Abstract
Objectives
Immune dysregulation contributes to the development of RA. Altered surface expression patterns of integrin adhesion receptors by immune cells is one mechanism by which this may occur. We investigated the role of β2 integrin subunits CD11a and CD11b in dendritic cell (DC) subsets of RA patients.
Methods
Total β2 integrin subunit expression and its conformation (‘active’ vs ‘inactive’ state) were quantified in DC subsets from peripheral blood (PB) and SF of RA patients as well as PB from healthy controls. Ex vivo stimulation of PB DC subsets and in vitro-generated mature and tolerogenic monocyte-derived DCs (moDCs) were utilized to model the clinical findings. Integrin subunit contribution to DC function was tested by analysing clustering and adhesion, and in co-cultures to assess T cell activation.
Results
A significant reduction in total and active CD11a expression in DCs in RA SF compared with PB and, conversely, a significant increase in CD11b expression was found. These findings were modelled in vitro using moDCs: tolerogenic moDCs showed higher expression of active CD11a and reduced levels of active CD11b compared with mature moDCs. Finally, blockade of CD11b impaired T cell activation in DC–T cell co-cultures.
Conclusion
For the first time in RA, we show opposing expression of CD11a and CD11b in DCs in environments of inflammation (CD11alow/CD11bhigh) and steady state/tolerance (CD11ahigh/CD11blow), as well as a T cell stimulatory role for CD11b. These findings highlight DC integrins as potential novel targets for intervention in RA.
Keywords: dendritic cells, integrins, rheumatoid arthritis, tolerogenic dendritic cells, immune regulation
Rheumatology key messages
Dendritic cell integrins are strikingly altered in the RA joint.
Mature and tolerogenic dendritic cells provide a model for this differential integrin response.
Opposing functions of integrin subunits CD11a and CD11b impact on T cell activation.
Introduction
In autoimmune diseases such as RA, genetic risk factors together with environmental factors lead to a breach in immune tolerance to self-antigens. The following asymptomatic phase, where T and B cells become activated and auto-antibody is produced, can last for months or years before presenting as clinical symptoms [1]. Currently, there is no cure for RA: therapeutics control disease activity and treat the symptoms [2], but do not restore immune tolerance.
Dendritic cells (DCs) are the key cell type that controls the balance between immune activation and tolerance. Through direct cellular interactions, DCs direct T cell tolerance under steady-state conditions and induce T cell activation against pathogens. It is likely that DCs contribute to both the initiation and perpetuation of RA (reviewed in [3]). First, DCs are the major antigen-presenting cell population, priming naïve T cells in secondary lymphoid tissues. In RA, these activated T cells are major contributors to local (joint) and systemic inflammation. Second, DCs contribute to the perpetuation of RA by acting locally in the inflamed joint. They present antigen within the joint tissue that promotes auto-reactive T cell responses [4], produce a myriad inflammatory factors that drive innate cell activation and effector responses [5, 6], and potentially contribute to the development of ectopic lymphoid structures [7]. Different DC subsets are specialized to direct different types of T cell response [8]: conventional DC1 (cDC1) are capable of cross presentation of antigen to CD8+ T cells; cDC2 are dynamic cells that initiate different types of CD4+ T cell responses (e.g. Th1, Th2, Th17) depending on the inflammatory signals they receive; whilst plasmacytoid DC (pDC) rapidly produce type I IFN, promoting CD8+ T cell activation. Each of these DC subsets is known to infiltrate the joint during RA, where their inflammatory phenotype suggests they contribute to pathology [9]. Altered behaviour of DCs is likely a key factor in the development of autoimmunity, but also presents a potential therapeutic opportunity.
Integrins are heterodimeric adhesion receptors present on the surface of cells that bind ligands on other cells and extracellular matrix components. The β2 integrin family, consisting of four members (the β2 subunit, CD18, paired with one of four α subunits αL CD11a, αM CD11b, αX CD11c, or αD CD11d), are expressed exclusively by leukocytes and control various aspects of DC function [10]. In mouse models, β2 integrins have been shown to directly regulate the activation status of DCs in steady state [11], thus restricting T cell priming [11] and limiting inflammation [12]. Additionally, β2 integrins expressed by DCs regulate contact dynamics with T cells thereby influencing T cell priming [13, 14]. Importantly, the involvement of β2 integrins in the regulation of DC subset function in humans is yet to be characterized. However, their role in immune regulation is supported by evidence from patients with β2 integrin deficiencies, termed leukocyte adhesion deficiencies, where increased incidence of IBD has been reported [15, 16].
Given the clear role for β2 integrins in regulating DC function, we hypothesized that the integrin expression and/or conformation profile in infiltrating DCs in the RA joint contributes to the failure of synovitis to resolve in this condition. Our ‘first in RA’ study demonstrates opposing expression of the integrin subunits CD11a and CD11b in DCs in environments of inflammation (including RA) and steady state/tolerance, potentially highlighting these integrin subunits as novel targets for intervention in RA treatment.
Methods
Subjects
RA patient volunteers were recruited from routine outpatient clinics at Gartnavel General Hospital (National Health Service Greater Glasgow and Clyde). All patients fulfilled classification criteria for RA according to international criteria [17] and provided full written informed consent. Healthy donors were recruited locally with full written informed consent. The study complies with the Declaration of Helsinki and was approved by the University of Glasgow Research Ethics Committee [reference numbers 2012073 for healthy control peripheral blood (PB); 14/WS/1035 and 16/WS/0207 for RA patient samples]. All samples were anonymized using a unique identifying number.
Cell isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from PB, SF or buffy coat by density gradient centrifugation utilizing Lymphoprep (Axis-Shield POC AS, Oslo, Norway) on the date of draw. To isolate naïve CD4+ T cells (CD4+CD45RO–), buffy coat was enriched for T cells using the Rosettesep Human CD4+ enrichment cocktail (Stemcell Technologies, Grenoble, France) before excluding CD45RO+ cells using anti-CD45RO magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. CD14+ monocytes were isolated by positive selection according to manufacturer guidelines (Miltenyi Biotec, Bergisch Gladbach, Germany).
PBMC stimulation
PBMCs were plated into 48 well plates (Corning, New York, NY, USA) at 1.5 × 106 cells/well and stimulated for 18 h at 37°C with 5% CO2: untreated, lipopolysaccharide (LPS; 100 ng/ml, Sigma-Aldrich, St Louis, MO, USA), IL-1β+TNF (both 10 ng/ml, Immunotools, Friesoythe, Germany), IL-10+TGF-β (both 10 ng/ml, Immunotools, Friesoythe, Germany). All stimulations were performed in duplicate.
Generation of moDCs
Monocyte-derived DCs (moDCs) were generated as previously described [18]. Briefly, isolated CD14+ monocytes were cultured in IL-4 and GM-CSF (50 ng/ml each; ImmunoTools, Friesoythe, Germany). Tolerogenic moDCs (tolDCs) received 10−6 M dexamethasone on day 3, which was refreshed on day 6 with the further addition of 10−10 M vitamin D3 and 0.1 µg/ml of LPS (Sigma-Aldrich, St Louis, MO, USA). Mature moDCs (matDCs) received 0.1 µg/ml LPS on day 6 of culture. Immature moDCs received no stimuli. All cells were harvested on day 7 after incubation on ice for 60 min to increase cell detachment.
Quantification of β2 integrin expression by flow cytometry
Staining of total and active β2 integrin subunits was performed at 37°C for 30 min. A negative control containing no integrin antibodies, a negative control containing isotype control antibodies and a positive control treated with phorbol myristate acetate (concurrently with antibody staining) were included in every experiment. All flow cytometry staining and washing steps were executed using cold FACS buffer, consisting of PBS plus 3% foetal calf serum, 0.01% sodium azide (Sigma-Aldrich, St Louis, MO, USA) and 1 mM EDTA, and in the presence of human IgG (Sigma-Aldrich, St Louis, MO, USA) and 1 mM magnesium chloride (Sigma-Aldrich, St Louis, MO, USA). For β2 integrin detection the following antibodies were used (Biolegend, San Diego, CA, USA; unless otherwise stated): CD11a active (MEM83, NovusBio, Littleton, CO, USA), CD11a total (HI111), CD11b active (CBRM1/5), and CD11b total (ICRF44). The following antibodies were used to define the populations of interest: CD1c, CD11c, CD16, CD141 (Biolegend, San Diego, CA, USA), CD14, CD45, HLA-DR (BD Biosciences, San Jose, CA, USA) and CD3, CD19, CD20 and CD56 (ebioscience, San Diego, CA, USA). The DC gating strategy is shown in supplementary Fig. S1, available at Rheumatology online. All flow cytometry samples were acquired using a BD Fortessa and data was analysed using Flowjo software (BD Biosciences, San Jose, CA, USA).
moDC–T cell co-cultures
Naïve CD4+ T cells were stained with 5 µM CellTrace Violet Proliferation Dye (CTV, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. moDC (matDC or tolDC; 5 × 104 cells/well) were co-cultured with allogeneic naïve CD4+ T cells (5 × 105 cells/well) in 48 well plates (Corning, New York, NY, USA) at 37°C, 5% CO2. Where indicated, 20 µg/ml CD11b blocking antibody or relevant isotype control (both Biolegend, San Diego, CA, USA) were added. CD11b blocking antibody/isotype control were replenished on day 4. On day 6, T cell proliferation was analysed by flow cytometry and supernatants were collected for IFNγ and IL-10 quantification by ELISA (Biolegend, San Diego, CA, USA) according to the manufacturer’s instructions.
Analysis of moDC clustering
moDC clustering was analysed on a Leica DMi8 microscope (Leica Microsystems, Wetzlar, Germany) with a live cell chamber maintaining 37°C and 5% CO2. matDCs and tolDCs were plated onto glass-bottomed 24-well plates (200 000 cells/well) and imaged 6 h later. A cellprofiler pipeline was designed to identify clusters of cells and single non-clustered cells for quantification of number, size and radius (supplementary Fig. S2, available at Rheumatology online). Clusters were defined as cells that had neighbouring cells with touching borders.
Adhesion assay
Fibronectin (10 µg/ml, R&D Systems, Minneapolis, MN, USA) was coated overnight at 4°C onto COSTAR 96 well high-binding assay plates (Corning, New York, NY, USA). Wells were blocked with 1% milk for 1 h 15 min at 37°C. moDCs were resuspended in adhesion medium consisting of RPMI 1640, 0.1% BSA, 20 mM HEPES (pH 7.25) and 2 mM magnesium chloride (all from Sigma-Aldrich, St Louis, MO, USAs), before being added to wells at 25 × 103 moDCs/well in duplicate or triplicate. Cells were allowed to adhere for 15 min at 37°C before plates were gently washed. Cell lysis and detection was performed according to Matthews et al. [19].
Statistical analysis
Prism software (Graphpad, San Diego, CA, USA) was used for all statistical analysis. All datasets were subjected to testing for normal distribution using the Shapiro–Wilk normality test. Normally distributed data underwent a paired or unpaired Student’s t-test (for two groups) or one-way ANOVA (for more than two groups). If data were not normally distributed, a Wilcoxon matched-pairs signed rank test was used if comparing two paired groups, otherwise a Mann–Whitney U test was used. Graphs show mean (s.d.) unless stated otherwise in the legend. P-values shown are as follows: *P < 0.05, **P < 0.01, ***P < 0.001.
Results
CD11a expression by DCs is strikingly reduced in the RA joint, while CD11b expression is increased
Expression of total integrins (i.e. low- and high-affinity forms) and active integrins (only the high-affinity conformation that can mediate binding) was measured in RA patients with active disease (DAS28 ESR >3.2), RA patients in remission (DAS28 ESR <2.6) and healthy controls. Three different DC populations were analysed, namely cDC1, cDC2 and pDC (gating strategy shown in supplementary Fig. S1, available at Rheumatology online), which are known to infiltrate the RA joint and promote T cell activation [9].
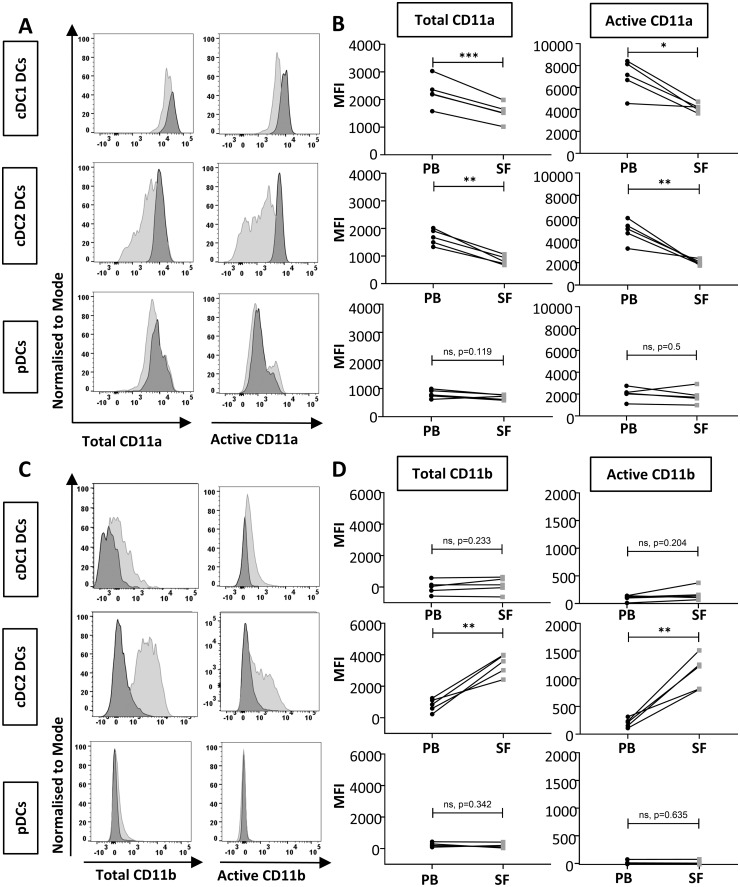
Integrin expression in circulating DC populations isolated from RA patients was equivalent to that in healthy controls, regardless of the integrin subunit in question (CD11a or CD11b), the conformation of the integrin (total or active), the DC population (cDC1, cDC2 or pDC) or the inflammatory burden (active RA or remission) (supplementary Fig. S3, clinical characteristics shown in supplementary Table S1, both available at Rheumatology online). However, analysis of matched SF and PB samples identified striking changes in integrin subunit expression by DCs (Fig. 1; clinical characteristics shown in supplementary Table S2, available at Rheumatology online). Expression of both total and active CD11a was significantly decreased in cDC1 and cDC2 cells, but not pDCs, isolated from SF compared with PB (Fig. 1A and B), whilst expression of total and active CD11b was significantly increased in SF compared with PB in cDC2 cells only (Fig. 1C and D). Together, these data indicate that β2 integrin α subunits CD11a and CD11b are differentially regulated in DCs in the RA joint.
Fig. 1.
Reduced CD11a and increased CD11b expression in cDC2s in SF compared with PB in RA
Expression of total and active CD11a (A, B) and CD11b (C, D) comparing PB (dark grey) versus matched SF (light grey) from the same RA patient in cDC1, cDC2 and pDCs. Representative histograms (A and C) and pooled data displaying MFI (B and D) are shown. n = 5 paired samples. As n was too small for normality testing, paired Student’s t-test was used to calculate statistical significance; *P < 0.05, **P < 0.01, ***P < 0.001. cDC: conventional dendritic cell; pDC: plasmacytoid dendritic cell; PB: peripheral blood; MFI: median fluorescence intensity.
CD11a expression is reduced in DCs in response to inflammatory stimuli
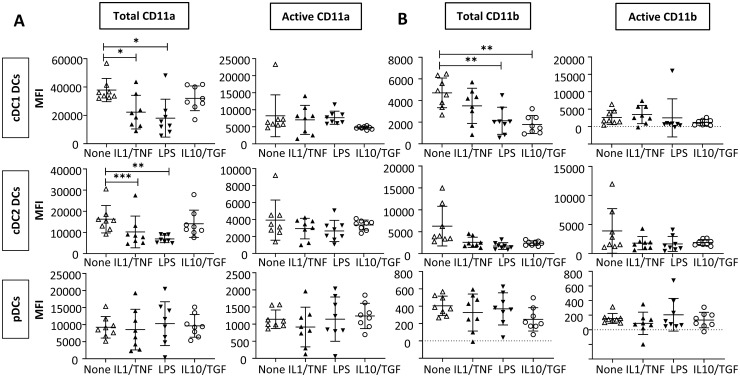
Next, we questioned whether these changes in integrin subunit expression could be modelled in vitro with short-term stimulation. Given that the joint is the main focus of inflammation in RA, we hypothesized that DCs would lose CD11a in response to pro-inflammatory stimuli and show an increase in CD11b, in line with our findings in SF DCs. To test this hypothesis, unsorted PBMCs from healthy subjects (containing immature DCs) were treated with pro- (LPS, IL-1/TNF-α) and anti- (IL-10/TGF-β) inflammatory stimuli, and the effect on expression of total and active integrin subunits was measured in each DC subset present within the PBMC population (Fig. 2).
Fig. 2.
CD11a expression is reduced in DCs in response to inflammatory stimuli
PBMCs were isolated from healthy controls and either left untreated (none) or stimulated with IL-1/TNF-α (10 ng/ml each), LPS (100 ng/ml) or IL-10/TGF-β (10 ng/ml each) for 18 h. cDC1, cDC2 and pDCs were gated as per the gating strategy shown in supplementary Fig. S1 available at Rheumatology online. MFI of expression of integrins CD11a (A) and CD11b (B) is shown. n = 8, pooled from two independent experiments. All data are normally distributed. Repeated measures matched one-way ANOVA was used to calculate statistical significance; *P < 0.05, **P < 0.01, ***P < 0.001. DC: dendritic cell; PBMC: peripheral blood mononuclear cell; LPS: lipopolysaccharide; cDC: conventional DC; pDC: plasmacytoid DC; MFI: median fluorescence intensity.
Total CD11a expression was significantly reduced in cDC1 and cDC2 DCs treated with the pro-inflammatory stimuli, LPS and IL-1/TNF-α when compared with the untreated control, while total CD11a expression was not affected by IL-10/TGF-β treatment (Fig. 2A), mirroring our observations in RA patient PB vs the inflammatory milieu of the joint. In contrast, CD11a conformation was not significantly affected by the addition of pro- or anti-inflammatory stimuli in either cDC1 or cDC2 DCs, with neither expression nor conformation affected in pDCs. These data indicate that CD11a expression is reduced in cDCs in inflammatory environments.
The predicted increase in total and/or active CD11b in response to inflammatory stimuli was not observed in any of the DC subsets. Indeed, total CD11b expression by cDC1 cell exposed to such stimuli was paradoxically reduced compared with that in untreated control cells (Fig. 2B). Total CD11b expression was also reduced in cDC1 cells treated with IL-10/TGF-β.
Together, these data suggest exposure to inflammatory stimuli have the potential to shape patterns of integrin expression in RA.
tolDCs display increased CD11a and reduced CD11b expression compared with matDCs
Due to the widespread use of moDCs as an ex vivo model of DCs, together with the recognized therapeutic potential of tolDCs in RA (reviewed in [20] and [21]), we next determined whether alterations in DC integrin subunit expression could be modelled in matDCs and tolDCs. matDCs and tolDCs were generated by in vitro culture of monocytes and their phenotype was confirmed by flow cytometry (supplementary Fig. S4A–C, available at Rheumatology online [22, 23]). Importantly, tolDCs were confirmed to induce reduced levels of T cell activation compared with matDCs (supplementary Fig. S4D, available at Rheumatology online).
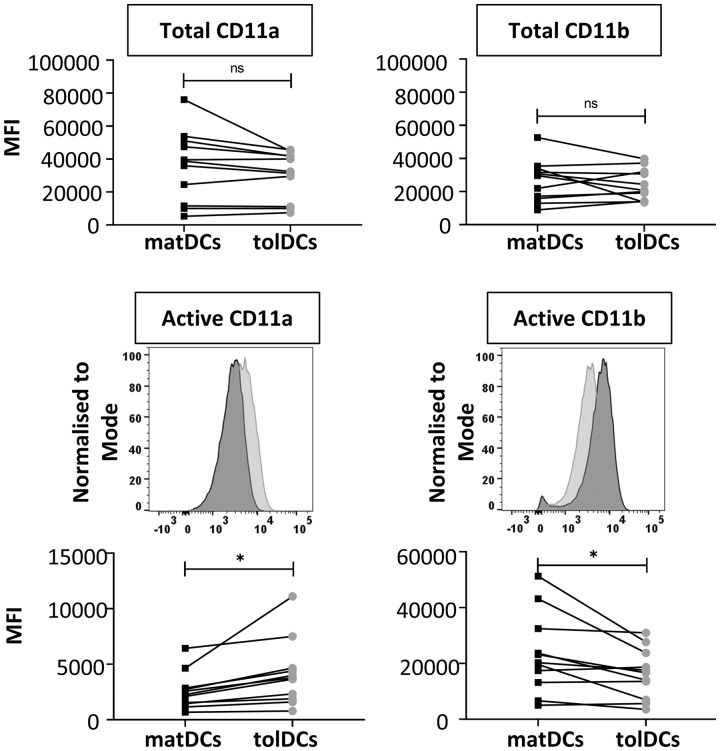
Expression of CD11a and CD11b was quantified in matDCs and tolDCs (Fig. 3). No differences in expression of total CD11a or total CD11b were observed between matDCs and tolDCs. However, tolDCs expressed significantly increased levels of active CD11a, but significantly reduced levels of active CD11b compared with matDCs. Together, these data support the notion that the inflammatory phenotype of DCs is reciprocally linked to their CD11a/b activity.
Fig. 3.
tolDCs display increased CD11a and reduced CD11b expression compared with matDCs
Monocyte-derived mature (matDCs) and tolerogenic (tolDCs) DC expression of β2 integrin subunits CD11a and CD11b is shown. Representative histograms are shown for active integrin subunit expression only, with matDCs in dark grey and tolDCs in light grey. n = 13. Total CD11a data are not normally distributed; Wilcoxon matched-pairs signed rank test was used to calculate statistical significance. All other data were normally distributed; paired Student’s t-test was used; *P < 0.05. DC: dendritic cell; MFI: median fluorescence intensity.
tolDCs show reduced clustering and adhesion compared with matDCs
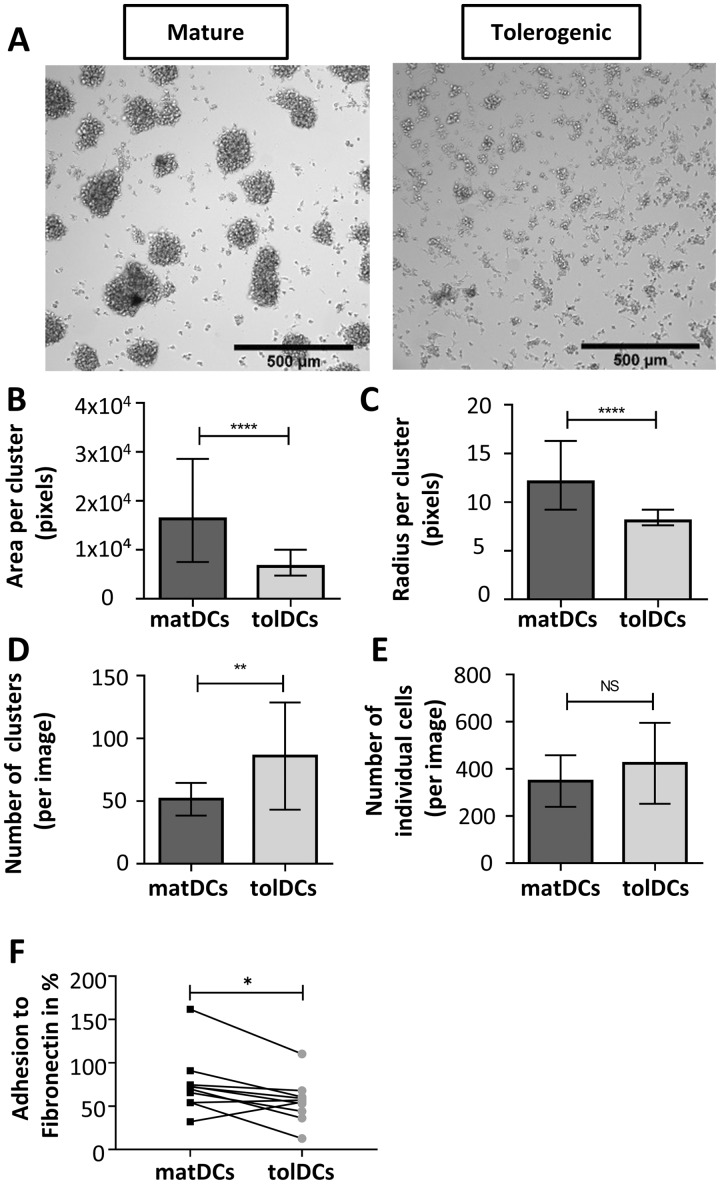
Using monocyte-derived matDCs and tolDCs as a model of endogenous DCs under inflammatory and tolerogenic conditions, respectively [24], we next determined the functional impact of differential expression of the integrin subunits, CD11a and CD11b, in DCs. During immune responses in vivo, DCs and T cells form clusters in secondary lymphoid tissues that promote long-lived immune interactions and T cell activation [25]. Therefore, the clustering behaviour of moDCs was quantified by imaging on glass slides (Fig. 4A;supplementary Fig. S2, available at Rheumatology online). matDCs showed increased clustering compared with tolDCs (Fig. 4A). Quantification of cluster size confirmed that matDCs form clusters with significantly larger area and mean radius compared with tolDCs (Fig. 4B and C). To further quantify clustering ability of different moDC subtypes, the number of clusters formed and number of unclustered cells was compared (Fig. 4D and E). The data show that matDCs formed significantly fewer clusters compared with tolDCs, but that they are large in size, as shown in Fig. 4B. The number of unclustered cells was equivalent between matDCs and tolDCs (Fig. 4E). These data demonstrate that clustering is significantly reduced in tolerogenic compared with mature moDCs.
Fig. 4.
tolDCs show reduced clustering and adhesion compared with matDCs
Clustering analysis (see supplementary Fig. S2 available at Rheumatology online) was performed on monocyte-derived mature (matDCs) and tolerogenic (tolDCs) dendritic cells plated on glass slides for 6 h. (A) Representative bright field microscopy images. (B) Area (number of pixels per cluster), where a cluster size cut-off was set as 40 μm (approx. 2× cell diameter). (C) Median radius, where a cluster size cut-off was set as 20 μm radius. (B and C) Mature = 1493, tolerogenic = 2405 individual clusters; not normally distributed; Mann–Whitney U test. (D, E) Number of clusters and unclustered cells. Mature n = 29, tolerogenic n = 28; data are normally distributed; Student’s t-test. (F) Adhesion of matDCs and tolDCs to fibronectin. n = 10; not normally distributed; Wilcoxon test. For (A–F), three independent experiments (different donors) were performed. Mean (s.d.) is shown except in (B) and (C), where median and interquartile range is shown. In all cases *P < 0.05, **P < 0.01, ***P < 0.001.
Considering the differences in integrin subunit expression by matDCs and tolDCs and the striking effects on cell clustering, we next measured moDC adhesiveness to the matrix component fibronectin. The adhesiveness of DCs to matrix is thought to inversely correlate with their ability to migrate to the T cell zones of secondary lymphoid tissues [11]. Adhesion to fibronectin is mediated by several integrin subunits, with CD11b having a higher affinity than CD11a [26]. Adhesion to fibronectin was reduced in tolDCs compared with matDCs (Fig. 4D).
Together, these data suggest that alterations in integrin activity in tolDC result in reduced cell–cell clustering and adhesion to fibronectin compared with matDC.
Blockade of CD11b on matDCs reduces their ability to induce T cell activation
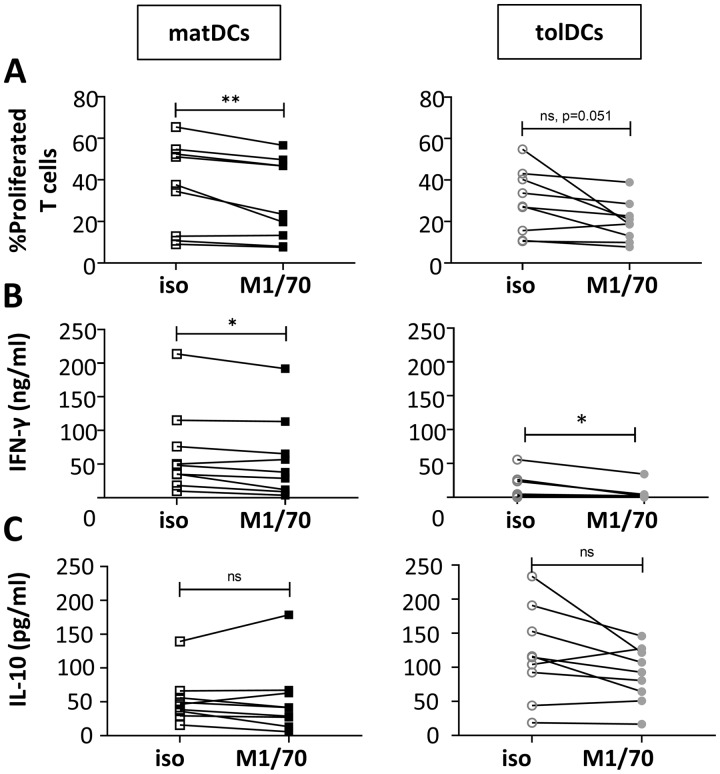
Finally, we investigated the functional impact of β2 integrin subunit expression by DCs on T cell activation. To this end, the integrin subunit CD11b, which, unlike CD11a, is not expressed by T cells (supplementary Fig. S5, available at Rheumatology online), was blocked in DC–T cell co-cultures containing either matDCs or tolDCs together with allogeneic naïve CD4+ T cells. Blockade of CD11b on matDCs resulted in significantly reduced T cell proliferation (Fig. 5A) and production of the Th1 cytokine IFNγ (Fig. 5B). A similar trend of reduced T cell proliferation was observed in tolDC cultures with CD11b blockade (Fig. 5A), and reduced IFNγ production was also observed (Fig. 5B). IL-10 production was not affected by CD11b blockade in either moDC–T cell co-culture (Fig. 5C), though the source of this IL-10 (DCs or T cells) was not confirmed.
Fig. 5.
Blockade of CD11b on matDCs reduces their ability to induce T cell activation
Anti-CD11b blocking antibody (M1/70) or isotype control (iso; rat IgG2bκ) was added to DC–T cell co-cultures. (A) T cell proliferation was quantified on day 6 of culture by analysis of Cell Trace Violet dilution. Concentrations of IFNγ (B) and IL-10 (C) in co-culture supernatants on day 6 were measured by ELISA. n = 9 moDCs, cultured with n = 3 naïve T cells. In (A) and (C), data are normally distributed; paired Student’s t-test was performed. In (B) data are not normally distributed; Wilcoxon test was performed. *P < 0.05, **P < 0.01. DC: dendritic cell; moDC: monocyte-derived DC; matDC: mature moDC; tolDC: tolerogenic moDC.
These data reveal that CD11b expressed by matDCs functions to promote T cell priming. In turn, these findings suggest that the reduction of active CD11b in tolDCs when compared with matDCs may contribute to their tolerogenic function.
Discussion
Using RA patient samples and in vitro DC models, this study demonstrates for the first time that β2 integrin subunits CD11a and CD11b have distinct and opposing roles in human DCs: high CD11a expression in steady state/tolerogenic DCs supports previous findings of an immunoregulatory role, while high CD11b was observed in inflammatory DCs and promoted T cell activation. These findings highlight the integrin expression phenotype of DCs as a potentially targetable regulator of tissue inflammation in immune-mediated diseases such as RA.
We consistently found reduced expression of CD11a (either total, active or both) by DCs in inflammatory contexts. This was true (i) in cDC1 and cDC2 cells from RA patient SF compared with circulating PB DCs; (ii) in circulating cDC1 and cDC2 cells treated with pro-inflammatory stimuli LPS or IL-1/TNF-α; and (iii) in monocyte-derived matDCs compared with tolDCs. The down-regulation of CD11a in response to both short-term stimulation with cytokines and pathogen-derived factors (LPS) in vitro and chronic inflammation in vivo (in RA patient SF) indicate that this is likely a general response to inflammation rather than an RA-specific phenomenon. In the future, it will be important to determine whether other factors present in the RA joint such as cytokines (e.g. IL-6), growth factors (e.g. GM-CSF), and host-derived stress molecules (e.g. HSPs) induce similar changes in integrin expression profiles in DCs. Our current model is that CD11a plays a regulatory role in DCs that is down-regulated in the context of inflammation. Although the number of studies is few, previous evidence does support a functional immunoregulatory role for CD11a on DCs: constitutive activation of CD11a on murine bone marrow-derived DCs reduced their ability to stimulate T cell proliferation both in vitro and in vivo [13]; meanwhile, high levels of ‘shed’ CD11a have been reported in synovial effusions [27], suggesting that CD11a is actively lost from the cell surface in response to inflammatory stimuli. As technical challenges prevented us from blocking DC CD11a in the moDC–T cell co-cultures (as T cells highly express CD11a; supplementary Fig. S5, available at Rheumatology online), further studies are required to fully explore the direct and indirect functional effects of CD11a expression by DCs in T cell activation and tolerance.
Conversely, the findings from our study highlight a pro-inflammatory role for CD11b expressed by DCs that actively promotes T cell activation. Expression of both total and active CD11b was significantly increased in cDC2 cells in the RA joint compared with PB, whilst active CD11b expression was elevated in matDCs compared with tolDCs. However, stimulation of PB DCs did not model this result, potentially due to limitations with the in vitro system, differences between DCs in healthy controls compared with RA patients, or that the upregulation of CD11b requires long-term (chronic) rather than short-term stimulation. Importantly, however, blockade of CD11b in moDC–T cell co-cultures reduced the ability of matDCs to activate T cells. This appears to be a novel finding. Indeed, several studies instead report a regulatory role for CD11b in DCs: DC CD11b reportedly inhibits T cell activation [14], whilst CD11b ligation in DCs reduces pro-inflammatory cytokine production and renders DCs more tolerogenic [28, 29]. Furthermore, CD11b has repeatedly been described as a negative regulator of Toll-like receptor (TLR) signalling [30, 31] and was shown to induce production of the anti-inflammatory cytokine IL-10 [32]. In mice, CD11b was shown to have an essential role in preventing Th17 responses, in inducing oral tolerance and in resistance to collagen-induced arthritis via the suppression of DC-derived IL-6 [33, 34]. Similarly, ligation of CD11b on human moDCs down-regulated pro-inflammatory cytokine production and efficiently restricted Th17 cell expansion [35].
Despite these numerous reports of CD11b involvement in immune regulation, our CD11b blockade data clearly demonstrate a functional relevance of CD11b in promoting T cell activation. In support of this, one previous study showed a reduction in T cell proliferation by CD11b-deficient DCs due to the inability of DCs to endocytose TLR4, indicating a role for CD11b as a positive regulator of TLR4 signalling [36]. However, as CD11b blockade occurred after LPS-induced maturation in our study, an impaired response via TLR4 is unlikely to be responsible for the reduction in T cell priming observed in the moDC–T cell co-cultures. Regardless, our findings of increased CD11b expression in SF compared with PB and in matDCs compared with tolDCs provide strong evidence for a pro-inflammatory role for CD11b in DCs.
One question that remains is how does CD11b expressed by DCs promote T cell activation? We predict that DC CD11b binds to T cell intercellular adhesion molecules (ICAMs). This CD11b–ICAM interaction may (i) increase the contact area and/or duration of DC–T cell interactions, thus increasing signal strength through the T cell receptor and co-stimulation (CD28) and increasing activation [25]; or (ii) lead to direct signalling downstream of ICAM that promotes T cell activation [37]. Note that these two scenarios are not mutually exclusive.
Furthermore, the mechanism(s) responsible for the opposing functions of CD11a and CD11b remains to be determined. The ligand repertoire of CD11a and CD11b is overlapping, but ligands that are unique to each integrin do exist [26]. Thus, it is possible that the opposing effects are due to binding to distinct ligands. Alternatively, CD11a and CD11b may initiate distinct signalling pathways. The CD11a intracellular domain has four identified phosphorylation sites, whereas CD11b cytoplasmic sequence is much shorter, with only one [38]. However, the possible functions of these CD11 chain phosphorylation sites remain unknown.
Our data raise the question of cause and effect: is integrin expression altered in response to environment and/or is integrin expression by DCs actively contributing to the initiation or progression of inflammation and autoimmunity? From the short-term stimulation of DCs (Fig. 2) it is clear that expression of CD11a is down-regulated in response to the local inflammatory milieu, whilst a previous study points to a role for DC CD11a in directly suppressing T cell responses [13]. For CD11b, on the other hand, the in vitro stimulation data were unable to conclude whether expression is altered in response to short-term treatment with pro- or anti-inflammatory stimuli. However, blockade of DC CD11b reduced T cell activation, suggesting that high CD11b expression by DCs in the inflamed RA joint may be contributing to disease progression. Thus, it is likely that DCs alter their integrin expression pattern in response to the local inflammatory milieu that drives their maturation and inflammatory or tolerogenic potential. Importantly, our study provides evidence that the integrin expression profile of cDCs actively contributes to their functional properties and influences CD4+ T cell responses, and thus may be contributing to joint pathology in RA. tolDCs are considered a promising strategy for the treatment of RA [39] owing to their ability to produce immunoregulatory cytokines such as TGF-β and IL-10, induce a tolerogenic T cell phenotype, and dampen immune responses induced by matDCs [18, 23]. Knowledge of integrin subunit expression and function in DCs in steady-state, tolerogenic and inflammatory environments may aid in the optimization of tolDC therapy. For example, based on the findings from this study, we predict DCs with high CD11a and low CD11b expression to be the most tolerogenic. Manipulation of integrins (e.g. by providing integrin subunit agonists or antagonists [10] during culture) may render tolDCs ‘better’ at tolerizing T cells, and thereby aid our progress towards a cure for RA.
In conclusion, we present for the first time data illustrating that β2 integrin family members, CD11a and CD11b, on DCs have distinct roles in RA pathophysiology: CD11a is highly expressed in steady state and tolerogenic DCs, where it may exhibit an immunoregulatory function, whereas CD11b is highly expressed in mature inflammatory DCs such as those found in the RA joint, and promotes T cell activation. Careful consideration of integrin expression and function in DCs should be taken when developing and optimizing DC-targeted therapies.
Supplementary Material
Acknowledgements
We thank the Flow Cytometry Core Facilities at the University of Glasgow and Newcastle University for assistance with panel design, and the Glasgow Imaging Facility for assistance with Cellprofiler pipeline development. V.L.M. and C.M.H. designed the project. L.S. performed the experiments and analysed the data. J.R. facilitated the collection of all clinical samples. A.G.P. provided valuable contribution to the RA clinical sample study design. L.S., V.L.M. and C.M.H. wrote the manuscript with input from all co-authors.
Funding: This work was supported by the Research into Inflammatory Arthritis Centre Versus Arthritis (RACE) (grant number 20298), Versus Arthritis (grant number 20848 to V.L.M.) and Tenovus Scotland (grant number S17-18 to V.L.M.).
Disclosure statement: The authors declare no conflicts of interest.
Data availability statement
The data underlying this article are available in the article and in its online supplementary material.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Smolen JS, Aletaha D, McInnes IB.. Rheumatoid arthritis. Lancet 2016;388:2023–38. [DOI] [PubMed] [Google Scholar]
- 2. Kohler BM, Gunther J, Kaudewitz D, Lorenz HM.. Current therapeutic options in the treatment of rheumatoid arthritis. J Clin Med 2019;8:938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lutzky V, Hannawi S, Thomas R.. Cells of the synovium in rheumatoid arthritis. Dendritic cells. Arthritis Res Ther 2007;9:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas R, Davis LS, Lipsky PE.. Rheumatoid synovium is enriched in mature antigen-presenting dendritic cells. J Immunol 1994;152:2613–23. [PubMed] [Google Scholar]
- 5. Leung BP, Conacher M, Hunter D. et al. A novel dendritic cell-induced model of erosive inflammatory arthritis: distinct roles for dendritic cells in T cell activation and induction of local inflammation. J Immunol 2002;169:7071–7. [DOI] [PubMed] [Google Scholar]
- 6. Lebre MC, Jongbloed SL, Tas SW. et al. Rheumatoid arthritis synovium contains two subsets of CD83-DC-LAMP- dendritic cells with distinct cytokine profiles. Am J Pathol 2008;172:940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Page G, Lebecque S, Miossec P.. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J Immunol 2002;168:5333–41. [DOI] [PubMed] [Google Scholar]
- 8. Collin M, Bigley V.. Human dendritic cell subsets: an update. Immunology 2018;154:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu MB, Langridge WHR.. The function of myeloid dendritic cells in rheumatoid arthritis. Rheumatol Int 2017;37:1043–51. [DOI] [PubMed] [Google Scholar]
- 10. Schittenhelm L, Hilkens CM, Morrison VL.. beta2 integrins as regulators of dendritic cell, monocyte, and macrophage function. Front Immunol 2017;8: 1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morrison VL, James MJ, Grzes K. et al. Loss of beta2-integrin-mediated cytoskeletal linkage reprogrammes dendritic cells to a mature migratory phenotype. Nat Commun 2014;5:5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Savinko TS, Morrison VL, Uotila LM. et al. Functional Beta2-integrins restrict skin inflammation in vivo. J Invest Dermatol 2015;135:2249–57. [DOI] [PubMed] [Google Scholar]
- 13. Balkow S, Heinz S, Schmidbauer P. et al. LFA-1 activity state on dendritic cells regulates contact duration with T cells and promotes T-cell priming. Blood 2010;116:1885–94. [DOI] [PubMed] [Google Scholar]
- 14. Varga G, Balkow S, Wild MK. et al. Active MAC-1 (CD11b/CD18) on DCs inhibits full T-cell activation. Blood 2007;109:661–9. [DOI] [PubMed] [Google Scholar]
- 15. D’Agata ID, Paradis K, Chad Z, Bonny Y, Seidman E.. Leucocyte adhesion deficiency presenting as a chronic ileocolitis. Gut 1996;39:605–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uzel G, Kleiner DE, Kuhns DB, Holland SM.. Dysfunctional LAD-1 neutrophils and colitis. Gastroenterology 2001;121:958–64. [DOI] [PubMed] [Google Scholar]
- 17. Aletaha D, Neogi T, Silman AJ. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 18. Anderson AE, Swan DJ, Wong OY. et al. Tolerogenic dendritic cells generated with dexamethasone and vitamin D3 regulate rheumatoid arthritis CD4+ T cells partly via transforming growth factor-beta1. Clin Exp Immunol 2017;187:113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matthews SA, San Lek H, Morrison VL. et al. Protein kinase D isoforms are dispensable for integrin-mediated lymphocyte adhesion and homing to lymphoid tissues. Eur J Immunol 2012;42:1316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phillips BE, Garciafigueroa Y, Trucco M, Giannoukakis N.. Clinical tolerogenic dendritic cells: exploring therapeutic impact on human autoimmune disease. Front Immunol 2017;8:1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hilkens CM, Isaacs JD.. Tolerogenic dendritic cell therapy for rheumatoid arthritis: where are we now? Clin Exp Immunol 2013;172:148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anderson AE, Swan DJ, Sayers BL. et al. LPS activation is required for migratory activity and antigen presentation by tolerogenic dendritic cells. J Leukoc Biol 2009;85:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harry RA, Anderson AE, Isaacs JD, Hilkens CM.. Generation and characterisation of therapeutic tolerogenic dendritic cells for rheumatoid arthritis. Ann Rheum Dis 2010;69:2042–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Domogalla MP, Rostan PV, Raker VK, Steinbrink K.. Tolerance through education: how tolerogenic dendritic cells shape immunity. Front Immunol 2017;8:1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Celli S, Lemaître F, Bousso P.. Real-time manipulation of T cell-dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+ T cell activation. Immunity 2007;27:625–34. [DOI] [PubMed] [Google Scholar]
- 26. Arnaout MA. Biology and structure of leukocyte beta 2 integrins and their role in inflammation. F1000Res 2016;5:2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evans BJ, McDowall A, Taylor PC. et al. Shedding of lymphocyte function-associated antigen-1 (LFA-1) in a human inflammatory response. Blood 2006;107:3593–9. [DOI] [PubMed] [Google Scholar]
- 28. Behrens EM, Sriram U, Shivers DK. et al. Complement receptor 3 ligation of dendritic cells suppresses their stimulatory capacity. J Immunol 2007;178:6268–79. [DOI] [PubMed] [Google Scholar]
- 29. Škoberne M, Somersan S, Almodovar W. et al. The apoptotic-cell receptor CR3, but not alphavbeta5, is a regulator of human dendritic-cell immunostimulatory function. Blood 2006;108:947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Han C, Jin J, Xu S. et al. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol 2010;11:734–42. [DOI] [PubMed] [Google Scholar]
- 31. Yee NK, Hamerman JA.. Beta(2) integrins inhibit TLR responses by regulating NF-κB pathway and p38 MAPK activation. Eur J Immunol 2013;43:779–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang L, Gordon RA, Huynh L. et al. Indirect inhibition of Toll-like receptor and type I interferon responses by ITAM-coupled receptors and integrins. Immunity 2010;32:518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ehirchiou D, Xiong Y, Xu G. et al. CD11b facilitates the development of peripheral tolerance by suppressing Th17 differentiation. J Exp Med 2007;204:1519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stevanin M, Busso N, Chobaz V. et al. CD11b regulates the Treg/Th17 balance in murine arthritis via IL-6. Eur J Immunol 2017;47:637–45. [DOI] [PubMed] [Google Scholar]
- 35. Nowatzky J, Manches O, Khan SA, Godefroy E, Bhardwaj N.. Modulation of human Th17 cell responses through complement receptor 3 (CD11b/CD18) ligation on monocyte-derived dendritic cells. J Autoimmun 2018;92:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ling GS, Bennett J, Woollard KJ. et al. Integrin CD11b positively regulates TLR4-induced signalling pathways in dendritic cells but not in macrophages. Nat Commun 2014;5:3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kohlmeier JE, Rumsey LM, Chan MA, Benedict SH.. The outcome of T-cell costimulation through intercellular adhesion molecule-1 differs from costimulation through leucocyte function-associated antigen-1. Immunology 2003;108:152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fagerholm SC, Hilden TJ, Gahmberg CG.. P marks the spot: site-specific integrin phosphorylation regulates molecular interactions. Trends Biochem Sci 2004;29:504–12. [DOI] [PubMed] [Google Scholar]
- 39. Bell GM, Anderson AE, Diboll J. et al. Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann Rheum Dis 2017;76:227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.