Abstract
Recent advances in protein profiling technology has facilitated simultaneous measurement of thousands of proteins in large population studies, exposing the depth and complexity of the plasma and serum proteomes. This revealed that proteins in circulation were organized into regulatory modules under genetic control and closely associated with current and future common diseases. Unlike networks in solid tissues, serum protein networks comprise members synthesized across different tissues of the body. Genetic analysis reveals that this cross-tissue regulation of the serum proteome participates in systemic homeostasis and mirrors the global disease state of individuals. Here, we discuss how application of this information in routine clinical evaluations may transform the future practice of medicine.
Challenges and Prospects of Personalized Medicine
Despite personalized medicine (see Glossary) being featured prominently in industry and academia, its promise has largely not been realized. There have been some notable successes, particularly in oncology [1–3] and often involving genetic variants in the target of a candidate therapy. However, patients in clinical practice are generally not prescribed therapeutics based on an individuaĺs higher probability of an effective response. There are many reasons for this lack of progress, but a significant one is that the utility of novel biomarkers for prediction of treatment response can only be validated in a clinical trial setting, making the investment lengthy, costly, and risky. With the occasional exception of alterations in a drug target itself, it remains a challenge to predict which measures will be directly associated with outcome or response and often scientists must resort to discovering such markers de novo in a potentially very large search space.
Given this challenge, a strategy to facilitate biomarker development is to use broad-based molecular measures that comprehensively capture the molecular state of individuals (Figure 1). Within such broad measures, the associations with past, current, and future disease states could be established, yielding a comprehensive molecular map of individuals that can be mined for specific treatment associated biomarkers. However, are such comprehensive, unified molecular measures touching all common diseases, and the tissues they involve, a possibility, or are we more likely to be trapped in a mishmash of different measurements and noncompatible modalities? As detailed here, proteins in serum have the desired attributes required for a comprehensive and unified approach to measuring the global molecular state of an individual: easy sample collection, comprehensive information captured across tissues, and direct connection to disease-relevant molecular pathways and activities [4]. Furthermore, we suggest that the serum proteome is of sufficient depth, comprehensiveness, and complexity that it can also be used to derive novel treatment specific biomarkers for most, if not all, common diseases.
Figure 1. The Development of a Broad-Based Molecular Biomarker Platform.

For a Figure360 author presentation of Figure 1, see the figure legend at https://doi.org/10.1016/j.molmed.2020.09.003. An overview of the development of a biomarker platform from a large-scale collection of deep phenotype and blood proteome data to the construction of protein networks in the serum. The protein networks connect to all tissues and most diseases as well as to different states of disease, yielding a comprehensive molecular map of individuals from which specific treatment associated biomarkers can be established. Once a reference set of protein networks and relationships to diseases is established, the grouping and disease status of new donors using their serum can be inferred.
Technological advances for high-throughput measurement of proteins in biological samples have facilitated this work [4–7], and aptamer-based affinity methods in particular have been an engine of recent discovery [4,8–11]. In this opinion, we do not address the platform technology for such measures but rather focus on what the availability of such global molecular measures might mean for the practice of medicine and drug discovery.
Serum Proteins in Coregulatory Networks Associate with Disease
The cardiovascular system controls appropriate blood flow to and from all tissues of the body to maintain global homeostasis in terms of physiological temperature, oxygen transport, and exchange of nutrients and waste [12]. Tissues and organs contribute components and capabilities to the whole organism by systemic coordination. How is higher order coordination across different tissues achieved? In addition to the nervous and lymphatic systems, blood is well placed to facilitate such coordination between tissues and cell types via the serum proteome [4]. Heterochronic parabiosis experiments that surgically joined the circulation of young and old mice indicate that serum proteins mediate numerous complex events in many organs, including heart and brain [13–18].
It is generally accepted that common diseases are not caused by single proteins acting in isolation but instead by networks of highly interacting proteins that sense genetic and environmental perturbations and can ultimately drive physiological states toward disease [19–22]. Such networks are found to be highly structured [4,23,24], where a small number of protein hubs are tightly connected to many other proteins, while most have few connections. Recent work provides support that systemic homeostasis in humans is facilitated by a complex web of cross-tissue regulatory loops involving serum protein networks connecting most or all tissues [4]. As reported, 4783 serum proteins were measured in a population-based cohort of 5457 individuals, where the relationships of those proteins to each other, to genetic variation, and to specific disease in the same individuals were assessed [4]. This work indicated that serum proteins exist in coregulatory network modules in a manner reminiscent of mRNA modules derived from solid tissues [20,21,24,25]. In contrast to single-tissue networks, those in blood proteins synthesized in many or all tissues of the body (Figure 2A). For example, a protein specifically synthesized in liver was as likely to be coregulated with one originating from brain, muscle, or kidney as to another protein from liver [4]. The central regulatory role of individual secreted proteins has been known for decades, including examples such as insulin and glucagon; yet, the full depth and complexity of regulation mediated by proteins transiting through blood has not been fully explored (Box 1).
Figure 2. Many Tissues Can Contribute Proteins to a Single Serum Protein Module.
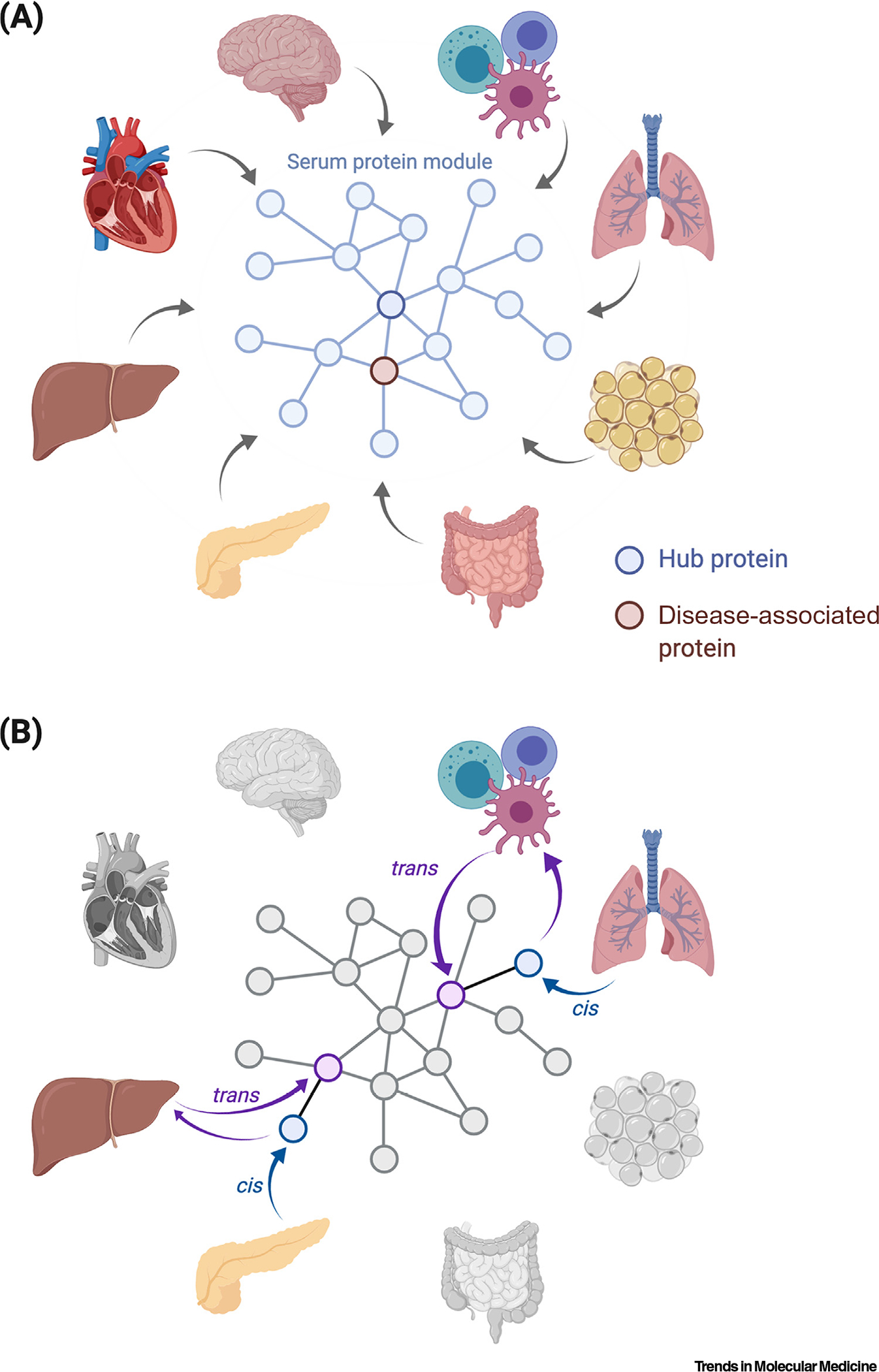
(A) An example of a module from the coregulatory serum protein network comprising proteins synthesized in different tissues of the body. The hub protein is connected to many other proteins and is strongly associated with disease and overall survival. (B) Cross-tissue cis and trans pairs of serum proteins reveal a web of regulatory loops that connects different tissues in the body and is apparent in the serum protein network. For instance, a genetic variant that controls a protein encoded by a nearby gene (cis) in pancreas is exported to the blood and that, in turn, may regulate another protein in a physically distant tissue, such as liver.
Box 1. Cross-Tissue Relationship of Proteins in Circulation.
The serum proteome is organized into different network modules that comprise proteins sharing similar functions and that were synthesized in different tissues of the body [4]. The pancreatic-specific hormones insulin and glucagon are well-known global regulators of blood glucose homeostasis [63], and further analyses have revealed additional secreted proteins, both upstream and downstream of these key actors, that are produced by distinct tissues, including enteroendocrine cells of the gut (incretins), adipose tissue (leptin), and stomach (ghrelin) [64–68]. In this manner, a complex web of interactions between different tissues and cell types is mediated by multiple serum proteins transiting through blood. When this homeostatic system is disrupted, pathologic conditions, such as obesity and diabetes, may arise.
The ability to measure the level of thousands of proteins in the serum of thousands of individuals has led to the discovery of many more such cross-tissue relationships of coregulated proteins [4]. For instance, a 378-protein serum protein module associated with metabolic syndrome, heart failure, tissue fibrosis, and overall survival [4] comprises proteins specifically synthesized in distinct tissues, including liver (ANGPTL3), skeletal muscle (BIN1), pancreas (IAPP), lung (SFTPC), testis (IGLL1), kidney (UMOD), and ovaries (WFIKKN2) [4]. The tissue of origin was determined using high tissue-specificity scores (Z >9.24, the top 0.5%) based on the GTEx transcript data covering 43 different tissues [4,36].
Proteins in circulation were strongly associated with common diseases, including, but not limited to, coronary heart disease, heart failure, obesity, type 2 diabetes mellitus, cancer, and diseases of muscle, kidney, liver, and brain [4,8,9,26]. These associations included diseases present at the time of blood collection (prevalent disease) and those that manifested after blood collection (incident disease). It was also found that the protein associations to disease extended to the network structure of the proteins, with the principal components of individual protein coregulatory modules being robustly associated with disease and survival [4]. In other words, the serum protein network, derived solely from protein variation data, robustly reflects the current and future disease states of the individuals.
Cross-Tissue Regulation of Serum Protein Networks
What then might be the function of the serum protein networks and how do they arise? A significant clue comes from the analysis of protein quantitative trait loci (pQTLs) (Box 2), where variation of proteins in blood and genetic variation in the same population were compared [4,11,27,28]. These analyses showed that serum proteins were frequently under genetic control individually and also at the level of protein networks [4]. This is where a genetic variant controlled a protein encoded by a nearby gene (cis) and/or one or more proteins the coding sequences of which are located elsewhere in the genome (trans), resulting in the following causal chain pQTL ➔ cis protein ➔ trans proteins ➔ protein network (Figure 2B and Box 2). Numerous studies of the genetics of gene expression have been carried out in large collections of human tissue samples to identify mRNA-based expression QTLs (eQTLs) [20,24,29–36], offering critical information regarding transcriptional regulation. Comparison between pQTLs and eQTLs revealed a significant overlap between these two key regulatory layers [4,11,28,37]. One notable difference was the large number of trans effects mediated by single pQTLs [4,11], which have not been observed to a similar extent at the transcriptional level (Box 2). Importantly, many proteins coregulated in trans share similar function and are part of the same network observed in both humans and mice [4,11,38].
Box 2. DNA Sequence Variants Affecting Protein Levels.
Naturally occurring DNA sequence variants associated with protein levels are called pQTLs. Protein QTLs are located either proximal to (cis pQTL) or distant (e.g., at another chromosome; trans pQTL) from the gene encoding the regulated protein. Large-scale proteomic studies in humans and model organisms revealed strong genetic effects on protein levels in both solid tissues and biofluids [4,11,38,69], which is in line with the reported heritability of protein levels in plasma [70]. Importantly, cis and trans-acting pQTLs detected in serum can be readily replicated across different populations and proteomic platforms [4]. Furthermore, pQTLs show a significant overlap with mRNA gene expression QTLs (eQTLs), yet with some notable differences [4,11]: in contrast to trans eQTL effects, which tend to be weak and hard to detect [20,24], trans pQTLs can be readily identified. One possible reason for this difference is that protein maturation encompasses multiple post-transcriptional steps, which may be genetically controlled in trans. Also, it is possible that, in some cases, trans effects may be secondary to a primary genetic effect on another trait, including, for instance, a transporter molecule such as HDL-cholesterol.
Recent studies uncovered thousands of cis–trans pairs of proteins in circulation [4,11]. For some of these pairs, each protein showed highly tissue-specific expression, often involving distinct tissues [4]. For instance, while CFHR5 (liver) levels in serum were controlled by a genetic variant near its coding region, the same variant controlled levels of DLL3 (brain), CFP (spleen), and HPX (liver) where the coding sequences in each case were on other chromosomes. Additionally, all four proteins were part of the same network module [4]. Therefore, these data are most simply explained by the causal chain SNP ➔ cis CFHR5 (liver) ➔ trans DLL3 (brain), CFP (spleen) and HPX (liver) ➔ network. This same analysis uncovered 1078 unique cis–trans pairs involving 43 tissues present in the GTEx analysis [4]. This work reveals that the network of cross-tissue regulation involving serum proteins is deep and complex and spans all tissues of the body.
Of relevance for this discussion, a large subset of cis/trans pairs have been identified where both proteins have tissue-specific expression, often involving distinct locations in the body [4,11] (Box 2). This observation suggests that an individual tissue synthesizes certain proteins that are exported to the blood and may then regulate protein synthesis and secretion in physically distant tissues (Figure 2B), revealing a rich web of regulatory loops that connect all tissues in the body. This also provides an explanation for the finding that co-expression networks contain proteins synthesized in many tissues [39,40]. In this sense, the network of proteins in serum can be considered as an integration of all the tissue-specific networks of the body.
Serum Protein Networks Link Genetics to Disease
The many large-scale genome-wide association studies (GWASs) over the past decade have documented >70 000 DNA variants associated with phenotypes related to complex disease [41]. These analyses revealed that each complex disease trait is associated with hundreds of risk loci of small effect sizes and commonly located outside coding regions (e.g., reviewed in [42]). Integration of intermediate traits, such as mRNA and/or protein levels, with genetics and disease traits aids in the identification of the causal candidates [4,19–21,24]. In fact, a profound overlap between the genetics of protein levels and complex diseases has been demonstrated [4,11,27,28,43], and in prepublication data [44–46]. Specifically, when GWAS risk loci for many complex diseases were linked to the serum proteome, they tended to regulate many proteins in cis and/or trans, many of which were found to cluster in the same network modules [4]. This indicates that the networks sense, process, and integrate multiple and variable signals from both the DNA and the environment, resulting in a unified systemic output.
How then do hundreds of different risk loci transmit their effects from many different tissues to affect complex systemic traits? Here, biological networks provide a framework for understanding the architecture of complex disease by capturing effects and regulatory mechanisms across multiple high-dimensional data sets [47–49]. For this discussion, we postulate that many of the genetic signals for common diseases with individual weak effects are cis and/or trans-acting pQTL pairs connecting different tissues and diverse disease-related effects via the serum protein network. This would explain why all common diseases were mirrored in the levels of proteins in sera; that is, that they are directly participating in the disease processes. It will be a major challenge for the future to disentangle the cause and effect between proteins in circulation and disease.
Serum Proteins and Holistic Molecular Medicine
Many approaches to biomarker development have been reported, including, for instance, measurement of RNAs, metabolites, peptides, and volatile organic compounds from various sources and associating such measures with clinically relevant phenotypes [50–57]. The increasing availability of genome-scale omics data and the parallel development of computational tools have allowed effective integration of the high-dimensional data at the level of molecular networks [24,58,59]. However, most of these studies have not been built around protein assay data. In fact, proteins have fundamental roles in all life processes and offer direct links to the pathways and activities associated with complex disease. The time and effort needed to develop a platform that can measure a large number of proteins accurately, specifically, and at reasonable cost will not be insubstantial, but the motivation to do so may be bolstered by the knowledge that a holistic view of the molecular state of individuals is present in the relative levels of serum proteins.
To illustrate the promise of this approach, unrelated individual donors were grouped together based solely on the particular serum protein network that each one contained [4]. For this analysis, the loading coefficients for the eigenproteins (principal components) for each of the 27 protein modules [4] were used. Similarities and differences between individual donors were simply assessed using pairwise donor-to-donor correlations using the module eigenprotein scores. The correlations between different donors were in most cases not significant, although, in some cases, this revealed groups of individuals that were either highly positively correlated or intriguingly highly negatively correlated (Figure 3A). Interestingly, most individuals had a least one significant correlation to another donor. Studies have shown that 75% of individual protein biomarkers for disease diagnosis vary significantly across the population depending on genetic and clinical factors that are unrelated to the disease of interest [60], which in turn often make them unsuitable as predictors of disease unless using personalized clinical cutoffs. By contrast, the collective behavior of protein modules, as captured at the individual donor level by loading coefficients, represents on average 45% of the variance of all proteins within a given cluster [4], offering novel disease information and robust biomarkers that are less affected by deviating levels of single proteins.
Figure 3. Serum Protein Networks to Cluster Donors.

(A) Using robust features of the protein networks (eigenvectors of protein modules), it is found that, at an individual-level, donors can be either positively, negatively, or not correlated to other donors. (B) The loading coefficients for the eigenproteins for each of the 27 serum protein modules capturing the variance of thousands of serum proteins can be measured in individuals and these patterns can be compared across the population to disease states to derive numeric tests. (C) Hypothetically, this information can be used to cluster individuals into groups and, since serum proteins also associate with disease, these groups specify disease subclusters in the population (the axis represents derived dimensions 1 and 2 used to cluster individuals). Abbreviations: CVD, cardiovascular disease; T2DM, type 2 diabetes mellitus.
This indicates that, at the global molecular level, individuals can be readily categorized and grouped and, given that modules are associated with disease, this clustering of individuals incorporates disease status. Thus, it appears entirely feasible to develop numeric-based methods to objectively classify and cluster individuals by their global common disease status, including past, current, and future maladies. Hypothetical examples of disease-related clustering of donors using this unified and global metric of protein levels in serum samples are shown in Figure 3B,C. In this manner, a large multiplex proteomic platform could objectively and numerically inform on the molecular state of each individual, where those same measures could be used to group together individuals in a holistic disease-relevant manner.
Serum Protein-Based Biomarker Platform: The Realization of Personalized Medicine
What might be the consequences of being able to routinely assess the holistic disease state of individuals? One obvious benefit will be comprehensive clinical assessments and early diagnoses. Shifting diagnoses toward an objective numeric test will provide the patient and doctor a glimpse into the future and potentially avoid missed diagnoses (Figure 4). A serum protein-based platform would inform on many diseases for the individual tested and, therefore, would provide a richness and depth that is currently missing, particularly in terms of the very early stages of disease emergence (Figure 4). Further development and refinement of the use of such a platform is needed, including broad testing of well-characterized populations, time-series and comparison with other omics data [61], and validation of biomarker sets for risk prediction of each disease.
Figure 4. Blood Proteins Offer a Holistic View of the Disease State of Individuals.

Numeric-based tests derived from the serum protein networks that inform on the wellness status of individuals tested offer a richness and depth of the disease state of individuals that is currently missing in clinical practice. Current medical records are generally based on clinical presentation of disease (A) and are sparse. By contrast, inference of current and future disease with serum proteins will be richer and more forward looking (B).
The early warning of the onset of some of the most prevalent causes of morbidity and mortality in industrialized societies could boost motivation for lifestyle changes and perhaps early treatment. Intuitively, early diagnosis opens the possibility of better outcomes. For example, if diagnosed at an early stage, most cancers can be surgically removed. However, much of the healthcare system has been necessarily built on treating patients after clinical signs of disease have developed. Will current therapies designed to ameliorate clinically presenting disease be useful in a preventative setting? Clinical trials to prevent the onset of disease would involve the treatment of otherwise healthy individuals where the bar for safety will be necessarily high and the outcome, lack of disease onset, must be measured over many years. These trials would involve large study populations and would likely be expensive. These and other questions will have to be answered by tomorrow’s researchers as they address the perhaps shifting paradigms of disease diagnosis and the possibility of early intervention.
A possible route to develop preventive treatments is to use early markers of later disease as proxies to reduce the time and expenses of preventive clinical trials. The same markers could also be used to focus studies on individuals at risk for disease onset. For instance, it has been proposed that changes in the estimated glomerular filtration rate (eGFR) can be used as a proxy for end-stage kidney disease [62]. In an analogous manner, it can be anticipated that monitoring blood protein profiles before and after treatment could also be used as short-term proxy for longer term outcomes. Thus, at both the individual and population levels, patient selection, early responses, and numeric reduction of current disease burden and, importantly, of future disease risk could be measured and, therefore, used to assess an early readout on the utility of any therapy. Might this also be used to assess at an early stage if certain therapies are effective? A new therapy could be assessed for its ability to reverse the molecular disease state and/or block further progression toward disease. Such readouts may also be used for portfolio management by comparing current and new therapies in terms of overlapping or novel activities. In the case of established therapies, serum proteins might provide an early readout of response or lack thereof at the individual level, allowing alternate treatments to be considered by doctors and patients.
Concluding Remarks
Much more remains to be discovered and tested, including qualification of validated biomarkers based on selected proteins, further exploration of serum protein based genetic and drug signatures, application to rare diseases, infectious diseases, and different environmental and ethnic backgrounds (see Outstanding Questions). However, at this point, it does appear that serum proteins exist in coregulatory networks spanning all tissues of the body, the protein levels are under genetic control, and network modules can be associated with all common diseases, whether those diseases occurred before or after the time of the blood draw. As such, they offer an opportunity to measure the detailed global molecular status of individuals and across time. If it is practically feasible for these measurements to be accurately and routinely collected, this could have large-scale impact on the practice of medicine and drug development by objectively assessing individuals and grouping diseased populations, before and following treatment, using an all-encompassing molecular measure.
Outstanding Questions.
The gatekeeper to using proteins in blood as biomarkers is the methods of measurement that can be applied for routine clinical practice. Aptamer-based technology has the benefits of measuring thousands of proteins in parallel, low sample requirements, and high specificity and selectivity. Here, the cost for broad sample testing is an important criterium for general use. Although this is not a large problem, proteomic platforms will always need to avoid measurement differences resulting directly from DNA-coding sequence changes. Ideally, additional technologies and platforms should be developed as complementary approaches.
Proteins in blood associate with all common diseases and future work will establish whether this can be extended to less common and even rare diseases, infectious diseases, and different environmental and ethnic backgrounds. Formal classifiers will be derived, tested, and compared with existing biomarkers. How well does this work prospectively and how well can we translate discoveries into clinical intervention are key questions. The ability to predict future, particularly acutely life-threatening disease, can be expected to both drive demand and result in pressures on drug companies and healthcare systems.
The current platform will also be used to fill the gap of personalized medicine of drug developers. There will be two general classes of such use: biomarkers indicative of drug success and companion biomarkers. The former may include assessment of the ability of a drug to reverse disease at the molecular level and comparison of the effects of various drugs for portfolio management purposes. The companion biomarkers are intended to be developed in parallel with the drug, where the final form of the test may differ from the discovery platform. The timing and economics of companion biomarkers will still be challenging, but the protein-based general platform will at the least facilitate their discovery.
The advent of detailed comprehensive molecular readouts of the state of individuals is a level of knowledge that science and medicine has not had access to, to date. It is likely that this new knowledge will drive innovation and fundamentally alter the relationships of patients to the medical system and their own health.
Highlights.
Parallel measurements of thousands of plasma and serum proteins in populations give an unprecedented view of the complexity of the protein content of blood.
Serum proteins exist in coregulated modules that are genetically controlled and to which all tissues contribute. Modules are closely associated with past, current, and future diseases of diverse etiologies.
Measurements of serum proteins en masse can facilitate the yet largely unrealized promise of personalized medicine and, where additional treatment-specific biomarkers of response or adverse events can be derived. This offers numeric classification of individuals.
With routine use in clinical practice, measurement of serum proteins could change the focus of medicine from treating illness to preventing its onset.
Clinician’s Corner.
Technological advances have allowed the parallel measurement of the levels of thousands of proteins in the sera of thousands of individuals. This has revealed a previously unrecognized depth of information contained in sera about the global disease state of individuals.
Serum proteins were found to exist in regulatory groups of modules comprising members synthesized in all tissues of the body. By combining tissue-specific expression with genetic markers, a web of regulatory relationships became apparent in which a protein synthesized in one tissue transits through serum and regulates events in another physically distant tissue. In other words, system-level coordination or homeostasis is facilitated to a significant degree by thousands of proteins in blood.
Given this, it is not surprising that changes in protein levels in blood report on, and may mediate, many common and at least some rare diseases, including but not restricted to cardiovascular and metabolic diseases, immune and infectious diseases, diseases of the brain, muscle, liver and kidney, and cancers. This includes diseases present in the individual at the time of blood draw (prevalent disease) and those that will appear in the future (incident disease).
This work indicates that a single test using highly accessible material gives a global readout of the body encompassing all tissues and diseases, which can be thought of as an individuaĺs comprehensive status report. The resultant information can be readily used to group unrelated individuals, to comprehensively readout their disease status now and in the future, and to monitor the onset of disease over time and the successful or otherwise response to treatment.
If used commonly in clinical settings, this may substantively change the practice of medicine toward prevention, facilitate truly personalized treatment, and change the focus of drug discovery from the treatment of clinically presenting disease to altering the onset of disease.
Acknowledgments
V.E. and Va.G. are supported by the Icelandic Research Fund (IRF grants 195761-051 and 184845-053).
Glossary
- Biomarker
a biological entity that can be measured and optimally quantified and that expresses the effect of exposure, condition, or disease.
- Coregulatory network module
a cluster of proteins coregulated or covarying across individuals. The covariance profile may be determined by coregulation at the transcriptional level and/or post-transcriptional coregulatory mechanisms, including, for instance, protein maturation, secretion, clearance, and stability.
- Disease
a medical condition of the organism or its parts that damages normal functioning showing symptoms not related to physical injury.
- Eigenprotein
collection of principal components of a given protein module identified through a singular value decomposition and transformation of the variable protein levels for any given module; can be thought of as the low-dimensional representation of all proteins in a module.
- Holistic disease state
because patients can present with more than one disease, the state of being is regarded in its wholeness.
- Homeostasis
maintaining a relatively constant (i.e., within pre-set limits) internal environment of the biological system.
- Incident disease
when new cases appear with a disease condition that arises after the first recruitment and blood draw. Disease processes may occur over many years before clinical presentation. Proteins associated with those disease processes may have altered levels due to genetics, environmental factors, and/or age, and, as such, may be predictive of future disease. Often these proteins differ from those proteins that are strictly associated with prevalent disease.
- Loading coefficient
estimated for each protein in its module, showing the correlation between the original individual variation in protein levels and a given principal component for an estimated eigenprotein.
- Personalized medicine
often called precision medicine, which implies fitting medical treatment to the individual patient-specific characteristics in responding to treatment.
- Prevalent disease
a disease condition that is present in individuals at the time of recruitment and blood draw.
- Serum proteome
a comprehensive collection of proteins in serum arising largely from active or passive secretion, ectodomain shedding, extracellular vesicles, lysis and/or cell death.
Footnotes
Disclaimer Statement
J.R.L. was, and L.L.J. is, an employee of, and own stocks in, Novartis.
References
- 1.Dalton WB et al. (2017) Personalized medicine in the oncology clinic: implementation and outcomes of the Johns Hopkins Molecular Tumor Board. JCO Precis Oncol. 2017. 10.1200/PO.16.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karapetis CS et al. (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 359, 1757–1765 [DOI] [PubMed] [Google Scholar]
- 3.Schork NJ (2015) Personalized medicine: time for one-person trials. Nature 520, 609–611 [DOI] [PubMed] [Google Scholar]
- 4.Emilsson V et al. (2018) Co-regulatory networks of human serum proteins link genetics to disease. Science 361, 769–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geyer PE et al. (2019) Plasma Proteome Profiling to detect and avoid sample-related biases in biomarker studies. EMBO Mol. Med. 11, e10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enroth S et al. (2018) Systemic and specific effects of antihypertensive and lipid-lowering medication on plasma protein biomarkers for cardiovascular diseases. Sci. Rep. 8, 5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwenk JM et al. (2017) The Human Plasma Proteome Draft of 2017: building on the Human Plasma PeptideAtlas from mass spectrometry and complementary Assays. J. Proteome Res. 16, 4299–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams SA et al. (2019) Plasma protein patterns as comprehensive indicators of health. Nat. Med. 25, 1851–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehallier B et al. (2019) Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 25, 1843–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emilsson V et al. (2019) Predicting health and life span with the deep plasma proteome. Nat. Med. 25, 1815–1816 [DOI] [PubMed] [Google Scholar]
- 11.Sun BB et al. (2018) Genomic atlas of the human plasma proteome. Nature 558, 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodwill AG et al. (2017) Regulation of coronary blood flow. Compr. Physiol. 7, 321–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conboy IM et al. (2005) Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 [DOI] [PubMed] [Google Scholar]
- 14.Villeda SA et al. (2011) The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pluvinage JV and Wyss-Coray T (2020) Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat. Rev. Neurosci. 21, 93–102 [DOI] [PubMed] [Google Scholar]
- 16.Loffredo FS et al. (2013) Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153, 828–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinha M et al. (2014) Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 344, 649–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katsimpardi L et al. (2014) Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schadt EE (2009) Molecular networks as sensors and drivers of common human diseases. Nature 461, 218–223 [DOI] [PubMed] [Google Scholar]
- 20.Emilsson V et al. (2008) Genetics of gene expression and its effect on disease. Nature 452, 423–428 [DOI] [PubMed] [Google Scholar]
- 21.Chen YQ et al. (2008) Variations in DNA elucidate molecular networks that cause disease. Nature 452, 429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barabási AL et al. (2011) Network medicine: a network-based approach to human disease. Nat. Rev. Genet. 12, 56–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravasz E et al. (2002) Hierarchical organization of modularity in metabolic networks. Science 297, 1551–1555 [DOI] [PubMed] [Google Scholar]
- 24.Zhang B et al. (2013) Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 153, 707–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandal MJ et al. (2018) Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 362, eaat8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enroth S et al. (2019) High throughput proteomics identifies a high-accuracy 11 plasma protein biomarker signature for ovarian cancer. Commun. Biol. 2, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao C et al. (2018) Genome-wide mapping of plasma protein QTLs identifies putatively causal genes and pathways for cardiovascular disease. Nat. Commun. 9, 3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suhre K et al. (2017) Connecting genetic risk to disease end points through the human blood plasma proteome. Nat. Commun. 8, 14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaffney DJ et al. (2012) Dissecting the regulatory architecture of gene expression QTLs. Genome Biol. 13, R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montgomery SB et al. (2010) Transcriptome genetics using second generation sequencing in a Caucasian population. Nature 464, 773–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimas AS et al. (2009) Common regulatory variation impacts gene expression in a cell type-dependent manner. Science 325, 1246–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stranger BE et al. (2007) Population genomics of human gene expression. Nat. Genet. 39, 1217–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers AJ et al. (2007) A survey of genetic human cortical gene expression. Nat. Genet. 39, 1494–1499 [DOI] [PubMed] [Google Scholar]
- 34.Dixon AL et al. (2007) A genome-wide association study of global gene expression. Nat. Genet. 39, 1202–1207 [DOI] [PubMed] [Google Scholar]
- 35.Schadt EE et al. (2003) Genetics of gene expression surveyed in maize, mouse and man. Nature 422, 297–302 [DOI] [PubMed] [Google Scholar]
- 36.Consortium GTEx (2015) Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348, 648–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu L et al. (2013) Variation and genetic control of protein abundance in humans. Nature 499, 79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chick JM et al. (2016) Defining the consequences of genetic variation on a proteome-wide scale. Nature 534, 500–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talukdar HA et al. (2016) Cross-tissue regulatory gene networks in coronary artery disease. Cell Syst. 2, 196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dobrin R et al. (2009) Multi-tissue coexpression networks reveal unexpected subnetworks associated with disease. Genome Biol. 10, R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buniello A et al. (2019) The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 47, D1005–D1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barroso I and McCarthy MI (2019) The genetic basis of metabolic disease. Cell 177, 146–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Folkersen L et al. (2017) Mapping of 79 loci for 83 plasma protein biomarkers in cardiovascular disease. PLoS Genet. 13, e1006706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng J et al. (2020) Phenome-wide Mendelian randomization mapping the influence of the plasma proteome on complex diseases. Nat. Genet. Published online September 7, 2020. 10.1038/s41588-020-0682-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Folkersen L et al. (2020) Genomic evaluation of circulating proteins for drug target characterisation and precision medicine. bioRxiv Published online April 6, 2020. 10.1101/2020.04.03.023804 [DOI] [Google Scholar]
- 46.Emilsson V et al. (2020) Human serum proteome profoundly overlaps with genetic signatures of disease. bioRxiv Published online May 8, 2020. 10.1101/2020.05.06.080440 [DOI] [Google Scholar]
- 47.Liu X et al. (2019) Trans effects on gene expression can drive omnigenic inheritance. Cell 177, 1022–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyle EA et al. (2017) An expanded view of complex traits: from polygenic to omnigenic. Cell 169, 1177–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Connor LJ et al. (2019) Extreme polygenicity of complex traits is explained by negative selection. Am. J. Hum. Genet. 105, 456–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.May A and Wang TJ (2008) Biomarkers for cardiovascular disease: challenges and future directions. Trends Mol. Med. 14, 261–267 [DOI] [PubMed] [Google Scholar]
- 51.Davis MD et al. (2019) Exhaled breath testing - a tool for the clinician and researcher. Paediatr. Respir. Rev. 29, 37–41 [DOI] [PubMed] [Google Scholar]
- 52.Khera AV et al. (2018) Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 50, 1219–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newgard CB (2017) Metabolomics and metabolic diseases: where do we stand? Cell Metab. 25, 43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakamura A et al. (2018) High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 554, 249–254 [DOI] [PubMed] [Google Scholar]
- 55.Dodgson SE (2018) There will be blood tests. Cell 173, 1–3 [DOI] [PubMed] [Google Scholar]
- 56.Cohen JD et al. (2018) Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359, 926–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kristensen SL et al. (2017) Prognostic value of N-terminal pro-B-type natriuretic peptide levels in heart failure patients with and without atrial fibrillation. Circ. Heart Fail. 10, e004409. [DOI] [PubMed] [Google Scholar]
- 58.Wang B et al. (2014) Similarity network fusion for aggregating data types on a genomic scale. Nat. Methods 11, 333–337 [DOI] [PubMed] [Google Scholar]
- 59.Yan J et al. (2018) Network approaches to systems biology analysis of complex disease: integrative methods for multi-omics data. Brief. Bioinform. 19, 1370–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Enroth S et al. (2014) Strong effects of genetic and lifestyle factors on biomarker variation and use of personalized cutoffs. Nat. Commun. 5, 4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Price ND et al. (2017) A wellness study of 108 individuals using personal, dense, dynamic data clouds. Nat. Biotechnol. 35, 747–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stevens PE and Levin A (2013) Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 158, 825–830 [DOI] [PubMed] [Google Scholar]
- 63.Castillo-Armengol J et al. (2019) Inter-organ communication: a gatekeeper for metabolic health. EMBO Rep. 20, e47903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turton MD et al. (1996) A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379, 69–72 [DOI] [PubMed] [Google Scholar]
- 65.Holst JJ and Orskov C (2004) The incretin approach for diabetes treatment: modulation of islet hormone release by GLP-1 agonism. Diabetes 53, S197–S204 [DOI] [PubMed] [Google Scholar]
- 66.Creutzfeldt WO et al. (1996) Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7–36) amide in type I diabetic patients. Diabetes Care 19, 580–586 [DOI] [PubMed] [Google Scholar]
- 67.Ferrario CR et al. (2016) Homeostasis meets motivation in the battle to control food intake. J. Neurosci. 36, 11469–11481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Emilsson V et al. (1997) Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion. Diabetes 46, 313–316 [DOI] [PubMed] [Google Scholar]
- 69.Albert FW et al. (2014) Genetics of single-cell protein abundance variation in large yeast populations. Nature 506, 494–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y et al. (2015) Quantitative variability of 342 plasma proteins in a human twin population. Mol. Syst. Biol. 11, 786. [DOI] [PMC free article] [PubMed] [Google Scholar]


