Abstract
Tea (Camellia sinensis (L.) O.Kuntze) is an industry-oriented economical crop in India. Among the sap sucking pests, tea mosquito bug (Helopeltis theivora) is one of the most serious pests causing heavy crop loss in tea plantation. Continuous use of chemical pesticides causes environmental pollution and health hazards besides developing pesticide residues in tea powder. The control of pests by bacterial metabolite is an alternative that may contribute to reduce or eliminate the chemical pesticide use. The use of chitinase as a biological control is an emerging field of research. In the present study, Chitinase (~ 25 kDa) was purified from Bacillus cereus C-13 strain using gel-filtration chromatography and further characterized for its optimum pH, temperature and substrate specificity. Bioefficacy of chitinase from B. cereus C-13 was compared with our previously reported Pseudomonas fluorescens MP-13 chitinase against H. theivora. Result concluded that, 100% and 78% mortality was observed by using P. fluorescens MP-13 chitinase and B. cereus C-13 chitinase, respectively. In future, bacterial chitinase can be utilized in eco-friendly pest management strategies.
Keywords: Agriculture, Bacterial metabolite, Crop loss, Mortality, Pest management
Introduction
In India, tea Camellia sinensis [L.] 0. Kuntz has a special place as an export-oriented economical crop. Tea is the most popular non-alcoholic beverage consumed worldwide, produced by processing the tender tea leaves and young buds. Being a monoculture nature of tea, it provides an appropriate environment for insect feeding. Tea mosquito bug (TMB), Helopeltis theivora Waterhouse (Hemiptera: Miridae), is one the most serious pests of tea plantation in India, also called as polyphagous pest since it affects a number of economically important crops in India. Nowadays, H. theivora has become the central problem in tea plantations. It attacks only the tender shoots for feeding and egg laying that is the actual crop of tea production. Crop loss due to H. theivora infestation is ~ 17%, occasionally crop loss is near total in case of severe infestation (Roy et al. 2015). Molecular characterization of tea mosquito bug infesting tea in South India was done using mitochondrial cytochrome c oxidase subunit I (mtCOI) gene sequencing which makes a platform for precise control measures (Suganthi et al. 2016).
One of the challenges confronting researchers worldwide is the development of new and sustainable ways of protecting agricultural crops from pests. In usual practice, tea planters adopted to use heavy synthetic pesticides viz., Alphamethrin, Bifenthrin, Clothianidin, Cypermethrin, Endosulfan to manage Helopeltis, however repeated spraying over a long period leads to environmental pollution, pesticide resistance (Roy et al. 2011), conversion of innocuous species into severe pests and mainly accumulation of undesirable pesticide residues in processed tea (Seenivasan and Muraleedharan 2011; Amaraweera and Wickramasinghe 2019). Sometimes, inappropriate exploitation of chemical pesticides causes some adverse effects in human beings; also extend to death due to pesticide poisonings (Langley and Mort 2012). Chemical residues persist for long days after application and the maintenance of pesticide residue level is one of the important criteria since tea is a food crop. Continuous application of chemical pesticides over H. theivora leads to pesticide resistance (Gurusubramanian et al. 2008). Hence, replacement of chemical pesticides by biopesticide is need of the hour by considering the target specific control measures, also to minimize the toxic residues in food crops (Hynes and Boyetchko 2006).
Characterization of chitinase is essential for understanding its role in insect toxicity and its stability in different pH, temperature, time and substrate specificity. The production of chitinase from Bacillus and Pseudomonas sp. has been reported (Faramarzi et al. 2009; Rishad et al. 2016), no work has been reported from tea rhizosphere. At present, searching a specific molecule as a biopesticides from microorganism is necessary for sustainable agriculture. Biocontrol of insect pests by aiming their chitin degradation is an exceptional target for its management. Chitin (C8H13O5N)n, an insoluble, most abundant polysaccharide which is an important structural part of insect cuticles (chitin occupies 50% of the insect cuticle), gut lining of peritrophic membrane and also plays a vital role in structural integrity (Bhattachrya et al. 2007). Chitinases, a major group of hydrolytic enzymes secreted by bacteria, involved in the enzymatic hydrolysis of chitin. As these degrade chitin into its monomeric or oligomeric components, it might be speculated that if sprayed on to the insect, either it penetrate the gut regions, cause perforations in the chitin containing peritrophic membrane which will result in feeding abnormalities or it disrupts the chitin containing cuticle region which subsequently causes abnormal moulting (Chandrasekaran et al. 2012).
In our previous reports, a total of 113 bacterial strains were isolated from different regions of tea rhizospheric soil, and evaluated for their chitinase activity (Suganthi et al. 2015). Among them, MP-13 (27.4 U/ml) & C-13 (26.3 U/ml) recorded the highest chitinolytic activity (Suganthi et al. 2015). Using 16S rRNA sequencing, MP-13 strain validated as Pseudomonas fluorescens & C-13 validated as B. cereus (Suganthi et al. 2018). In our earlier report, Pseudomonas fluorescens MP-13 found to produce ~34 kDa chitinase enzyme (Suganthi et al. 2015), the bioefficacy of chitinase from MP-13 was also reported (Suganthi et al. 2017) and the data was referred for comparison in the present study. Present investigation aimed to isolate the chitinase from B. cereus C-13, compare the biocontrol efficacy of Bacillus and Pseudomonas chitinase against H. theivora, check the mortality levels in H. theivora populations under in vitro condition and finally to identify the potent chitinase producer. This study can further be utilized to develop an effective and eco-compatible bioformulation for the management of Helopeltis populations in tea.
Materials and methods
Purification and characterization of B. cereus C-13 chitinase
Chitinase precipitation and dialysis
A single bacterial colony of B. cereus C-13 was inoculated in nutrient broth with 1.5% colloidal chitin and allowed to grow for 48 h in a shaker at 37 °C. Grown culture was centrifuged at 10,000 rpm for 15 min to collect the culture filtrate. The cell free supernatant was saturated with different range concentration of ammonium sulfate (50% to 80% (w/v)) and protein was precipitated by centrifugation at 10,000 rpm for 30 min at 4 °C. The pellet was dissolved in 50 mM phosphate buffered saline (PBS) pH 7.0. Excess salt and organic solvents were removed from the solution, the precipitated protein was dialyzed against the same buffer for at least 12 h at 4 °C.
Chitinase purification and SDS-PAGE analysis
Gel-filtration chromatography was carried out by using sephacryl CL-250 packed column (1.5 × 4.5 cm). The column was equilibrated with 20 mM PBS buffer pH 7.0 until the pH of the matrix remain constant. Sample (30 ml) was loaded into the column and eluted with flow rate of 0.2 ml/min, in order to allow the chitinase to bind to the matrix. Then, the column was washed with 100 ml of PBS buffer pH 7.0. The collected fraction was assayed for chitinase activity by using colloidal chitin as a substrate by following Sun et al. (2006) method. Positive chitinase fractions were collected and lyophilized.
SDS-PAGE was carried out by the method described by Laemmli, 1970. A stacking gel with 4% acrylamide was layered on top of a 15% separating gel in a sigma slap gel apparatus. Samples with an equal amount of protein were dissolved in a sample buffer and used for electrophoresis. Medium range molecular weight marker (Genei, Bangalore) was used as the standard. The protein bands were visualized by staining with Commassie brilliant blue overnight. The accurate molecular weight of chitinase enzyme was determined with the help of standard commercial protein ladder (Fermentas, USA).
Chitinase enzyme characterization
Before checking the bioefficacy of chitinase against Helopeltis, purified chitinase was partially characterized by determining the optimum temperature, time, pH and substrate specificity. The effect of incubation time on chitinase production was studied by growing B.cereus C-13 strain in nutrient agar medium up to 6 days. The effect of pH was determined by incubating the purified chitinase at different pH levels (3 to 10) using acetate buffer (50 mM) for pH 3–5, phosphate buffer (50 mM) for pH 6–7 and borate buffer for pH 8–10 (Park et al. 2000). Incubating the enzyme solution for 1 h at various pH levels at predetermined temperature and the stability of enzyme was documented. Optimum temperature for the chitinase activity was studied in the range of 20–100 °C under standard assay conditions. The enzyme activity was determined under standard assay conditions using colloidal chitin as the substrate (Viswanathan and Samiyappan 2000). Substrate concentration was also optimized by incorporating different concentrations of colloidal chitin (0.5 to 3%) in the medium under standard assay conditions. Following incubation, the release of N-acetylglucosamine was measured and the relative activity was calculated using colloidal chitin as control. All the experiments were repeated three times with five replications. The results were subjected to statistical analysis (ANOVA followed by DMRT).
Collection and rearing of H. theivora
H. theivora populations were collected from the tea field, insects were immediately transferred onto uniform sized 1-year old potted tea plants covered on moistened cotton kept in a plastic container at 25 ± 1 °C, 75 ± 5% relative humidity. Withered and drying leaves were regularly replaced in moistened cotton kept in a tray since shoot contains the insect egg with careful monitoring. Nymphs were collected from the egg and maintained in the pot containing young tea shoots at 25 ± 1 °C, 75 ± 5% relative humidity. Adults generated from the nymph were used for bioassay.
Bioassay of chitinases against H. theivora
Chitinase enzyme from B. cereus C-13 was evaluated against H. theivora populations. For laboratory bioassay, mature tea shoots were selected from the tea field and uniform sized tea shoots (13 cm from the top) were placed on moist cotton in a glass bottle. Tea shoots were sprayed with 2 ml of three different concentrations (0.012, 0.024 and 0.048 U/ml) of B. cereus C-13 chitinase using a glass atomizer (50 ml, Vensil). Tea shoots sprayed with distilled water served as untreated control (Chandrasekaran et al. 2012). The leaf shoots were left to dry for 30 min and ten adults of H. theivora were transferred directly onto each tea shoot set. Insect mortality was observed after 24, 48, 72 and 96 h. All experiments were conducted using five replicates of a three concentration design with distilled water as control and average mortality determined. Data on the mortality of H. theivora on various treatments are subjected to analysis of variance and means were separated by Duncan’s Multiple Range Test (DMRT).
Results
Purification and characterization of B. cereus C-13 chitinase
Chitinase enzyme was purified by using gel-filtration chromatography from B. cereus C-13 strain and exhibited a molecular weight of ~ 25 kDa on SDS PAGE (Fig. 1). In our earlier report, P. fluorescens MP-13 chitinase was purified, separated by SDS-PAGE with the molecular weight ~ 34 kDa (Suganthi et al. 2015). Further the partially purified chitinase isolated from B. cereus C-13 was characterized in terms of pH, time, temperature and substrate specificity. By considering the time course for chitinase production, maximum activity (17.3 U/ml) in the culture liquid of B. cereus C-13 was observed after 72 h of cultivation (Fig. 2). In contrast, chitinase production was declined (6.5 U/ml) during 96–120 h of cultivation. Since, bacterial species requires prolonged time to produce chitinase by decomposing chitin. The effect of pH on enzyme activity is shown in Fig. 3. Chitinase enzyme showed an optimum activity at pH 6. Chitinase activity was considerably higher at pH 7, indicating that chitinase tends to be active in alkaline condition. The temperature effect of the chitinase activity (Fig. 4) was also performed which indicated that the activity was increased at temperature 30–40 °C (9.6 U/ml) and reduced considerably at a higher temperature 60–100 °C (1.2 U/ml). Also enzyme activity was reduced at 20 °C (4.8 U/ml). Standardization of colloidal chitin concentration is yet another important parameter in the chitinase production. High chitinase activity (11.5 U/ml) was observed when 1.5% colloidal chitin was added to the culture medium (Fig. 5). The ANOVA and DMRT analysis showed a significant difference in the overall chitinase activity U/ml between the time periods (p < 0.001), Temperature (p < 0.001), pH (p < 0.001) and Substrate concentration (p < 0.001) (Table 2).
Fig. 1.
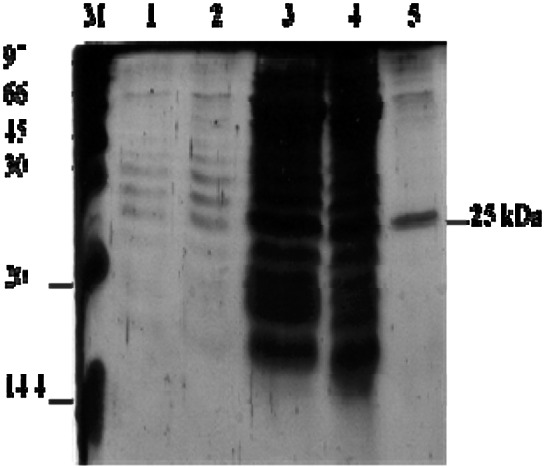
SDS-PAGE analysis of chitinase from B. cereus (C-13). Lane M—Protein marker; Lane 1–3—culture supernatant; Lane 4—enzyme after 70% ammonium sulphate precipitation; Lane 5—chitinase purified by gel-filtration chromatography
Fig. 2.

Time course of chitinase production by B. cereus C-13 in a medium supplemented with colloidal chitin as a sole carbon source. Mean plots showed that estimated mean values is changing over different time course
Fig. 3.
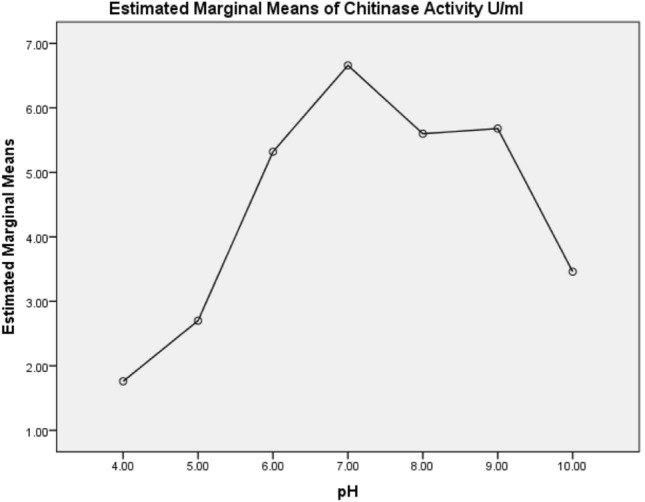
Effect of different pH on chitinase production by B. cereus C-13 Mean plots showed that estimated mean values is changing over different pH concentration
Fig. 4.

Effect of different temperature on chitinase production by B. cereus C-13 Mean plots showed that estimated mean values is changing over different temperature
Fig. 5.

Production of chitinase by B. cereus C-13 in different concentrations of colloidal chitin Mean plots showed that estimated mean values is changing over substrate concentration
Table 2.
ANOVA and Duncan’s showed a significant difference in the overall Chitinase Activity U/ml between the time periods, Temperature, pH and Substrate
| Tests of between-subjects effects | |||||
|---|---|---|---|---|---|
| Dependent variable: Chitinase activity U/ml | |||||
| Source | Type III Sum of squares | df | Mean square | F | p value |
| Time | 576.892 | 4 | 144.223 | 1912.772 | <0.001 |
| Temperature | 405.480 | 8 | 50.685 | 385.926 | <0.001 |
| pH | 98.775 | 6 | 16.462 | 167.496 | <0.001 |
| Substrate conc. | 360.702 | 5 | 72.140 | 609.637 | <0.001 |
| Error | 1.508 | 20 | 0.075 | ||
| Total | 3483.610 | 25 | |||
| Corrected total | 578.400 | 24 | |||
R Squared = 0.997 (Adjusted R Squared = 0.997)
Comparitive bioefficacy of chitinase against H. theivora
Efficacy of chitinase from B. cereus C-13 (Bc C-13) was tested in different concentrations (0.012 U/ml, 0.024 U/ml & 0.048 U/ml) on the adults of H. theivora. Comparative bioassay results are given in Table 1 (Pf MP-13 data was referred from our previous report Suganthi et al. 2017). Results shows that, after 24 h of Bc C-13 chi treatment (0.012 U/ml), the percentage of insect mortality was 12% and the trend of pest mortality increased as 22% at 48 h, 30% at 72 h and 36% at 96 h. At the same time, Pf MP-13 chi showed the mortality of 24% at 24 h, the percentage of mortality increased to 44% after 72 h. Whereas in case of treating the insect with 0.024 U/ml of Pf MP-13 chi, maximal of 60% mortality at 72 and 96 h was noticed, however Bc C-13 chi at the same concentration showed 42% and 54% at 72 and 96 h respectively. When chitinase concentration was increased to 0.048 U/ml, percent mortality of H. theivora was noticed as 100% after 72 h by using Pf MP-13 chi, on the other hand Bc C-13 chi showed only 62%, 78% mortality at 72 h and 96 h respectively (Table 1). There was no insect mortality observed in the control. When the concentration of chitinase was increased, stable increase in insect mortality was observed. Results acquired from the present study indicated that chitinase from both bacterial strains were most effective against sucking pest H. theivora. When compared to Bc C-13 chi, Pf MP-13 chi showed the highest insecticidal activity against H. theivora (Table 2).
Table 1.
Comparision of mortality rate caused by B. cereus C-13 chi and P. fluorescens MP-13 chi against H. theivora
| Bc C-13 chi & Pf MP-13 chi* (U/ml) | Percentage of mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | |||||
| Bc C-13 chi | Pf MP-13 chi | Bc C-13 chi | Pf MP-13 chi | Bc C-13 chi | Pf MP-13 chi | Bc C-13 chi | Pf MP-13 chi | |
| Control | 0 ± 0.0a | 0 ± 0.0a | 0 ± 0.0a | 0 ± 0.0a | 0 ± 0.0a | 0 ± 0.0a | 0 ± 0.0a | 0 ± 0.0a |
| 0.012 | 12 ± 2.0b | 24 ± 4.0b | 22 ± 2.0b | 28 ± 4.8b | 30 ± 3.1b | 44 ± 4.0b | 36 ± 2.4b | 44 ± 4.0b |
| 0.024 | 34 ± 5.4b | 30 ± 5.1b | 38 ± 4.4c | 50 ± 5.1c | 42 ± 8.3c | 60 ± 0.0c | 54 ± 5.4c | 60 ± 0.0c |
| 0.048 | 48 ± 2.0c | 48 ± 4.8c | 54 ± 2.4d | 72 ± 4.8d | 62 ± 2.0d | 100 ± 0.0d | 78 ± 3.7d | 100 ± 0.0d |
Values are means of five replicates ± SE; means followed by the same letter in a vertical column do not differ significantly at p = 0.05 according to Duncan’s Multiple Range Test (DMRT). Bc C-13 chi (B. cereus C-13 chitinase) & Pf MP-13 chi (Pseudomonas florescens MP-13 chitinase)
*Suganthi et al. (2017)
Discussion
H. theivora (Hemiptera: miridae), is one of the most serious pests of tea plantation since it attacks only the young tea shoots and buds which is the actual crop of tea. Among different hydrolytic enzymes, chitinases play a significant role in the management of pest incidence by hydrolyzing chitin, a major cuticle and cell wall constituent in the majority of the insect pests. Bacteria viz., Streptomyces (Blaak and Schrempf 1995), Serratia marcescens (Synstad et al. 2008), Aeromonas punctata and A. hydrophila (Kuddus and Ahmad 2013), Bacillus (Rishad et al. 2016), etc. reported for its potential chitinase production. The molecular weight of chitinases varies from 20 to 90 kDa (Hamid et al. 2013). Only one type of chitinases produced by such bacteria but others reported to produce several types with various size. For example, several chitinases of molecular sizes 21, 38, 43, 54 and 62 kDa was produced by S. marcescens (Duzhak et al. 2002). However, only one chitinase with the molecular mass of 25 kDa was detected in SDS-PAGE analysis of B. cereus C-13 used in this study (Fig. 1).
In the present study, purified chitinase showed a number of interesting features. Maximum chitinase production was obtained after 48–72 h by B. cereus C-13. Similarly, maximum chitinase production by B. alvei, B. cereus and B. sphaericus occurred after 48 h of incubation, then it subsequently declined (Wang and Hwang 2001) and the bacteria Massilia timonae was reported to produce high chitinase after 36 h (Faramarzi et al. 2009). Chitinase production gradually decreased after 72 h, this may be attributed to production of proteolytic enzymes that may hydrolyse chitinase or chitin decomposition leading to accumulation of GlcNAc or inhibitory metabolites production or nutrition depletion (Gomaa 2012). Chitinase production by Bacillus sp. was declined after 5 days which was attributed to the increase in pH due to the release of carbonate salts and protein into the medium (Gomaa 2012).
Chitinase maintains its activity at neutral to alkaline pH values (6–9), confirming its activity in alkaline insect gut. Also, Gomaa (2012) reported maximum chitinase production from B. thuringiensis and B. licheniformis achieved at pH 7 and 8, respectively. Bacterial chitinases are most effectively functional in insect outer skeleton, alkaline mid gut or digestive tracts where the chitin is present but plant chitinolytic enzymes, needs an acidic environment for its activity; there was no activity under alkaline pH. However, herbivorous insects, in general, have alkaline midguts (Zhe-fu et al. 1992). B. cereus strain CH2 showed an optimum chitinase activity in the temperature range of 40 °C (Li et al. 2008). Chitinase from B. cereus C-13 used in this study was active at 30–50 °C after 30 min. Considering the thermo stability of chitinase, it can be used under field conditions to control insect pests. To enhance the production of chitinase, standardization of colloidal chitin concentration is yet another important parameter. Chitinase activity was substantially higher at 1.5% colloidal chitin when compared to other concentrations and identical results were also reported by Gomaa (2012). Chitin in a colloidal form is easily utilized by bacteria which degrade the chitin into monomers and oligomeric components essential substrate for growth and development of bacteria.
Biological control agents have received increased attention as safe and environmental friendly alternative to the use of high volume of chemical pesticides (Cook 1996). Bacterial biocontrol agents as such have been reported to control several plant pests including Manduca sexta and Plutella xylostella (Martin and Blackburn 2007), Aphis gossypii (Melatti et al. 2010). Also reports discuss that use of whole bacteria as a spray cause only low leathality in agricultural pests (Tsagou et al. 2004; Roobakkumar et al. 2011), further continuous use will end up with insect resistance (Wirth et al. 2005). Among 400 strains of Bacillus sp. screening, only two strains S29 and S1168 were most efficient against cotton aphid (A. gossypii Glover) and showed only 50% mortality (Melatti et al. 2010).
Instead of spraying whole living microorganisms to manage pest incidence, in recent years, research has focused on the use of selective metabolite from bacteria, because they are generally considered as target specific, safer and environment friendly (Shternshis et al. 2002; Berini et al. 2019). Bacterial chitinase has been evaluated for less number of agricultural insect pests (Hamid et al. 2013; Berini et al. 2019). It is believed that the chitinase cause perforations in the chitin containing cuticle region or peritrophic membrane of the insect pests, leads to insect death. Hence, chitinase act as both contact and systemic toxic component to kill the insects (Broadway et al. 1998). Till now, no investigations were carried out against H. theivora using bacterial chitinase. In this study, mortality in H. theivora populations was observed, this may due to the chitinase perforations in the peritrophic membrane of insect gut. The damage in the insect gut might have resulted in nutritional imbalance due to numerous processes viz., water excretion or retention, food acquisition, which further leads to slow growth and ultimately leads insect death.
Numerous reports are available for the degradation of chitin containing peritrophic membrane using bacterial chitinase. Initially, Brandt et al. 1978 noticed that chitinase cause perforations in gut peritrophic membrane of Orgyia pseudotsugata, thereby facilitating the entry of toxic materials or pathogens into the hemocoel of susceptible insects. Ding et al. 1998 demonstrated the bioassay against tobacco budworm, Heliothis virescens using tobacco leaves excised from transgenic tobacco plants expressing the Manduca sexta chitinase, results shows that total mass of larvae fed on chitinase-negative leaves was nearly six fold higher than the mass of larvae fed on chitinase-positive leaves. Khajuria et al. 2010 provide a strong evidence to understand the role of chitinase on chitin degradation of insect, subsequently affect the growth and development of European corn borer larvae.
It is evident that among different concentrations tested, P. fluorescens MP-13 chitinase (0.048 U/ml) can induce 100% mortality within 96 h in adults of H. theivora (Suganthi et al. 2017). Similarly, recent report discuss that enzyme toxin of P. fluorescens efficiently hydrolysed the chitin in cuticular region as well as in the peritrophic membrane and also binds to the gut regions of mosquito, Culex quinquefasciatus causes the swelling of internal organs and enlargement of vacuoles, followed by lysis of epithelial cells, and the death of mosquito larvae (Brammacharry and Paily 2012). Mendonsa et al. 1996 reported lytic enzymes from the bacteria, Myrothecium verrucaria effectively degrade the chitin of insect cuticles of Aedes aegypti, 100% mortality was observed after 48 h. In another study, Prasanna et al. 2012 reported that insect bioassay using chitinase from Bacilus laterosporus Lak1210 reduced the larval survival of diamondback moths (Plutella xylostella) on cabbage leaves.
Culture filtrate of P. fluorescens effectively used to kill the vector mosquitoe species viz., An. stephensi, C. quinquefasciatus and Ae. Aegypti at pupal stage, concluded that culture filtrate may contains some exotoxins (Prabakaran et al. 2003). While using the formulation developed from a metabolite of P. fluorescens against C. quinquefasciatus larvae and pupae under field condition, 100% elimination of larvae and pupae was observed after 12 days (Sadanandane et al. 2003). In another study, formulated chitinase sprayed on strawberry leaves and fruits which further prevent the infestation of common insects (Koga 2005). In another study, combination of CryIC and endochitinase was successfully used against Spodoptera larvae cause perforations in peritrophic membranes, further insect mortality was observed (Regev et al. 1996). Similary, in this study even very low concentration of chitinase (0.048 U/ml) from Bacillus cereus C-13 showed 78% mortality in H. theivora populations.
Since tea is a beverage, the replacement of chemical pesticides by alternate control measures is expected to make a significant contribution for tea consumers, by reducing the pesticide residues in tea. In conclusion, first time, metabolite like bacterial chitinase exhibited remarkable insect mortality in H. theivora populations. Chitinases can be implemented to manage insect pests by over-expression of the desired chitinase gene, chitinase formulation, evaluation of shelf life and field trial. Also, intensive research is necessary to understand the mechanism of bacterial chitinase during insect toxicity. Chitinase based biopesticide could also be effectively utilized for the effective management of different tea pests/pathogens.
Acknowledgments
We gratefully acknowledge the Director, UPASI Tea Research Institute, Valparai, TamilNadu, India for positive encouragements and facilities. First author sincerely acknowledge Dr. K.N. Chandrashekara for his valuable support and suggestions during the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amaraweera OHH, Wickramasinghe I. Analysis of pesticide residue concentration in exported quality ceylon black tea by GC-MS. J Food Nutr Health. 2019;2:1–6. [Google Scholar]
- Berini F, Casartelli M, Montali A, Reguzzoni M, Tettamanti G, Marinelli F. Metagenome-sourced microbial chitinases as potential insecticide proteins. Front Microbiol. 2019;10:1358. doi: 10.3389/fmicb.2019.01358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattachrya D, Nagpure A, Gupta RK. Bacterial chitinases: properties and potential. Crit Rev Biotechnol. 2007;27:21–28. doi: 10.1080/07388550601168223. [DOI] [PubMed] [Google Scholar]
- Blaak H, Schrempf H. Binding and substrate specificities of a Streptomyces olivaceoviridis chitinase in comparison with its proteolytically processed form. Eur J Biochem. 1995;229:132–139. doi: 10.1111/j.1432-1033.1995.tb20447.x. [DOI] [PubMed] [Google Scholar]
- Brammacharry U, Paily K. Chitinase like activity of metabolites of Pseudomonas fluorescens Migula on immature stages of the mosquito, Culex quinquefasciatus (Diptera: Culicidae) Afr J Microbiol Res. 2012;6:2718–2726. [Google Scholar]
- Brandt CR, Adang MJ, Spence KD. The peritrophic membrane: ultrastructural analysis and function as a mechanical barrier to microbial infection in Orgyia pseudotsugata. J Invertebrate Pathol. 1978;32:12–24. [Google Scholar]
- Broadway RM, Gongora C, Kain WC, Sanderson JP, Monroy JA, Bennett KC, Warner JP, Hoffmann MP. Novel chitinolytic enzymes with biological activity against herbivorous insects. J Chem Ecol. 1998;24:985–998. [Google Scholar]
- Chandrasekaran R, Revathi K, Nisha S, Kirubakaran SA, Sengottayan S. Physiological effect of chitinase purified from Bacillus subtilis against the tobacco cutworm Spodoptera litura Fab. Pesticide Biochem Physiol. 2012 doi: 10.1016/j.pestbp.2012.07.002. [DOI] [Google Scholar]
- Cook RJ. Assuring the safe use of microbial biocontrol agents: a need for policy based on real rather than perceived risks. Can J Plant Pathol. 1996;18:439–445. [Google Scholar]
- Ding X, Gopalakrishnan B, Johnson LB, White FF, Wang X, Morgan TD, Kramer KJ, Muthukrishnan S. Insect resistance of transgenic tobacco expressing an insect chitinase gene. Trans Res. 1998;7:77–84. doi: 10.1023/a:1008820507262. [DOI] [PubMed] [Google Scholar]
- Duzhak AB, Panfilova ZI, Vasyunina EA. Synthesis of extracellular chitinase by wild-type B-10 and mutant M-1 strains of Serratia marcescens. Appl Biochem Microbiol. 2002;38:214–221. [PubMed] [Google Scholar]
- Gomaa EZ. Chitinase production by Bacillus thuringiensis and Bacillus lichenformis: their potential in antifungal biocontrol. J Microbiol. 2012;50:103–111. doi: 10.1007/s12275-012-1343-y. [DOI] [PubMed] [Google Scholar]
- Faramarzi MA, Fazeli M, Tabatabaei Yazdi M, Adrangi S, Jami AL, Ahmadi K, Tasharrofi N, Aziz MF. Optimization of cultural conditions for production of chitinase by a soil isolate of Massilia timonae. Biotechnol. 2009;8:93–99. [Google Scholar]
- Gurusubramanian G, Sarmah M, Rahman A, Roy S, Bora S. Pesticide usage pattern in tea ecosystem, their retrospects and alternative measures: a review. J Environ Biol. 2008;29:813–826. [PubMed] [Google Scholar]
- Hamid R, Khan MA, Ahmad M, Ahmad MM, Abdin MZ, Musarrat J, Javed S. Chitinases: an update. J Pharm Bioallied Sci. 2013;5:21–29. doi: 10.4103/0975-7406.106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RK, Boyetchko SM. Research initiatives in the art and science of biopesticide formulations. Soil Biol Biochem. 2006;38:845–849. [Google Scholar]
- Khajuria C, Buschman LL, Chen MS, Muthukrishnan S, Zhu KY. A gutspecific chitinase gene essential for regulation of chitin content of peritrophic matrix and growth of Ostrinia nubilalis larvae. Insect Biochem Mol Biol. 2010;40:621–629. doi: 10.1016/j.ibmb.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Koga D. Application of chitinase in agriculture. J Min Metal Mat. 2005;15:33–36. [Google Scholar]
- Kuddus M, Ahmad I. Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. J Genet Eng Biotechnol. 2013;11:39–46. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langley RL, Mort SA. Human exposures to pesticides in the United States. J Agromed. 2012;17:300–315. doi: 10.1080/1059924X.2012.688467. [DOI] [PubMed] [Google Scholar]
- Li JG, Jiang ZQ, Xu LP, Sun FF, Guo JH. Characterization of chitinase secreted by Bacillus cereus strain CH2 and evaluation of its efficacy against Verticillium wilt of eggplant. Biocontrol. 2008;53:931–944. [Google Scholar]
- Martin PAW, Blackburn MB. Using combinatorics to screen Bacillus thuringiensis isolates for toxicity against Manduca sexta and Plutella xylostella. Biol Control. 2007;42:226–232. [Google Scholar]
- Melatti VM, Praca LB, Martins ES, Sujii E, Berry C, Monnerat RG. Selection of Bacillus thuringiensis strains toxic against cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae) Bioassay. 2010;5:1–4. [Google Scholar]
- Mendonsa ES, Vartak PH, Rao JU, Deshpande MV. An enzyme from Myrothecium verrucaria that degrades insect cuticles for biocontrol of Aedes aegypti mosquito. Biotechnol Lett. 1996;18:373–376. [Google Scholar]
- Park SH, Lee JH, Lee HK. Purification and characterization of chitinase from a marine bacterium, Vibrio sp. 98CJ11027. J Microbiol. 2000;38:224–229. [Google Scholar]
- Prabakaran G, Paily KP, Padmanabhan V, Hoti SL, Balaraman K. Isolation of Pseudomonas fluorescens metabolite/exotoxin active against both larvae and pupae of vector mosquitoes. Pest Manag Sci. 2003;59:21–24. doi: 10.1002/ps.610. [DOI] [PubMed] [Google Scholar]
- Prasanna L, Eijsink VGH, Meadow R, Gaseidnes S. A novel strain of Brevibacillus laterosporus produces chitinases that contribute to its biocontrol potential. Appl Microbiol Biotechnol. 2012;6:1–11. doi: 10.1007/s00253-012-4019-y. [DOI] [PubMed] [Google Scholar]
- Regev A, Keller M, Strizhov N, Sneh B, Prudovsky E, Chet I, Ginzberg I, Koncz-Kalman Z, Koncz C, Schell J, Zilberstein A. Synergistic activity of a Bacillus thuringiensis d-endotoxin and a bacterial endochitinase against Spodoptera littoralis larvae. Appl Environ Microbiol. 1996;62:3581–3586. doi: 10.1128/aem.62.10.3581-3586.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rishad K, Rebello S, Nathan VK, Shabanamol S, Jisha M. Optimised production of chitinase from a novel mangrove isolate, Bacillus pumilus MCB-7 using response surface methodology. Biocatal Agric Biotechnol. 2016;5:143–149. [Google Scholar]
- Roobakkumar A, Babu A, Vasantha Kumar D, Jasin Rahman V, Sarkar S. Pseudomonas fluorescens as an efficient entomopathogen against Oligonychus coffeae Nietner (Acari: Tetranychidae) infesting tea. J Entomol Nematol. 2011;3(5):73–77. [Google Scholar]
- Roy S, Mukhopadhyay A, Gurusubramanian G. Resistance to insecticides in field-collected populations of tea mosquito bug (Helopeltis theivora Waterhouse) from the Dooars (North Bengal, India) tea cultivations. J Entomol Res Soci. 2011;13:37–44. [Google Scholar]
- Roy S, Muraleedharan N, Mukhapadhyay A, Handique G. The tea mosquito bug, Helopeltis theivora Waterhouse (Heteroptera: Miridae): its status, biology, ecology and management in tea plantations. Int J Pest Manag. 2015 doi: 10.1080/09670874.2015.1030002. [DOI] [Google Scholar]
- Sadanandane C, Reddy CMR, Prabakaran G, Balaraman K. Field evaluation of Pseudomonas fluorescens against Culex quinquefasciatus larvae and pupae. Acta Trop. 2003;87:341–343. doi: 10.1016/s0001-706x(03)00154-2. [DOI] [PubMed] [Google Scholar]
- Seenivasan S, Muraleedharan NN. Survey on the pesticide residues in tea in south India. Environ Monit Assess. 2011;176:365–371. doi: 10.1007/s10661-010-1589-y. [DOI] [PubMed] [Google Scholar]
- Shternshis MV, Ovchinnikova LA, Duzhak AB, Tomilova OG. The efficiency of the viral and bacterial entomopathogens formulated with chitinase for biocontrol of lepidopteran cabbage pests. Arch Phytopathol Plant Prot. 2002;35:161–169. [Google Scholar]
- Suganthi M, Arvinth S, Raj Kumar R. Detection of chitinase activity and its characterization from Pseudomonas fluorescens of tea rhizosphere. J Plant Crops. 2015;43:236–239. [Google Scholar]
- Suganthi M, Arvinth S, Chandrashekara KN, Raj Kumar R. Molecular characterization of tea mosquito bug from tea growing regions of India. Mitochondrial DNA. 2016;27:3504–3506. doi: 10.3109/19401736.2015.1066369. [DOI] [PubMed] [Google Scholar]
- Suganthi M, Arvinth S, Chandrashekara KN, Raj Kumar R, Senthilkumar P. Molecular characterization of bacterial biocontrol agents and their chitinase genes from tea soil. Indian J Exp Biol. 2018;56:395–401. [Google Scholar]
- Suganthi M, Senthilkumar P, Arvinth S, Chandrashekara KN. Chitinase from Pseudomonas fluorescens and its insecticidal activity against Helopeltis theivora. J Gen Appl Microbiol. 2017;63(4):222–227. doi: 10.2323/jgam.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liu W, Han B, Zhang J, Liu B. Purification and characterization of two types of chitosanase from a Microbacterium sp. Biotechnol Lett. 2006;28:1393–1399. doi: 10.1007/s10529-006-9101-z. [DOI] [PubMed] [Google Scholar]
- Synstad B, Vaaje-Kolstad G, Cederkvist FH, Saua SF, Horn SJ, Eijsink VG, Sorlie M. Expression and characterization of endochitinase C from Serratia marcescens BJL200 and its purification by a one-step general chitinase purification method. Biosci Biotechnol Biochem. 2008;72:715–723. doi: 10.1271/bbb.70594. [DOI] [PubMed] [Google Scholar]
- Tsagou V, Lianou A, Lazarakis D, Emmanouel N, Aggeli G. Newly isolated bacterial strains belonging to Bacillaceae (Bacillus sp.) and Micrococcaceae accelerate death of the honey bee mite, Varroa destructor (V. jacobsoni), in laboratory assays. Biotechnol Lett. 2004;26:529–532. doi: 10.1023/b:bile.0000019563.92959.0e. [DOI] [PubMed] [Google Scholar]
- Viswanathan R, Samiyappan R. Antifungal activity of chitinases produced by some fluorescent pseudomonas against Colletotrichum falcatum Went causing rot disease in sugarcane. Microbiol Res. 2000;155:1–6. doi: 10.1016/S0944-5013(01)80009-4. [DOI] [PubMed] [Google Scholar]
- Wang SL, Hwang JR. Microbial reclamation of shellfish wastes for the production of chitinases. Enzyme Microb Technol. 2001;28:376–382. doi: 10.1016/s0141-0229(00)00325-2. [DOI] [PubMed] [Google Scholar]
- Wirth MC, Jiannino JA, Federici BA, Walton WE. Evolution of resistance toward Bacillus sphaericus or a mixture of B. sphaericus + Cyt1A from Bacillus thuringiensis, in the mosquito, Culex quinquefasciatus (Diptera: Culicidae) J Invert Pathol. 2005;88:154–162. doi: 10.1016/j.jip.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Zhe-fu L, Danqi W, Annuo L, Wefqin Z. Chitinases from seeds of Zea mays and Coix lachryma-jobi L. purification and some properties. Proc Biochem. 1992;27:83–88. [Google Scholar]


