Abstract
Bone marrow derived progenitor cells and macrophages are known to migrate into the retina in response to inflammation and neovascularization. These migratory cells might play important regulatory roles in the pathogenesis of neovascularization, a common complication observed in diabetic retinopathy (DR), retinopathy of prematurity (ROP) and retinal vein occlusion (RVO). Hypoxia-inducible factor 1 alpha (HIF-1α) has been shown to contribute to the pathogenesis of retinal inflammation and neovascularization. However, contributions of monocyte-derived macrophages to neovascularization are largely unknown. We hypothesized that selective visualization of these microglia/macrophages could be a powerful method to predict the onset of neovascularization and its progression at molecular level. In this report we described the synthesis of a new hybrid nanoparticle to visualize HIF-1α mRNA selectively in microglia/macrophages in a mouse model of neovascularization. HIF-1α expression was confirmed in MRC-1 positive macrophages as well as in CD4 positive T-cells and CD19 positive B-cells using single cell RNA sequencing (scRNAseq) data analysis. The imaging probes (AS- or NS-shRNA-lipid) were synthesized by conjugating diacyl-lipids to short hairpin RNA with an anti-sense sequence complimentary to HIF-1α mRNA, and a fluorophore that is quenched by a black-hole quencher (BHQ). We believe that imaging mRNA selectively in tissue specific microglia/macrophages could be a powerful method to predict the onset of neovascularization, its progression and response to therapy.
Keywords: HIF-1α mRNA, oxygen-induced retinopathy, neovascularization, shRNA-lipid, imaging
Graphical Abstract
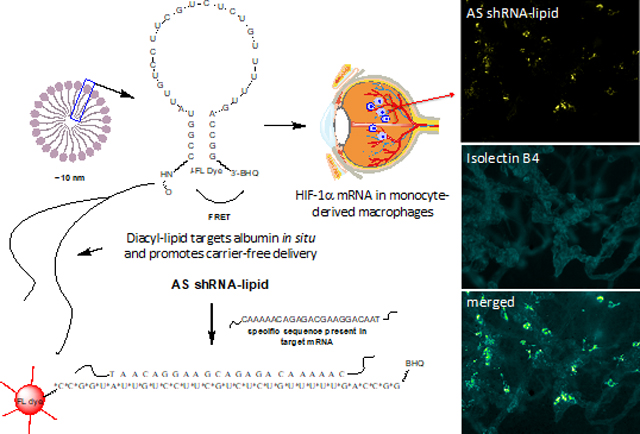
Introduction
Neovascularization (NV) is a common complication in all proliferative retinopathies, including diabetic retinopathy (DR), retinopathy of prematurity (ROP) and retinal vein occlusion (RVO). Though, the pathogenesis of neovascularization is largely unknown, ischemia-induced retinal hypoxia and the release of hypoxia-dependent vascular endothelial growth factor (VEGF), in addition to other vasoactive and/or proinflammatory factors are the central importance.1 Circulating endothelial progenitor cells and macrophages migrate into the retina in response to neovascularization.2–5 However, the exact role of these migratory cells and macrophages in neovascularization is largely unknown.6 In response to neovascularization, such as that occurring in proliferative retinopathies, microglia/macrophages release pro-angiogenic and proinflammatory mediators that may contribute to neovascularization.7 Reports have shown that adult myeloid progenitor cells migrate to the avascular retina to facilitate revascularization in a mouse model of oxygen-induced retinopathy (OIR).8,9 Precisely, myeloid-specific hypoxia-inducible factor 1 alpha (HIF-1α) expression is required for this effect.10 We hypothesized that visualizing mRNA selectively in these tissue specific microglia/macrophages could be a powerful method to predict the onset, progression and resolution of retinal neovascularization. However, visualizing mRNA specifically in these small numbers of macrophages in the retina remains challenging.
In situ hybridization (ISH) is a powerful method to visualize intracellular mRNA localization in excised tissues.11,12 However, in situ hybridization methods require the use of fixed tissues or endogenously labelled target mRNA for imaging and tracking. In a recent report, we have shown that gold-mediated targeted delivery of oligonucleotides facilitates the real-time imaging of mRNA in living cells.13 In the current study, we have designed and synthesized anti-sense probes conjugated to diacyl-lipids (AS-shRNA-lipid) for targeted imaging of HIF-1α mRNA that are associated with bone marrow derived cells in retinal neovascularization without using any added toxic transfection reagents.
RESULTS
Design and synthesis of shRNA-lipid conjugates.
Short-hairpin RNA (shRNA) oligonucleotides were designed computationally and synthesized using an automated solid phase synthesizer. For increased stability of the shRNA, 2’-O-methylribonucleotides (2’-OMe) were used for the oligonucleotide synthesis. A fluorescence dye at the 5’ end and a quencher (black-hole quencher or BHQ) at the 3’ end of the oligonucleotide were incorporated. Lipid conjugates were synthesized in two additional steps as described in the method section.14 After purification, the pure shRNA-lipid conjugates are likely to spontaneously form lipid-micelle structures in isotonic solution as shown in Figure 1. From the dynamic light scattering (DLS) measurements of the shRNA-lipid conjugates and transmission electron microscopy (TEM) imaging, we confirmed that shRNA-lipids are forming spherical nanoparticles of around 10 nm. The polydispersity index (PDI) value of >0.2 suggest a broad size range within the population. This multi-species nanoformulation may be due to a series of PEG lengths as observed in ESI-TOF MS data of the shRNA-lipid conjugates as shown in Figure S4 contributing to high PDI. However, the mRNA recognition moiety remains on the surface of these nanoparticles and the diacyl-lipid remains in the core, allowing direct hybridization with specific sequence in target mRNA as shown in Figure 2 and Figure S5.
Figure 1:
Schematic drawing and hybridization motif of shRNA-lipid conjugates showing the design incorporating anti-sense sequence complementary to the HIF-1α mRNA. The shRNA-lipid conjugates are stabilized using 2’-OMe nucleotides. A fluorescence dye (FL Dye) and a black hole quencher (BHQ) are incorporated onto opposite ends of the shRNA construct as shown. Upon hybridization to the target sequence, the intramolecular quenching is compromised, and fluorescence occurs, thus reporting the hybridization event. Since the shRNA-lipid conjugates contain a hydrophilic and a lipophilic components, it is likely that they form lipid-micelle structures. From the dynamic light scattering (DLS) measurements and transmission electron microscopy (TEM) imaging, we observed that shRNA-lipids from spherical nanoparticles of around 10 nm. The mRNA recognition moiety resides on the surface of the nanoparticles and the lipid-core remains inside.
Figure 2:
In vitro localization of HIF-1α mRNA targeted AS-shRNA-lipid in mouse Müller cells (MMC) using fluorescence microscopy. Expression of HIF-1α mRNA was induced in MMCs by exposing the Cells under hypoxia. DAPI was used to counterstain the nucleolus (blue). (A-D) AS-shRNA-lipid derived fluorescence was minimally observed in normoxic MMCs and the fluorescence was significantly increased in hypoxic MMCs, supporting the expression of HIF-1α mRNA in hypoxic MMCs. (E) Comparative assessment of fluorescence intensities in normoxic and hypoxic MMCs, showing increased fluorescence in hypoxic cells. Fluorescence intensities were measured computationally using ImageJ software (n = 6). Statistical significance *** P<0.0001.
In vitro imaging of HIF-1α mRNA using AS-shRNA-lipid.
We have used AS-shRNA-lipid for imaging HIF-1α mRNA expression in murine Müller cells (MMC) and primary retinal microvascular endothelial cells (MRMEC). Cells were treated under hypoxia and normoxia conditions in presence of AS-shRNA-lipid to monitor HIF-1α mRNA expression. We observed that AS-shRNA-lipid derived fluorescence was minimally detectable in normoxic MMCs and fluorescence was significantly increased in hypoxic MMCs (Figure 2), suggesting that HIF-1α mRNA expression could be induced in MMCs under hypoxia. Interestingly, AS-shRNA-lipid derived fluorescence was observed in normoxic as well as in hypoxic MRMECs, suggesting that HIF-1α mRNA might be constitutively expressed in cultured MRMECs and remain unchanged after hypoxia treatment (Figure S5). These observations are consistent with previously reported results in vascular endothelial cells.15 Thus, AS-shRNA-lipid could be used for imaging endogenous mRNA in cultured retinal cell and potentially other cell types. Furthermore, a non-sense probe, (NS-shRNA-lipid conjugate) showed minimal fluorescence in hypoxic cells as shown in Figure S1. The NS-shRNA-lipid probe was designed and synthesized using scrambled sequence of the anti-sense probe with same G-C content as confirmed from a series of MS-data as shown in Figure S4.
Myeloid-specific HIF-1α expression in mouse oxygen-induced retinopathy (OIR).
In an effort to characterize the active cell population and associated HIF-1α expression profiles, we sought to use single cell RNA sequencing (scRNAseq) data analysis to identify a very small number of MRC-1 positive macrophages (<1%)16 in blood samples from oxygen-induced retinopathy (OIR) (Figure 3). We observed that HIF-1α expression is associated with these MRC-1 positive macrophages in mouse OIR. Also, the MRC-1 positive cells are minimally present in healthy controls. These observations are consistent with previously reported results describing the association of HIF-1α with myeloid-specific progenitor cells.10 Interestingly, we observed that the number of CD4 positive T cells decreased (Figure 3A), and CD19 positive B cells increased in P17 OIR compared to normal healthy controls (Figure 3B). These observations are indicative of poor immune system in OIR animals, which suggest the possibility of “systemic” toxic effect of high oxygen treatment in human ROP patients during their early life. Furthermore, most of the CD4 positive T cells and CD19 positive B cells are also positive for HIF-1α in OIR as well as in room air control animals (Figure 3D).
Figure 3:

Characterization of CD4 positive T cells, CD19 positive B cells, MRC-1 positive macrophages and expression of HIF-1α using single cell RNAseq data analysis in isolated white blood cells from P17 mouse OIR and RA control mice. (A-C) Representative aggregated t-SNE plot showing higher numbers of CD4 positive T cells in room air (RA) control group (A); higher numbers of CD19 positive B cells in oxygen-induced retinopathy (OIR) group (B); macrophage mannose receptor C-1 (MRC1) positive cells mostly in OIR group (C). (D) HIF-1α expression was observed in T cells, B cells and MRC1 positive macrophages. t-SNE plot was created using Loupe Cell Browser program.
Imaging HIF-1α mRNA using AS-shRNA-lipid in mouse OIR retina.
After confirming the association of HIF-1α mRNA with MRC-1 positive myeloid cells in mouse OIR, we used our newly synthesized AS-shRNA-lipid conjugate to track bone marrow derived cells in the OIR retina (Figure 4). After intraperitoneal injection in OIR animals, we observed that AS-shRNA-lipid yielded a strong punctate fluorescence in cells that were associated with neovascularization, presumably due to hybridization with HIF-1α mRNA.5 Reports have shown that transplanted bone marrow derived myeloid progenitor cells differentiated into microglia in the OIR retina.10 Based on these observations, we then sought to co-localize the AS-shRNA-lipid associated fluorescence with microglia/macrophages both in the P17 OIR (Figure 5), and room air (RA) control retinas (Figure 6); we used IBA1 as a marker for retinal microglial/macrophages.5 We observed two morphologically distinct microglial populations in the OIR retina (Figure S2); resident microglia/macrophages reside on the surface of the superficial capillary plexus of the OIR retina, whereas resting microglia resides in the deep capillary plexuses. Furthermore, AS-shRNA-lipid-derived fluorescence was observed in microglia/macrophages that reside on the superficial capillary plexus (Figure 5D-E). We did not observe AS-shRNA-lipid derived fluorescence in ramified IBA1-positive cells that resided around the deep capillary plexus of the OIR retina as shown in in Figure S2B. Even though, we observed the expression of HIF-1α in CD4 positive T cells and CD19 positive B cells in room air control animals, we did not observe AS-shRNA-lipid derived fluorescence in these control retinas as shown in Figure 6. Furthermore, we did not observe AS-shRNA-lipid-derived fluorescence in the retinal microvascular endothelial cells or in other cell-types that might also be positive for HIF-1α mRNA suggesting the possibility of probe-internalization outside of the retina and transported to the retina in response to neovascularization in OIR animals. Depletion of the circulating macrophages in OIR using intraperitoneal injection of clodronate-liposome significantly reduced the AS-shRNA-lipid dependent fluorescence in P17 OIR retina (Figure 7), suggesting that after intraperitoneal injection, shRNA-lipid conjugates could be internalized into the macrophages outside the ocular tissues as observed in lymph node in OIR animals (Figure S7) and then migrate to the retina. This migration could be a response to neovascularization and could be used as measure for severity of retinopathy and disease progression as well as treatment response. Furthermore, a non-sense probe, (NS-shRNA-lipid conjugate) showed minimal fluorescence in the OIR retina as shown in Figure S3. The NS-shRNA-lipid probe was designed and synthesized from scrambled sequence of the anti-sense probe and thus has almost same molecular mass as shown in Figure S4.
Figure 4:
Imaging HIF-1α mRNA selectively in neovascularization in mouse P17 OIR retina. Isolectin B4 was used to visualize the retinal vasculatures. The OIR mice received intraperitonel injections of AS-shRNA-lipid conjugates. Eighteen hours post-injection, retinal tissues were analyzed ex vivo. (A-B) The shRNA-lipid conjugates incorporating anti-sense sequence complementary to HIF-1α mRNA was localized in cells that are also associated with neovascular tufts (arrows). (C-D) Higher magnification of A-B showing strong fluorescence punctate patterns presumably due to hybridization of AS-shRNA-lipid with HIF-1α mRNA in cells in the OIR retina. (E-F) AS-shRNA-lipid derived fluorescence was also observed in cells that are localized around the neovascular tufts (arrows) in the P17 OIR retinas. Scale bar 30 µm in A-B and 20 µm in C-F.
Figure 5:
Co-localization of AS-shRNA-lipid derived fluorescence with IBA1 positive cells in the P17 mouse OIR retina. (A-C) Isolectin B4 was used to visualize the retinal vasculatures (green); IBA1 was used to stain the microglial cells (magenta). (D-E) AS-shRNA-lipid derived fluorescence was localized in cells that are also associated with IBA1 positive cells (white arrows), suggesting that IBA1 positive microglia/macrophages (magenta color) could express HIF-1α mRNA that are also associated with neovascularization.41 (F-G) AS-shRNA-lipid derived fluorescence were not observed in ramified IBA1-positive cells (yellow arrows) in the OIR retina.
Figure 6:
The AS-shRNA-lipid conjugates were minimally detectable in IBA1 positive ramified/resting microglia (magenta color) in room air (RA) raised healthy control retinas. Isolectin B4 was used to visualize the retinal vasculatures. Microglia were observed on the surface of superficial capillary plexus (SCP), middle capillary plexus (MCP) and deep capillary plexus (DCP) and are ramified. AS-shRNA-lipid conjugates were not detectable in SCP (A-D); and were not detectable in MCP or DCP (E-L). Scale bar 50 µm.
Figure 7:
Depletion of macrophages using clodronate-liposome reduces the AS-shRNA-Lipid derived fluorescence in P17 OIR mouse retinas compared to control PBS-injected OIR retinas. AS-shRNA-lipid derived fluorescence was monitored in mice with intraperitoneal injection of AS-shRNA-lipid. IBA1 was used to visualize the microglia/macrophages in the retina and IB4 was used to visualize the vasculatures. (A) A large number of IBA1 positive cells were observed in the control PBS-injected OIR retinas. (B) Although a large number of IBA1 positive cells were observed in the clodronate-liposome injected retina, AS-shRNA-lipid derived fluorescence significantly decreased in the clodronate-liposome injected retinas as shown in C.
To clarify the mode of labeling using non-phagocytic cells in presence of AS-shRNA-lipid conjugate, we used microvascular endothelial cells (MRMECs). AS-shRNA-lipid conjugate are readily internalized by the MRMECs as shown in Figure S5, and could be used for imaging mRNA in non-phagocytic cells as well. In addition, to characterize pathway mechanism for delivery of AS(or NS)-shRNA-lipid conjugates into primary retinal cells, inhibitors or stimulators of endocytosis or macropinocytosis were examined as shown in Table 1 and Figure 8 and Figure S6. For this assay, both AS- and NS-shRNA-lipid conjugates were synthesized using only the fluorescence dye (Cy5) at the 5’ end, without the black hole quencher (BHQ). Cells were exposed to different inhibitors and stimulators at different concentrations as shown in Table 1. First we investigated the clathrin-mediated endocytosis pathway by inhibiting the clathrin-mediated endocytosis using sucrose,17 and compared the internalization to the control as well as comparing the response by blocking temperature dependent internalization18 as shown in Figure 8A-B. We observed that shRNA-lipid internalization was significantly inhibited in presence of sucrose, suggesting that the internalization could be clathrin-mediated pathway. However, similar effect was also observed in presence of wortmannin, an inhibitor of macropinocytosis,19 as well as monensin, an inhibitor of endosomal acidification and maturation,20 giving the possibility of internalization through multiple pathways. Interestingly, poly-L-lysine allows the probe internalization through disruption of cell membrane association,21 and the internalization is independent of the sequence present in the shRNA-lipids (Figure S6). Other inhibitor of macropinocytosis, such as amiloride,22 and stimulation of macropinocytosis, such as phorbol esters,23,24 perturbation of caveolae by filipin,25,26 increased endosomal pH by chloroquine,27 inhibition of clathrin by chlorpromazine,28 all showed no significant effect on probe internalization (Figure S6).
Table1:
Influence of Inhibitor/activator on intracellular delivery of shRNA-lipid.
| Treatment | Concentration* | Effect |
|---|---|---|
| Chlorpromazine Hydrochloride | 10 μM | inhibitory interaction with clathrin |
| Filipin | 5 μg/mL | perturbation caveolae by sequestering cholesterol |
| Sucrose | 100 mM | inhibition of clathrin-mediated endocytosis |
| Wortmannin | 500 nM | inhibition of macropinocytosis through blocked PI-3 kinase |
| Amiloride Hydrochloride | 10 μM | inhibition of macropinocytosis by blocked Na+/H+ pump |
| Phorbol ester | 10 nM | stimulation of macropinocytosis |
| Monensin | 5 μM | prevention of endosomal acidification and maturation |
| Chloroquine | 100 μM | increase of endosomal pH |
| poly-L-lysine | 10 nM | disruption of cell membrane association |
| Low temperature (4 °C) | - | blocking energy dependent processes |
Concentrations used in this study.
Figure 8:
Effect of inhibitors/stimulator on shRNA-lipid internalization into the primary retinal microvascular endothelial cells (MRMEC). shRNA-lipid conjugates (both AS- and NS-) were synthesized using only the fluorescence dye (Cy5) at the 5’ end without using the black hole quencher (BHQ). Thus, the probes are not quenched and are always ON. Cells were exposed to different inhibitors or stimulators for 30 min prior to addition of the shRNA-lipid conjugates. Plate based fluorescence assays and fluorescence imaging were performed two hour post-incubation to monitor probe internalization. (A) shRNA-lipid internalization is inhibited in presence of clathrin-mediated endocytosis inhibitor (sucrose), suggesting the possibility of clathrin-mediated endocytosis of shRNA-lipid, * P<0.05 (n=4). (B) Inhibition of endocytosis (missing punctate structures) could be visualized using confocal imaging (yellow arrows). Scale bar 25 μm. (C) In addition, shRNA-lipid internalization is independent of the macropinocytosis pathways as observed from phorbol esters treatment; and also internalization is independent of filipin-sensitive caveolae-mediated transport.
DISCUSSIONS
Macrophages were observed in the retina during hyaloid degeneration and in response to neovascularization such as that occurring in proliferative diabetic retinopathy.29 Regulated expression of HIF-1α by macrophages was demonstrated more than a decade ago.10 In addition, HIF-1α can be induced in monocytic-cells differentiated into macrophages, suggesting that inflammation might initiate phenotypic differentiation of monocytes. However, the exact role of macrophages in neovascularization is largely unknown. It is known that, tissue macrophages play a key role to promote vasculogenesis as well as angiogenesis.30 These observations suggest that molecular imaging of specific mRNA biomarkers in tissue specific microglia/macrophages could uncover the role of this distinct cell-population in the pathogenesis of proliferative retinopathy. Small interfering RNA (siRNA), antisense-DNA and micro RNA technologies are attractive for mRNA interference.31 However, these agents have short half-lives and often require toxic transfection reagents. Therefore, these methods are not suitable for real-time in vivo imaging of mRNA. Previously, we have shown that siRNA-lipid conjugates could facilitate the delivery of siRNA into cells and tumor tissues without requiring any toxic transfection reagents.14 In the current study we have used AS-shRNA-lipid for molecular imaging of mRNA in mouse OIR without using any added transfection reagents. For diagnostic purposes, topical or systemic delivery of shRNA is vital for clinical applications. Part of the strategy for the application of shRNA-lipid conjugates stems from the albumin binding capacity conferred by the lipid moiety.14 Albumin is the most abundant serum protein (>40 mg/mL) and has a circulation half-life of about 20 days,32 making it a natural chaperone for systemic delivery of shRNA conjugates.33 Our data indicate that this chaperone activity greatly facilitates delivery of our shRNA-lipid conjugates to target tissues.
Flourescein angiography (FA), optical coherence tomography angiography (OCTA) and OCTA combined with FA (OCTA-FA) are techniques capable of imaging pre-retinal neovascularization. Retinal neovascular tufts are formed by angiogenesis in which endothelial cells proliferate, migrate and extend through the basal lamina, and lumina formation is delayed until adjacent neovascular structures anastomose. Therefore, a significant percentage of neovascularization may go undetected when applying imaging modalities that depend on blood flow. In vivo mRNA imaging may offer a solution to detect non-patent vessels by targeting diverse cell population in neovascularization. It can also be adapted to discern diverse cell phenotypes that comprise neovascularization such as endothelial cells (EC), M1 and M2 macrophages, endothelial progenitor cells, hematopoietic stem cells and mesenchymal stem cells, by selecting cell-specific transcripts. However, in vivo imaging techniques capable of distinguishing diverse cell phenotypes in retinal neovascularization do not exist. In the current study, we have investigated HIF-1α mRNA as a potential marker for imaging retinal neovascularization in OIR mice. We have chosen to use mouse OIR because this model yields a robust neovascular response and it is highly amenable to optical imaging of the retina. Strong evidence from our data in Figure 4 and 5 suggest that AS-shRNA-lipid could selectively target the monocytes in neovascularization, thus suggesting the efficacy of this new imaging modality. Although, our scRNAseq data indicated that CD4+ T-cells and CD19+ B-cells are also positive for HIF-1α, our ex vivo images suggested that our AS-shRNA–lipid-dependent fluorescence activity was largely activated in cells positive for the monocyte/macrophage marker IBA1. These observations suggest that after intraperitoneal injection, the AS-shRNA-lipid is internalized by circulating monocytes that are recruited to OIR retina where they extravasate and incorporate into neovascular lesions. Based on these data we suggest that our probe may be used to image retinal neovascularization. Additionally, it may also be suitable to estimate levels of neovascularization. For example, Zhu et al demonstrated that the number of macrophages in the mouse OIR retina varied over the post-oxygen normoxic exposure period, increasing from P13 to P15, peaking at P18 and then declining at P21 and P24.34 These post-oxygen exposure times overlapped with the development, peak and decline of the neovascular-response in the OIR mice in that study. These findings suggest future time-course experiments to determine whether differences in AS-shRNA-lipid specific florescence could be used to monitor changes in disease level and/or response to therapy.
In summary, we have developed a novel method to track specific cell population in ocular tissues using antisense short-hairpin RNA conjugated to diacyl-lipids (AS-shRNA-lipid). We believe that molecular imaging of inflammatory cytokines and tracking specific cell populations will help physicians to predict neovascularization, a common complication observed in proliferative retinopathies.
METHODS
All chemicals were purchased form Sigma-Aldrich (St. Louis, MO) and used as received unless otherwise noted. The mouse primary retinal microvascular endothelial cells (MRMEC) were obtained from Cell Biologics Inc (IL, USA). Primary mouse Müller cells (MMC) were isolated from adult C57BL/6 mice following our previously described method.35 Custom designed 2’-O-methyl-protected short hairpin RNA (shRNA) and custom oligonucleotides were purchased from Integrated DNA Technologies Inc. (IA, USA).
Design and synthesis of AS-HIF-1α-(or NS)-shRNA-lipid conjugates:
The shRNA was computationally designed via energy minimization to achieve the formation of the hairpin structure. Each of the optimized shRNA-oligonucleotides was coupled to a Cy3 dye (fluorophore) and C6 amino group to facilitate conjugation to the diacyl-lipid. The 3’ end was coupled to a BHQ2. The 2’-O-methyl-protected short hairpin RNA (shRNA) that incorporate anti-sense sequence complementary to mouse HIF-1α mRNA, position 2327 to 2349 (NM_010431.2) was synthesized and purified using HPLC system. A scrambled sequence of the anti-sense sequence (NS-shRNA) was also synthesized and characterized using MS-analysis as shown in Figure S4. The anti-sense sequences was extensively BLAST searched to determine no significant overlap with any other mouse mRNA sequence. The same was performed on the non-sense sequence to confirm non-specific binding. The anti-sense and the non-sense sequences are located within the loop of the hairpin structure as shown in Figure 1. A self-complementary sequence was incorporated to form the stem of the shRNA hairpin. Finally, the shRNA was conjugated to the diacyl-lipid according to our previously described method.14 Excess reagents were removed by centrifugal filtration using a filter with a 10K molecular weight cut-off (Amicon Ultracel 10K from Millipore, Billerica, MA). The freshly conjugated lipid-oligonucleotides were washed three times with PBS (Life Technologies Corporation; Grand Island, NY) and stored at 4 °C until used. The shRNA-lipid conjugates can spontaneously form spherical nanoparticles.
Dynamic light scattering (DLS):
LS measurements were performed on a Malvern Zetasizer Nano ZS (Malvern Instruments, Inc.; Westborough, MA). Particle measurements were performed at a concentration of 10 μM shRNA-lipid in PBS (Life Technologies Corp.; Carlsbad, CA). These measurements were performed in triplicate.
Transmission electron microscopy (TEM):
shRNA-lipid probes were mounted on 300-mesh copper grids and stained with 2% uranyl acetate. Samples were subsequently imaged on the Philips/FEI Tecnai T12 electron microscope (Hillsboro, OR) at various magnifications.
Animals:
Multi-timed pregnant C57BL/6 female mice were purchased from Charles River Laboratories. All animal procedures used in this study were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Primary cell culture:
MRMECs were cultured in T-75 cell culture flasks (Thermo Fisher Scientific; Wilmington, MA) coated with attachment factor (Cell Systems; Danvers, MA) and in growth medium consisting of endothelial basal medium (EBM; Lonza; Walkersville, MD) supplemented with 2% FBS (Lonza) and endothelial cell growth supplements (EGM SingleQuots; Lonza). Primary MMCs were cultured in T-75 cell culture flasks (Thermo Fisher Scientific; Wilmington, MA) coated with attachment factor (Cell Systems; Danvers, MA) and in low glucose DMEM growth medium consisting of 10% FBS (Lonza), 1x GlutaMAX and 1x penicillin-streptomycin. All cultures were incubated at 37 °C, 5% CO2 and 95% relative humidity (20.9% oxygen). The cells were cultured in 96-well plates and treated with shRNA-lipid in complete growth medium. Hypoxia was induced following our previously described method.36 Briefly, cells were treated with shRNA-lipid diluted in complete media and the assay plates were placed into a humidified hypoxic chamber. Ambient air was displaced with a mixture of 5% CO2 and 95% N2 at a flow rate of 20 L/min for 5 min according to manufacturer instructions and published methods.37 The chamber was clamped and placed at 37 °C for the appropriate treatment time. For probe internalization assays, cells were treated with the inhibitors or stimulators for 30 minutes with doses as shown in Table 1. After the treatment, cells were treated with shRNA-lipid in complete cell culture medium, for two hours and then a plate-based fluorescence assay was performed to analyze intracellular delivery.
Ex vivo imaging of mRNA in mouse OIR:
To generate the OIR mouse model, dams with their pups were treated with 75% oxygen for 5 days from postnatal day 7 (P7) to P12.38 On P12, pups were removed from the hyperoxic chamber and stayed with the nursing mother in normal air condition for additional 5 days. At P17 AS-(or NS)-shRNA-lipid conjugates in sterile saline were injected intraperitoneally at a dose of 0.5 mg/kg. After 18 hours, AS-(or NS)-shRNA-lipid dependent fluorescence imaging was performed ex vivo. Briefly, animals were sacrificed, enucleated and the globes were fixed in 10% neutral buffered formalin (NBF). Retinas were dissected and blocked/permeabilized in 10% donkey serum with 1% Triton X-100 and 0.05% Tween 20 in TBS for 2 hours and were then counter-stained for IB4 and IBA-1 conjugated to Alexafluor-dyes (Life Technologies; Grand Island, NY). The tissues were then mounted with Prolong Gold mounting medium with DAPI (Life Technologies; Grand Island, NY). Images were taken using an epifluorescence Nikon Eclipse Ti-E inverted microscope (Melville, NY).
Macrophage depletion using intraperitoneal injection of clodrosome and imaging using intraperitoneal Injection of AS-shRNA-lipid:
Clodrosome (0.1 mL/10g, Encapsula NanoSciences LLC.; Brentwood, TN) or PBS as control was injected intraperitoneally into P14 mouse OIR pups and in consideration of its depletion efficacy after two days,39 AS-shRNA-lipid was injected intraperitoneally (0.5 mg/kg). Eighteen hours later, animals were sacrificed and retinal tissues were stained and analyzed for IBA1 and IB4, as described above.
Isolation and single cell RNAseq analysis of bone marrow-derive cells from mouse OIR and RA pups:
Peripheral blood samples were collected from deeply anesthetized OIR and age-matched RA control pups. Bone marrow-derived mononuclear cells were isolated from fresh blood within 2 hours of collection, using Ficoll-Paque density gradient centrifugation as described.40 Each sample (targeting 5,000 cells/sample) was processed for single cell 5’ RNA sequencing utilizing the 10X Chromium system. Libraries were prepared using P/N 1000006, 1000080, and 1000020 following the manufacturer’s protocol. The libraries were sequenced using the NovaSeq 6000 with 150 bp paired end reads. RTA (version 2.4.11; Illumina) was used for base calling and analysis was completed using 10X Genomics Cell Ranger software v2.1.1. or Loupe Cell Browser.
STATISTICS
Data were expressed as mean ± % SDM and statistical differences among groups were determined by one-way analysis of variance (ANOVA) using Prism 6 (Graph- Pad, San Diego, CA) followed by Bonferroni post hoc test to determine significant differences between specific groups. A ‘p’ value <0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health grants R01EY029693 (to MIU) and R01EY023397 (to MIU and JSP), a Grant from the Knights Templar Eye Foundation, Inc. (to MIU), NIDDK MICROMouse funding program DK076169 and DK115255 (to MIU), a Grant from BrightFocus Foundation (to MIU) and a Grant from the Carl Marshall Reeves & Mildred Almen Reeves Foundation, Inc. (to JSP), Vanderbilt Diabetes Research and Training Center Core grant (P30 DK020593-34-39), Vanderbilt Vision Research Center NEI Core Grant (P30-EY008126) and an Unrestricted Grant from Research to Prevent Blindness, Inc. The authors thank G. McCollum for critical reading and important suggestions for this manuscript.
Footnotes
Supplementary information
The Supplementary information is available free of charge via the Internet.
• Supplementary Information contains Figures S1–S7, including the MS-data for the oligonucleotides and shRNA-lipid conjugates after purification.
Conflicts of interest: None
REFERENCES
- 1.Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res 2008; 27(4): 331–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritter MR, Banin E, Moreno SK, Aguilar E, Dorrell MI, Friedlander M. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J Clin Invest 2006; 116(12): 3266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies M, Eubanks J, Powers M. Microglia and macrophages are increased in response to ischemia-induced retinopathy in the mouse retina. Mol Vis 2006; 12(53–54): 467–77. [PubMed] [Google Scholar]
- 4.Gao X, Wang YS, Li XQ, et al. Macrophages promote vasculogenesis of retinal neovascularization in an oxygen-induced retinopathy model in mice. Cell Tissue Res 2016; 364(3): 599–610. [DOI] [PubMed] [Google Scholar]
- 5.Kataoka K, Nishiguchi KM, Kaneko H, van Rooijen N, Kachi S, Terasaki H. The Roles of Vitreal Macrophages and Circulating Leukocytes in Retinal Neovascularization. Invest Ophth Vis Sci 2011; 52(3): 1431–8. [DOI] [PubMed] [Google Scholar]
- 6.Naug HL, Browning J, Gole GA, Gobe G. Vitreal macrophages express vascular endothelial growth factor in oxygen-induced retinopathy. Clin Exp Ophthalmol 2000; 28(1): 48–52. [DOI] [PubMed] [Google Scholar]
- 7.Shen J, Xie B, Dong A, Swaim M, Hackett SF, Campochiaro PA. In vivo immunostaining demonstrates macrophages associate with growing and regressing vessels. Invest Ophthalmol Vis Sci 2007; 48(9): 4335–41. [DOI] [PubMed] [Google Scholar]
- 8.Otani A, Kinder K, Ewalt K, Otero FJ, Schimmel P, Friedlander M. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med 2002; 8(9): 1004–10. [DOI] [PubMed] [Google Scholar]
- 9.Grant MB, May WS, Caballero S, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med 2002; 8(6): 607–12. [DOI] [PubMed] [Google Scholar]
- 10.Ritter MR, Banin E, Moreno SK, Aguilar E, Dorrell MI, Friedlander M. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J Clin Invest 2006; 116(12): 3266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson C, Grundberg I, Soderberg O, Nilsson M. In situ detection and genotyping of individual mRNA molecules. Nat Methods 2010; 7(5): 395–7. [DOI] [PubMed] [Google Scholar]
- 12.Tyagi S, Kramer FR. Molecular beacons: Probes that fluoresce upon hybridization. Nat Biotechnol 1996; 14(3): 303–8. [DOI] [PubMed] [Google Scholar]
- 13.Uddin MI, Jayagopal A, Wong A, McCollum GW, Wright DW, Penn JS. Real-time imaging of VCAM-1 mRNA in TNF-alpha activated retinal microvascular endothelial cells using antisense hairpin-DNA functionalized gold nanoparticles. Nanomedicine 2018; 14(1): 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarett SM, Werfel TA, Lee L, et al. Lipophilic siRNA targets albumin in situ and promotes bioavailability, tumor penetration, and carrier-free gene silencing. Proc Natl Acad Sci U S A 2017; 114(32): E6490-E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao W, Gao Y, Zhang G, Song L, Sun B, Shi J. Hypoxia-induced expression of HIF-1alpha and its target genes in umbilical venous endothelial cells of Tibetans and immigrant Han. Comp Biochem Physiol C Toxicol Pharmacol 2005; 141(1): 93–100. [DOI] [PubMed] [Google Scholar]
- 16.Macosko EZ, Basu A, Satija R, et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015; 161(5): 1202–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veldhoen S, Laufer SD, Trampe A, Restle T. Cellular delivery of small interfering RNA by a non-covalently attached cell-penetrating peptide: quantitative analysis of uptake and biological effect. Nucleic Acids Res 2006; 34(22): 6561–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn WA, Hubbard AL, Aronson NN, Jr. Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver. J Biol Chem 1980; 255(12): 5971–8. [PubMed] [Google Scholar]
- 19.Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J 1993; 296 ( Pt 2): 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mollenhauer HH, Morre DJ, Rowe LD. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim Biophys Acta 1990; 1031(2): 225–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drin G, Cottin S, Blanc E, Rees AR, Temsamani J. Studies on the internalization mechanism of cationic cell-penetrating peptides. J Biol Chem 2003; 278(33): 31192–201. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann PR, deCathelineau AM, Ogden CA, et al. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol 2001; 155(4): 649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hewlett LJ, Prescott AR, Watts C. The coated pit and macropinocytic pathways serve distinct endosome populations. J Cell Biol 1994; 124(5): 689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller HU. Diacylglycerols and PMA are particularly effective stimulators of fluid pinocytosis in human neutrophils. J Cell Physiol 1990; 145(3): 465–71. [DOI] [PubMed] [Google Scholar]
- 25.Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol 1998; 141(4): 905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol 1994; 127(5): 1217–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pless DD, Wellner RB. In vitro fusion of endocytic vesicles: Effects of reagents that alter endosomal pH. J Cell Biochem 1996; 62(1): 27–39. [DOI] [PubMed] [Google Scholar]
- 28.Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol 1993; 123(5): 1107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshikawa Y, Yamada T, Tai-Nagara I, et al. Developmental regression of hyaloid vasculature is triggered by neurons. Journal of Experimental Medicine 2016; 213(7): 1175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fantin A, Vieira JM, Gestri G, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 2010; 116(5): 829–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001; 411(6836): 494–8. [DOI] [PubMed] [Google Scholar]
- 32.Neumann E, Frei E, Funk D, et al. Native albumin for targeted drug delivery. Expert Opin Drug Del 2010; 7(8): 915–25. [DOI] [PubMed] [Google Scholar]
- 33.Kratz F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J Control Release 2008; 132(3): 171–83. [DOI] [PubMed] [Google Scholar]
- 34.Zhu YJ, Zhang L, Lu Q, et al. Identification of different macrophage subpopulations with distinct activities in a mouse model of oxygen-induced retinopathy. Int J Mol Med 2017; 40(2): 281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanni SE, McCollum GW, Penn JS. Genetic deletion of COX-2 diminishes VEGF production in mouse retinal Muller cells. Exp Eye Res 2010; 91(1): 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uddin MI, Kilburn TC, Yang R, McCollum GW, Wright DW, Penn JS. Targeted Imaging of VCAM-1 mRNA in a Mouse Model of Laser-Induced Choroidal Neovascularization Using Antisense Hairpin-DNA-Functionalized Gold-Nanoparticles. Mol Pharm 2018; 15(12): 5514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uddin MI, Evans SM, Craft JR, et al. In Vivo Imaging of Retinal Hypoxia in a Model of Oxygen-Induced Retinopathy. Sci Rep 2016; 6: 31011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994; 35(1): 101–11. [PubMed] [Google Scholar]
- 39.Shi YY, Wang YS, Zhang ZX, et al. Monocyte/macrophages promote vasculogenesis in choroidal neovascularization in mice by stimulating SDF-1 expression in RPE cells. Graefes Arch Clin Exp Ophthalmol 2011; 249(11): 1667–79. [DOI] [PubMed] [Google Scholar]
- 40.Lee MN, Ye C, Villani AC, et al. Common Genetic Variants Modulate Pathogen-Sensing Responses in Human Dendritic Cells. Science 2014; 343(6175): 1119-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lastres P, Bellon T, Cabanas C, et al. Regulated Expression on Human Macrophages of Endoglin, an Arg-Gly-Asp-Containing Surface-Antigen. Eur J Immunol 1992; 22(2): 393–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









