Highlights
-
•
Pediatric spontaneous pneumothorax is relatively a rare condition.
-
•
A metachronous pneumothorax whether ipsilateral or contralateral side is even rarer.
-
•
Current literature is deficient in a solid consensus for management of this type of pneumothorax.
-
•
Video-assisted thoracoscopic surgery is an excellent therapeutic tool for pediatric pneumothorax.
Keywords: Pediatric spontaneous pneumothorax, Metachronous recurrence, Management, VATS blebectomy
Abstract
Introduction
Pediatric primary spontaneous pneumothorax (PSP) is defined as the presence of air in the pleural cavity without underlying lung disease or thoracic trauma. Metachronous recurrence of PSP whether ipsilateral or contralateral is rare. Apical bullae and sub-pleural blebs are found in the majority of PSP patients. As in adults, surgery is indicated in cases with prolonged air leak. Video-assisted thoracoscopic surgery (VATS) is increasingly performed in children and has been reported to be both safe and effective.
Presentation of the case
An 11-years-old girl had bilateral attacks of PSP, the second attack happened one after the first one and this later was associated with her menarche. Chest CT scan detected bilateral apical blebs.
Discussion
Contralateral recurrence in pediatric PSP is a low probability. The decision for surgery in the pediatric age group is a matter of controversy as there are no strict pediatric guidelines for management of PSP. Currently, VATS is superior to open surgery. Pediatric Catamenial pneumothorax is not well described in the literature.
Conclusions
Contralateral recurrence of PSP in children is rarer. No guidelines exist for the management of these cases. The association of pediatric PSP with menarche is not well described in the current literature.
1. Introduction
Primary spontaneous pneumothorax (PSP) is defined as the presence of air in the pleural cavity of patients without underlying lung disease or thoracic trauma. While recurrent primary spontaneous pneumothorax (RPSP) denotes a metachronous pneumothorax whether ipsilateral or contralateral side. PSP usually affects young and tall male patients, often adolescents with a slim build. PSP is uncommon pathology under the age of 18 and it is almost very rare to be bilateral.
The incidence of PSP in the pediatric population is 3.4/100,000 children (neonatal pneumothorax excluded), male predominance ranging from 2:1 to 9:1 with a peak in ages 14–17. Female preponderance appearing under age 8 years is reported by many researchers [[2], [3], [4]]. Pediatric PSP which occurs without a family history as in the majority (90%) of cases is named as Sporadic Pneumothorax [1,[2], [3], [4],5].
The pathophysiology of PSP remains unknown, PSP in children is thought to be caused either by an acute increase in trans-pulmonary pressure or defects in visceral pleural [1,2]. Apical bullae and sub-pleural blebs are found in the majority of PSP patients which in turn results in air leaks through visceral pleura [1,2]. Lopez et al. identified blebs/bullae intra-operatively in 98% of the cases [3], mainly in apices of upper lobes. No exact genetic abnormality identified in PSP cases but some genetic studies of sporadic pneumothorax cohorts have focused on mutation in the folliculin (FLCN) gene that has been mapped to the short arm of chromosome 17 which is the same gene for Birt-Hogg-Dubé syndrome [5,6].
Metachronous contralateral recurrence of PSP in children is not widely reported in the current literature, most of the management guidelines for such as recurrence still following adult consensuses for treatment [[3], [4], [5]]. The Association of recurrence and menarche in female children is not thoroughly discussed in the literature.
Sudden onset mild to moderate pleuritic chest pain with breathlessness is the main clinical features, but some patients are asymptomatic and have mild dry cough and shoulder pain if the pneumothorax is large [[1], [2], [3]].
Diagnosis of pediatric PSP is based on; history and clinical examination, chest X-ray (CXR) being the mainstay of diagnosis. There is no consensus about the radiological size of pneumothorax in pediatric age, and it is based on adult criteria of from the British Thoracic Society (BTS), American College of Chest Physicians (ACCP) a large pneumothorax is defined as ≥3 cm of air between the pleural line and apical chest wall (apex-to-cupola distance), or ≥2 cm between the entire lateral lung edge and the chest wall, at the level of the hilum [4].
In pediatric patients sizing of pneumothorax compared to the size of the whole chest could be used [5,7]. Chest ultrasound (US) is an effective modality for the diagnosis of pneumothorax in pediatric age. Vasques et al. showed 45.5% sensitivity, 98.6% specificity, and 96.0% accuracy in the skilled operator's hands and the pneumothoraces that were not detected were small and asymptomatic [5]. Though US of the chest has the sensitivity and specificity of chest ultrasound is 95% and 100%, respectively in adults but there is no guideline regarding pediatric age [5,7].
The results of a recent meta-analysis revealed a higher sensitivity and similar specificity in the use of ultrasonography compared with CXR. Pooled sensitivity and specificity were 0.88 and 0.99, for US, and 0.52 and 1.00, respectively, for CXR. Furthermore, because of its portability and the absence of ionizing radiations, many authors point out its usefulness, especially for children and adolescents. On the other hand, the use of US as a reliable tool for the diagnosis of PSP, is limited because its accuracy is strongly dependent on the operator’s skill [7]. Chest CT scan is a highly specific and sensitive diagnostic tool in PSP but it should be used with caution in the pediatric age group [2,4].
Management options for PSP in children are extrapolated from adult guidelines, but conservative and needle aspiration are not well supported by many authors. As in adults surgery is indicated in cases with prolonged air leak [[2], [3], [4], [5], [6], [7]] days after intercostal drainage. Such surgery has progressed from the era of posterolateral thoracotomy to trans-axillary mini-thoracotomy and finally, Video-assisted thoracoscopic surgery (VATS) is increasingly performed in children and has been reported to be both safe and effective by many authors [[3], [4], [5],7,8].
Our case is an 11 years old girl that presented in two occasions with bilateral attacks of PSP to our university hospital emergency departement, one of them associated with her menarche. Her PSPs treated with Video-assisted thoracoscopic surgery (VATS) blebectomy. This work was reported in line with the SCARE criteria [9].
2. Presentation of the case
In May 2019, a previously healthy 11 years old girl student was referred from pediatric hospital after she was presented sudden onset shortness of breath and chest pain for 2 h duration, her condition started to worsen to a point that she couldn’t finish a sentence and she had some casual coughs which were dry.
On arrival to our emergency room she and the parents denied having any prior trauma nor any chronic illness, she is not on any chronic medications nor has taken any drug before her condition after initial resuscitation, her vital signs were: her peripheral oxygen saturation: 88% on room air, the pulse rate: sinus tachycardia of 125 beats per minute, brachial arterial pressure: 110/65 mmHg, respiratory rate: 28 cycles/minute.
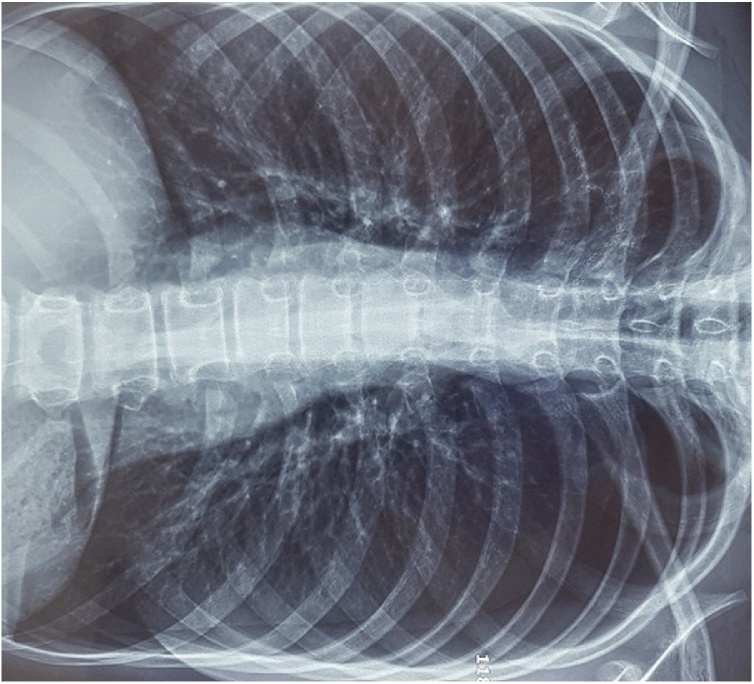
On chest examination: decreased chest wall movement on the right side, hyper- resonant on percussion diminished breath sounds on the right side and no added sounds on auscultation left side being normal, the trachea was central and precordium had normal examination except for tachycardia. Supplemental oxygen started by nasal cannula and Oxygen saturation raised to 95% then a standing poster-anterior chest X-ray (PA) view was obtained and showed right side large pneumothorax with underlying lung collapse, mediastinum was in normal position with normal left lung (Fig. 1A).
Fig. 1.
A: Chest X-ray PA view shows large right pneumothorax with signs of impending tension.
B: Total re-expansion of the right lung with chest tube in proper position.
C: Chest CT scan shows bilateral apical subpleural blebs.
Based on this clinical vital un-stability, the decision for tube thoracostomy was made, a size 14 French intercostal drain inserted in the 4th intercostal space midaxillary line and connected to an underwater seal system.
After an initial great amount of air leak the patient's condition dramatically improved, SPO2: 98% on room air and a post tube Chest X-ray was obtained which showed near total lung re-expansion in day zero (Fig. 1B).
After this further history taking revealed that this female hadn’t had her menarche yet, no personal and family history of any respiratory illness, she denies being admitted for any prior condition neither medical nor surgical. Her BMI of 16.5, She was admitted in our ward and multiple investigations were sent (including Alpha-1 antitrypsin enzyme) all came back with in the normal limits.
After 7 days in our ward with the encouragement of physiotherapy and medical therapy, the patient was clinically stable but still had a persistent air leak, a chest CT with IV contrast was obtained at this time and showed bilateral upper lobes apical segment bleb and non-expanded right lung (Fig. 1C).
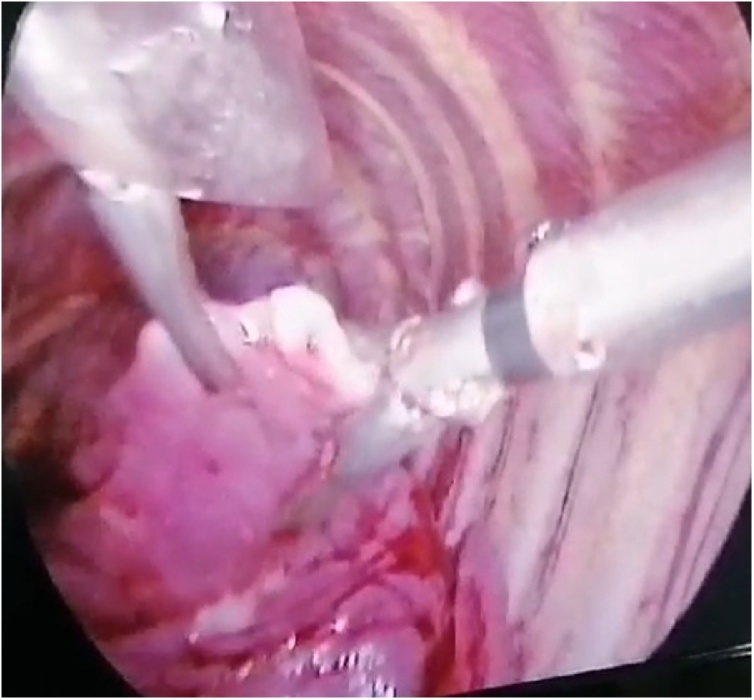
The decision for surgical intervention was made by using VATS bleb resection and pleural abrasion. Under general anesthesia VATS reveled ruptured right upper lobe apical bleb which was excised, the diaphragm was checked for any depositions, then mechanical pleural abrasion was performed, chest tube placed, we didn’t use talc because we think in the age group still not advisable (Fig. 2). She was discharged home in the first postoperative day after checking her chest X-ray, with recommendations and advice in case of other attacks.
Fig. 2.
Left upper lobe apical bleb, thoracoscopic view.
In June 2020 this patient was readmitted in our department with a one-day history of shortness of breath and chest pain. This time patient stated that she had her first menstruation (menarche) 24 h before her condition, she had some casual coughs, no fever nor rigor. On examination: the patient was not in distress and not cyanotic, the trachea was central. Chest examination revealed diminished breath sounds on the left side of the chest with no added sounds, right had good breath sounds. Chest x-ray revealed large left-side pneumothorax, and right side normal (Fig. 3A).
Fig. 3.
Left metachronous large pneumothorax.
This time the patient was stable hemodynamically to be prepared for urgent surgical intervention through VATS was admitted overnight under close observation. VATS blebectomy for left upper lobe apical segment performed with mechanical abrasion to the pleura. This time searching for any endometrial deposits was done but was negative, a small size chest tube placed, she was admitted to our surgical ward, she went home in day one postoperative. The position of the blebs is demonstrated schematically in Fig. 4.
Fig. 4.
Schematic presentation for the site of the blebs.
Because of the age of our patient, a written consent for publishing was obtained from her parents.
3. Discussion
PSP is rarely encountered in children, it mostly found in teenage males with a male to female ratio found to be 1.6:1 to 8:1 in a case series of 78 children ratio was 7.7:1 [8]
In pediatric PSP usually, there is no underlying lung disease other than subpleural blebs [10,11]. Attacks are commonly are happens during June, and fewer in May [8]. Recurrence found to be as significant in most observational studies reaching up to 50% especially those treated with observation and needle exsufflation [8].
In our case, left side pneumothorax nearly one year before the contralateral attack latter happening in May, there is no consensus regarding the exact percentage of recurrence of contralateral pneumothorax in pediatric age group, what is available is in adult patients. Contralateral recurrence is a low probability and it’s a significant reason for morbidity, a significant point in these patients is that most of them have BMI < 18.5 and have blebs or bullae on HRCT contralateraly [10]. The same finding was present in our case.
Our patient underwent conservative therapy in the first attack for seven days per pediatric guidelines, even so, this is still debatable and surgeons still in a split decision [[6], [7], [8],10,11].
The decision for surgery in the pediatric age group is a matter of controversy in the currently published literature. Williams et al. surveyed 287 surgeons with a 33% response rate. For the first episode of PSP, 57% of surgeons opt for chest tube drainage only, 4% for upfront video-assisted thoracoscopic surgery (VATS), 3% for needle aspiration, and 29% for only oxygen administration [12]. More evidence is needed for the creation of a better pediatric guideline.
Leys et al. in 2020 made a new approach for the treatment of PSP with initial risk measurement with needle thoracentesis and moving directly for operation as they found recurrence after VATS was only 15% when other studies that even more than 60% recurrence who were treated conservatively [13].
In a comparative study done for prophylactic contralateral resection of blebs for patients with PSP that accidentally were found to have asymptomatic contralateral apical blebs, the results were satisfying and the degree of recurrence was dramatically decreased [8], but this study included a small number of patients and other studies are focusing on the current disease rather than future recurrences [8].
Initially, in our patient, contralateral bleb was found but was kept conservative and instructions were given to the family in avoiding any positive pressure situation.
The decision for open thoracotomy or VATS is still controversial. The ACCP guidelines recommend a thoracoscopic approach both for PSP and SSP [6], but a study by Barker et al. in 2003 showed fewer recurrence in open (1%) in comparison to VATS [14]. In 2018 total of 1040 patients underwent a study for the decision of early VATS rather than chest tube management, early VATS is associated with a decreased hospital stay, charges, and readmissions. For those managed initially with chest tube alone, the likelihood of requiring operation increases with each day hospitalized, and early conversion to operative management should be considered in patients with persistent pneumothorax or air leak [13,14].
Vanderschueren suggested thoracoscopic intervention for PSP based on his criteria to four types; Type I: Normal findingsno abnormalities, Type II: Presence of pleural adhesions, Type III: blebs/bullae <2 cm in diameter, Type IV: blebs/bullae >2 cm in diameter [15]. The most important limitations of this study were the chemical pleurodesis by talc which is not a good practice in pediatric age.
These guidelines are all for adult Pneumothoraces, most of the pediatric studies showed that VATS is superior to open regarding peri-operative findings and complication a comparison study was performed and noted even VATS blebectomy plus mechanical pleurodesis than chemical pleurodesis [8,11,13].
In 2020 the first level 3 meta-analytic study was conducted regarding the management of spontaneous pneumothorax in children, showing that there is a lack of evidence for concrete management of pneumothorax as the studies are still yet to be informative enough to create a management plan for spontaneous pneumothorax in children, but the clear message of this meta-analysis is that early surgical intervention is superior to conservative management [16].
Catamenial pneumothorax (CP), is pneumothorax occurring during the perimenstrual period, it has been recognized for several decades. Differences in its definition stem from the various definitions of the “perimenstrual period”, which encompasses 72 h before and after menstrual bleeding [17]. Our case had her menarche 24 h before her contralateral left side pneumothorax.
Visouli et al. have proposed extending this period to 96 h with an incidence of less than 3–6% among women suffering from spontaneous pneumothorax with age be in 14–44 years [18]. Another significant unique fact about our patient is that her second left PSP happened within 24 h of her menarche.
It’s well known that only 5% of all CPs could occur on the left side and their little discussion about CP with first menstruation, though our patient yet to fit the criteria of CP her condition could be related to her first menstruation [18,19].
4. Conclusions
PSP is rare in the pediatric population, and to be complicated by contralateral recurrence is even rarer. No strict inter-society guidelines exist for the management of these cases. The association of pediatric PSP with menarche is not well established in the current literature.
Declaration of Competing Interest
The authors report no declarations of interest.
Sources of funding
No fund received for this case report.
Ethical approval
Ethical approval is not required for case reports in our institution.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
A written consent obtained from both parents of the patient for anonymous publication of all the data.
Author contribution
Aram Baram: Surgeon in charge, study design, follow-up, data collection, manuscript writing and revision, final approval.
Yad N. Othman: study design, follow-up, data collection, manuscript writing and revision.
Rzgar Ghareeb Muhammed: follow-up, data collection.
Zryan Salar Majeed: Drawing of the illustration, drafting and revision.
Dezhin Faeq Rashid: follow-up, data collection, manuscript revision.
Fitoon Falah: follow-up, data collection, manuscript revision.
Hiwa Sherzad: follow-up, data collection, manuscript revision.
Zhyan Khalil Mahmood: follow-up, data collection, manuscript revision.
Rebwar Ghareeb Hama: follow-up, data collection, manuscript revision.
Registration of research studies
NA.
Guarantor
Aram Baram is the Guarantor for this manuscript.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
We would like to acknowledge all our personnel who assisted in serving our patients.
Footnotes
All the patient and authors details removed from all the figures.
Contributor Information
Aram Baram, Email: aram.baramm@gmail.com, aram.baram@univsul.edu.iq.
Yad N. Othman, Email: yad.osman@gmail.com.
Rzgar Ghareeb Muhammed, Email: rzgar.haji.ghareeb@gmail.com.
Zryan Salar Majeed, Email: zryanmejio@gmail.com.
Dezhin Faeq Rashid, Email: dezhynsolae@gmail.com.
Fitoon Falah, Email: dr.fitoonfalah@yahoo.com.
Hiwa Sherzad, Email: hiwasherzad@aol.com.
Zhyan Khalil Mahmood, Email: zhyan8913@yahoo.com.
Rebwar Ghareeb Hama, Email: rebwar1974@yahoo.com.
References
- 1.Kuo P.Y., Nong B.R., Huang Y.F., Chiou Y.H. Primary spontaneous pneumothorax in children: a literature review. Pediatr. Respirol. Crit. Care Med. 2018;2:25–31. doi: 10.4103/prcm.prcm_3_18. [DOI] [Google Scholar]
- 2.Dotson Kurtis, Timm Nathan, Gittelman Mike. Is spontaneous pneumothorax really a pediatric problem? A national perspective. Pediatr. Emerg. Care. 2012;28(April (4)):340–344. doi: 10.1097/PEC.0b013e31824d9a65. [DOI] [PubMed] [Google Scholar]
- 3.Lopez Monica, Fallon Sara, Lee Timothy, Rodriguez J., Brandt Mary, Mazziotti Mark. Management of the pediatric spontaneous pneumothorax: is primary surgery the treatment of choice? Am. J. Surg. 2014;208 doi: 10.1016/j.amjsurg.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Maconochie Ian K., Howell Andrew, Walton Emily. Spontaneous pneumothorax in children: the problem with rare presentations. Arch. Dis. Child. 2015;100(October (10)):903–904. doi: 10.1136/archdischild-2015-308309. Epub 2015 Jul 3. [DOI] [PubMed] [Google Scholar]
- 5.Vasquez D.G., Berg G.M., Srour S.G., Ali K. Lung ultrasound for detecting pneumothorax in injured children: preliminary experience at a community-based Level II pediatric trauma center. Pediatr. Radiol. 2020;50(3):329–337. doi: 10.1007/s00247-019-04509-y. [DOI] [PubMed] [Google Scholar]
- 6.Boone Philip M., Scott Rachel M., Marciniak Stefan J., Henske Elizabeth P., Raby Benjamin A. The genetics of pneumothorax. Am. J. Respir. Crit. Care Med. 2019;199(June (11)):1344–1357. doi: 10.1164/rccm.201807-1212CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahmarde Hamid, Parooie Fateme, Salarzaei Morteza. Accuracy of ultrasound in diagnosis of pneumothorax: a comparison between neonates and adults—a systematic review and meta-analysis. Can. Respir. J. 2020 doi: 10.1155/2019/5271982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furia Simone, Breda Cristiano. Primary spontaneous pneumothorax in children and adolescents: a systematic review. Pediatr. Med. 2019;2:12. doi: 10.21037/pm.2019.04.01. [DOI] [Google Scholar]
- 9.Agha R.A., Borrelli M.R., Farwana R. The SCARE 2018 statement: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Bialas R.C., Weiner T.M., Phillips J.D. Video-assisted thoracic surgery for primary spontaneous pneumothorax in children: is there an optimal technique? J. Pediatr. Surg. 2008;43(12):2151–2155. doi: 10.1016/j.jpedsurg.2008.08.041. [DOI] [PubMed] [Google Scholar]
- 11.Huang T.W., Lee S.C., Cheng Y.L. Contralateral recurrence of primary spontaneous pneumothorax. Chest. 2007;132(4):1146–1150. doi: 10.1378/chest.06-2772. [DOI] [PubMed] [Google Scholar]
- 12.Williams K., Baumann L., Grabowski J., Lautz T.B. Current practice in the management of spontaneous pneumothorax in children. J. Laparoendosc. Adv. Surg. Tech. A. 2019;29(4):551–556. doi: 10.1089/lap.2018.0629. [DOI] [PubMed] [Google Scholar]
- 13.Leys C.M., Hirschl R.B., Kohler J.E. Changing the paradigm for management of pediatric primary spontaneous pneumothorax: a simple aspiration test predicts need for operation. J. Pediatr. Surg. 2020;55(January (1)):169–175. doi: 10.1016/j.jpedsurg.2019.09.043. Epub 2019 Oct 24. [DOI] [PubMed] [Google Scholar]
- 14.Barker A., Marartos E.C., Edmonds L. Recurrence rates of video assisted thoracoscopic versus open surgery in the prevention of recurrent pneumothoraces: a systematic review of randomized and non randomized trials. Lancet. 2007;370:329–335. doi: 10.1016/S0140-6736(07)61163-5. [DOI] [PubMed] [Google Scholar]
- 15.Vanderschueren R.G. The role of thoracoscopy in the evaluation and management of pneumothorax. Lung. 1990;168:1122–1125. doi: 10.1007/BF02718252. [DOI] [PubMed] [Google Scholar]
- 16.Miscia Maria Enrica, Lauriti Giuseppe, Lisi Gabriele, Riccio Angela, Chiesa Pierluigi Lelli. Management of spontaneous pneumothorax in children: a systematic review and meta-analysis. Eur. J. Pediatr. Surg. 2020;30(February (1)):2–12. doi: 10.1055/s-0039-3402522. Epub 2020 Jan 3. [DOI] [PubMed] [Google Scholar]
- 17.Marjański T., Sowa K., Czapla A., Rzyman W. Catamenial pneumothorax - a review of the literature. Kardiochir. Torakochirurgia Pol. 2016;13(2):117–121. doi: 10.5114/kitp.2016.61044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visouli A.N., Zarogoulidis K., Kougioumtzi I. Catamenial pneumothorax. J. Thorac. Dis. 2014;6(Suppl 4):S448–S460. doi: 10.3978/j.issn.2072-1439.2014.08.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams K., Oyetunji T.A., Hsuing G., Hendrickson R.J., Lautz T.B. Spontaneous pneumothorax in children: national management strategies and outcomes. J. Laparoendosc. Adv. Surg. Tech. A. 2018;28(2):218–222. doi: 10.1089/lap.2017.046. [DOI] [PubMed] [Google Scholar]