Abstract
This review of research on behavioral discrimination of nicotine and how it informs public health policy for reducing risk of tobacco dependence is adapted from the author’s American Psychological Association Division 28 (Psychopharmacology and Substance Abuse) 2020 Med Associates Brady/Schuster Award lecture. The author’s initial programmatic clinical research on nicotine is introduced, especially efforts to develop and validate a novel method of acute nicotine dosing. After the public health rationale for characterizing the discriminative stimulus effects of nicotine in humans are described, details from two separate programs of research on nicotine discrimination in humans are presented. The first, conducted with nicotine dosing by nasal spray, documented that humans could discriminate nicotine administered rapidly, examined nicotine’s neuropharmacological specificity, identified discrimination threshold dose in smokers and nonsmokers, and explored other conditions that might alter ability to discriminate its effects. The second, more recent program focused on threshold doses for discrimination of nicotine by cigarette smoking, a program that was very difficult to do until the past decade, and how nicotine’s self-reported “reward” and preference via choice behavior relate to its discriminability. Differences due to menthol and degree of tobacco dependence were also examined. For each of these two programs, the main findings of selected studies are noted, followed by very recent work on nicotine discrimination and choice that informs FDA’s efforts to formulate public policy to improve health and reduce the nearly half million American deaths per year due to persistent tobacco use.
Keywords: nicotine, behavioral discrimination, smoking reward, choice, nicotine threshold
“To lower nicotine too much might end up destroying the nicotine habit in a large number of consumers and prevent it from ever being acquired by new smokers.”
– British American Tobacco Company (1959) internal document.
Introduction
As long feared (but publicly denied) by the tobacco industry (Henningfield et al. 2006; Wayne & Carpenter, 2009), greatly reducing nicotine intake from smoking could prevent onset of dependence in teens and foster cessation among adult smokers. In this way, the prevalence of dependent smoking would gradually decline to the point it no longer caused substantial adverse health effects in the general adult population (e.g. Apfelberg et al. 2018). This notion was formally introduced by Neal Benowitz and Jack Henningfield, first in 1994 and updated in 2013 (Benowitz & Henningfield, 1994; 2013), based partly on research with non-dependent smokers (Shiffman, 1989). The announcement of FDA’s proposed rule (FDA, 2018), to consider lowering the maximum nicotine content allowed in commercial cigarettes, made this proposal no longer just an idea, but now potential public health policy. Very recently, it was estimated that, had FDA implemented this policy in 1985, 16 million smoking-attributed deaths could have been prevented (Levy et al., in press).
Although a large and diverse body of research is needed by FDA to support any policy on setting a maximum nicotine level (Carter et al. 2009), testing of acute responses to nicotine dosing can help guide future clinical research. For example, directions for new clinical studies intended to inform a reduced nicotine standard could benefit from testing responses to low nicotine cigarettes that address Benowitz and Henningfield’s 2013 suggestion that “these doses would not produce psychoactive and rewarding effects.” As will be summarized here, these responses can be assessed by testing behavioral discrimination of smoked nicotine, and the “rewarding” effects of that acute nicotine intake and resulting preference for cigarettes varying in nicotine content. This paper will provide an overview of the author’s research on nicotine discrimination in humans, initially documenting and characterizing it and then addressing these other self-report and behavioral responses to smoked nicotine intake by humans. Two separate programs of research on behavioral discrimination of nicotine are summarized, first via nasal spray and the second with low and very low nicotine content research cigarettes, along with the subjective rewarding and choice effects of smoked nicotine. The paper also explains how these procedures may help preparation of larger clinical research to guide the establishment of a maximum allowable nicotine content in commercial cigarettes. Most of this text is based on the author’s lecture as part of receiving the APA Division 28 award in 2020 for career research contributions in the field of psychopharmacology and substance abuse, named the Med Associates Brady/Schuster Award, recognizing pioneers in this broad area of research, Drs. Joseph V. Brady and Charles R. Schuster. The recorded lecture was available online at the APA website as part of the August 2020 annual meeting.
Background on Nicotine Discrimination
Essentially by definition, psychoactive drugs of abuse primarily are reinforcing and dependence producing because of their interoceptive stimulus effects (i.e. perceived in the brain), causing subjective arousal or calmness, head rush, or other feelings associated with pleasure or relief (Porter et al., 2018). How to objectively determine whether those effects of a drug have been perceived in a research participant is difficult. Because non-humans were not able to verbalize effects of being administered different drug conditions, behavioral drug discrimination procedures were first developed in the 1960s using preclinical models (Porter et al., 2018). This work showed that behavioral responses contingent upon dosing with one drug condition or the other could objectively verify that the stimulus effects of a drug condition were perceived. In general, behavioral discrimination of psychoactive drugs in non-humans (as well as humans; see below) is based on the ability of individuals to perceive a change in their subjective (internal) state produced by the drug. That change, also labeled the drug’s interoceptive stimuli, allows the individual to discriminate between administered drug conditions, such as active drug from placebo (vehicle), one drug from a different drug, between different doses of the same drug (very relevant in the smoked nicotine section of this review), etc. Those interoceptive drug stimuli act as cues to direct behavior, and the pattern of that change in internal state can be used to train individuals to respond in specific ways to provide an objective means of documenting that state has been perceived (Shoaib & Perkins, 2020; Siegel, 1991).
After establishment of preclinical studies of behavioral discrimination due to a variety of drugs, research on drug discrimination in humans began for several reasons, including translational research intended to show that very well-controlled findings with non-humans generalized to humans, and to characterize that drug’s stimulus effects via self-report and additional behavioral responses. Other clinical uses of discrimination research were then developed, such as to evaluate new drugs for their abuse potential, if substitutable for known abused drugs, or identify possible CNS actions of new compounds suggesting they may have efficacy in treating substance abuse (e.g. Bolin et al. 2018; LeSage et al. 2009; Meert, 1991).
Persistent Public Health Problem of Nicotine Dependence
Before proceeding to outline our research on nicotine discrimination, it is worth stepping back briefly to summarize the continuing enormous toll taken by tobacco use on the health of Americans. In that way, the potential public health significance of clinical nicotine research may be better appreciated. A common manner of expressing the magnitude of the tobacco smoking problem is to report a single current value for the total prevalence of smoking in the U.S. adult population. A decline of two-thirds in that prevalence since the 1964 Surgeon General’s report on smoking (USDHEW 1964), from 42% to 14% today, appears to show steady, if slow, progress toward eliminating cigarette smoking in the U.S. (Drope et al., 2018). However, reliance on one prevalence value is misleading for a few reasons. First, the total number of American smokers has declined over the last half century only modestly, from about 50 million to now under 40 million, and they still consume 230 billion cigarettes per year (Proctor, 2020).
The second, and probably bigger, reason cigarette smoking is not on its way to disappearing as a public health calamity is the wide disparity in success of reducing the prevalence and consequences of smoking (Drope et al., 2018). At smoking’s peak prevalence among Americans in the 1950s, there were few differences in characteristics between those who did vs. did not smoke, since close to half of all adults smoked. Yet, the overall decline since then has been very selective, as smoking prevalence dropped sharply among the well-off, in terms of their education, income, lack of comorbid problems, etc., and is now well under 10% for those subgroups. By contrast, prevalence across the decades has remained persistently high and relatively flat among those with vulnerabilities that make living more difficult, especially those who also have severe mental illness, other substance abuse, lack of education or income, etc. For example, nearly a majority of adult smokers in the U.S. today have a current or history of mental illness, and almost all have at least one of the vulnerabilities linked to higher smoking prevalence (Drope et al., 2018; Prochaska et al., 2017).
Thus, because the total number of Americans who smoke has not decreased much, and most severe disease risks due to tobacco use often lag smoking onset by about 30 years (Lubin & Caporaso, 2006), the health impact of smoking in the U.S. has not declined at all. The death toll continued to climb after the 1964 Surgeon General’s report (USDHEW 1964) and is peaking only now (Thun et al. 2013; USDHHS 2014), remaining at nearly a half million per year in the U.S. over the last decade, far more than that from any other drug dependence problem. As horrendous a death toll as that is, total deaths worldwide are now over 8 million, with over 1 billion still smoking tobacco. These facts point to decades more of millions of preventable deaths per year globally and an “unacceptably” slow rate of decline in tobacco use (USDHHS 2014) without major innovations in prevention and treatment (Renteria et al. 2016). Aside from new effective interventions (Lerman et al., 2007), those innovations include significant changes in public health policies to regulate tobacco products (WHO 2015; Zeller 2019).
Origins of Our Clinical Research on Nicotine Discrimination
As with virtually any research, our work builds on that by others, particularly preclinical studies of nicotine discrimination, by John Rosecrans (Rosecrans, 1989) and Ian Stolerman (Stolerman et al. 1987), as well as clinical research on discrimination of other drugs, by George Bigelow (Bigelow & Preston, 1989) and Chris-Ellyn Johanson (1991), both of whom also happen to be prior recipients of this same APA Division 28 career research award. Given the focus of this review on nicotine discrimination in humans, their foundational contributions cannot be given proper recognition here, but some of their early work will occasionally be noted.
I first learned about the critical influence of contextual factors on responses to drug administration from a graduate psychology course in behavioral pharmacology, and about careful assessment of behavioral and psychophysiological responses from my PhD mentor Don Fowles at the Univ of Iowa (Perkins, 1984), as well as on clinical internship at the Univ of Mississippi Medical Center (Perkins, Dubbert, et al. 1986). As a result of that background, I entered the post doctoral program in Cardiovascular Behavioral Medicine at the Dept of Psychiatry of the Univ of Pittsburgh, with Leonard Epstein and Dick Jennings, aided by clinical pharmacologist Richard Stiller. When we started conducting research on acute effects of nicotine in 1984, before the work on nicotine discrimination, one key problem was how to administer doses of nicotine per se in tightly controlled fashion that would be delivered to the brain quickly, as with smoking. At that time, it had just become apparent that smoking commercial brands differing in nicotine “yield” did not allow manipulations of controlled doses for reasons explained in the second main research program below, on nicotine via smoking. For that reason, the primary goal of my postdoc training was to develop and test a nasal spray method of rapidly administering nicotine doses, and a saline placebo, all matched on exteroceptive stimulus effects. Similar to our later studies with smoking, detailed in the second part of this paper, we administered doses in 4 spray administrations, two to each nostril, with 30 sec between those pairs of sprays. We found clearly dose-dependent blood levels from a subset of participants in our first study to validate this method (Perkins, Epstein, et al., 1986) and comparable cardiovascular and subjective responses to similar nicotine dosing via spray vs. smoking (Perkins, Sexton, et al. 1994).
Once this procedure had been shown to administer nicotine doses in rapid controlled fashion, we then were able to use it in several lines of basic behavioral and clinical research into factors maintaining nicotine dependence. As hard as it is to believe in 2020, few indoor restrictions on smoking existed then and research on the abuse liability of nicotine was just becoming a higher priority for NIDA funding support (Henningfield 2011; Hughes & Liguori 2000); previously, nicotine research support had been largely relegated to other NIH institutes focused on the health consequences of chronic smoking (e.g. NHLBI, NCI). Thus, the priority before the mid-1980s was not on factors responsible for onset or persistence of nicotine dependence, but on the disease processes that dependence caused. This shift in NIDA priority was further accelerated by the 1988 Surgeon General’s report on Nicotine Addiction (USDHHS 1988). Our early projects assessing acute effects of rapid nicotine delivery to humans included: 1) Nicotine’s increase in energy expenditure under naturalistic daily activity level vs. rest, to help explain why smoking reduces body weight (Perkins, Epstein et al., 1989); 2) Early clinical testing of the efficacy of nicotine nasal spray use in aiding smoking cessation (Perkins, Grobe et al. , 1992); note that our nasal spray was similar to, but developed completely independently of, the Nicotrol spray approved by FDA to aid smoking cessation a decade after our initial studies (Hjalmarson et al. 1994); 3) Sex differences in how reinforcing and rewarding men and women find doses of nicotine per se, isolated from non-nicotine stimuli of smoking. (Perkins, 1996); and 4) Studies showing presence of chronic and acute tolerance to nicotine, and how chronic tolerance does not resolve even years after having quit smoking (Perkins, Gerlach, et al. 2001). Researchers studying acute effects of nicotine or other drugs within preclinical (e.g., Corrigall & Coen 1989) or clinical (e.g. Benowitz et al. 1983; Griffiths et al., 1982; Zacny & Stitzer, 1988) models also provided early inspiration and support for taking on these research topics.
Yet, the research program I chose to outline for this Award talk and paper was a different one, to assess behavioral discrimination of nicotine dosing in humans and factors affecting that discrimination responding. I initiated this program beginning in 1994 (Perkins, DiMarco, et al. 1994) after gaining an appreciation of the clinical discrimination research with other drugs, especially that by Bigelow (Bigelow & Preston 1989) and Johanson (1991), and realizing a virtual absence of clinical discrimination research with nicotine (mostly due to the aforementioned lack of controlled dosing of nicotine in rapid form).
Basic Procedures for Assessing Behavioral Discrimination of Nicotine
Basically, our nicotine discrimination testing procedure is simple, adapted from the preclinical and clinical research by others noted previously. The primary way in which we adapted those procedures was to combine the training and testing trials into a single session, versus the many sessions across multiple days typical of prior human discrimination testing with other drugs, such as oral stimulants or sedatives, which require much longer durations between trials due to the pharmacokinetics of those drugs administered by pills or capsules (Bolin et al., 2018). In our procedure with nicotine (Perkins, 2011), we first confirm overnight abstinence from smoking and then proceed to the experimental session.
First are “training” trials, where participants are administered two drug conditions (e.g. active vs placebo) in random order, only one of them per trial, to learn to perceive the difference in how each condition makes them “feel”. Each drug condition is identified by some neutral label (such as letter codes, “A” vs “B”), and participants are told to pay close attention to what they perceive in the minutes after administration so they can learn to tell the difference between the two conditions during subsequent trials, where they will be able to earn extra payment every time they are correct. Then, in the “testing” trials, they are administered those same drug conditions, again one or the other in random order across trials, but they are not told which is which. They are instructed to continue paying attention to what they perceive after each condition, complete self-report items (see section on discrimination of nicotine via smoking), and to then state 2-3 mins after dosing whether what they just got was “A” or “B”. They then continue to get these same two drug conditions one at a time to test whether they have acquired that ability to discriminate the two. Successful discrimination is correctly identifying which is which on at least 5 of the 6 trials (i.e. >80%, based on prior research; see Takada, 1996). All of our clinical studies described in this paper were approved by the University of Pittsburgh IRB.
Program of Behavioral Discrimination of Nicotine via Nasal Spray
Documenting nicotine discrimination in humans
Our first study in 1994 demonstrated that humans, just like non-humans (e.g. Rosecrans 1989; Stolerman 1987), can discriminate different doses of nicotine per se. Briefly, we first trained smokers to discriminate between 12 μg/kg nicotine (about 1.0 mg for 82 kg participant) vs. saline in session 1, and all were able to learn the discrimination. (Note that an additional subgroup was tested for ability to discriminate these spray doses within 10 secs, rather than 2 mins, after dosing and was unable to do so. This test was done to verify their discrimination responding was due to the spray’s interoceptive stimulus effects, unable to be perceived that quickly, and not to immediate exteroceptive stimuli or other non-CNS effects of nicotine spray administration, which proved to be the case.) Then, in session 2, we confirmed retention of that discrimination from session 1, and then administered intermediate nicotine spray doses (unidentified) in random order across trials to assess “how much like spray A or spray B” that intermediate spray was. Two measures were obtained in each trial, the first a quantitative task, instructing participants to gauge in continuous fashion how similar this new spray was to “spray A or spray B” from session 1. After dosing, they were given 10 plastic poker chips and asked to distribute those 10 into separate “bins” within a small box, with bins marked prominently with one of the two letter codes (“A”, “B”), according to how much the drug they just received was “like spray A” or “like spray B”; the number of chips placed in the bin marked with the letter code of active drug was the amount of drug-appropriate responding. This matched “drug-appropriate” lever responding in the animal studies. The second task was a quantal task, requiring them to identify the spray as just one or the other option in dichotomous fashion (as in session 1), by circling “A” or “B” on a form, even if the dose was not quite one or the other.
As shown in Figure 1, the proportion of nicotine-appropriate responses was strongly dose-dependent. (Although documenting cross-species consistency in drug effects can be challenging, behavioral drug discrimination has generally been quite consistent between non-human animals and humans, as also shown in the figure by the nicotine-appropriate lever pressing results from an early study by the Rosecrans group; Chance et al. 1977). Somewhat surprisingly, the ED50, or the dose at which 50% of participants identify it as “A” and 50% identify it as “B” in the quantal task, was fairly low at 3 μg/kg, one-fourth the training dose of 12 μg/kg, meaning they appeared to be sensitive to even low nicotine doses. The ED50 depends on the training conditions during the study but may be relevant to identifying a minimum dose for discriminating nicotine, or threshold dose, as discussed later.
Figure 1.
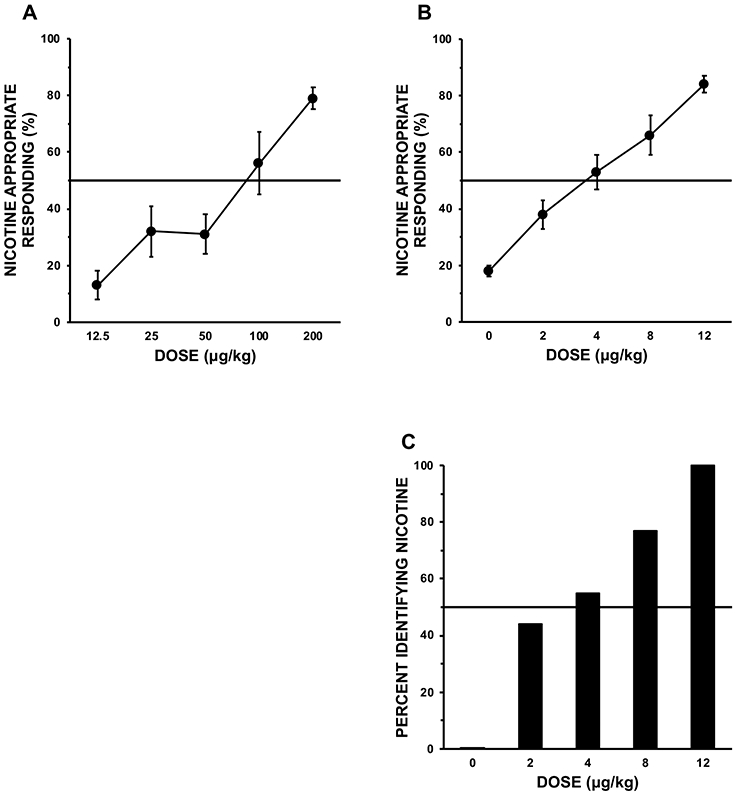
Nicotine-appropriate responding (as a percentage of all) due to intermittent nicotine doses during quantitative testing (top) in: rodents (n=8) trained to discriminate 0 vs 200 μg/kg s.c. (A), and human smokers (n=18) trained to discriminate 0 vs 12 μg/kg via nasal spray (B). Also shown is absolute proportion of the human smokers identifying the spray letter code consistent with nicotine in quantal testing (C). Horizontal lines show 50% of nicotine-appropriate responding, or 50% of participants. Adapted from part of Figure 2 in Chance, et al. (1977), and from Figure 1 in Perkins, DiMarco, et al. (1994). With kind permission from Springer Nature.
Central mediation and specificity of nicotine discrimination: Cross-species consistency
Drug discrimination research can be used to show selectivity in the neural sites of action of a drug. In an early study with rodents, Stolerman et al. (1984) found that pretreatment with mecamylamine, an antagonist at both central and peripheral nicotine receptors, blocks discrimination of nicotine, while hexamethonium, a peripheral antagonist only, does not. Their prior classic study with human smokers (Stolerman et al. 1973), showing subjective and behavioral effects of smoking altered by mecamylamine but not pentolinium (another peripheral antagonist), provided initial support for the importance of the central effects of nicotine, inspiring a subsequent program of preclinical studies on discrimination responding. More recently, a study with primates also showed that mecamylamine blocks discrimination of nicotine, while several drugs that are peripheral antagonists only do not (Cunningham et al., 2019). Thus, this research with non-human animals documents the actions of nicotine at its CNS receptors and not outside the CNS are responsible for its discriminative stimulus effects, or those psychoactive effects perceived in the brain (Smith & Stolerman, 2009; Wiley et al., 1995).
Because consistency in CNS mediation of nicotine’s stimulus effects from animals to humans seemed quite important, we conducted a similar clinical study (Perkins, Sanders, et al. 1999). In abstinent smokers able to reliably discriminate 0 (placebo) vs 20 μg/kg nicotine in session 1, we tested different pre-treatment effects on quantitative responding across five doses from 0 to 20 μg/kg during subsequent sessions in a within-subjects comparison. In piloting, we compared pre-treatment with mecamylamine vs. saline, showing the expected blockade due to mecamylamine. Then, in the main study, we added a third pre-treatment condition, that involving the peripheral nicotine antagonist trimethaphan. Results were similar to the preclinical studies noted above, showing that this nicotinic blockade of discrimination responding across intermediate doses occurred with the central blocker mecamylamine only, not the peripheral blocker or saline. To our knowledge, this was the very first time formal behavioral discrimination of any psychoactive drug had been shown in humans to be CNS-mediated.
In another very general suggestion of cross-species consistency in this area, much research shows that pre-treatment with other drugs not acting at the same receptors as nicotine do not appear to alter subsequent nicotine discrimination responding. As an example, preclinical research with midazolam pre-treatment prior to nicotine discrimination testing showed generally little change in that subsequent nicotine discrimination responding (Stolerman, et al., 1987). When we tested this notion in humans in two of our clinical studies, neither alcohol nor caffeine pre-treatment showed much effect in subsequent nicotine discrimination responding across doses (Perkins, Fonte, et al. 2005a; 2005b).
Threshold dose for nasal spray nicotine discrimination
The final study with nicotine spray presented here produced findings that directly led to the second program of research, on discriminating very low doses of nicotine via smoking, to be covered next. One of the key questions in research on nicotine dependence long has been, what is the smallest dose of nicotine that can be discriminated from placebo? That would indicate a minimum amount of nicotine necessary to perceive its effects in the brain, which may be key to the amount needed to produce reinforcement and onset of dependence (e.g., Sofuoglu & LeSage, 2012). Another key question is, do smokers and nonsmokers differ in their ability to discriminate those nicotine effects?
Thus, in a study aimed at identifying threshold doses for discrimination, participants engaged in very similar repeated sessions of training and testing for ability to discriminate gradually lower doses of nicotine versus placebo via spray until a participant could not discriminate the two, which we labeled their “subthreshold” dose. In the last session, we then repeated testing with the next highest dose versus placebo, which they had previously discriminated successfully, to confirm the participant could discriminate those two, which we labeled their “threshold” dose (Perkins, Fonte, et al. 2001). Three main conclusions from this study were: 1) the range of threshold doses was very wide, differing 100-fold, from 0.2 to 20 μg/kg; 2) median thresholds were very low, and 3) smokers and nonsmokers did not differ in median threshold, at 3 and 2 μg/kg, respectively, similar to the ED50 value we saw in our very first study (see Fig 1, although that study involved a lower training dose than this study).
Other testing conditions altering behavioral discrimination responding
Other factors affecting nicotine discrimination responding were tested as part of this first program, such as the importance of training dose and 2- or 3-choice options in the quantitative testing task. A 3-choice option allows participants to respond that the administered drug condition was like “neither A nor B”, with “C” also provided as a “novel response” option (such as when an active drug or drug dose very different from the “A” and “B” training conditions is administered; Perkins, D’Amico et al. 1996; Perkins, Fonte, et al. 1999). Aside from further showing consistency between preclinical and clinical nicotine discrimination research, differences in nicotine-appropriate responding due to variable training doses and testing response options show that specific results from behavioral drug discrimination testing do not reflect immutable properties of drugs, or magnitudes or even patterns of a drug’s CNS actions. They remind us that all behavioral responses to acute drug administration are partly determined by the environmental context (e.g. Perkins & Stitzer, 1998; Siegel, 1991), such as training conditions.
Research on Behavioral Discrimination of Nicotine via Cigarette Smoking
In 2005, this work on discrimination of nicotine in humans stopped, mostly because the next obvious question--whether these results would be seen with nicotine dosing administered by smoking—was not readily testable. Smoking is obviously the most common and deadly form of using nicotine, and research to identify the threshold dose for discriminating nicotine via cigarette smoking, rather than spray, would have enormous implications for public health policy aimed at reducing nicotine content in commercial cigarettes, thereby lessening dependence onset or maintenance (Benowitz & Henningfield 2013). The rationale here is that smoked nicotine doses too low to be discriminable from a smoked product with no nicotine (e.g. as with herbal cigarettes, which are not dependence producing) are not likely to foster initiation or maintenance of nicotine dependence (Perkins, 2019; Wayne and Carpenter, 2009).
However, there is virtually no relationship between a commercial cigarette’s stated nicotine “yield” and the resulting increase in blood nicotine, as shown by Neal Benowitz (Benowitz, et al., 1983) and others, except for ultra-low yield cigarettes, which are associated with somewhat lower nicotine intake (USDHHS, 2001). This is largely the reason we had to develop our nasal spray method of administering controlled nicotine doses in the first place. In brief, so-called “Light” brands advertised as low in nicotine yield were engineered by perforations in the paper wrapping to dilute the smoke inhaled, not by reducing nicotine content in tobacco. Smokers easily defeated this dilution manipulation, by intentionally or unintentionally covering the holes (Kozlowski and O’Connor 2002), which were solely designed to lower nicotine detection by FTC smoke machines, not human smokers. As a result, the Family Smoking Prevention and Tobacco Control Act (2009) prohibits “Light” brand labels because they make a deceptive marketing claim. Drug discrimination, and most other controlled research on acute drug responses, is impaired without careful control over dose administration (Glennon & Young, 2011). Faced with this severe limitation in 2005, we shifted our research focus to improving methods for efficiently evaluating evidence of efficacy for smoking cessation in novel drug compounds (e.g. Perkins & Lerman, 2014), as well as the conditions under which nicotine can relieve negative affect (Perkins et al., 2010), and to translating study of nicotine’s reinforcement enhancing effects from preclinical to clinical models (e.g. Perkins et al., 2017).
Background on Adapting Methods for Use with Cigarettes
Although long-term changes in self-administration with nicotine reduction in cigarettes are most relevant here, acute studies can also help inform FDA (e.g. FDA 2018; Hatsukami et al. 2010). To conduct this research on nicotine via Spectrum cigarettes, we had to adapt all our nicotine discrimination procedures from the spray studies above to use with cigarettes, which I will try to summarize. These included: 1. Very strict control over dosing via smoking, 2. A choice procedure to assess nicotine preference, or relative reinforcement, 3. Subjective ratings of perceived rewarding effects of nicotine via smoking, and 4. Other procedural changes.
1. Smoked nicotine dosing.
a. Spectrum low and very low nicotine research cigarettes.
The first innovation that made discrimination (or assessing any responses) of controlled dosing of nicotine via smoking possible was the availability of Spectrum research cigarettes (Hatsukami et al. 2013; Richter et al. 2016), manufactured by altering the nicotine content of the tobacco used, not by manipulating the holes in the wrapping paper. Thanks to the 2009 Tobacco Control Act, FDA was granted authority over regulating tobacco products, including reducing their nicotine. As a result, NIDA contracted with 22nd Century to manufacture cigarettes with explicit contents at the low or very low end of the range of nicotine possible from tobacco, with nicotine contents of about 17 mg/g (typical of commercial brands), down to 11, 5, 2, 1 and 0.4 mg/g. Note that the 0.4 mg/g (very low nicotine content, or VLNC) was only about 2% of the nicotine content in typical current brands (Carmines & Gillman, 2019).
Also worth pointing out is that these Spectrum research cigarettes were already made in standardized fashion that precluded the possibility that the discriminative stimulus, self-reported rewarding, and choice effects of smoking them may be due to factors other than nicotine content. All stimuli involved in administering alternative drug conditions, other than their pharmacology, have to be matched so that behavioral discrimination responding is influenced by the drug’s pharmacology and not by those non-pharmacological stimuli (e.g. Kamien et al. 1995). To illustrate, we recently compared subjective responses to acute ad lib smoking of one’s own brand, between those blinded vs unblinded to the brand they were given to smoke. We found significantly greater ratings of subjective “liking”, “how much nicotine”, etc., in the unblinded group, despite equal total smoke intake between groups. This result showed that simply knowing the brand name enhanced perceptions of smoking reward (Perkins & Karelitz 2019). In a second example, smokers randomized to one of the four 2 x 2 groups in the balanced-placebo design (Marlatt & Rohsenow, 1980) were: 1) told the cigarette was either nicotine (Quest 1; 0.6 mg) or denicotinized (Quest 3; 0.05 mg), and 2) actually given the nicotine or denic cigarette, such that dosing was either consistent or inconsistent with what they were told. Reward ratings mostly depended on what they were told about the nicotine content, not what they were actually given (Perkins et al. 2008). In short, Spectrum cigarettes simplify blinding of participants to a cigarette’s actual dose and to their dose expectancies.
b. Control of puff number and topography.
As noted, Spectrum research cigarettes allow much better control of nicotine dosing due to their engineered nicotine content, with which smokers do not compensate by increasing topography to try and defeat their low nicotine content (Mercincavage et al., 2018). Nevertheless, the very tight doses needed in drug discrimination testing required additional steps to standardize smoke intake. We further controlled nicotine dosing with Spectrum by using our validated and reliable puffing procedure, which instructs participants on precisely when and for how long to take each puff to target intake at 60 ml per puff (typical of that during ad lib puffing). That procedure carefully standardizes intake from smoking, as well as from vaping e-cigarettes (Perkins & Karelitz, 2020a).
2. Forced choice procedure, for assessing preference.
More importantly here, we also wanted to relate discrimination responding to preference for one or the other nicotine dose, to see if ability to discriminate related to choice behavior. We reasoned that if someone could not discriminate between cigarettes differing in nicotine, how could they find one more reinforcing than the other? That seemed obvious to us, and almost trivial, but there had not been much clinical research on this link with any drug and none with nicotine that we knew of, and others in this research field cited findings that questioned whether that was necessarily the case (e.g. Lamb et al., 1991). So, well before this line of research with Spectrum cigarettes, we adapted a “forced choice” procedure based on methods pioneered by the group at the Univ of Chicago (deWit, 1991), led then by Dr. Charles R. Schuster (e.g. Schuster & Johanson, 1988), for whom this APA Division 28 award is partly named. Our procedure was first validated in 1995, using nicotine administered via nasal spray and via smoking (Perkins, Grobe, et al. 1996). It subsequently has been shown sensitive to individual differences in, and some medication pre-treatments on, the reinforcing effects of nicotine via smoking (Blendy, et al. 2005; Ray, et al. 2006; Rukstalis et al. 2005). We recently published a comprehensive update of the method’s use over the subsequent 25 years (Perkins & Karelitz, in press).
To briefly describe our choice procedure, now added after the end of discrimination testing trials, both of the cigarettes are presented concurrently, now labeled “A” and “B” as in the training trials. The participant is told to take exactly 4 puffs using the above puffing procedure, as in all prior trials, but they are to choose how many of those puffs to take from which cigarette (i.e., all 4 from one, all 4 from the other, or some mix of the two). The total number of puffs chosen from the higher nicotine cigarette (vs the 0.4 mg/g VLNC in each session), out of 8 choice opportunities across the two choice trials, was used to index the preference, or relative reinforcing effect, of that nicotine cigarette (Perkins & Karelitz, in press).
3. Acute Cigarette Perceptions (ACP) scale of self-reported smoking “reward”
A third update of procedures for assessing nicotine discrimination was to develop and validate a self-report measure of nicotine “reward,” to relate the discrimination behavior to other types of responding that may help explain why Spectrum cigarettes differing in nicotine content can or cannot be discriminated. We focused on five immediate pleasurable perceptions of nicotine in the minutes after puffing on a cigarette (each on a 0-100 VAS), based on separate items from our past studies with spray and smoking (e.g. Perkins et al., 2004), asking: “How much…. Nicotine, Liking, Satisfying, Flavor, or Strong” was that cigarette. This Acute Cigarette Perceptions (ACP) scale is viewed generally as an index of positive reward from the dose of nicotine via smoking a particular cigarette. For example, to further validate the ACP (Perkins et al., 2018), we found that the greater the subsequent choice of a 17 mg/g nicotine Spectrum cigarette vs the 0.4 mg/g VLNC, the greater the difference in these ACP ratings during initial exposure to each cigarette. A more recent and better controlled study found that the difference in ACP between the full range of cigarettes higher in nicotine, each vs the VLNC, predicted the magnitude of that subsequent greater preference for the higher nicotine cigarette in the choice procedure (Karelitz & Perkins, submitted). This is consistent with our understanding of drug preference, that choice is driven by the immediate pleasurable effects that drug condition elicits. Again, this seems obvious but has not often been demonstrated in systematic fashion, especially by nicotine dose in smoking given inability to control dose before advent of Spectrum cigarettes.
4. Discrimination testing procedures adapted for tobacco smoking dose administration
As with our earlier nasal spray study on nicotine threshold, these sessions were identical across days, as the 0.4 mg/g VLNC was compared with just one of the Spectrum cigarettes containing more nicotine contents, 17, 11, 5.3, 2.3, or 1.3 mg/g, until participants could not discriminate between the two cigarettes. The cigarette tested versus the VLNC in that session was then determined to be that participant’s “subthreshold” dose. The last session repeated the prior session’s testing of the next higher nicotine cigarette to confirm they could discriminate it from the VLNC, identifying it as the participant’s “threshold” dose.
Virtually all procedures for assessing behavioral discrimination of nicotine via spray, in above studies, were the same with nicotine via Spectrum cigarettes, with a few slight exceptions. First, based on piloting (Perkins, Kunkle, et al. 2016a; 2016b), we found that doubling the training trials increased the rate of smokers subsequently showing ability to discriminate the two most widely varying nicotine content cigarettes (16 vs 0.4 mg/g) during testing trials. That ability increased from 50% of those given just two (one for each dose) to 89% of those given four (two for each dose, in random order) training trials prior to testing trials. Second, although all participants in the spray and cigarette studies arrived to sessions abstinent overnight, we limited smoking exposure to 4 puffs per trial (equivalent to one-third of a full cigarette; Hatsukami et al., 1988), to prevent smoking satiation by the end of the session. Total smoke exposure across the four training, six testing, and two choice trials (see 2, above) amounted to 48 puffs, or four full cigarettes, over the 3.5-hour session. This rate of exposure, about one cigarette/hour, was intentionally no more than the amount of smoking, and far less than the amount of nicotine intake, that abstinent smokers typically consume over a morning (Mooney et al., 2006). With curtailing of smoke/nicotine exposure to 4 puffs/trial, the interval between trials was shortened to 15 mins, allowing additional time to insert the two choice trials following completion of discrimination training and testing trials, as described above.
Smoked Nicotine Discrimination Threshold in Menthol vs Non-menthol Dependent Smokers
Our first primary study with Spectrum cigarettes compared nicotine discrimination threshold for non-menthol versus menthol dependent smokers (Perkins et al., 2017a). Spectrum nicotine levels are generally matched for non-menthol and menthol versions, allowing those who prefer one or the other to be tested with the flavor they prefer. All completed study sessions until they were unable to discriminate one of higher nicotine content cigarettes compared with the VLNC, to identify subthreshold and threshold cigarettes, producing median values for groups by menthol flavoring. Those unable to discriminate even the first comparison, between 17 mg/g and the VLNC, were also included in analyses so that threshold results were not biased by excluding those who may require even higher doses to discriminate.
Results first showed a wide range of threshold doses, as each nicotine content was a threshold for some of the smokers able to discriminate, similar to our prior spray study on threshold (Perkins, Fonte, et al. 2001) and confirming individual differences in sensitivity to nicotine’s stimulus effects. Second, threshold tended to be higher for menthol vs. non-menthol (medians of 17 mg/g vs 11 mg/g, respectively), partly because twice as many menthol as non-menthol smokers could not discriminate even the highest nicotine content, suggesting less sensitivity to the discriminative stimulus effects of smoked nicotine when combined with menthol. Yet, the few who were able to discriminate the lowest nicotine content above the VLNC, at about 1.3 mg/g, were menthol smokers. So, conclusions based on menthol per se are uncertain from these data. Most importantly overall in this first study, relative to the VLNC, the threshold cigarette did, and the subthreshold cigarette did not, differ on ratings of ACP items of “how much nicotine’’ or “liking”, and on choice between each cigarette and the VLNC. Also, these ACP and choice responses did not differ by menthol. In short, ability to discriminate a cigarette’s nicotine content was necessary to show increases in subjective reward and in choice of cigarettes, consistent with hypotheses, but menthol did not alter these associations. Nevertheless, these responses to nicotine dose should be assessed in conjunction with other tobacco constituents or additives, such as acetaldehyde, ammonia, etc., which may alter the pattern of responses (Henningfield, 2011).
Smoked Nicotine Discrimination Threshold in Dependent vs Non-dependent Smokers
Our next study compared nicotine discrimination threshold between dependent versus a smaller subgroup of non-dependent smokers (Perkins et al., 2017b). (Only non-menthol smokers were compared, due to very small numbers of non-dependent menthol smokers recruited.) Non-dependent smokers do not meet DSM dependence criteria and smoke no more than a few cigarettes/day, suggesting their ability to maintain only a very modest amount of nicotine exposure prevents the onset of dependence (Shiffman, 1989). This pattern is partly what led to the proposal by Benowitz and Henningfield (1994) to mandate reduction of nicotine content in cigarettes, so that all smokers would be able to consume only the small amount of nicotine that non-dependent smokers now consume daily.
Results showed no differences in discrimination threshold between dependent and nondependent smokers, both having medians of 11 mg/g, which was also very similar to the lack of differences in nasal spray nicotine threshold with smokers and nonsmokers (Perkins, Fonte, et al. 2001). We also saw no differences due to dependence status in self-reported “how much nicotine”, indicating similar ability to perceive the cigarette’s nicotine content between groups. Yet, the dependent smokers did, and the non-dependent smokers did not, “Like” or choose the threshold cigarette over the VLNC. The lack of choice and “liking” of the higher nicotine cigarette in non-dependent smokers is consistent with their lack of dependence on nicotine. Our finding supports the view that, relative to dependent smokers, smoking reinforcement in these non-dependent smokers is motivated less by nicotine’s acute psychoactive effects and more by smoking’s non-nicotine stimulus effects, matched across Spectrum cigarettes (Richter et al. 2016), although other stimulus control factors may also be responsible (Shiffman et al., 2015). Thus, both dependent and non-dependent smokers can perceive differences in nicotine dose, but only dependent smokers “like” and are driven to obtain higher nicotine amounts.
As indicated in Figure 2, our main conclusion from both studies is that discriminability of a cigarette’s nicotine content is necessary to find it reinforcing, but it is not sufficient (Perkins, 2019). Differences in choice and “liking” also require that a smoker be dependent on nicotine, which is explicitly characterized by persistent nicotine seeking. So, one implication, we believe, is that a public health policy that requires a dropping of nicotine content in commercial cigarettes to that no more than the subthreshold dose of a dependent smoker should lead that smoker to not perceive the rewarding or reinforcing effects of that difference in nicotine from the 0.4 mg/g VLNC. As such, they would be no more likely to choose between those two cigarettes, both limited in reward or reinforcement, greatly reducing overall nicotine exposure and, thus, dependence onset or persistence (Benowitz & Henningfield, 2013).
Figure 2.

Means (and SEM) for self-reported Acute Cigarette Perceptions (ACP) composite ratings (A, C) and for choice (B, D) of each participant’s discrimination subthreshold nicotine and threshold nicotine cigarettes, each compared to the 0.4 mg/g VLNC, for dependent smokers (top, n=42) and for non-dependent smokers (bottom, n=7). (*** p<.001). Partly adapted from Figures 2 and 3 in Perkins (2019). With kind permission from Oxford University Press.
Consideration of Choice Threshold
In follow-up post hoc analyses focusing just on the choice results from all nicotine comparison sessions in these discrimination studies, we compared choice between each of the 5 higher nicotine Spectrum cigarettes versus the 0.4 mg/g VLNC, regardless of a participant’s discrimination threshold. Taking all dependent smokers together as a group, they chose cigarettes at or above 5.3 mg/g. By contrast, the smaller subgroup of non-dependent smokers did not choose any of the higher nicotine cigarettes more than the 0.4 VLNC, even the highest nicotine Spectrum content, 17 mg/g (Perkins & Karelitz, in press). Further supporting the idea that a reinforcing dose of nicotine requires that it be discriminable, 80% of the group of dependent smokers had discrimination thresholds of 5.3 mg/g or greater, and subsequent group means for greater cigarette choice behavior were found at or above 5.3 mg/g. Yet, this analysis was limited because the sessions involved comparisons across the different nicotine content cigarettes in fixed orders, not in random order, and did not include all participants since all discontinued testing sessions as soon as their discrimination threshold and subthreshold doses were identified.
Because of those limitations, we recently completed a follow-up study involving only the training and choice trials, skipping the discrimination testing trials (Perkins & Karelitz, 2020b). That change in eliminating the 6 testing trials allowed us to double the number of choice trials to 4, so that 16 choice opportunities were provided, and 8.0 was chance. Also, all participants got all five nicotine dose comparisons with the 0.4 mg/g VLNC, in random order across sessions, to assess at what nicotine content choice increases over the VLNC, in a “bottom-up” type comparison. Results, shown in Figure 3, were very similar to the prior analysis above, as choice vs. the VLNC again was greater for cigarettes at or above 5.3 mg/g, as were the increases in ACP subjective ratings. Findings suggest the mean group threshold for nicotine “reward” and choice (ignoring discrimination, which was not tested), relative to the VLNC, is as low as 5.3 mg/g. Moreover, in exploratory analyses from this study to potentially model a trial in which smokers attempt to switch from their typical brand down to one of the very low nicotine cigarettes, we thought a “top-down” comparison would be relevant. That is, at what smaller nicotine content would choice relative to the 0.4 mg/g VLNC decline, compared to the 18 mg/g “moderate” nicotine content (similar to typical commercial brands)? We found that mean difference in ACP ratings and in choice were significantly less for cigarettes below 5.3 mg/g. To summarize, the increase in choice above the 0.4 mg/g VLNC was significant at 5.3 mg/g and above, while the reduction in choice below the 18 mg/g typical regular nicotine cigarette was significant for nicotine levels at 2.3 mg/g and below (i.e. below the 5.3 mg/g).
Figure 3.

Mean ACP difference (A) and mean (+ 95% C.I.) number of puff choices (B) from each higher nicotine content cigarette, vs. the 0.4 mg/g VLNC. Choices are out of a fixed total of 16 per paired cigarette comparison. Horizontal dashed line at 8.0 indicates no difference in choice from the 0.4 mg/g VLNC (i.e., 50% of 16). Non-overlapping 95% C.I. with 8.0 indicates significantly greater choice for that content vs. VLNC. In both graphs, horizontal brackets indicate significant differences between 18.7 mg/g vs. the lower nicotine content cigarettes in ACP or choice. (***p < .001, *p < .05). Partly adapted from Figure 1 in Perkins & Karelitz (2020b). With kind permission from Springer Nature.
That last finding is consistent with a recent study assessing change in smoking due to switching to lower nicotine cigarettes, suggesting our acute choice results could have clinical predictive relevance. In a large clinical trial of 780 smokers not interested in quitting, all were randomly assigned to either receive their usual brand or switch to Spectrum cigarettes with one of five different lower Spectrum nicotine contents, provided at no cost, to smoke continuously for 6 weeks (Donny et al., 2015). In comparisons of changes in cigarettes/day, there appeared to be two clusters of groups, one with daily consumption remaining high around the level of the group continuing with their usual brand, which comprised those randomized to smoke cigarettes with 5.2 and 15.8 mg/g. The second cluster showed a slight but significant decline in daily consumption across weeks, consisting of those randomized to smoke cigarettes with only 2.4 mg/g and lower (i.e. all those below the 5.2 mg/g). Thus, despite how different our smaller and acute studies of choice are from this large trial by Donny et al., we found a split in relative magnitude of reinforcement from these Spectrum cigarettes using our acute choice procedure that was comparable to the split found in the trial’s weeks-long ad lib cigarettes/day assessment.
Conclusions and Future Directions
Note that our results on median thresholds for discrimination may not be directly helpful in informing FDA policy on nicotine reduction or regulation, which would need to be based on evidence of the nicotine content in cigarettes that is below the discrimination or choice threshold for nearly all smokers in the population, not just for the average smoker, pending further research. For that reason, I returned to the frequencies for the different nicotine discrimination thresholds across the studies, including the participants who were unable to discriminate (Perkins, 2019). The threshold that was below that for at least 90% of the dependent smokers we tested was the lowest cigarette above the 0.4 mg/g VLNC, the 1.3 mg/g. So, although much more research is needed, a difference of less than 1 mg/g may be a starting point for research directly aimed at determining how low a reduction in nicotine content of cigarettes is needed to minimize risk of dependence in most smokers. Moreover, if each is smoked ad lib with 2-3 times as many puffs as the 4 puffs per trial in our studies, a better target for a population “subthreshold” cigarette may be closer to Benowitz and Henningfield’s (1994) estimate of 0.5 mg nicotine per cigarette. In Dec 2019, FDA permitted 22nd Century Group, the maker of Spectrum, to market commercial cigarettes with nicotine contents between 0.2 and 0.7 mg/cigarette (FDA, 2019).
The research program outlined here documents that acute testing of discrimination and choice of nicotine via cigarette smoking is feasible, that discriminability of a nicotine dose in dependent smokers is necessary for choice and “liking” of that dose; and that results of this testing may help inform the design and planning of larger and longer clinical assessments, perhaps including switching trials. Given a virtual absence of other research on nicotine discrimination threshold in humans, far more study is needed to determine more specific threshold doses across the entire smoking population, a key to guiding FDA nicotine reduction policy. Limitations in the research described here, gaps that remain in identifying a population threshold dose, and suggestions for future research include:
1) No zero nicotine “true placebo” tobacco cigarette exists, as current US law prohibits eliminating all nicotine from commercial tobacco products (FDA, 2018), although such no-nicotine placebos are not illegal for research purposes. In any case, all our comparisons were limited by being versus the 0.4 mg/g VLNC. Even though the VLNC is just 2% of the nicotine in typical commercial brands, that low an amount of nicotine may elicit some stimulus effects that can be perceived (Brody et al., 2008), relative to smoking a no nicotine cigarette. A no nicotine tobacco cigarette should be made available for researchers to conduct adequately controlled study of the acute effects of nicotine per se from smoking. That way, they could identify what cigarette nicotine content is required for discrimination and choice due to smoked nicotine alone.
2) As noted, to minimize satiation across sessions, we limited exposure to 4 puffs per trial. Because ad lib smoking averages about 8-12 puffs per cigarette, true nicotine discrimination or choice thresholds likely are at even lower nicotine contents per cigarette than those we found here, when it is smoked ad lib. Replication of our results should be done with thresholds due to nicotine obtained from a full cigarette, although practical factors may limit the number of trials per session before smoking satiation interferes with assessments.
3) We tested only healthy dependent and nondependent smokers. Smokers with some of the vulnerabilities noted in the Introduction, who are far more prevalent among today’s smokers, are very important to assess, to determine generalizability of nicotine thresholds for discrimination and choice across the current population of adult smokers.
4) As suggested by the clinical trial of cigarette switching over 6 weeks (Donny et al., 2015), similar discrimination and choice testing should examine the duration of differences in perceptions between cigarettes differing in nicotine content, which may change with greater familiarity across continuing use.
5) Future research should be able to easily apply these methods to assessing discrimination of nicotine via new methods of consumption, such as electronic cigarettes (Perkins et al., 2019), and other marketed products with rapid delivery of acute nicotine, including heat-not-burn tobacco (e.g. St. Helen et al., 2018). Similar research in novel products evaluated as treatments to aid smoking cessation may also help identify low nicotine doses (subthreshold) that are neither discriminable nor reinforcing via choice in acute tests, pointing toward their likely lack of abuse liability. However, switching from smoked to non-smoked nicotine, of substantial interest as a harm reduction approach (Notley et al., 2018), may be more difficult to test via our discrimination procedures due to problems blinding participants to administration methods and resulting differences in exteroceptive stimuli. Yet, direct comparisons of the subjective rewarding and, perhaps, choice responses to nicotine administered by smoked vs. nonsmoked products might be possible (e.g. Perkins, Sexton, et al. 1994).
In conclusion, the ability to discriminate the stimulus effects of nicotine is necessary for it to be reinforcing or “liked.” Yet, discriminability is not sufficient, as nicotine reinforcement or liking also requires being a dependent smoker able to discriminate that dose of nicotine. Nondependent smokers show equal ability to discriminate nicotine doses but do not find smoking them more reinforcing or liked than smoking cigarettes virtually devoid of nicotine. Determining minimally reinforcing doses in clinical studies with larger and more diverse samples, as well as longer duration of access to products with those doses, may inform FDA policy. Such research may also provide directions for further examination of mechanisms accounting for persistent self-administration of all commercial nicotine products in those dependent on nicotine, and it may help determine the degree to which nicotine content of such products should be reduced to minimize risk of dependence (Perkins, 2019). Finally, this area of research provides perhaps an ideal illustration of how behavioral pharmacology research effectively informs regulatory authorities seeking to formulate policies intended to reduce risks of harm and thereby improve public health (FDA 2018).
Public Significance statement:
Research on the behavioral discrimination of nicotine in humans documents translation of results from preclinical models and confirms nicotine is only reinforcing, and thus prone to foster dependence, at doses in which the user can objectively perceive the drug’s effects in the brain. Identifying nicotine amounts that are clearly below doses that can be perceived by nearly all in the population may help inform federal regulatory efforts to mandate reduction in nicotine content of tobacco products. That objective would be to limit the risk that teens will become dependent smokers and make it easier for already dependent smokers to permanently quit.
Disclosures and Acknowledgments
The research described in this paper is directly adapted from the author’s American Psychological Association Division 28 (Psychopharmacology and Substance Abuse) 2020 Med Associates Brady/Schuster Award lecture. Information on the development and early testing of behavioral discrimination of nicotine via nasal spray was presented at many scientific conferences in the 1990s, especially as part of an invited symposium, “Drug discrimination in relation to gender and species differences” at the Fifth International Meeting on Drug Discrimination in Behavioural Neuroscience, Antwerp Belgium, September 1998. Data from the later studies on discriminating nicotine via low nicotine cigarette smoking were presented at the annual meetings of the Society for Research on Nicotine and Tobacco in 2015 through 2019.
This research was supported by NIH grants R01 DA08578, R01 DA035774, R21 DA035968, and a supplement to U54 DA031659. Preparation of this review was also supported by consulting of KAP to Technical Resources International, Inc. (TRI), Bethesda MD, for the purpose of developing procedures to conduct clinical research assessing nicotine dose discrimination in marketed cigarettes, under FDA Contract to TRI, No. 75F40120P00166. (The content of this talk is solely the responsibility of the author and does not necessarily represent official views of NIH, the Food and Drug Administration, or any other organization.)
The author thanks Nicole Kunkle, Carolyn Fonte, and Joshua L. Karelitz, among other research assistants, for their expert help in conducting the individual studies described here. Thanks are also given to J.L. Karelitz for his overseeing analyses and presentation of the later studies of discrimination and choice of nicotine administered by research cigarettes, including the adapted figures displayed in this paper.
Footnotes
The author states no conflicts of interest are apparent in this review paper.
References
- Apelberg BJ, Feirman SP, Salazar E, et al. (2018). Potential public health effects of reducing nicotine levels in cigarettes in the United States. New England Journal of Medicine, 378(18), 1725–1733. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hall SM, Herning RI, Jacob P, Jones RT, & Osman AL (1983). Smokers of low-yield cigarettes do not consume less nicotine. New England Journal of Medicine, 309, 139–142. 10.1056/NEJM198307213090303. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, & Henningfield JE (1994) Establishing a nicotine threshold for addiction. New England Journal of Medicine, 331, 123–125. 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, & Henningfield JE (2013). Reducing the nicotine content to make cigarettes less addictive. Tobacco Control, 22(suppl 1): i14–i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow GE, & Preston KL (1989) Drug discrimination: Methods for drug characterization and classification. In Fischman MW & Mello NK (eds), Testing for Abuse Liability of Drugs in Humans. NIDA Research Monograph 92, USDHHS: Washington DC. [PubMed] [Google Scholar]
- Blendy JA, Strasser A, Walters CL, Perkins KA, Patterson F, Berkowitz R, & Lerman C (2005). Reduced nicotine reward in obesity: cross comparison in human and mouse. Psychopharmacology, 180, 306–315. DOI: 10.1007/s00213-005-2167-9. [DOI] [PubMed] [Google Scholar]
- Bolin BL, Alcorn JL, Reynolds AR, Lile JA, Stoops WW, & Rush CR (2018). Human drug discrimination: elucidating the neuropharmacology of commonly abuse illicit drugs. In Porter JH, Prus AJ (eds) The Behavioral Neuroscience of Drug Discrimination. Current Topics in Behavioral Neuroscience, 39, 261–296. Doi: 10.1007/7854_2016_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British American Tobacco Company, RDW (1959). Complexity of the PA 5A machine and variables pool. Minnesota Trial Exhibit 10,392, State of Minnesota: et al. v. Philip Morris, Incl, et al. (dated June, 1959). [Google Scholar]
- Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J, London ED, Olmstead RE, Rose JE, & Mukhin AG (2009). Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. International Journal of Neuropsychopharmacology, 12, 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmines E, & Gillman IG (2019). Comparison of the yield of very low nicotine content cigarettes to the top 100 United States brand styles. Beitrage zur Tabakforschung International/Contributions Tobacco Research, 28, 253–266. Doi: 10.2478/cttr-2019-0005. [DOI] [Google Scholar]
- Carter LP, Stitzer ML, Henningfield JE, O’Connor RJ, Cummings KM, & Hatsukami DK (2009). Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiology, Biomarkers, & Prevention, 18(12), 3241–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance LT, Murphin D, Krynock GM, & Rosecrans JA (1977). A description of the nicotine stimulus and tests of its generalization to amphetamine. Psychopharmacology, 55, 19–26. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, & Coen KM (1989). Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology, 99, 473–478. [DOI] [PubMed] [Google Scholar]
- Cunningham CS, Moerke MJ, & McMahon LR (2019). Discriminative stimulus effects of mecamylamine and nicotine in rhesus monkeys: central and peripheral mechanisms. Pharmacology, Biochemistry, & Behavior, 179, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWit H (1991). Preference procedures for testing the abuse liability of drugs in humans. British Journal of Addiction. 86, 1579–1586. [DOI] [PubMed] [Google Scholar]
- Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, et al. (2015). Randomized trial of reduced-nicotine standards for cigarettes. New England Journal of Medicine, 373, 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drope J, Liber AC, Cahn Z, et al. (2018). Who’s still smoking? Disparities in adult cigarette smoking prevalence in the United States. CA Cancer J Clin, 68: 106–115. [DOI] [PubMed] [Google Scholar]
- Family Smoking Prevention and Tobacco Control Act (2009), 21 U.S.C. § 387 https://www.govinfo.gov/content/pkg/PLAW-111pub131/pdf/PLAW-111pub131.pdf (accessed 7/23/2020). [Google Scholar]
- FDA (2018). Tobacco Product Standard for Nicotine Level of Combusted Cigarettes. A proposed rule. Document citation: 83 FR 11818, pages 11818–11843. Document number: 2018-05345. https://www.federalregister.gov/documents/2018/03/16/2018-Q5345/tobacco-product-standard-for-nicotine-level-of-combusted-cigarettes (accessed 3/16/2018). [Google Scholar]
- FDA (2019). FDA permits sale of two new reduced nicotine cigarettes through premarket tobacco product application pathway. https://www.fda.gov/news-events/press-announcements/fda-permits-sale-two-new-reduced-nicotine-cigarettes-through-premarket-tobacco-product-application. (accessed 12/18/19). [Google Scholar]
- Glennon RA, & Young R (2011). Drug Discrimination: Application to Medicinal Chemistry and Drug Studies. New York: John Wiley & Sons. ISBN: 978-0-470-43352-2. [Google Scholar]
- Griffiths RR, Henningfield JE, & Bigelow GE (1982). Human cigarette smoking: manipulation of number of puffs per bout, interbout interval and nicotine dose. Journal of Pharmacology and Experimental Therapeutics, 220, 256–265. [PubMed] [Google Scholar]
- Hatsukami DK, Heishman SJ, Vogel RI, Denlinger RL, et al. (2013). Dose-response effects of Spectrum Research Cigarettes. Nicotine and Tobacco Research, 15, 1113–1121. Doi: 10.1093/ntr/nts247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Perkins KA, LeSage MG, Ashley DL, Henningfield JE, Benowitz NL, Backinger C, & Zeller M (2010). Nicotine reduction revisited: science and future directions. Tobacco Control, 19, 436–445. DOI: 10.1136/tc.7009.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Pickens RW, Svikis DC, & Hughes JR (1988). Smoking topography and nicotine blood levels. Addictive Behaviors, 13, 91–95. [DOI] [PubMed] [Google Scholar]
- Henningfield JE (2011). Tobacco psychopharmacology and public health policy: it takes a community. Experimental and Clinical Psychopharmacology, 19, 249–262. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Rose CA, & Zeller M (2006). Tobacco industry litigation position on addiction: continued dependence on past views. Tobacco Control, 15 (suppl 4), iv27–iv36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalmarson A, Franzon M, Westin A, & Wiklund O (1994). Effect of nicotine nasal spray on smoking cessation. Archives of Internal Medicine, 154, 2567–2572. [PubMed] [Google Scholar]
- Hughes JR, & Liguori A (2000). A critical view of past NIH research funding on tobacco and nicotine, Nicotine & Tobacco Research, 2, 117–120. 10.1080/713688134 [DOI] [PubMed] [Google Scholar]
- Johanson C-E (1991). Discriminative stimulus effects of psychomotor stimulants and benzodiazepines in humans. In Glennon RA, Jarbe TUC, Frankenheimer J (eds) Drug Discrimination: Applications to Drug Abuse Research, NIDA Research Monograph 116, USDHHS: Rockville MD, pp.181–196. [PubMed] [Google Scholar]
- Kamien JB, Bickel WK, Oliveto AH, Higgins ST, Hughes JR, Richards AT, & Badger GJ (1995). Placebo-effects contribute to differences in the acquisition of drug discrimination by humans: a retrospective analysis. Behavioural Pharmacology, 6, 187–194. [PubMed] [Google Scholar]
- Karelitz JL, & Perkins KA (2020). Acute sensory perceptions may predict relative reinforcing effects of smoked nicotine. Manuscript submitted July 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski LT, O'Connor RJ (2002). Cigarette filter ventilation is a defective design because of misleading taste, bigger puffs, and blocked vents. Tobacco Control, 11(suppl 1), i40–i50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RJ, Preston KL, Schindler CW, Meisch RA, Davis F, Katz JL, Henningfield JE, & Goldberg SR (1991). The reinforcing and subjective effects of morphine in post-addicts: a dose-response study. Journal of Pharmacology & Experimental Therapeutics, 259(3): 1165–1174. [PubMed] [Google Scholar]
- Lerman C, LeSage MG, Perkins KA, O’Malley SS, Siegel SJ, Benowitz NL, Corrigall WA (2007). Translational research in medication development for nicotine dependence. Nature Reviews: Drug Discovery, 6, 746–762. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Shelley D, Ross JT, Carroll FI, & Corrigall WA (2009). Effects of the nicotinic receptor partial agonists varenicline and cytisine on the discriminative stimulus effects of nicotine in rats. Pharmacology, Biochemistry, & Behavior, 91, 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DT, Cummings KM, Heckman BW, Li Y, Yuan Z, Smith TT, & Meza R (in press). The public health gains had cigarette companies chosen to sell very low nicotine cigarettes. Nicotine & Tobacco Research, online 2020, doi: 10.1093/ntr/ntaa128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin JH, & Caporaso NE (2006). Cigarette smoking and lung cancer: modeling total exposure and intensity. Cancer Epidemiology, Biomarkers, & Prevention, 15(3), 517–23. doi: 10.1158/1055-9965.EPI-05-0863 [DOI] [PubMed] [Google Scholar]
- Marlatt GA, & Rohsenow DJ (1980). Cognitive processes in alcohol use: Expectancy and the balanced placebo design. In Mello NK (Ed.), Advances in substance abuse: Behavioral and biological research (Vol. 1, pp. 159–199). Greenwich, CT: JAI Press. [Google Scholar]
- Meert TF (1991). Application of drug discrimination with drugs of abuse to develop new therapeutic agents. In Glennon RA, Jarbe TUC, Frankenheimer J (eds) Drug Discrimination: Applications to Drug Abuse Research, NIDA Research Monograph 116, USDHHS: Rockville MD, pp.307–323. [DOI] [PubMed] [Google Scholar]
- Mercincavage M, Lochbuehler K, Wileyto EP, Benowitz NL, Tyndale RF, Lerman C, & Strasser A (2018). Association of reduced nicotine content cigarettes with smoking behaviors and biomarkers of exposure among slow and fast nicotine metabolizers: a nonrandomized clinical trial. JAMA Open, 1(4):e181346. doi: 10.1001/jamanetworkopen.2018.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney M, Green C, & Hatsukami D (2006). Nicotine self-administration: cigarette versus nicotine gum diurnal topography. Human Psychopharmacology, 21(8), 539–548. [DOI] [PubMed] [Google Scholar]
- Notley C, Ward E, Dawkins L, & Holland R (2018). The unique contribution of e-cigarettes for tobacco harm reduction in supporting smoking relapse prevention. Harm Reduction Journal, 15, 31. doi: 10.1186/s12954-018-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA (1984). Heart rate change in Type A and Type B males as a function of response cost and task difficulty. Psychophysiology, 21, 14–21. [DOI] [PubMed] [Google Scholar]
- Perkins KA (1996). Sex differences in nicotine versus non-nicotine reinforcement as determinants of tobacco smoking. Experimental and Clinical Psychopharmacology, 4, 166–177. [Google Scholar]
- Perkins KA (2011). Nicotine discrimination in humans. Chapter 15 in Glennon RA, Young R (eds), Drug Discrimination: Application to Medicinal Chemistry and Drug Studies. New York: John Wiley & Sons, pp. 463–481. ISBN: 978-0-470-43352-2. [Google Scholar]
- Perkins KA (2019). Research on behavioral discrimination of nicotine may inform FDA policy on setting a maximum nicotine content in cigarettes. Nicotine & Tobacco Research, 21(suppl 1), S5–S12. doi: 10.1093/ntr/ntz136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Ciccocioppo M, Conklin C, Milanak M, Grottenthaler A & Sayette M (2008). Mood influences on acute smoking responses are independent of nicotine intake and dose expectancy. Journal of Abnormal Psychology, 117, 79–93. [DOI] [PubMed] [Google Scholar]
- Perkins KA, D'Amico D, Sanders M, Grobe JE, Scierka A, & Stiller RL (1996). Influence of training dose on nicotine discrimination in humans. Psychopharmacology, 126, 132–139. [DOI] [PubMed] [Google Scholar]
- Perkins KA, DiMarco A, Grobe JE, Scierka AC, & Stiller RL (1994). Nicotine discrimination in male and female smokers. Psychopharmacology, 116, 407–413. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Dubbert PM, Martin JE, Faulstich M & Harris J (1986) Cardiovascular reactivity to psychological stress in aerobically trained versus untrained mild hypertensives and normotensives. Health Psychology, 5, 407–421. http://psycnet.apa.org/fulltext/1987-27404-001.pdf [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Stiller RL, Jennings JR, Christiansen C, & McCarthy T (1986). An aerosol spray alternative to cigarette smoking in the study of the behavioral and physiological effects of nicotine. Behavior Research Methods, Instruments, & Computers, 18, 420–426. [Google Scholar]
- Perkins KA, Epstein LH, Marks BL, Stiller RL, & Jacob RG (1989) Effect of nicotine on energy expenditure during light physical activity. New England Journal of Medicine, 320, 898–903. PMID: 2927460 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Blakesley-Ball R, Stolinski A, & Wilson AS (2005a). The influence of alcohol pre-treatment on the discriminative stimulus, subjective, and relative reinforcing effects of nicotine. Behavioural Pharmacology, 16, 521–529. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Sanders M, Meeker J, & Wilson A (2001). Threshold doses for nicotine discrimination in smokers and nonsmokers. Psychopharmacology, 155, 163–170. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Sanders M, White W, & Wilson AS (1999). Effects of training dose and two- versus three-choice testing procedure on nicotine discrimination responding in humans. Psychopharmacology, 145, 418–425. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Stolinski A, Blakesley-Ball R, & Wilson AS (2005b). The influence of caffeine on nicotine’s discriminative stimulus, subjective, and reinforcing effects. Experimental and Clinical Psychopharmacology, 13, 275–281. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Broge M, Grobe JE, Sanders M, Fonte C, Vender J, Cherry C, & Wilson A (2001). Dissociation of nicotine tolerance from tobacco dependence in humans. Journal of Pharmacology & Experimental Therapeutics, 296, 849–856. [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Stiller RL, Fonte C, & Goettler JE (1992). Nasal spray nicotine replacement suppresses cigarette smoking desire and behavior. Clinical Pharmacology & Therapeutics, 52, 627–634. PMID: 1458772. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Weiss D, Fonte C, & Caggiula A (1996). Nicotine preference in smokers as a function of smoking abstinence. Pharmacology, Biochemistry, & Behavior, 55(2): 257–263. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Herb T, Karelitz JL (2019). Discrimination of nicotine content in electronic cigarettes. Addictive Behaviors, 91, 106–111. Doi: 10.1016/j.addbeh.2018.05.027. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Jacobs L, Ciccocioppo M, Conklin CA, Sayette M, & Caggiula A (2004). The influence of instructions and nicotine dose on the subjective and reinforcing effects of smoking. Experimental and Clinical Psychopharmacology, 12, 91–101. [DOI] [PubMed] [Google Scholar]
- Perkins KA, & Karelitz JL (in press). A forced choice procedure to assess the acute relative reinforcing effects of nicotine dose per se in humans. Nicotine & Tobacco Research. doi: 10.1093/ntr/ntz224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, & Karelitz JL (2020a). A procedure to standardize puff topography during evaluations of acute tobacco or electronic cigarette exposure. Nicotine and Tobacco Research, 22, 689–698. doi: 10.1093/ntr/nty261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, & Karelitz JL (2020b) Differences in acute reinforcement across reduced nicotine content cigarettes. Psychopharmacology, 237(6), 1885–1891. Doi: 10.1007/s00213-020-05509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, & Karelitz JL (2019). Acute perceptions of preferred cigarettes when blinded to brand. Tobacco Control, 28(3), 311–316. doi: 10.1136/tobaccocontrol-2018-054388. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, & Boldry MA (2017). Nicotine acutely enhances reinforcement from non-drug rewards in humans. Frontiers in Psychiatry (Addictive Disorders section), 8, 65. doi: 10.3389/fpsyt.2017.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Conklin CA, Sayette MA, & Giedgowd GE (2010). Acute negative affect relief from smoking depends on the affect measure and situation, but not on nicotine. Biological Psychiatry, 67, 707–714. DOI: 10.1016/j.biopsych.2009.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, & Kunkle N (2018). Evaluation of menthol per se on acute perceptions and behavioral choice of cigarettes differing in nicotine content. Journal of Psychopharmacology, 32(3), 324–331. doi: 10.1177/0269881117742660 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Kunkle N, & Karelitz JL (2017a). Threshold dose for behavioral discrimination of cigarette nicotine content in menthol vs. non-menthol smokers. Psychopharmacology, 234, 1255–1265. DOI: 10.1007/s00213-017-4563-3 . [DOI] [PubMed] [Google Scholar]
- Perkins KA, Kunkle N, & Karelitz JL (2017b). Preliminary test of cigarette nicotine discrimination threshold in non-dependent versus dependent smokers. Drug & Alcohol Dependence, 175, 36–41. doi: 10.1016/j.drugalcdep.2017.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Kunkle N, Michael VC, Karelitz JL, & Donny EC (2016a). Assessing discrimination of nicotine in humans via cigarette smoking. Nicotine & Tobacco Research, 18, 1830–1836. DOI: 10.1093/ntr/ntw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Kunkle N, Karelitz JL, Michael VC, & Donny EC (2016b). Threshold dose for discrimination of nicotine via cigarette smoking. Psychopharmacology, 233, 2309–2317. doi: 10.1007/s00213-016-4281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA & Lerman C (2014). An efficient early Phase 2 procedure to screen medications for efficacy in smoking cessation. Psychopharmacology, 231, 1–11, DOI: 10.1007/s00213-013-3364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Sanders M, Fonte C, Wilson AS, White W, Stiller R, & McNamara D (1999). Effects of central and peripheral nicotinic blockade on human nicotine discrimination. Psychopharmacology, 142, 158–164. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Sexton JE, Reynolds WA, Grobe JE, Fonte C, & Stiller RL (1994). Comparison of acute subjective and heart rate effects of nicotine intake via tobacco smoking vs. nasal spray. Pharmacology, Biochemistry, & Behavior, 47, 295–299. [DOI] [PubMed] [Google Scholar]
- Perkins KA, & Stitzer M (1998) Behavioral pharmacology of nicotine. In Tarter RL, Ammerman RT, & Ott P (eds), Handbook of substance abuse: neurobehavioral pharmacology. New York: Ayllon & Bacon, pp. 299–317. [Google Scholar]
- Porter JH, Prus AJ, & Overton DA (2018). Drug Discrimination: historical origins, important concepts, and principles. In Porter JH, Prus AJ (eds) The Behavioral Neuroscience of Drug Discrimination. Current Topics in Behavioral Neuroscience, 39, 3–26. Doi: 10.1007/7854_2018_40. [DOI] [PubMed] [Google Scholar]
- Prochaska JJ, Das S, & Young-Wolff KC (2017). Smoking, mental illness, and public health. Annual Review of Public Health 38, 165–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor RN (2020). The cigarette: a political history—the past, present, and future of U.S. tobacco. JAMA, 324(1), 10–11. Doi: 10.1001/jama.2020.8237. [DOI] [PubMed] [Google Scholar]
- Ray R, Jepson C, Patterson F, Strasser AA, Rukstalis M, Perkins K, Lynch K, O'Malley S, Berrettini W, & Lerman C (2006). Association of OPRM1 Asn40Asp variant with the relative reinforcing value of nicotine in female smokers. Psychopharmacology, 188, 355–363. DOI: 10.1007/s00213-006-0504-2 [DOI] [PubMed] [Google Scholar]
- Renteria E, Jha P, Forman D, & Soerjomataram I (2016). The impact of cigarette smoking on life expectancy between 1980 and 2010: a global perspective. Tobacco Control, 25: 551–557. doi: 10.1136/tobaccocontrol-2015-052265. [DOI] [PubMed] [Google Scholar]
- Richter P, Pappas RS, Bravo R, et al. (2016). Characterization of SPECTRUM variable nicotine research cigarettes. Tobacco Regulatory Science, 2(2), 94–105. Doi: 10.18001/TRS.2.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosecrans JA, & Chance WT (1977). Cholinergic and noncholinergic aspects of the discriminative stimulus properties of nicotine. In: Lal H (ed) Discriminative stimulus properties of drugs. Plenum, New York, pp 155–185. [Google Scholar]
- Rosecrans JA (1989). Nicotine as a discriminative stimulus: a neurobehavioral approach to studying central cholinergic mechanisms. Journal of Substance Abuse, 1, 287–300. [PubMed] [Google Scholar]
- Rukstalis M, Jepson C, Strasser A, Lynch KG, Perkins KA, Patterson F & Lerman CA (2005). Naltrexone reduces the relative reinforcing value of nicotine in a cigarette smoking choice paradigm. Psychopharmacology, 181, 41–48. [DOI] [PubMed] [Google Scholar]
- Schuster CR, & Johanson CE (1988). Relationship between the discriminative stimulus properties and subjective effects of drugs. In Colpaert FC, & Balster RL (eds), Transduction Methods of Drug Stimuli. Psychopharmacology Supplement Series, Berlin: Springer-Verlag, pp. 161–175. [DOI] [PubMed] [Google Scholar]
- Shiffman S (1989) Tobacco “chippers”: individual differences in tobacco dependence. Psychopharmacology, 97, 539–547 [DOI] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Ferguson SG (2015). Stimulus control in intermittent and daily smokers. Psychology of Addictive Behaviors, 29(4), 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, & Perkins KA (2020). Preclinical and clinical research on the discriminative stimulus effects of nicotine. Neuropharmacology, 170:108063. DOI: 10.1016/j.neuropharm.2020.108063. [DOI] [PubMed] [Google Scholar]
- Siegel S (1991). Tolerance: role of conditioning processes. In Glennon RA, Jarbe TUC, Frankenheimer J (eds) Drug Discrimination: Applications to Drug Abuse Research, NIDA Research Monograph 116, USDHHS: Rockville MD, pp. 213–229. [PubMed] [Google Scholar]
- Smith BJ, & Bickel WK 1999. The novel-response procedure in humans. Pharmacology, Biochemistry, & Behavior, 64(2), 245–250. [DOI] [PubMed] [Google Scholar]
- Smith JW, & Stolerman IP (2009). Recognising nicotine: the neurobiological basis of nicotine discrimination. In Henningfield JE, London E, Pogun S (eds) Nicotine Psychopharmacology, New York: Springer, pp. 295–333. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, & LeSage MG (2012). The reinforcement threshold for nicotine as a target for tobacco control. Drug and Alcohol Dependence, 125, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helen G St., Jacob P, Nardone N, & Benowitz NL (2018). IQOS: examination of Philip Morris International’s claim of reduced exposure. Tobacco Control, 27(suppl 1):s30–s36. Doi: 10.1136/tobaccocontrol-2018-054321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP (1999) Inter-species consistency in the behavioural pharmacology of nicotine dependence. Behavioural Pharmacology; 10:559–580. [DOI] [PubMed] [Google Scholar]
- Stolerman IP (1987). Psychopharmacology of nicotine: stimulus effects and receptor mechanisms. In: Iversen LL, Iversen SD, & Snyder SH (eds) Handbook of psychopharmacology, vol 19. Plenum, New York, pp 421–465. [Google Scholar]
- Stolerman IP, Garcha HS, Pratt JA, & Kumar R (1984). Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology, 84, 413–419. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Goldfarb T, Fink R, & Jarvik ME (1973). Influencing cigarette smoking with nicotine antagonists. Psychopharmacologia, 28(3), 247–259. 10.1007/BF00429305 [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Rauch RJ, & Norris EA (1987). Discriminative stimulus effects of a nicotine-midazolam mixture in rats. Psychopharmacology, 93, 250–256. [DOI] [PubMed] [Google Scholar]
- Takada K (1996). Drug discrimination studies in humans: a review of methodologies. Methods and Findings in Experimental Clinical Pharmacology, 18 (suppl 1), 187–196. [Google Scholar]
- Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, Hartge P, & Gapstur SM (2013). 50-year trends in smoking-related mortality in the United States. New England Journal of Medicine, 368, 351–364. Doi: 10.1056/NEHMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Dept of Health, Education and Welfare (USDHEW, 1964) Smoking and health: report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington: USDHEW, PHS, Center for Disease Control, 1964. PHS Publication No. 1103. [Google Scholar]
- U.S. Department of Health and Human Services. (USDHHS, 1988). The Health Consequences of Smoking: Nicotine Addiction. A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. DHHS Publication No. (CDC) 88–8406. [Google Scholar]
- US Department of Health and Human Services (USDHHS, 2001). Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine. Smoking and Tobacco Control Monograph No. 13, Bethesda MD. Public Health Service, National Cancer Institute. NIH Publication No 02-5047. [Google Scholar]
- US Department of Health and Human Services (USDHHS, 2014). The Health Consequences of Smoking–50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [Google Scholar]
- Wayne GF, & Carpenter CM (2009). Tobacco industry manipulation of nicotine dosing, in Henningfield JE, London E, & Pogun S (Eds) Nicotine Psychopharmacology. New York: Springer-Verlag, pp. 457–485. [DOI] [PubMed] [Google Scholar]
- Wiley JL, James JR, & Rosecrans JA (1995). Discriminative stimulus properties of nicotine: approaches to evaluating potential nicotinic receptor agonists and antagonists. Drug Development Research, 38, 222–230. [Google Scholar]
- World Health Organization (WHO) (2015). Advisory Note: Global nicotine reduction strategy. WHO Study Group on Tobacco Product Regulation. Geneva: WHO Press. [Google Scholar]
- Zacny JP, & Stitzer ML (1988). Cigarette brand-switching: effects on smoke exposure and smoking behavior. Journal of Pharmacology & Experimental Therapeutics, 246(2), 619–627. [PubMed] [Google Scholar]
- Zeller M (2019). The future of nicotine regulation: key questions and challenges. Nicotine & Tobacco Research, 21, 331–332. Doi: 10.1093/ntr/nty200. [DOI] [PubMed] [Google Scholar]


