Abstract
A 10-month-old lurcher with history of recurrent skin problems, presented with tachypnoea which had progressively become severe. Investigations included haematology, serum biochemistry, blood coagulation profile, diagnostic imaging, bronchoscopy and bronchoalveolar lavage (BAL). Cytological evaluation of the BAL revealed the presence of Pneumocystis cysts. The patient was euthanased on humane grounds prior to treatment against Pneumocystosis. To the best of our knowledge this is the first case of Pneumocystosis in a lurcher puppy.
Keywords: Pneumocystis, Lurcher, Puppy, Pulmonary, Tachypnoea
1. Introduction
Pneumocystis, an elusive microorganism which was originally thought to be a protozoan due to its biological behavior [1], has in the recent years been reassigned to the Fungal Kingdom and more specifically to the largest phylum within the fungi, Ascomycota and the subphyla Tarphrinomycotina [2]. Even though Pneumocystis spp was discovered in the early 1900's [3], Pneumocystis infection in humans was first reported in 1942 [4]. Pneumocystis pneumonia became well known in the 1980's as it was one of the most common clinical presentations in humans with acquired immune deficiency syndrome (AIDS) [5]. Pneumocystis organisms are extracellular fungi that reside in the lungs of humans and animals. These are usually of low virulence [6]. It affects most mammalian species, including dogs in which it causes opportunistic pneumonia [7].
Canine pneumocystosis is usually found in young, less than a year of age, dogs. It has been reported in only a handful of breeds with the dachshund and Cavalier King Charles Spaniel being overrepresented [8,9]. It is usually linked with immunodeficiency of the host. Regarding pathogenesis, after inhalation the cysts arrive at the alveoli where they proceed with their life cycle, multiply and proliferate in the alveolar spaces causing alveolo-capillary blockage and decreased gaseous exchange [7].
We report here the first case of Pneumocystosis in a lurcher puppy.
1.1. Case presentation
A 10-month-old male neutered lurcher was referred for investigation of an approximately 5-week progressively worsening tachypnoea. The patient lived in the UK and had never travelled abroad. Additional history had been waxing and waning non-pruritic skin disease, with erythema, scaling, papules, pustules and otitis externa, which had been treated with topical therapy, courses of broad-spectrum antibiotics and non-steroidal anti-inflammatory drugs (NSAID). More specifically the patient had completed the mandatory vaccination protocol by the age of 4 months. He had been on preventative medication for endo and ectoparasites with a combination of fipronil and praziquantel. He was fed on a commercial age appropriate dry food. At 2 months of age he presented with scaly skin and papules on his ventral abdomen but mainly on his groin. At 3 months of age he presented with bilateral otitis externa which consisted of erythema and papules on the inner pinna. Treatment with a topical preparation containing fucidic acid, framycetin sulphate, nystatin and prednisolone in both ears was successful. A month later the patient presented again due to deterioration of the lesions previously seen on his groin and ventral abdomen which in addition to scales, erythema and papules, pustules were now noted. A 10 day-course of oral amoxiclav (12.5mg/kg, every 12 hours) was introduced along with meloxicam. Initial resolution of what was suspected to be superficial pyoderma was followed by relapse with the initial therapy continued for a further 10 days. At 6 months of age he presented with bilateral conjunctivitis of unknown origin; mild green discharge was noted on both sides. Treatment with local fucidic acid was successful. Further discussions of the referring vet (RV) and the owner revealed that the patient enjoyed scavenging and would occasionally eat slugs. Due to concerns of lungworms, he was started with monthly topical application with a preparation containing moxidectin and imidacloprid. During this period the patient was castrated uneventfully. Two months later, at the age of 8 months, he was administered an intranasal kennel cough vaccine. A week later the patient progressively became malaise and tachypnoeic and presented with diarrhoea and hyporexia. Clinical examination had not revealed any significant abnormalities and treatment trial with a 5-day course of metronidazole and spiramycin was unrewarding. At this stage his owners reported a respiratory rate of >100 breaths per minute (br.pm) even at rest. Thoracic radiographs taken by the RV were suggestive of bronchopneumonia (Day 0). Oral treatment with amoxiclav (12.5mg/kg, every 12 hours) and carprofen (2mg/kg/day) were initiated but made no significant difference. By Day 5 his appetite had improved slightly but still remained tachypnoeic. His owners reported that tachypnoea was worse in the evening times when compared to morning (>100 br.pm versus 80 br.pm). On Day 7 due to continuous lack of response to the treatment, the RV added oral enrofloxacin (5mg/kg/day) and furosemide (2mg/kg every 12 hours) to the treatment regimen. By Day 9 it had become more obvious that the patient had lost weight. On Day 10 and due to further deterioration as patient had started becoming inactive, easily tired, with a respiratory rate of >120 br.pm, the RV obtained new thoracic radiographs which demonstrated the presence of pneumomediastinum. Therapeutic thoracocentesis was carried out and 300ml of air removed from his thorax. Simultaneously a diagnostical transtracheal lavage was carried out and samples submitted for culture. By Day 12 the patient was no different and he was subsequently referred as a matter of urgency due to further worsening of tachypnoea and failure to respond to the above therapeutic approach.
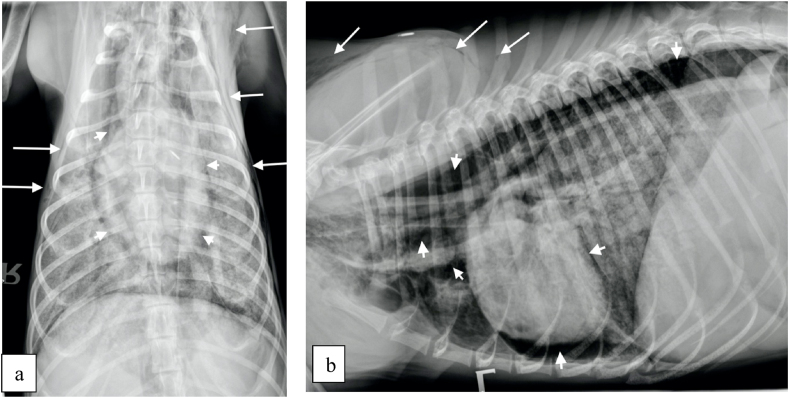
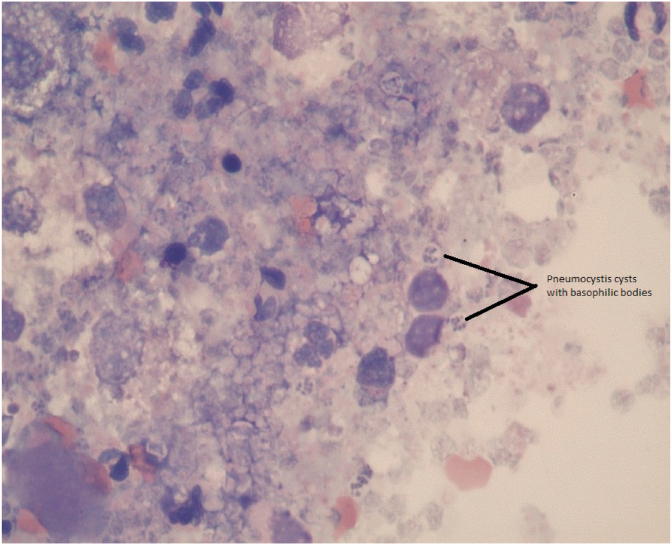
On presentation (Day 12) the patient was lethargic, severely tachypnoeic (>120 br.pm) and had a body condition score of 3/9. Clinical examination revealed a heart rate of 180 beats per minute (bpm) that was synchronous with his pulse, which was subjectively strong. He had congested mucous membranes with a capillary refill time less than 2 seconds and normal rectal temperature. Thoracic auscultation revealed normal heart sounds and bilaterally subjectively harsh respiratory sounds throughout all the pulmonary fields. No peripheral lymphadenomegaly was noted. Abdominal palpation was unremarkable. A faecal sample was obtained for parasitological examination and a blood sample was obtained for haematology, serum biochemistry and electrolytes and blood coagulation profile. Pending results, which are summarized in Table 1, Table 2, the patient was placed on flow-by oxygen. Briefly, left shift neutrophilia, and elevated total bilirubin (TBIL), gamma-glutamyl transfarase (GGT), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) concentrations were the most significant findings. Both pro-thrombin time (PT) and partial thromboplastin time (PTT) were within normal range (Table 1). Based on the above changes in the liver enzymes further investigation with abdominal imaging was pursued. The only abnormality on abdominal ultrasound was diffuse generalized hyperechoic hepatic changes. 3-view thoracic radiographs (right, left and ventro-dorsal) revealed a generalized broncho-interstitial pattern with alveolar patches visible throughout the lung fields (Fig. 1). Pneumomediastinum (short arrows), increasing the visibility of the margins of the cardiac silhouette, aorta and cranial mediastinal vessels, and subcutaneous emphysema (long arrows) were also visible (Fig. 1). Following thoracic imaging bronchoscopy was further carried out. Briefly, the trachea appeared within normal limits, and both left and right bronchial stems revealed mild erythematous changes. Bronchoalveolar lavage (BAL) was performed for cytological evaluation and culture. Following the procedures, the patient recovered but remained tachypnoeic and treated with oxygen. Due to further deterioration (Day 13) leading to severe dyspnea and cyanotic mucus membranes, his owners elected for euthanasia while results were still pending. There was no post mortem investigation and the final diagnosis was made based on the BAL cytology result, which showed pyogranulomatous inflammation with Pneumocystis carinii infection (Fig. 2). Culture from the same sample did not yield any growth. Faecal examination using the Baermann technique was negative for helminth eggs, protozoa and larvae.
Table 1.
Haematology and Coagulation profile.
| Parameter | Result | Normal range |
|---|---|---|
| RBC | 7.88 X 1012/L | 5.65–8.87 |
| HCT | 50.3% | 37.3–61.7 |
| HGB | 17.3 g/L | 13.1–20.5 |
| MCV | 63.8 fL | 61.6–73.5 |
| MCH | 22.0 pg | 21.2–25.9 |
| MCHC | 34.4 g/dL | 32.0–37.9 |
| RDH | 20.3% | 13.6–21.7 |
| %RETIC | 1.7% | |
| RETIC | 137.1 K/μL | 10–110 |
| WBC | 42.04 X 109/L | 5.05–16.76 |
| %NEU | 60.7% | |
| %LYM | 23.3% | |
| %MONO | 13.0% | |
| %EOS | 2.9% | |
| %BASO | 0.1% | |
| NEU | 25.51 X 109/L | 2.95–11.64 |
| LYM | 9.80 X 109/L | 1.05–5.10 |
| MONO | 5.46 X 109/L | 0.16–1.12 |
| EOS | 1.22 X 109/L | 0.06–1.23 |
| BASO | 0.05 X 109/L | 0.00–0.10 |
| PLT | 256 K/μL | 148–484 |
| PT | 12 seconds | 11–17 |
| PTT | 87 seconds | 72–102 |
Table 2.
Serum biochemistry.
| Parameter | Result | Normal range |
|---|---|---|
| GLUCOSE | 4.89 mmol/L | 4.28–8.34 |
| UREA | 5.1 mmol/L | 2.5–10.4 |
| CREATININE | 58 μmol/L | 27–106 |
| PHOSPHORUS | 1.73 mmol/L | 1.65–3.36 |
| CALCIUM | 2.01 mmol/L | 1.95–3.15 |
| TOTAL PROTEIN | 57 g/L | 48–72 |
| ALBUMIN | 28 g/L | 21–36 |
| GLOBULIN | 29 | 23–38 |
| ALT | > 3000 U/L (after serum sample dilution) | 8–75 |
| ALKP | No result readable even after serum sample dilution | 46–337 |
| GGT | 97 U/L | 0–2 |
| TOTAL BILIRUBIN | 28 μmol/L | 0–14 |
| CHOLESTEROL | 6.93 mmol/L | 2.58–10.32 |
| AMYLASE | 745 U/L | 300–1300 |
| LIPASE | 2302 U/L | 100–1500 |
| SODIUM | 151 mmol/L | 145–157 |
| POTASSIUM | 4.7 mmol/L | 3.5–5.5 |
| CHLORIDE | 111 mmol/L | 105–119 |
Fig. 1.
Thoracic radiographs; ventro-dorsal (a) and left lateral views (b).
Fig. 2.
Cytology from the Bronchoalveolar lavage demonstrating multiple Pneumocystis cysts (two of which are noted with a black line).
2. Discussion
This report describes a case of canine pneumocystosis in a lurcher puppy following a report of an adult onset of Pneumocystosis in a Whippet cross breed dog [10]. The patient in this current report had presented with respiratory compromise and was subsequently diagnosed with pulmonary Pneumocystis infection. He was euthanized, due to significant deterioration, shortly after the investigation and therefore specific treatment was not instituted. Based on this investigation, cytology from a BAL revealed the presence of cysts compatible with Pneumocystis carinii. In humans and due to the difficulty in culturing Pneumocystis spp in the lab, microscopical identification of the organism is required for the diagnosis of Pneumocystis pneumonia from sputum specimens, bronchoalveolar fluid or lung tissue [11]. Microscopic identification of the organism from appropriate respiratory tract specimen is also used in dogs for the diagnosis of Pneumocystis pneumonia [13]. PCR appears to have high sensitivity though and has been increasingly used for the diagnosis of Pneumocystis pneumonia in humans [12]. Similarly, diagnosis of canine pneumocystosis by utilizing PCR has shown promising results and has gained popularity in the recent years [14]. Sample from the bronchoalveolar fluid from this case was not submitted for PCR, as one was not readily available at the time. It is unclear how this patient acquired the organism. Pneumocystis can colonize the respiratory tract of healthy individuals who can act as reservoirs of the fungus. It can then be transferred to immunocompromised host (most likely airborne transmission) who can then develop Pneumocystis pneumonia [15].
The majority of dogs with Pneumocystosis are immunocompromised [9]. This patient's globulin concentration was within mid-normal range. Although not run, serum electrophoresis could have been helpful in identifying any reductions in the individual immunoglobulin concentrations. According to the history this patient had presented on several occasions due to skin infections and appeared to develop pneumocystosis after receiving the intra-nasal kennel cough vaccine. Thus, an immunodeficiency cannot therefore be excluded. The serum biochemistry also revealed significant increases in ALT, ALP (which was not readable even after sample's dilution), GGT and total bilirubin while the ultrasonographic appearance of the liver was abnormal. Even though hypoxia can have major impact to visceral organs and cause significant compromise, it is possible that this patient could have had concurrent hepatic pneumocystosis or infectious hepatitis from an unknown causative agent e.g. bacterial or viral hepatitis or leptospirosis. Even though the patient was up to date with his vaccinations the above scenario cannot entirely be excluded. Further testing (e.g. Leptospira PCR/serology), liver fine needle aspiration and/or biopsies could have provided more information. More recently, dissemination of Pneumocystis to various tissues, including the liver, was identified in a dog with pneumocystosis [16]. Interestingly in another report, hepatocellular necrosis developed in a dog following subcutaneous injection of an intra-nasal vaccine preparation for kennel cough [17].
Pneumocystis spp is a fungal microorganism that can cause severe pulmonary infection and is usually associated with immunodeficiency of the host. Regardless of the breed, pneumocystosis should be part of the differential diagnosis in young dogs with similar presentation and lack of improvement following trials with commonly used-antibiotics. Further studies are necessary in order to identify the connection between pneumocystosis and its presentation in only a handful of canine breeds.
Declaration of competing interest
There are none.
Acknowledgements
The authors would like to thank David Buckeridge from Synlab VPG Exeter (previously known as Torrance Diamond Diagnostic Services) for providing the cytology image for this paper and Pete Mantis from Dick White Referrals for his valuable contribution towards the interpretation of the radiographs.
References
- 1.Stringer J.R. Pneumocystis carinii: what is it, exactly? Clin. Microbiol. Rev. 1996;9(4):489–498. doi: 10.1128/cmr.9.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James T.Y., Kauff F., Schoch C.L. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature. 2006;443(7113):818–822. doi: 10.1038/nature05110. [DOI] [PubMed] [Google Scholar]
- 3.Chagas C. Nova trypanozomiaze humana: estudos sobre a morpholojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen, n. spec, ajente etiolojico de nova entidade morbida de hornern. Mem Inst Oswaldo Cruzi. 1909;1:159–218. [Google Scholar]
- 4.Van der Meer M.G., Brug S.L. Infection à Pneumocystis chez l’ homme et chez les animaux. Ann Soc Belg Med Trop. 1942;22 301–, 9. [Google Scholar]
- 5.Masur H., Michelis M.A., Greene J.B. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N. Engl. J. Med. 1981;305(24):1431–1438. doi: 10.1056/NEJM198112103052402. [DOI] [PubMed] [Google Scholar]
- 6.Walzer P.D. The ecology of pneumocystis: perspectives, personal recollections, and future research opportunities. J. Eukaryot. Microbiol. 2013;60(6):634–645. doi: 10.1111/jeu.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson P.J., Wotton P., Eastwood J., Swift S.T., Jones B., Day M.J. Immunoglobulin deficiency in cavalier king Charles spaniels with pneumocystis pneumonia. J. Vet. Intern. Med./American College of Veterinary Internal Medicine. 2006;20(3):523–527. doi: 10.1892/0891-6640(2006)20[523:idickc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Lobetti R. Fungal and algal diseases: pneumocystosis. In: Greene C.E., editor. Infectious Diseases of the Dog and Cat. fourth ed. Elsevier/Saunders; St. Louis, Mo: 2012. pp. 689–695. [Google Scholar]
- 9.Lobetti R. Common variable immunodeficiency in miniature dachshunds affected with Pneumonocystis carinii pneumonia. J. Vet. Diagn. Invest.: official publication of the American Association of Veterinary Laboratory Diagnosticians, Inc. 2000;12(1):39–45. doi: 10.1177/104063870001200107. [DOI] [PubMed] [Google Scholar]
- 10.Weissenbacher-Lang C., Fuchs-Baumgartinger A., Klang A., Kneissl S., Pirker A., Shibly S. Pneumocystis carinii infection with severe pneumomediastinum and lymph node involvement in a Whippet mixed-breed dog. J. Vet. Diagn. Invest. 2017;29(5):757–762. doi: 10.1177/1040638717710237. [DOI] [PubMed] [Google Scholar]
- 11.Thomas C.F., jr., Limper A.H. Pneumocystis pneumonia. N. Engl. J. Med. 2004;350:2487–2498. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 12.Fan L.C., Lu H.W., Cheng K.B., Li H.P., Xu J.F. Evaluation of PCR in bronchoalveolar lavage fluid for diagnosis of Pneumocystis jirovecii pneumonia: a bivariate meta-analysis and systematic review. PloS One. 2013;8 doi: 10.1371/journal.pone.0073099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lobetti R.G., Leisewitz A.L., Spencer J.A. Pneumocystis carinii in the miniature dachshund: case report and literature review. J. Small Anim. Pract. 1996;37(6):280–285. doi: 10.1111/j.1748-5827.1996.tb02380.x. [DOI] [PubMed] [Google Scholar]
- 14.Danezi P., Ravagnan S., Johnson L.R., Furlanello T., Milani A., Martin P. Molecular diagnosis of Pneumocystis pneumonia in dogs. Med. Mycol. 2017 Nov 1;55(8):828–842. doi: 10.1093/mmy/myx007. [DOI] [PubMed] [Google Scholar]
- 15.Gigliotti F., Harmsen A.G., Wright T.W. Characterization of transmission of Pneumocystis carinii f. sp. muris through immunocompetent BALB/c mice. Infect. Immun. 2003;71(7):3852–3856. doi: 10.1128/IAI.71.7.3852-3856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakashita T., Kaneko Y., Izzati U.Z., Hirai T., Fuke N., Torisu S. Disseminated pneumocystosis in a toy poodle. J. Comp. Pathol. 2020 Feb;175:85–89. doi: 10.1016/j.jcpa.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Toshach K., Jackson M.W., Dubielzig R.R. Hepatocellular necrosis associated with the subcutaneous injection of an intranasal Bordetella bronchiseptica-canine parainfluenza vaccine. J. Am. Anim. Hosp. Assoc. 1997;33(2):126–128. doi: 10.5326/15473317-33-2-126. Mar-Apr. [DOI] [PubMed] [Google Scholar]