Abstract
Polyautoimmunity implicates that some autoimmune diseases share common etiopathogenesis. Some studies have reported an association between multiple sclerosis (MS) and vitiligo; meanwhile, other studies have failed to confirm this association. We performed a systemic review and meta-analysis to examine the association of MS with vitiligo. We searched the MEDLINE and Embase databases on March 8, 2020 for relevant case–control, cross-sectional, and cohort studies. The Newcastle–Ottawa Scale was used to evaluate the risk of bias of the included studies. Where applicable, we performed a meta-analysis to calculate the pooled odds ratio (OR) for case–control/cross-sectional studies and risk ratio for cohort studies with 95% confidence interval (CI). Our search identified 285 citations after removing duplicates. Six case–control studies with 12,930 study subjects met our inclusion criteria. Our meta-analysis found no significant association of MS with prevalent vitiligo (pooled OR 1.33; 95% CI 0.80‒2.22). Analysis of the pooled data failed to display any increase of prevalent vitiligo in MS patients compared with controls. Ethnic and genetic factors may play an important role for sporadically observed associations between MS and vitiligo. Future studies of this association should therefore consider stratification by ethnic or genetic factors.
Subject terms: Vitiligo, Multiple sclerosis, Epidemiology, Epidemiology
Introduction
Multiple sclerosis (MS) is an inflammatory, demyelinating disease primarily confined to the central nervous system (CNS)1. The clinical characteristics of MS include ophthalmoplegia, trigeminal neuralgia, limb weakness, and sensory and cognitive impairment2. The global prevalence varies from 3 to 200 per 100,000, depending on geographic latitude and ethnicity3. The etiology of MS remains unclear but may involve B and T cell-mediated inflammation, oxidative stress, cerebrospinal venous insufficiency, and neurodegeneration4.
Vitiligo is an acquired depigmenting dermatosis characterized by well-defined chalky white macules or patches. People of all ages and both sexes appear to be equally affected. The prevalence of vitiligo in the general population ranges from 0.06% to 2.28%5. The etiology of vitiligo, including its genetic, autoimmune, and biochemical mechanisms, has been studied extensively6.
Patients with immune-mediated inflammatory disorders are at increased risk of having more than one type of autoimmune disease7. The most common autoimmune comorbidities in MS patients are reportedly psoriasis8 and thyroid disease, with a prevalence of 7.7% and 6.4%, respectively9. Vitiligo has been associated with diabetes mellitus, thyroid disease, alopecia areata, and pernicious anemia10. MS may share similarity with vitiligo regarding immunological, environmental, and genetic factors. However, studies examining the association between MS and vitiligo have reported inconsistent results. We therefore aimed to systemically appraise available data regarding the association of MS with vitiligo.
Methods
We performed a systematic review and meta-analysis of observational studies on the association between MS and vitiligo, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines11. The study protocol was registered with the PROSPERO (CRD42018112959; see https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=112959).
Inclusion and exclusion criteria
We included case–control, cross-sectional, and cohort studies quantifying the association of MS with vitiligo, and only studies that included a case group of patients with MS and a control group of individuals without MS. The study outcome was the odds ratio (OR) and risk ratio (RR) of vitiligo in association to MS for case–control/cross-sectional and cohort studies, respectively. We excluded studies that did not include a control group or lacked sufficient data relevant to the calculation of OR or RR.
Literature search and study selection
A literature search of the MEDLINE and Embase databases was performed on March 8, 2020. The search strategy is listed in Table 1. Two authors (MS and CN) independently screened the titles and abstracts of the articles retrieved by the search and obtained the full text of potentially eligible studies to evaluate if they met the inclusion criteria. If the two authors had different judgement, such discrepancies were resolved by consulting a third author (CC).
Table 1.
Search strategy.
| Database | Search strategy |
|---|---|
| MEDLINE | #1. exp Multiple Sclerosis/ |
| #2. multiple sclerosis.mp | |
| #3. disseminated sclerosis.mp | |
| #4. or/#1-#3 | |
| #5. exp Vitiligo/ | |
| #6. vitiligo.mp | |
| #7. leucoderma.mp | |
| #8. leukoderma.mp | |
| #9. or/#5-#8 | |
| #10. #4 and #9 | |
| Embase | #1. ‘multiple sclerosis'/exp OR 'multiple sclerosis' |
| #2. ‘disseminated sclerosis' | |
| #3. #1 OR #2 | |
| #4. ‘vitiligo'/exp OR ‘vitiligo' | |
| #5. ‘leucoderma' | |
| #6. ‘leukoderma' | |
| #7. #4 OR #5 OR #6 | |
| #8. #3 AND #7 |
Data extraction
For each included study, we extracted data including first author, publication year, country, study design, confounders that were matched or adjusted in the statistical analysis, number of patients in the case and control groups, the definition of cases and controls, the selection of controls, and quantitative estimates on the association of MS with vitiligo.
Assessment of risk of bias
The risk of bias of included studies was assessed using the Newcastle–Ottawa Scale (NOS)12. Two authors (MS and CC) interpreted the NOS results of each included article. Three domains were evaluated for cross-sectional and case–control studies, including selection of the studies (adequate definition and representativeness of the cases, selection and sufficient definition of the controls), comparability, and exposure (ascertainment, the same method for ascertainment of cases and controls, non-response rate). Similarly, three domains were evaluated for the cohort studies, including selection of the studies, comparability, and outcome. Each domain could be rated as ‘low risk’, ‘uncertain risk’, or ‘high risk’. For example, if a study controlled for confounding factors such as age and sex by either matching or statistical adjustment, we considered it at low risk of bias for comparability.
Statistical analysis
The Review Manager 5.3 software was used to perform the meta-analysis13. For case–control/cross-sectional studies, we calculated the pooled OR of vitiligo in MS patients. Heterogeneity was assessed by using the I2 statistic. If the I2 value is more than 50%, it represents moderate heterogeneity14. If I2 was lower than 50%, we chose a fixed-effect model meta-analysis. If I2 was higher than 50%, we performed a random-effects model meta-analysis15. Potential publication bias was assessed by examining funnel plots when at least 10 studies had been included16,17. All statistical tests were two-sided, and a probability (P) value < 0.05 was considered statistically significant.
Results
Characteristics of included studies
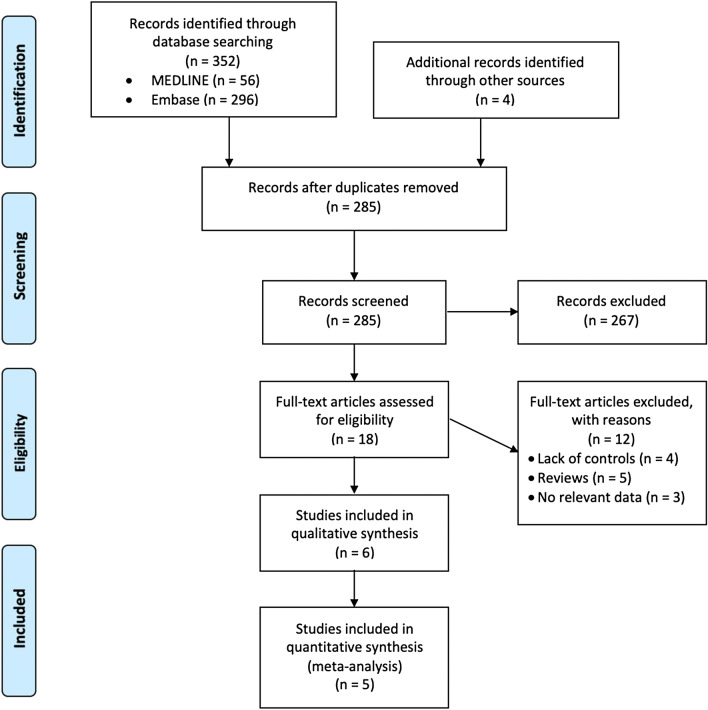
After removing duplicates, our systematic search identified 285 studies. Ultimately, a total of six case–control studies with 12,930 study subjects that investigated the association of MS with vitiligo met our inclusion criteria18–23. We found no relevant cross-sectional or cohort studies. The selection process and reasons for exclusion are illustrated in Fig. 1. The characteristics of the included studies are summarized in Table 2. These studies were all case–control studies performed in Western countries. The sample size ranged from 101 to 5,296, and the mean age of study subjects varied between 39.0 and 55.2 years.
Figure 1.
PRISMA study flow diagram.
Table 2.
Characteristic of included studies.
| First author, year, country | Study design | Diagnostic criteria: MS identified by | Outcome definition: vitiligo identified by | Cases (% female and mean age) | Controls (% female and mean age) | Results |
|---|---|---|---|---|---|---|
| Crude OR (95% CI) | ||||||
| Seyfert, 1990, Germany19 | Case–control study (matched by age) | Poser criteria | Medical chart review | 101 | 97 | (0/101)/(0/97) |
| Handerson, 2000, Australia20 | Case–control (Medical chart review/ Standardized administered questionnaires) | ICD selection code 340, 205 from Royal Brisbane Hospital Department of Neurology, database from 1992 to 1997, diagnosis of clinical definite or laboratory-support | Mailed questionnaire Controls were selected from persons living in the same street as the cases | 117 (71%, 46.2 years) | 221 (60%, 51.0 years) | (0/117)/(2/219) |
| Laroni, 2006, Italy21 | Case–control (1:1 ratio, matched by gender, and age) | Diagnosis by Poser criteria and the patient was followed up by neurologist | Medical chart review | 245 (68%, 39 ± 11 years) | 245 (61%, 37 ± 12 years) | 0.33 (0.03, 3.20) |
| Ramagopalan, 2007, Canada22 | Case–control (Medical chart review/Standardized administered questionnaires) | Poser and/or McDonald 2005 criteria | Medical chart review/Standardized administered questionnaires | 5031 (72%, 55.2 years) | 2707 spousal controls (MS simplex family: 23%, 59 years; MS multiplex family: 33%, 56.5 years) | 1.57 (0.82–3.04) |
| Langer-Gould, 2010, United States/ Northern California23 | Case–control (1:5 ratio, matched by gender, age and Kaiser membership characteristic) | Diagnostic codes entered by neurologists and primary care physicians, MS- specific medications, MRI and or lumbar puncture (codes/criteria not specified) | At least two diagnostic codes entered by a specialist/ KPNC (codes not specified) | 5296 (75%, 54.5 years) | 26,478 (75%, 54.5 years) | 0.86 (0.33–2.23) |
| Deretzi, 2015, Greek18 | Case–control (matched by gender and age) | McDonald 2005 criteria | Medical chart review, Standardized questionnaires by three neurologists in a structured face-to-face interview | 2140 (68%, 33.7 ± 10.2 years) | 1580 (65%, not reported) | 10.40 (1.37, 79.15) |
CI confidence interval, MS multiple sclerosis, OR odds ratio.
The risk of bias evaluation for included case–control studies is summarized in Fig. 2. All studies had utilized the diagnostic criteria for MS, such as Poser criteria and McDonald criteria of 200524,25. Two studies were rated as having a high risk of bias in the representativeness of cases because the study individuals were from a single hospital19,20. One study was rated as having a high risk of bias as to the selection of controls because of recruiting only from a single hospital19. Two studies were rated as having a high risk of bias regarding the comparability of cases and controls due to the lack of control for age or gender20,22. The study by Handerson et al. was rated as having a high risk of bias with regard to the method of ascertainment of cases and controls, which had been based on written self-reporting, and also with regard to non-response rate, since the non-response rates differed markedly between the two groups (82% in cases, 68% in the control group)20.
Figure 2.
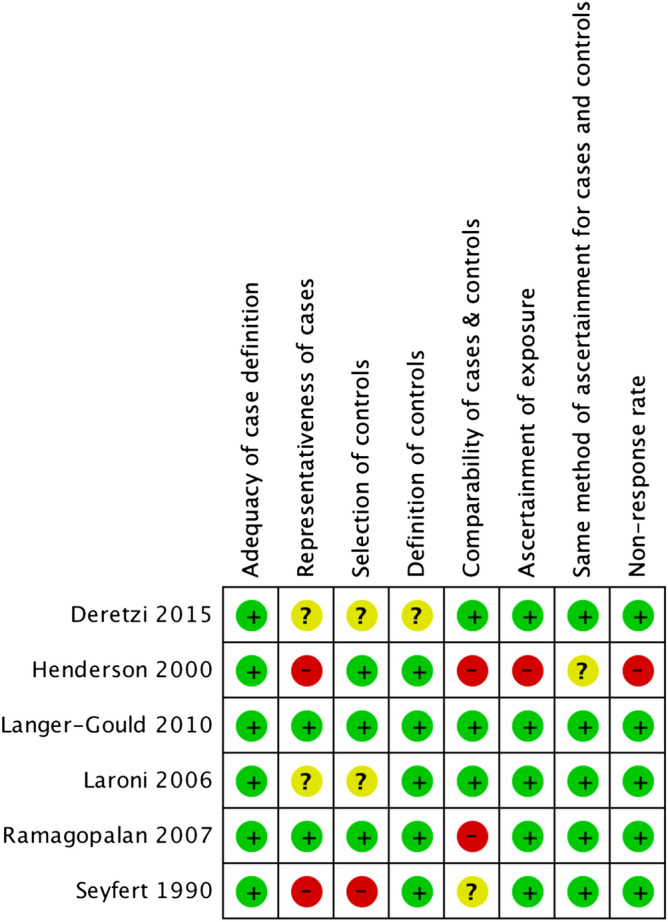
Risk of bias of included case–control studies.
Odds for prevalent vitiligo in patients with multiple sclerosis in case–control studies
In the Seyfert 1990 study, there were no cases of MS with vitiligo19; therefore, we could not calculate the OR. Only one study18 reported a positive association of MS with prevalent vitiligo. As shown in Fig. 3, our meta-analysis was based on five case–control studies, involving a total of 12,829 MS patients and 31,231 controls18,20–23. No significant association of MS with prevalent vitiligo was found (pooled OR 1.33; 95% confidence interval [CI] 0.80‒2.22; I2 = 44%). No publication bias was detected based on the symmetry of the funnel plot (Fig. 4).
Figure 3.
Odds for prevalent vitiligo in multiple sclerosis patients.
Figure 4.

Funnel plot of multiple sclerosis and prevalence of vitiligo. The symmetric distribution suggested no publication bias. However, the number of included studies was less than 10.
Discussion
Polyautoimmunity in patients with MS has been proposed. Different types of autoimmune diseases may have shared pathways and may therefore be associated with each other26. By using a Greek population, Deretzi and colleagues found a significantly higher prevalence of vitiligo in MS patients than in controls18. However, the present meta-analysis did not support an association between MS and vitiligo. As discussed below, the discrepancy in observations pertaining to the association between MS and vitiligo might reflect various factors such as immunologic, environmental, and genetic factors.
The pathophysiology of MS includes peripheral and CNS-compartmentalized inflammatory mechanisms. CD4 + T cells and CD8 + T cells cause neuro-axonal injury both peripherally and in the CNS. Dysregulation of the blood–brain barrier by pro-inflammatory cytokines, interleukin (IL)-17, and chemokines promotes neurodegeneration. Dysfunction of regulatory T cells (Tregs) and an abnormal B cell cytokine response can lead to an aberrant T-cell response27. Genome-wide studies show that IL-2 receptor α (IL2RA) gene and IL-7 receptor α gene play a role in the pathogenesis of MS28.
The pathogenesis of vitiligo includes genetic, autoimmune, and biochemical mechanisms. Increasing evidence in recent years suggests that vitiligo is caused by cytotoxic response of CD8 + T cells that induce apoptosis of melanocytes29. Gene expression profiling in lesional skin of patients and a mouse model of vitiligo indicates an increase expression of interferon gamma and interferon gamma induced genes30. Oxidative stress may also contribute to melanocyte destruction31. Studies also suggest that lesional skin has increased levels of interferon-gamma, IL-10, and IL-176. Genome-wide studies show susceptible genes associated with vitiligo including NLRP1, XBP1, and IL2RA genes32. From a pathophysiology perspective, although the major target cells and antigens are different, MS and vitiligo may have a shared pathologic pathway including increased levels of interferon gamma and IL-17, oxidative stress, and IL2RA gene.
Potential environmental factors associated with MS include smoking33, vitamin D deficiency34, and decreased exposure to sunlight35. Vitamin D plays a role in immune regulation and gene expression. It has a role in the induction of B lymphocyte apoptosis and pro-inflammatory cytokine suppression36. Sunlight is the main source of vitamin D, and an inverse association among exposure to sunlight and the incidence of MS has been identified35. Vitamin D also plays an important role in the development of secondary autoimmune illnesses in patients with vitiligo37.
MS is regulated by multi-genetic and epigenetic factors38,39. Human leukocyte antigen (HLA) studies and genome-wide association studies (GWAS) from different ethnic groups have identified several genes associated with MS40,41. A study using a similar methodology has been performed on vitiligo patients42. Disease-associated genetic loci shared between MS and vitiligo have been identified40,43. Individuals who carry the HLA-DRB1 gene may have an increased risk of developing both MS and vitiligo44,45. In Greece, the HLA-DRB1 gene has been observed to be significantly more common in MS patients than healthy controls46. However, after reviewing studies with data on MS and vitiligo in Greece, current evidence is insufficient to explain why MS patients in Greece have a higher odds for vitiligo compared with other ethnic groups. The diversity of different genetic and epigenetic factors in different ethnicities may, at least in part, explain the inconsistent results reported regarding the association between MS and vitiligo.
Treatments of MS include that for acute relapses and long-term disease modifying therapies. Steroid pulse therapy for 3 to 5 days is applied to control the acute relapses of MS. Disease modifying therapies including injection of interferon beta, glatiramer acetate, natalizumab, alemtuzumab, ocrelizumab, and oral medications such as fingolimod, teriflunomide, dimethyl fumarate, and cladribine can reduce the annualized relapse rate and slow disability progression47. Interferon beta was mainly used to treat MS from 1993 to 2010 before the first oral medication fingolimod came out. Alemtuzumab was launched into the market in November 2014. Despite reports on onset of vitiligo following treatments with interferon beta-1a and alemtuzumab, no evidence indicated that prevalent vitiligo in MS patients was affected during these time periods48,49.
This systematic review has limitations. First, as all the included studies were from Western countries, the results of this review may not be extrapolated to other ethnic groups. Second, in our study, the enrolled studies did not report what kinds of treatment the patients received, and it could be an important source of bias that may affect the association between MS and vitiligo. Therefore, we advocate studies on different ethnic groups involving MS probands and their family members, and studies that consider treatments of MS. Third, we identified no relevant cohort studies and thus the risk for incident vitiligo among patients with MS remains unclear.
In conclusion, the current evidence does not support an association of MS with prevalent vitiligo. To further explore the association, future national studies could provide more information about genetic and environmental influence on the association between MS and vitiligo. GWAS studies of vitiligo or MS in Greece may provide the clue to explain the association between MS and vitiligo. It is still worth being aware of the possibility of vitiligo in MS patients.
Author contributions
C.C. conceived this study. M.S. and C.C. performed data extraction and analysis. M.S. and C.C. drafted the manuscript. C.N. and K.C. commented on the manuscript and agreed with the final version. C.C. is the guarantor of the study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am. J. Manage. Care. 2013;19:S15–20. [PubMed] [Google Scholar]
- 2.Thompson AJ, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 3.Rosati G. The prevalence of multiple sclerosis in the world: an update. Neurol. Sci. 2001;22:117–139. doi: 10.1007/s100720170011. [DOI] [PubMed] [Google Scholar]
- 4.Miljković D, Spasojević I. Multiple sclerosis: molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2013;19:2286–2334. doi: 10.1089/ars.2012.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int. J. Dermatol. 2012;51:1206–1212. doi: 10.1111/j.1365-4632.2011.05377.x. [DOI] [PubMed] [Google Scholar]
- 6.Mohammed GF, Gomaa AH, Al-Dhubaibi MS. Highlights in pathogenesis of vitiligo. World J. Clin. Cases. 2015;3:221. doi: 10.12998/wjcc.v3.i3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson D, Jr, et al. Co-occurrence and comorbidities in patients with immune-mediated inflammatory disorders: an exploration using US healthcare claims data, 2001–2002. Curr. Med. Res. Opin. 2006;22:989–1000. doi: 10.1185/030079906X104641. [DOI] [PubMed] [Google Scholar]
- 8.Liu CY, et al. Association of multiple sclerosis with psoriasis: a systematic review and meta-analysis of observational studies. Am. J. Clin. Dermatol. 2019;20:201–208. doi: 10.1007/s40257-018-0399-9. [DOI] [PubMed] [Google Scholar]
- 9.Marrie RA, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult. Scler. J. 2015;21:263–281. doi: 10.1177/1352458514564491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: a comprehensive overview: part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J. Am. Acad. Dermatol. 2011;65:473–491. doi: 10.1016/j.jaad.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterson, J., Welch, V., Losos, M. & Tugwell, P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (2011 Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.).
- 13.Review Manager (RevMan)[Computer Program] Version 5.3. Copenhagen: The Cochrane Colaboration. 2014.
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods. 2006;11:193. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 18.Deretzi G, et al. Polyautoimmunity in a Greek cohort of multiple sclerosis. Acta. Neurol. Scand. 2015;131:225–230. doi: 10.1111/ane.12308. [DOI] [PubMed] [Google Scholar]
- 19.Seyfert S, Klapps P, Meisel C, Fischer T, Junghan U. Multiple sclerosis and other immunologic diseases. Acta. Neurol. Scand. 1990;81:37–42. doi: 10.1111/j.1600-0404.1990.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 20.Henderson RD, Bain CJ, Pender MP. The occurrence of autoimmune diseases in patients with multiple sclerosis and their families. J. Clin. Neurosci. 2000;7:434–437. doi: 10.1054/jocn.2000.0693. [DOI] [PubMed] [Google Scholar]
- 21.Laroni A, et al. Multiple sclerosis and autoimmune diseases: epidemiology and HLA-DR association in North-east Italy. J. Neurol. 2006;253:636–639. doi: 10.1007/s00415-006-0084-4. [DOI] [PubMed] [Google Scholar]
- 22.Ramagopalan SV, et al. Autoimmune disease in families with multiple sclerosis: a population-based study. Lancet Neurol. 2007;6:604–610. doi: 10.1016/S1474-4422(07)70132-1. [DOI] [PubMed] [Google Scholar]
- 23.Langer-Gould A, Albers K, Van Den Eeden S, Nelson L. Autoimmune diseases prior to the diagnosis of multiple sclerosis: a population-based case-control study. Mult. Scler. J. 2010;16:855–861. doi: 10.1177/1352458510369146. [DOI] [PubMed] [Google Scholar]
- 24.Poser CM, Brinar VV. Diagnostic criteria for multiple sclerosis. Clin. Neurol. Neurosurg. 2001;103:1–11. doi: 10.1016/S0303-8467(00)00125-6. [DOI] [PubMed] [Google Scholar]
- 25.Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann. Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Agarwal P. A pathway-based view of human diseases and disease relationships. PLoS ONE. 2009;4:e4346. doi: 10.1371/journal.pone.0004346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filippi M, et al. Multiple sclerosis (primer) Nat. Rev. Dis. Primers. 2018;4:49–49. doi: 10.1038/s41572-018-0050-3. [DOI] [PubMed] [Google Scholar]
- 28.Consortium, I. M. S. G Risk alleles for multiple sclerosis identified by a genomewide study. N. Engl. J. Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 29.Harris JE, et al. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+ T-cell accumulation in the skin. J. Invest. Dermatol. 2012;132:1869–1876. doi: 10.1038/jid.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rashighi, M. et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci. Transl. Med.6, 223ra223–223ra223 (2014). [DOI] [PMC free article] [PubMed]
- 31.Xie H, et al. Vitiligo: How do oxidative stress-induced autoantigens trigger autoimmunity? J. Dermatol. Sci. 2016;81:3–9. doi: 10.1016/j.jdermsci.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Spritz RA. Six decades of vitiligo genetics: genome-wide studies provide insights into autoimmune pathogenesis. J. Invest. Dermatol. 2012;132:268–273. doi: 10.1038/jid.2011.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Degelman ML, Herman KM. Smoking and multiple sclerosis: a systematic review and meta-analysis using the Bradford Hill criteria for causation. Mult. Scler. Relat. Disord. 2017;17:207–216. doi: 10.1016/j.msard.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Speer G. Impact of vitamin D in neurological diseases and neurorehabilitation: from dementia to multiple sclerosis. Part I: the role of vitamin D in the prevention and treatment of multiple sclerosis. Ideggyogy Sz. 2013;66:293–303. [PubMed] [Google Scholar]
- 35.Sakoda A, et al. Environmental risk factors for multiple sclerosis in Japanese people. Mult. Scler. Relat. Disord. 2020;38:101872. doi: 10.1016/j.msard.2019.101872. [DOI] [PubMed] [Google Scholar]
- 36.Ghasemi N, Razavi S, Nikzad E. Multiple sclerosis: pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J. 2017;19:1. doi: 10.22074/cellj.2016.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silverberg JI, Silverberg AI, Malka E, Silverberg NB. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J. Am. Acad. Dermatol. 2010;62:937–941. doi: 10.1016/j.jaad.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 38.Consortium, I. M. S. G Evidence for polygenic susceptibility to multiple sclerosis—the shape of things to come. Am. J. Hum. Genet. 2010;86:621–625. doi: 10.1016/j.ajhg.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gourraud P-A, et al. A genome-wide association study of brain lesion distribution in multiple sclerosis. Brain. 2013;136:1012–1024. doi: 10.1093/brain/aws363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt H, Williamson D, Ashley-Koch A. HLA-DR15 haplotype and multiple sclerosis: a HuGE review. Am. J. Epidemiol. 2007;165:1097–1109. doi: 10.1093/aje/kwk118. [DOI] [PubMed] [Google Scholar]
- 41.Oksenberg JR. Decoding multiple sclerosis: an update on genomics and future directions. Expert Rev. Neurother. 2013;13:11–19. doi: 10.1586/14737175.2013.865867. [DOI] [PubMed] [Google Scholar]
- 42.Shen C, et al. Genetic susceptibility to vitiligo: GWAS approaches for identifying vitiligo susceptibility genes and loci. Front. Genet. 2016;7:3. doi: 10.3389/fgene.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hadjigeorgiou GM, et al. Replication study of GWAS risk loci in Greek multiple sclerosis patients. Neurol. Sci. 2019;40:253–260. doi: 10.1007/s10072-018-3617-6. [DOI] [PubMed] [Google Scholar]
- 44.Strassner JP, Harris JE. Understanding mechanisms of autoimmunity through translational research in vitiligo. Curr. Opin. Immunol. 2016;43:81–88. doi: 10.1016/j.coi.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hollenbach JA, Oksenberg JR. The immunogenetics of multiple sclerosis: a comprehensive review. J. Autoimmun. 2015;64:13–25. doi: 10.1016/j.jaut.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kouri I, et al. HLA associations with multiple sclerosis in Greece. J. Neurol. Sci. 2011;308:28–31. doi: 10.1016/j.jns.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 47.Tintore M, Vidal-Jordana A, Sastre-Garriga J. Treatment of multiple sclerosis—success from bench to bedside. Nat. Rev. Neurol. 2019;15:53–58. doi: 10.1038/s41582-018-0082-z. [DOI] [PubMed] [Google Scholar]
- 48.Kocer B, Nazliel B, Oztas M, Batur H. Vitiligo and multiple sclerosis in a patient treated with interferon beta-1a: a case report. Eur. J. Neurol. 2009;16:e78. doi: 10.1111/j.1468-1331.2009.02563.x. [DOI] [PubMed] [Google Scholar]
- 49.Ruck T, et al. Vitiligo after alemtuzumab treatment: secondary autoimmunity is not all about B cells. Neurology. 2018;91:e2233–e2237. doi: 10.1212/WNL.0000000000006648. [DOI] [PMC free article] [PubMed] [Google Scholar]