Graphical abstract

Keywords: Loeffler endocarditis, Eosinophilic myocarditis, Hypereosinophilia
Highlights
-
•
Loeffler endocarditis is a rare eosinophilic myocardial fibrosing disease.
-
•
Classic TTE findings include apical obliteration with diffuse endocardial thickening.
-
•
Anticoagulation is reasonable if there is evidence of thromboembolism.
Introduction
Eosinophilic myocarditis (EM) represents a rare spectrum of heart disease that remains mysterious as to etiology and pathogenesis. Its presentation is quite variable and includes a fibrotic stage known as Loeffler endocarditis. We describe a case of EM in a patient with paraneoplastic eosinophilia in the setting of metastatic renal cell carcinoma (RCC).
Case Presentation
A 65-year-old man with a history of hypertension and tobacco use disorder was initially admitted for right parietal stroke in August 2018. He underwent right carotid endarterectomy, was started on clopidogrel, and was discharged the following day. His laboratory studies on admission were notable for a white-cell count of 15.5 × 109/L and an absolute eosinophil count of 2.17 × 109/L (14%).
As an outpatient, he was later found to have progressive leukocytosis of 27.5 × 109/L, with absolute cell differential as follows: neutrophils 14.4 × 109/L, lymphocytes 1.4 × 109/L, monocytes 1.4 × 109/L, eosinophils 10.1 × 109/L, and basophils 0.2 × 109/L. Given his risk factors and concern for malignancy, he was evaluated by an oncologist in February 2019. Bone marrow biopsy showed mildly hypercellular marrow, but results of flow cytometry and genetic analysis including fluorescence in situ hybridization were negative. Contrast-enhanced computed tomography of the chest, abdomen, and pelvis showed an 8.1-cm mass suspicious for right RCC without evidence of metastatic disease (Figure 1). Additionally, the right ventricular (RV) apex was noted to have an abnormal appearance concerning for possible chronic infarct with pseudoaneurysm (Figure 2).
Figure 1.

Right renal mass measuring approximately 8.1 cm concerning for RCC (arrow).
Figure 2.

A small amount of contrast accumulating in the apical right ventricle (arrow), suspicious for chronic infarct with small pseudoaneurysm.
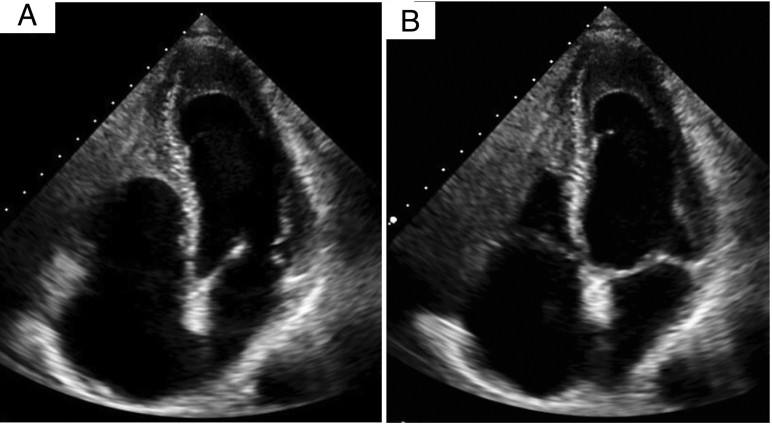
In preparation for right nephrectomy, the patient underwent preoperative cardiovascular evaluation in April 2019. Transthoracic echocardiographic (TTE) imaging revealed a hypoplastic right ventricle suggestive of Ebstein's anomaly versus RV apical infarction (Figure 3, Video 1). Nuclear myocardial perfusion imaging (regadenoson) showed a small degree of apical and anterolateral ischemia. He underwent left heart catheterization, and an 80% left anterior descending coronary artery stenosis was treated with a drug-eluting stent along with balloon angioplasty of a diagonal branch.
Figure 3.
First TTE study in April 2019 with mildly reduced ejection fraction (45%–50%) in systole (A) and diastole (B). This study described a hypoplastic right ventricle suggestive of Ebstein's anomaly vs prior RV apical infarction. See Video 1.
Between the time of the heart catheterization and nephrectomy in May 2019, the patient's white-cell count ranged from 38 × 109/L to 45 × 109/L, with an absolute eosinophil count of 17.4 × 109/L to 18.6 × 109/L. Pathology from the nephrectomy was consistent with clear cell RCC. Postoperatively, absolute eosinophil counts significantly decreased to a range of 0.5 × 109/L to 2.0 × 109/L.
Because of worsening volume overload postoperatively, a limited TTE study was performed and showed a moderately reduced left ventricular ejection fraction of 30% and left ventricular apical thrombus versus apical hypertrophy versus Loeffler endocarditis (Figure 4, Video 2). Transesophageal echocardiography the following day showed biventricular apical echodensities suggestive of Loeffler endocarditis, with possible thrombus in the right ventricle (Figure 5, Video 3). Warfarin was added to the patient's medical regimen.
Figure 4.
Limited TTE examination with ejection fraction of 30% to 35%, apical clot vs apical hypertrophy vs Loeffler endocarditis, normal to small RV size with decreased function, moderate right atrial enlargement, and mild tricuspid regurgitation. See Video 2.
Figure 5.
Midesophageal aortic valve long-axis (left) and four-chamber (right) views on transesophageal echocardiography showing ejection fraction of 30% and biventricular apical echodensities suggestive of Loeffler endocarditis. See Video 3.
In July 2019, surveillance imaging revealed metastatic disease in the abdomen and pelvis. He presented later that month with altered mental status and was found to have metastatic brain lesions. Cardiology was consulted to assess the risks and benefits of holding warfarin and clopidogrel given his risk for intracranial hemorrhage. Review of prior imaging and clinical course led the cardiology consult team to strongly suspect Loeffler endocarditis/EM rather than overt apical thrombi. Repeat TTE imaging again confirmed the biventricular apical echodensities, most consistent with EM (Figure 6, Video 4). Discontinuation of warfarin and clopidogrel was recommended. Soon after initiating stereotactic radiosurgery for management of the brain metastases, the patient was again hospitalized for altered mental status in August 2019. He was transitioned to comfort care and died later that month.
Figure 6.
Contrast-enhanced TTE examination in July 2019 shows endocardial thickening of the left ventricle (arrow A) as well as obliteration of the RV apex (arrow B), confirming Loeffler endocarditis. See Video 4.
Discussion
EM (also known as eosinophilic heart disease) can present as an acute or chronic condition and is expansive in its presentation, ranging from nearly asymptomatic to severe heart failure secondary to acute fulminant myocarditis.1 It is a rare cause of myocardial injury, but the true incidence and prevalence of the disease remain unclear. A retrospective study of EM in 2017 found that a total of 264 cases had been published.2 Among 112 biopsy-confirmed cases of myocarditis, it was the cause in 6% in a review conducted in 2006.3 It has been observed in 0.5% of unselected autopsy series of patients with heart failure and in 0.1% of cases among a cohort of patients biopsied for suspected myocarditis.4 The prevalence of EM in patients undergoing heart transplantation has been reported to be 3% to 7%.5
In about half of cases, EM is associated with an exposure or another factor believed to play some role in the pathogenesis. Among these include various drugs that may induce a hypersensitivity reaction (e.g., antibiotics, anticonvulsants, antipsychotics, diuretics), parasitic infections (e.g., Strongyloides, Echinococcus, Schistosoma, Toxocara, Trichinella), immune-mediated disorders (e.g., eosinophilic granulomatosis with polyangiitis), vaccinations (e.g., diphtheria, tetanus toxoid, smallpox, polio), and malignancy.2,6,7 When eosinophilia becomes significant enough to cause organ dysfunction, it is typically called hypereosinophilic syndrome (HES), which is characterized by eosinophil counts > 1,500/μL (1.5 × 109/L). It may arise secondary to an underlying disorder (reactive HES) or as a primary myeloproliferative neoplasm, or it may be idiopathic.4 Affected organs may include the skin, lungs, heart, gastrointestinal tract, and brain. Cardiac involvement in HES is estimated to occur in up to 60% of cases.7
Classically, EM is described as having three distinct pathologic stages: necrotic (acute), thrombotic (intermediate), and fibrotic (chronic).2 However, the disease usually manifests as an overlap of these stages without a clear sequential order. Its presentation as an acute fulminant myocarditis is often referred to as acute necrotizing EM, whereas its manifestation as a chronic restrictive cardiomyopathy is called Loeffler endocarditis. The term endomyocardial fibrosis is very similar but is generally reserved for fibrotic eosinophilic heart disease afflicting indigent populations in tropical climates, and peripheral eosinophilia is often absent.8 Loeffler endocarditis was named after the Swiss physician William Löffler, who first described eosinophil-induced cardiac damage in 1936. At that time, it was referred to as endocarditis parietalis fibroplastica and required only two criteria for diagnosis: the presence of fibrosing mural endocarditis and blood eosinophilia.9
Although a definitive diagnosis requires endomyocardial biopsy demonstrating tissue infiltration by eosinophils, there are imaging clues that can help diagnose EM, particularly its fibrotic forms (i.e., Loeffler endocarditis and endomyocardial fibrosis). Obliteration of the apex (left ventricle, right ventricle, or both) by thrombus and/or fibrosis is suggestive of the chronic stages of EM and can be detected on echocardiography.9,10 Eosinophilic infiltration has also been known to involve the valves and chordae tendineae.1 Cardiac magnetic resonance imaging is the most useful noninvasive test and may be used to localize sites for biopsy.2 The pattern of diffuse, nearly circumferential subendocardial delayed enhancement, more prominent at the midventricular to apical levels, is associated with eosinophilia-related subendocardial injury or fibrosis.11
Initial management of EM should be focused on providing resuscitative therapy as needed to prevent and manage the hemodynamic collapse the disease has the potential to induce. Targeted therapies should be considered when an exposure and/or disorder with a known association with EM is present. For example, antihelminthic agents (e.g., albendazole) may be used if related to a parasitic infection. Tyrosine kinase inhibitors (e.g., imatinib) have been used in the treatment of idiopathic HES. Mepolizumab is a monoclonal antibody against interleukin-5 that has been used in management of eosinophilic granulomatosis with polyangiitis and idiopathic HES.2 Immunosuppressive agents such as corticosteroids, azathioprine, and cyclophosphamide are sometimes administered to help blunt the cytotoxic effects of eosinophils as well.
Anticoagulation is indicated in patients with evidence of thromboembolism. However, the decision to anticoagulate is often challenging because of its limited evidence in Loeffler endocarditis. Additionally, as seen in our case, imaging results can be equivocal, and the patient's risk for bleeding must be taken into account. In cases of Loeffler endocarditis found to have ventricular thrombi, warfarin use has been associated with favorable outcomes in left ventricular remodeling.12,13 In one case caused by idiopathic HES, there was resolution of apical thickening on 3-month follow-up echocardiography after the patient was placed on imatinib and warfarin.14 When anticoagulation is pursued, the balance between its risks and benefits must be continuously reassessed. Close monitoring at approximately 3- to 6-month intervals has been recommended by some with the duration of anticoagulation determined by the activity of the patient's endomyocardial disease.1 If controlled and if echocardiography reveals the absence of thrombus or resolution of thrombus, then it is considered reasonable to discontinue anticoagulation.
This case provides an example of the clinical and echocardiographic features of EM and more specifically Loeffler endocarditis. Although the apical obliteration seen on echocardiography was initially suspicious for a thrombus, further assessment and studies were more supportive of fibrosis of the apex. There was no valvular involvement observed. The eosinophilia is believed to have been a paraneoplastic syndrome caused by the patient's RCC, which has been previously described.15 This was corroborated by the improvement in the absolute eosinophil count levels after nephrectomy was performed. However, to the best of our knowledge, this is the first report of Loeffler endocarditis in a patient with RCC. Because of the progression of the malignancy, the patient unfortunately succumbed to metastatic disease just a few months after surgery.
Conclusion
Our patient was found to have profound peripheral eosinophilia and was subsequently diagnosed with metastatic RCC. The best explanation for the eosinophilia appears to be a paraneoplastic manifestation of the RCC, which has been known to occur. He also had a fairly rare presentation of EM that was misinterpreted in several different ways on imaging (hypoplastic right ventricle and Ebstein's anomaly, RV infarct, and RV thrombus). Careful review of the patient's overall clinical course, however, revealed that his cardiac imaging findings and heart failure were likely due to EM/Loeffler endocarditis. Although rare, EM should be considered in the differential diagnosis for cardiomyopathy of unclear etiology, especially in the setting of eosinophilia and the echocardiographic findings described in this case.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2020.05.016.
Supplementary Data
Apical four-chamber view demonstrates an abnormal RV apex.
Repeat apical four-chamber view demonstrates RV dysfunction, a dilated right atrium, and mild tricuspid regurgitation.
Midesophageal four-chamber view demonstrates apical lesions and a reduced ejection fraction.
Apical four-chamber view demonstrates RV apical obliteration and left ventricular endocardial thickening.
References
- 1.Hernandez C.M., Arisha M.J., Ahmad A., Oates E., Nanda N.C., Nanda A. Usefulness of three-dimensional echocardiography in the assessment of valvular involvement in Loeffler endocarditis. Echocardiography. 2017;34:1050–1056. doi: 10.1111/echo.13575. [DOI] [PubMed] [Google Scholar]
- 2.Brambatti M., Matassini V.M., Adler E.D., Klingel K., Camici P.G., Ammirati E. Eosinophilic myocarditis: characteristics, treatment, and outcomes. J Am Coll Cardiol. 2017;70:2363–2375. doi: 10.1016/j.jacc.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Magnani J.W., Danik H.J., Dec J.W., Jr., DiSalvo T.G. Survival in biopsy-proven myocarditis: a long-term retrospective analysis of the histopathologic, clinical, and hemodynamic predictors. Am Heart J. 2006;151:463–470. doi: 10.1016/j.ahj.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 4.Al Ali A.M., Straatman L.P., Allard M.F., Ignaszewski A.P. Eosinophilic myocarditis: case series and review of literature. Can J Cardiol. 2006;22:1233–1237. doi: 10.1016/s0828-282x(06)70965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshizawa S., Sugiyama Kato T., Mancini D., Marboe C. Characteristics of patients with advanced heart failure having eosinophilic infiltration of the myocardium in the recent era. Int Heart J. 2013;54:146–148. doi: 10.1536/ihj.54.146. [DOI] [PubMed] [Google Scholar]
- 6.Nutman T.B. Evaluation and differential diagnosis of marked, persistent eosinophilia. Immunol Allergy Clin North Am. 2007;27:529–549. doi: 10.1016/j.iac.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto H., Hashimoto T., Ohta-Ogo K., Ishibashi-Ueda H., Imanaka-Yoshida K., Hiroe M. A case of biopsy-proven eosinophilic myocarditis related to tetanus toxoid immunization. Cardiovasc Pathol. 2018;37:54–57. doi: 10.1016/j.carpath.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Weller P.F., Bubley G.J. The idiopathic hypereosinophilic syndrome. Blood. 1994;83:2759–2779. [PubMed] [Google Scholar]
- 9.Jennings R.C., Pengelly C.D. Endocarditis parietalis fibroplastica (Löffler's disease) Postgrad Med J. 1968;44:251–254. doi: 10.1136/pgmj.44.509.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginsberg F., Parrillo J.E. Eosinophilic myocarditis. Heart Failure Clin. 2005;1:419–429. doi: 10.1016/j.hfc.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Ommen S., Seward J., Tajik A. Clinical and echocardiographic features of hypereosinophilic syndromes. Am J Cardiol. 2000;86:110–113. doi: 10.1016/s0002-9149(00)00841-9. [DOI] [PubMed] [Google Scholar]
- 12.Felker G.M., Boehmer J.P., Hruban R.H., Hutchins G.M., Kasper E.K., Baughman K.L. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36:227–232. doi: 10.1016/s0735-1097(00)00690-2. [DOI] [PubMed] [Google Scholar]
- 13.Lofiego C., Ferlito M., Rocchi G., Biagini E., Perugini E., Branzi A. Ventricular remodeling in Loeffler endocarditis: implications for therapeutic decision making. Eur J Heart Fail. 2005;7:1023–1026. doi: 10.1016/j.ejheart.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Lee S., Choi C.U., Kim E.J., Na J.O. Regression of biventricular Loeffler's endocarditis after early treatment. Eur Heart J Cardiovasc Imaging. 2017;18:610. doi: 10.1093/ehjci/jex017. [DOI] [PubMed] [Google Scholar]
- 15.Todenhöfer T., Wirths S., von Weyhern C.H., Heckl S., Horger M., Hennenlotter J. Severe paraneoplastic hypereosinophilia in metastatic renal cell carcinoma. BMC Urol. 2012;12:7. doi: 10.1186/1471-2490-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Apical four-chamber view demonstrates an abnormal RV apex.
Repeat apical four-chamber view demonstrates RV dysfunction, a dilated right atrium, and mild tricuspid regurgitation.
Midesophageal four-chamber view demonstrates apical lesions and a reduced ejection fraction.
Apical four-chamber view demonstrates RV apical obliteration and left ventricular endocardial thickening.