Abstract
Objectives
Ulcerative colitis is a systemic inflammatory disease which primarily involves the gut but presented by numerous extraintestinal manifestations. The effect of ulcerative colitis on knee cartilage has not been evaluated up to the present. In the current study, we aimed to investigate the possible relationship between the presence of ulcerative colitis and femoral cartilage thickness.
Design
Sixty-two patients with confirmed diagnosis of ulcerative colitis and 70 healthy controls aged 18 to 50 years referred to the gastroenterology outpatient department between January 2018 and January 2019 participated in this cross-sectional study. The measurements were made by ultrasonography with the patient in a supine position and the knees in complete flexion. Demographic, clinical, endoscopic and laboratory data were collected for all the subjects.
Results
The groups of ulcerative colitis and control group were similar with regard to sex, mean age, weight, height, body mass index, extremity dominancy, and existence of knee pain (P > 0.05). Medial femoral condyles, intercondylar areas, and lateral femoral condyles of both right and left knees had thinner cartilage thickness in ulcerative colitis group than control group (P < 0.001).
Conclusion
Knee cartilage was thinner in subjects with mild activity ulcerative colitis than in healthy controls. Decreased knee cartilage thickness may be an indicator of extraintestinal manifestation in patients with mild activity ulcerative colitis. This association between ulcerative colitis and knee cartilage degeneration may be effective in early detection of possible risk factors and potential treatment strategies for both ulcerative colitis and specific subtypes of knee osteoarthritis.
Keywords: ulcerative colitis, extraintestinal manifestation, knee, femoral cartilage, cartilage thickness, ultrasonography
Introduction
Ulcerative colitis is a systemic inflammatory disease that primarily involves the gut but is accompanied by numerous extraintestinal manifestations (EIM). 1 Approximately 50% of patients with inflammatory bowel disease (IBD) deal with at least 1 EIM during the course of disease. 2 The development of EIM decreases the quality of life, increases the morbidity and may result in disability. 2
Rheumatological and musculoskeletal manifestations cover a remarkable portion of EIMs, which have a complex pathogenesis and have not been fully understood. These mechanisms include ectopic expression of gut-specific chemokines and adhesion molecules, T cell flowing conducted by non-specific adhesion molecules, microbial antigen translocation and/or cross-reactivity, circulating antibodies, or self-directed inflammatory events sharing prevalent genetic or environmental risk factors with IBD. 3 The role of inflammation in the ethiopathogenesis of osteoarthritis (OA) has contradictory results with a general acceptance that the well-balanced quantities of inflammatory pathways are necessary for cartilage homeostasis and tissue repairs. The increased inflammatory mediators contribute to ethiopathogenesis of cartilage degeneration and OA. 4 So we hypothesize that the probability of early femoral cartilage degeneration and eventual OA may be an extraintestinal manifestation of ulcerative colitis or vice versa, ulcerative colitis may be a risk factor for OA.
As far as we know, the effect of ulcerative colitis on knee joint cartilage has not been assessed up to present. Ultrasonographic (US) evaluation of knee joint cartilage thickness appears to be an effective and valid method to evaluate cartilage degeneration. The aim of the current study was to investigate the possible relationship between the presence of ulcerative colitis and knee cartilage thickness.
Materials and Methods
Patient Selection
Sixty-two patients with confirmed diagnosis of ulcerative colitis and 70 healthy controls aged 18 to 50 years referred to the gastroenterology outpatient department between January 2018 and January 2019 participated in this cross-sectional study. Demographic and clinical properties of the subjects including sex, age, height, weight, body mass index (BMI), and extremity dominancy were recorded. Patients diagnosed with ulcerative colitis by clinical, endoscopic, and histopathological findings, who have been followed up for at least 1 year, and who did not use immunosuppressive drugs—antitumor necrosis factor (TNF), steroids, azothiopurines, and so on—were included in the study. Participants with a background of another systemic disease (and chronic medication use), knee injury, septic/inflammatory arthritis, any surgeries of lower extremities or ulcerative colitis, smoking, varus and valgus alignment or any intra-articular treatment within the last 6 months were not included.
Clinical activity assessment was made by modified Truelove and Witts criteria of the ulcerative colitis. According to these criteria only patients with mild activity were included in the study. Patients with moderate and severe activity (the patients who needed immunosuppressive therapies) were excluded.
Written informed consents form were filled up by participants in the study. Approval of the study was obtained from local ethics committee of Health Sciences University/Istanbul, Turkey.
Measurements of Femoral Articular Cartilage
Linear probe was used to evaluate femoral articular cartilage thickness (M Turbo, Sonosite) by the same physiatrist who had at least 5 years of experience in musculoskeletal US and was blind to patient groups.
General rules on US assessment of OA pathology were applied, which included the following: choosing the highest frequency that allows the visualization of the target area, adopting a multiplanar scanning technique to document US findings indicative of OA on at least 2 perpendicular planes of scanning and performing dynamic examination during flexion–extension movement.5,6 Scanning protocol for a tailored US assessment included the evaluation of the articular cartilage involvement, the identification and measurement of the osteophytes and the detection of joint inflammation. The weight-bearing surfaces of the femoral condyle were scanned in the suprapatellar region with the knee fully flexed.
Femoral articular cartilage thickness was evaluated with the subject in supine position and knees in complete flexion. The probe was located (in the transverse plane) axially over the exterior side of patella. Right and left knee cartilages were evaluated from the middle area of lateral condyle (LFC), medial condyle (MFC), and intercondylar area (ICA). The measurement of cartilage thickness performed by the extent of the straight hyperechoic edge at the bone-cartilage interface and the thin hyperechoic edge at the cartilage–synovial space interface ( Fig. 1 ).
Figure 1.
Ultrasonographic view (suprapatellar transverse view) demonstrating the knee cartilage evaluations. Right and left knee cartilage thickness was measured from the middle area of lateral femoral condyle (LFC), medial femoral condyle (MFC), and intercondylar area (ICA). Cartilage thickness evaluated by the extent of the straight hyperechoic edge at the bone-cartilage interface and the thin hyperechoic edge at the cartilage–synovial space interface. L, left; R, right.
Statistical Methods
Statistical analysis was done using SPSS version 15.0. (IBM SPSS Statistics, IBM Corp, Armonk, NY, USA). The power of the study was 90%. Descriptive statistics (percentage, mean, median, distributions) of the study group were determined. Kolmogorov-Smirnov test was used to determine conformity to normal distribution. Student t test was used for normal distributed parameters and Mann-Whitney U test was used for parameters that were not normally distributed. Categorical data were investigated by chi-square test. A value of P < 0.05 was considered statistically significant.
Results
Demographic and clinical data of the participants are presented in Table 1 . The groups of ulcerative colitis and control group were similar with regard to sex, mean age, weight, height, BMI, extremity dormancy, and existence of knee pain (P > 0.05).
Table 1.
Demographic and Clinical Data of the Study Groups.
| Patient Characteristics | Ulcerative Colitis ( n = 62) | Control ( n = 70) | P |
|---|---|---|---|
| Age, years, mean ± SD | 35.1 ± 9.2 | 35.3 ± 9.2 | 0.929 |
| Gender, male/female, n | 42/20 | 44/26 | 0.557 |
| Weight, kg, mean ± SD | 72.03 ± 13.96 | 75.09 ± 15.15 | 0.233 |
| Height, cm, mean ± SD | 171.8 ± 8.7 | 169.9 ± 8.3 | 0.227 |
| Body mass index, kg/m2, mean ± SD | 24.30 ± 3.53 | 25.83 ± 3.93 | 0.050 |
| Extremity dominancy, right/left, n | 54/8 | 66/4 | 0.152 |
| Existence of knee pain, yes/no, n | 20/42 | 14/56 | 0.108 |
The comparisons of the knee cartilage thickness evaluations of groups are given in Table 2 . Medial femoral condyles, ICAs, and LFCs of both right and left knees had thinner cartilage thickness in ulcerative colitis group than control group (p<0,001).
Table 2.
Comparison of Knee Cartilage Thickness Evaluations (mm) in Patients with Ulcerative Colitis and Control Groups.
| Femoral Cartilage Thickness | Ulcerative Colitis (n = 62), Mean ± SD | Control Group (n = 70), Mean ± SD | P |
|---|---|---|---|
| Right knee | |||
| Medial femoral condyle | 1.91 ± 0.26 | 2.19 ± 0.21 | <0.001 |
| Intercondylar area | 1.94 ± 0.27 | 2.14 ± 0.23 | <0.001 |
| Lateral femoral condyle | 1.90 ± 0.21 | 2.17 ± 0.19 | <0.001 |
| Left knee | |||
| Medial femoral condyle | 1.90 ± 0.28 | 2.18 ± 0.24 | <0.001 |
| Intercondylar area | 1.95 ± 0.27 | 2.15 ± 0.22 | <0.001 |
| Lateral femoral condyle | 1.92 ± 0.20 | 2.15 ± 0.19 | <0.001 |
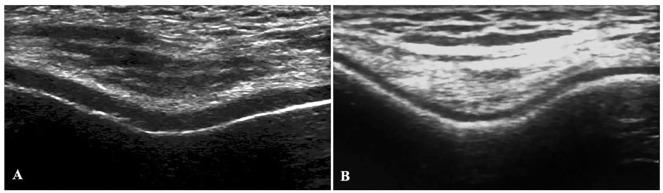
A representative image of US from control and ulcerative colitis patients for knee cartilage is presented in Fig. 2 .
Figure 2.
A representative ultrasonographic image from control and ulcerative colitis patients: (A) control group, knee cartilage is regular and smooth; (B) ulcerative colitis group, knee cartilage is thinner and more irregular than the control group.
Signs of joint inflammation were also compared between the ulcerative colitis and control groups. Both joint effusion (n = 12 of 62, 19% in ulcerative colitis group and n = 8 of 70, 11% in control group) and synovial proliferation (n = 26 of 62, 42% in ulcerative colitis group and n = 26 of 70, 37% in control group) were more common in ulcerative colitis patients, but there were no significantly difference between groups (P = 0.69, P = 0.37).
Discussion
The aim of the present study was to evaluate the effects of ulcerative colitis on knee cartilage thickness. Knee cartilage thickness was evaluated with US, which has previously been shown to be practical in the determination of cartilage degeneration and knee OA.7,8 US also provides a quantitative assessment of joint cartilage thickness and indicates loss in the sharpness of the cartilage margin, which is practical in early determination of knee OA.
The conclusions of the present work are supported by data of a single technique—US. Therefore, we believe it is important to state its advantages over limitations. US has limitations such as the lack of a standardized measuring method and validated scoring system, operator dependent imaging and reliability, establishment of adequate acquisition parameters. On the other hand, US has major advantages over the other modalities in that it is a highly accurate, reliable, reproducible, fast, economic, and patient-friendly method.9 -11 Also US has lower demands for processing, lack of radiological exposure, and provides valuable practical information about the current status of the knee joint. 9 Maeguchi et al. 9 noticed that US grading of the articular cartilage was sufficiently reliable to assess degeneration that the actual thickness of the cartilage was strongly correlated with the US. Cao et al. 10 compared US with magnetic resonance imaging (MRI) and arthroscopy (gold standard) and reported similar diagnostic performance compared to routine MRI for knee cartilage defects. The authors concluded that US was more accessible, easier to perform, and less expensive than MRI. 10 Steppacher et al. 11 investigated the accuracy, reproducibility, and reliability of ultrasonographic cartilage thickness measurement using contrast-enhanced micro-computed tomography for validation. The authors indicated US as a highly accurate, reliable, reproducible, fast, and patient-friendly modality that can detect early joint degeneration and facilitate decision making in joint preserving surgery.
Joint and musculoskeletal involvements have well-known associations with IBD. 12 EIM of IBD is defined as an inflammatory entity that is triggered by the inflammation in the intestine but is observed outside of the gut. 13 Karmiris et al. 14 reported that the patients with Crohn’s disease had a higher prevalence of EIMs than patients with ulcerative colitis. They also demonstrated the association between EIM and an aggressive course of Crohn’s disease. However, in their study, peripheral arthritis which was the most common objectively captured EIM (16.7% of patients) had a prevalence similar in Crohn’s disease and ulcerative colitis. Also Hiller et al. indicated that 34.6% out of 1438 ulcerative colitis patients had arthritis/arthralgia. 15 They stated that it was substantial to distinguish the predisposing factors in the condition of EIM in IBD patients to establish relevant and timely management of both the primary disorder and EIM. One interesting finding of the current study was that both joint effusion and synovial proliferation—as signs of joint inflammation—were more common in ulcerative colitis patients, but there were no significantly difference between groups (P = 0.69, P = 0.37). We believe that these findings may be related to that all the ulcerative colitis patients had mild activity and the group was relatively small.
Nevertheless, pathogenesis of musculoskeletal manifestations in IBD is very complex and unclear. 16 For example, environmental factors may influence development and outcomes of inflammatory bowel disease. 17 Yet, immunological mechanisms and independent inflammatory conditions with similar genetic or environmental risk factors with IBD plays the major role. 3 Upregulation of inflammatory adhesion molecules and chemokines allows capture of effector cells and facilitates their recruitment into extraintestinal areas. Also, T cells from the intestinal mucosa of IBD patients express chemokine receptors that provide entrance to other areas. Low-grade inflammatory process in the extraintestinal areas ensures the uptake of effector cells produced in the gut and moreover intensifies the inflammation. In addition to that, circulating antibodies, immune complex mediated inflammation, increased levels of interleukin-1-β (IL-1-β) and TNF-α has been proposed to contribute to EIM. 3
On the other hand, cartilage degeneration and osteoarthritis are also believed to have immunologic mechanisms besides others. The key inducers of catabolic processes in OA are IL-1 and TNF-α. 18 These inflammatory cytokines are associated with disease progression in both ulcerative colitis and OA. Both IL-1 and TNF-α trigger the synthesis of chondrocyte for degrading enzymes in cartilage, which are essentially ADAMTS metalloproteinases (MMP-1, MMP-3, MMP-13).
Patients with ulcerative colitis frequently suffer from skeletal abnormality, such as reduced bone mineral density, increased fracture risk, and/or joint inflammation. This pathological process is characterized by altered immune cell activity and elevated inflammatory cytokines in the bone marrow microenvironment due to disrupted gut immune response. Ulcerative colitis is recognized as an immune malfunction driven by multiple factors, including cytokines and signaling molecules. However, the mechanism by which intestinal inflammation magnified by gut-residing actors stimulates skeletal abnormality remains to be elucidated. On the other hand, osteoarthritis is a complex, chronic disease that affects primarily the weight-bearing joints of humans and other mammals. 19 During progression of the disease, all compartments of the joints undergo structural, functional, and metabolic changes that involve cellular elements as well as components of the extracellular matrix. The current evidence clearly suggests a link between increased levels of chemokines, cytokines and IBD pathogenesis. Several studies have confirmed the link between joint and gut inflammation. 19 It seems likely that both bacterial antigens and reactive T-cell clones, activated into the gut home the joint. However, the exact immunological mechanisms linking gut and joint inflammation are not fully understood. Various environmental (gut bacteria-dysbiosis) and host factors (migration of activated gut-T cells and macrophages) leading to initiation of inflammation in genetically predisposed individuals may act as triggers of inflammatory responses against gut and joints components. Additionally, it has been shown that macrophages from the gut of IBD patients are able to adhere in endothelial cells of synovial tissue, further enhancing the activation of T cells locally.
Subchondral bone and the synovium play an important role in the initiation and progression of OA.4,18 The subchondral bone in OA undergoes an uncoupled remodeling process, which is notably characterized by macrophage infiltration and osteoclast formation. Metabolic and mechanical factors can lead to synovitis in OA. Synovial tissue is highly vascularized and thus exposed to systemic influences such as low-grade inflammation. Synovitis can induce cartilage damage by fibrin deposition, pannus formation, or via inflammatory cytokine expression and triggers osteopenia, notably in early OA stages. Thus, besides mechanical impairment, underlying systemic factors trigger bone remodeling and might be taken into consideration in the treatment of OA.
The effectiveness of biological agents in EIM treatment may contribute to reveal the obscure inflammatory pathways. Anti-TNF-α drugs have high treatment success for arthritis, cutaneous and ocular EIM. This brought speculation about TNF-α-related mechanisms in EIM pathogenesis. 3 Peyrin-Biroulet et al. 20 stated that infliximab and adalimumab had good response rates for the treatment of skin, ocular, and musculoskeletal EIM and some favorable outcomes on hematological or vascular EIM and osteoporosis. Batmaz et al. 21 evaluated ankylosing spondylitis patients for femoral cartilage thickness and determined that anti-TNF users had thicker femoral cartilage measurements then anti-TNF naive patients. Indeed, we excluded anti-TNF/immunosuppressive users because of these relationships, which we speculate that anti-TNF treatments may also be beneficial for cartilage degeneration and osteoarthritis. Vavricka et al. 22 observed 71.8% of IBD patients had a clinical response for the underlying EIM to the anti-TNF therapy. Authors stated that anti-TNF might be an effective option in the management of EIM. Ulcerative colitis patients in our study had been mostly using oral and rectal mesalazine. Mesalazine is the first-line treatment in ulcerative colitis. Mesalazine is a drug with low potential side effects, but it must be noted that in a study by Sehgal et al., 23 arthralgia had been seen 0.5% to 7% as a side effect of mesalazine. They had also recorded that the side effects of mesalazine is not dose dependent.
Identification and management of EIMs are an important challenge in IBD practice. We consider it is unclear that whether our current findings are directly related to intestinal inflammation or these 2 entities share a genetic sensitivity with an impaired immune response to external stimuli. 1 Prospective studies with answers to these questions may introduce more effective treatment options.
Several anti-cytokines licensed for use in ulcerative colitis have also been studied in OA. Such cytokines may implicate innate immunity, including macrophages in OA development. 24 Multidirectional research into the mechanisms of IBD and OA has been rapidly growing in the recent years. 25 New inflammatory pathways and possible mechanisms of action have been disclosed, potentially leading to new-targeted therapy. However, the great disease heterogeneity and the overwhelming contribution of environmental risk factors has not modified yet the disease management. Despite a large amount of data regarding the pathogenesis, the translational results are still poor and there is a significant gap in understanding the direct consequence of the identified pathways. However, the discovered mechanisms explain only a small portion of disease variance. The possibility for the future of a better prediction of disease course, response to therapy and therapy-related adverse events may allow a more efficient and personalized strategy.
The relatively small sample size was the first limitation of this study. In addition, the current study was planned as a cross-sectional study that has its own limitation, which is the lack of causality assessment between factors. Also, it would have been better if we had the data of disease durations of patients with ulcerative colitis for investigating the correlations between femoral cartilage thickness and disease durations. Nevertheless, we think our results are significant to imply the relationship between ulcerative colitis and femoral cartilage degeneration.
In conclusion, the current study showed that knee cartilage is thinner in subjects with mild activity ulcerative colitis then in healthy controls. This association between ulcerative colitis and cartilage degeneration may be useful to identify targeted prevention and potential treatment strategies for ulcerative colitis and specific knee OA subtypes. Further studies are needed to evaluate the mechanisms and causality assessment between these entities.
Footnotes
Authors’ Note: The datasets generated and/or analyzed during the current study are not publicly available due ethical statement, but a limited and fully anonymized dataset containing the individual patient data that support the main analyses is available from the corresponding author on reasonable request.
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: We are confirming that the protocol for the research project has been approved by Ethics Committee of Health Science University, Istanbul within which the work was undertaken and that it conforms to the provisions of the Declaration of Helsinki (as revised in Brazil 2013).
Informed Consent: All participants provided written informed consent.
Trial Registration: Not applicable.
ORCID iD: Tolga Duzenli  https://orcid.org/0000-0002-6279-1018
https://orcid.org/0000-0002-6279-1018
References
- 1. Greuter T, Vavricka SR. Extraintestinal manifestations in inflammatory bowel disease—epidemiology, genetics, and pathogenesis. Expert Rev Gastroenterol Hepatol. 2019;13(4):307-17. [DOI] [PubMed] [Google Scholar]
- 2. Juillerat P, Manz M, Sauter B, Zeitz J, Vavricka SR; Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology. Therapies in inflammatory bowel disease patients with extraintestinal manifestations. Digestion. 2020;101(Suppl 1):83-97. [DOI] [PubMed] [Google Scholar]
- 3. Hedin CRH, Vavricka SR, Stagg AJ, Schoepfer A, Raine T, Puig L, et al. The pathogenesis of extraintestinal manifestations: implications for IBD research, diagnosis, and therapy. J Crohns Colitis. 2019;13(5):541-54. [DOI] [PubMed] [Google Scholar]
- 4. Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patil P, Dasgupta B. Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther Adv Musculo-skelet Dis. 2012;4(5):341-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Möller I, Bong D, Naredo E, Filippucci E, Carrasco I, Moragues C, et al. Ultrasound in the study and monitoring of osteoarthritis. Osteoarthritis Cartilage. 2008;16(Suppl 3):S4-7. [DOI] [PubMed] [Google Scholar]
- 7. Lee CL, Huang MH, Chai CY, Chen CH, Su JY, Tien YC. The validity of in vivo ultrasonographic grading of osteoarthritic femoral condylar cartilage: a comparison with in vitro ultrasonographic and histologic gradings. Osteoarthritis Cartilage. 2008;16(3):352-8. [DOI] [PubMed] [Google Scholar]
- 8. Yoon CH, Kim HS, Ju JH, Jee WH, Park SH, Kim HY. Validity of the sonographic longitudinal sagittal image for assessment of the cartilage thickness in the knee osteoarthritis. Clin Rheumatol. 2008;27(12):1507-16. [DOI] [PubMed] [Google Scholar]
- 9. Maeguchi K, Ito H, Morita Y, Furu M, Fujii T, Azukizawa M, et al. How precisely does ultrasonographic evaluation reflect the histological status of the articular cartilage of the knee joint? J Orthop. 2018;15(2):636-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao J, Zheng B, Meng X, Lv Y, Lu H, Wang K, et al. A novel ultrasound scanning approach for evaluating femoral cartilage defects of the knee: comparison with routine magnetic resonance imaging. J Orthop Surg Res. 2018;13(1_suppl):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steppacher SD, Hanke MS, Zurmühle CA, Haefeli PC, Klenke FM, Tannast M. Ultrasonic cartilage thickness measurement is accurate, reproducible, and reliable-validation study using contrast-enhanced micro-CT. J Orthop Surg Res. 2019;14(1_suppl):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kelly OB, Li N, Smith M, Chan J, Inman RD, Silverberg MS. The prevalence and clinical associations of subclinical sacroiliitis in inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(6):1066-71. [DOI] [PubMed] [Google Scholar]
- 13. Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(8):1982-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karmiris K, Avgerinos A, Tavernaraki A, Zeglinas C, Karatzas P, Koukouratos T, et al. Prevalence and characteristics of extra-intestinal manifestations in a large cohort of Greek patients with inflammatory bowel disease. J Crohns Colitis. 2016;10(4):429-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hiller A, Biedermann L, Fournier N, Butter M, Vavricka SR, Ciurea A, et al. The appearance of joint manifestations in the Swiss inflammatory bowel disease cohort. PLoS One. 2019;14(4):e0211554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chams S, Badran R, Sayegh SE, Chams N, Shams A, Hussein IH. Inflammatory bowel disease: looking beyond the tract. Int J Immunopathol Pharmacol. 2019;33:2058738419866567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vedamurthy A, Ananthakrishnan AN. Influence of environmental factors in the development and outcomes of inflam-matory bowel disease. Gastroenterol Hepatol (N Y). 2019;15(2):72-82. [PMC free article] [PubMed] [Google Scholar]
- 18. Lambova SN, Müller-Ladner U. Osteoarthritis—current insights in pathogenesis, diagnosis and treatment. Curr Rheumatol Rev. 2018;14(2):91-7. [DOI] [PubMed] [Google Scholar]
- 19. Francisco V, Pérez T, Pino J, López V, Franco E, Alonso A, et al. Biomechanics, obesity, and osteoarthritis. The role of adipokines: when the levee breaks. J Orthop Res. 2018;36(2):594-604. [DOI] [PubMed] [Google Scholar]
- 20. Peyrin-Biroulet L, Van Assche G, Gomez-Ulloa D, García-Álvarez L, Lara N, Black CM, et al. Systematic review of tumor necrosis factor antagonists in extraintestinal manifestations in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2017;15(1_suppl):25-36.e27. [DOI] [PubMed] [Google Scholar]
- 21. Batmaz I, Kara M, Tiftik T, Çapkin E, Karkucak M, Serdar ÖF, et al. Ultrasonographic evaluation of femoral cartilage thickness in patients with psoriatic arthritis. J Back Musculo-skelet Rehabil. 2016;29(4):703-8. [DOI] [PubMed] [Google Scholar]
- 22. Vavricka SR, Gubler M, Gantenbein C, Spoerri M, Froehlich F, Seibold F, et al. Anti-TNF treatment for extraintestinal manifestations of inflammatory bowel disease in the Swiss IBD Cohort Study. Inflamm Bowel Dis. 2017;23(7):1174-81. [DOI] [PubMed] [Google Scholar]
- 23. Sehgal P, Colombel JF, Aboubakr A, Narula N. Systematic review: safety of mesalazine in ulcerative colitis. Aliment Pharmacol Ther. 2018;47(12):1597-609. [DOI] [PubMed] [Google Scholar]
- 24. Hügle T, Geurts J. What drives osteoarthritis? Synovial versus subchondral bone pathology. Rheumatology (Oxford). 2017;56(9):1461-71. [DOI] [PubMed] [Google Scholar]
- 25. Watt FE, Gulati M. New drug treatments for osteoarthritis: what is on the horizon? Eur Med J Rheumatol. 2017;2(1_suppl):50-8. [PMC free article] [PubMed] [Google Scholar]