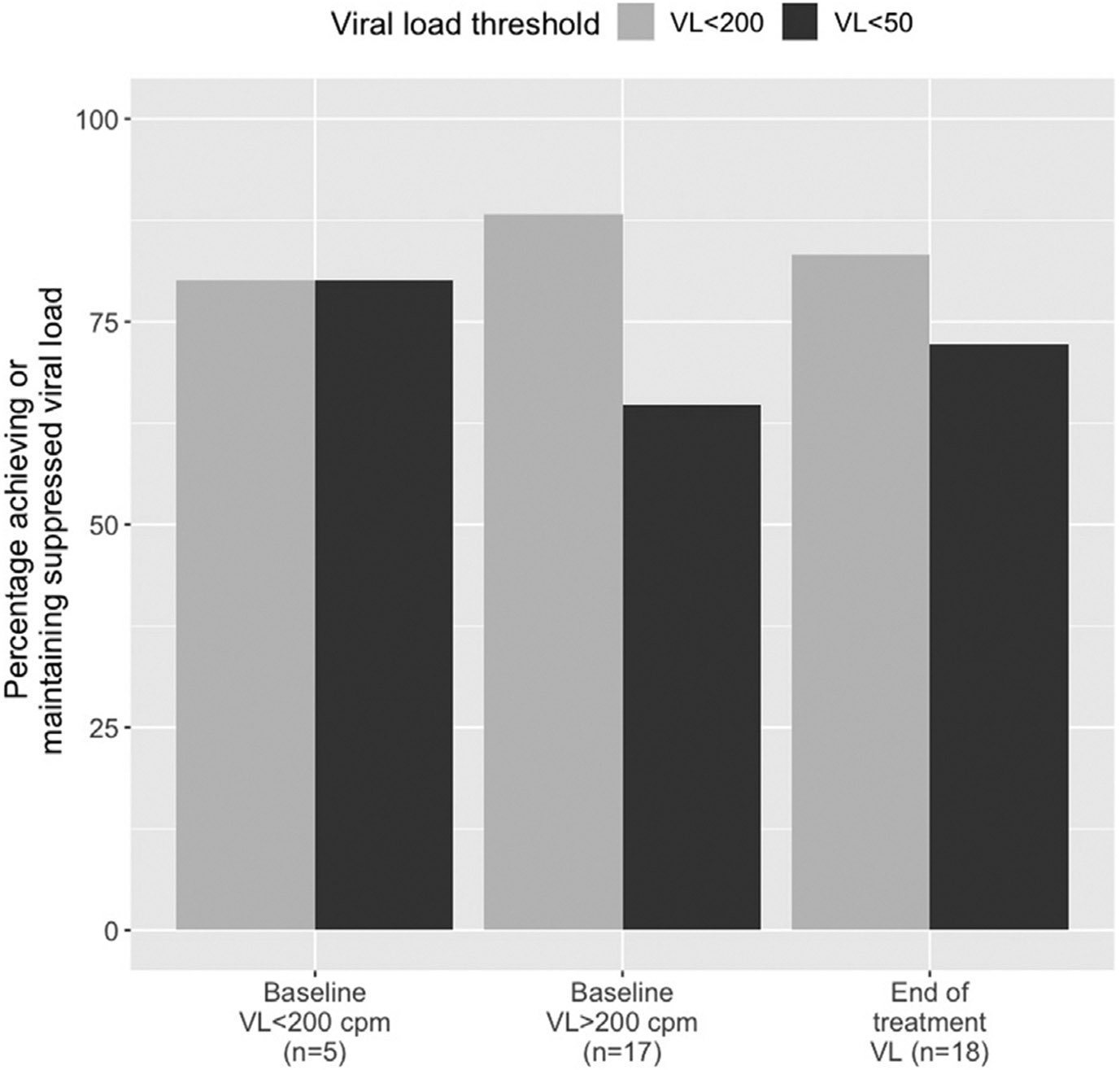
Percentage of participants who achieved or maintained an HIV viral load <200 cpm (light grey) or <50 cpm (dark grey) during TAF–RFB co-administration, according to baseline viral load at the start of co-administration. The percentage of participants with viral suppression at the end of rifabutin treatment was available for 18 participants (combined from both groups).
Abbreviations: VLviral load; cpmcopies per ml.
Three PWH did not achieve or maintain a viral load <200 cpm. Chart review indicated that two persons had self-discontinued ART, leading to an elevated viral load; the remaining patient was lost to follow-up prior to being able to evaluate viral suppression. Excluding these PWH who self-discontinued therapy or were lost to follow-up, 19/19 (100%) participants met the combined primary outcome goal of achieving or maintaining HIV viral load <200 cpm.
Discussion
This article reports the HIV virological outcomes of 22 PWH receiving TAF-containing ART and RFB co-administration for the treatment of a mycobacterial infection. Of the 19 PWH who had adequate data available and reported adherence to ART, 19 (100%) achieved or maintained an HIV viral load <200 cpm. These results suggest that TAF–RFB co-administration is efficacious and leads to the suppression of HIV viral replication.
Prior studies of TAF–rifampicin co-administration demonstrated that although absorption was significantly reduced, intracellular levels of TFV-DP remained approximately four times higher than those following standard dose TDF without rifampicin (Cerrone et al., 2019). One further study investigated the pharmacokinetics of twice-daily TAF dosing during rifampicin administration and found that despite double the dose, the 24 -h intracellular TFV-DP concentrations were reduced by 24% compared to once-daily TAF dosing in the absence of rifampicin (Custodio et al., 2017). The present study is the first to provide data on the clinical outcomes of TAF–RFB co-administration.
This study had limitations: it was retrospective in nature, did not include pharmacokinetics/pharmacogenomics, and had a limited sample size. There was loss to follow-up and two PWH self-discontinued ART. Despite these limitations, it was found that the majority of PWH who were adherent to therapy achieved or maintained HIV viral suppression.
In conclusion, this study found evidence to suggest that TAF–RFB co-administration is efficacious and results in viral suppression in the majority of PWH. Further work should evaluate pharmacokinetics to confirm this finding that TAF-containing HIV regimens can be administered with RFB.
Funding
This work was supported by the National Institutes of Health (T32AI007384—29).
Footnotes
Ethical approval
The study protocol was reviewed and approved by the UCSD Human Research Protections Program (#191448CX).
Conflict of interest
TCSM has received funding from Gilead Sciences. All other authors declare no conflict of interest.
References
- Cerrone M, Alfarisi O, Neary M, Marzinke MA, Parsons TL, Owen A, et al. Rifampicin effect on intracellular and plasma pharmacokinetics of tenofovir alafenamide. J Antimicrob Chemother 2019;74(6):1670–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custodio J, West S, Lutz J, Vu A, Xiao D, Collins S, et al. Twice daily administration of tenofovir alafenamide in combination with rifampin: potential for tenofovir alafenamide use in HIV-TB coinfection. Milan, Italy: European AIDS [142_TD $DIFF]Conference (EACS); 2017. [Google Scholar]
- Eron JJ, Orkin C, Gallant J, Molina JM, Negredo E, Antinori A, et al. A week-48 randomized phase-3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naive HIV-1 patients. AIDS 2018;32(11):1431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Post FA, Arribas JR, Eron JJ Jr., Wohl DA, Clarke AE, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS 2019;33(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz JD, Kirby BJ, Wang L, Song Q, Ling J, Massetto B, et al. Cytochrome P450 3A Induction Predicts P-glycoprotein Induction; Part 2: Prediction of Decreased Substrate Exposure After Rifabutin or Carbamazepine. Clin Pharmacol Ther 2018;104(6):1191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services; 2020. Available from: https://files.aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Accessed May 21, 2020. [Google Scholar]
- Sax PE, Zolopa A, Brar I, Elion R, Ortiz R, Post F, et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquired Immune Deficiency Syndromes (1999) 2014;67(1):52–8. [DOI] [PubMed] [Google Scholar]
- Wang H, Lu X, Yang X, Xu N. The efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens for HIV-1 therapy: Meta-analysis. Medicine 2016;95(41). [DOI] [PMC free article] [PubMed] [Google Scholar]


