Abstract
Here, we report a case of an infectious pseudoaneurysm at the root of the innominate artery, compressing the trachea, that resulted in massive hemorrhage due to rupture of the innominate artery. The patient, a 31-year-old man, had complained of persistent fever for 40 days and severe dyspnea for 1 week. Contrast-enhanced computed tomography imaging of neck and thorax showed a pseudoaneurysm originating from the root of the innominate artery that was severely compressing the main trachea. A hybrid surgery strategy was applied. We first implanted a covered stent in the root of the innominate artery. Then, we performed a left-to-right carotid−carotid bypass with a great saphenous vein graft. Finally, we performed a median thoracotomy in which both the pseudoaneurysm and the previously implanted covered stent were successfully extracted. The patient lost strength in the right upper limb muscle on postoperative day 2 but recovered to baseline strength after 3 months. A hybrid surgical technique may represent a practical solution for such conditions.
Keywords: Infectious innominate pseudoaneurysm, hybrid surgery, endovascular repair, saphenous vein graft patency, median thoracotomy, extra-anatomic bypass, anti-infection therapy
Introduction
Infectious innominate artery pseudoaneurysm accounts for 2.7% of pseudoaneurysm cases.1 It is mainly caused by infective endocarditis, blunt chest trauma, or iatrogenic factors such as a central venous puncture or cannulation.2 Late pseudoaneurysm can also be a potential complication of thoracic vascular endostenting.3 The pseudoaneurysm can lead to devastating complications, including persistent infection, compression of adjacent tissues and organs, or massive hemorrhage due to rupture. Once it forms, the patient should receive anti-infection therapy and undergo surgery as soon as possible.4
Conventionally, local lesion excision and extra-anatomic bypass are beneficial for patients because of the low infective recurrence rate.5 However, as new developments in endovascular devices have become available, our ability to treat complex disease processes has increased. For patients who are poor candidates for open surgery because of the high risk and the potential rupture of the pseudoaneurysm, an endovascular covered stent can be implanted into the lesion.6 However, the appropriate surgical procedure remains controversial at present. Hybrid surgery is an option that combines open surgery and an endovascular procedure.
Here, we report a case of infectious innominate artery pseudoaneurysm compressing the main trachea that was resolved by hybrid surgery. We hope to raise awareness of this uncommon disease and, importantly, highlight a better surgical option.
Case presentation
A 31-year-old man who presented with persistent fever for 40 days and severe dyspnea for 1 week was admitted to our hospital. He had a surgical history of a retroperitoneal tumor excision 6 months previously, and postoperative pathology noted muscle and vascular tissues. The patient had not had an artery puncture process in the past. Physical examination revealed a peak temperature of 39°C, blood pressure of 110/70 mm Hg, and a heart rate of 120 beats per minute. The patient’s respiratory rate was 20 breaths per minute and oxygen saturation (SpO2) was 98%. A right subclavian pulsatile mass could be easily palpated. The patient was unable to lie on his back because of the severe dyspnea. Immunological markers were normal and blood test results were as follows: white blood cell count 11 × 109/L, neutrophils 87%, and C-reactive protein 40 mg/L, indicating a possible infection. Blood tests were conducted four times: at admission, when the patient’s temperature was >38°C, during the chills attack, and following 1 week of anti-infection therapy; all blood culture results were negative. Instant contrast-enhanced computed tomography (CT) imaging of the neck and thorax showed a pseudoaneurysm measuring 5.2 × 6.5 × 8.2 mm, originating from the root of the innominate artery, and severely compressing the main trachea by at least 80% (Figure 1). Imipenem, a broad-spectrum antibiotic, was administered as basic therapy; in addition, blood pressure was controlled at less than 120 mm Hg, nutritional support was given, and treatment to improve the airway was administered (e.g., oxygen and aerosol inhalation). A week later, the patient’s temperature and infectious markers returned to normal. However, his dyspnea deteriorated markedly, causing cough, chest distress, and hypoxemia, and surgery was deemed urgent.
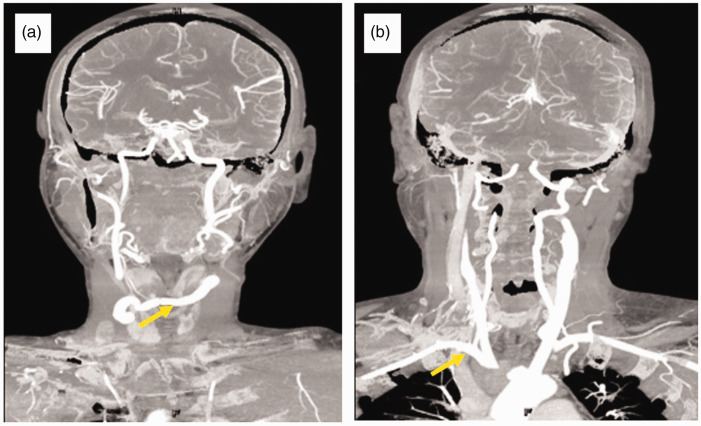
Figure 1.
Computed tomography images showing (a) the pseudoaneurysm originating from the root of the innominate artery (yellow arrow), and (b) the trachea that was severely compressed by the pseudoaneurysm (yellow arrow).
The patient was placed under general anesthesia in a hybrid operating room. Through the left femoral artery, a 260-cm-long, 0.035-inch guidewire (Terumo Medical Corporation, Tokyo, Japan) was placed into the ascending aorta under fluoroscopic control. Angiography showed obvious contrast extravasation and a pseudoaneurysm that originated from the root of the innominate artery, not involving the aorta arch or bifurcation of the innominate artery (Figure 2a). The same guidewire was carefully advanced through the injured portion into the internal carotid artery. The diameters of the proximal and distal innominate artery were 9.5 and 8 cm, respectively. The bilateral carotid arteries were patent. The guidewire was replaced by a stiff guidewire (Hi-Torque, Abbott, CA, USA) for better support. A covered stent graft (Viabahn, 11 × 8 mm, W. L. Gore and Associates, Newark, DE, USA) was delivered into the innominate artery to cover the vessel break, the distal end of which was 5 mm inside the aortic arch. Complete exclusion of the pseudoaneurysm sac was then observed by repeat angiography and no contrast extravasation occurred (Figure 2b).
Figure 2.
Arteriography showing (a) the rupture of the root in the innominate artery and contrast extravasation (yellow arrow), and (b) the covered stent implantation (Viabahn, 11 × 8 mm, W. L. Gore and Associates, Newark, DE, USA ) that prevented rupture of the innominate artery (yellow arrow).
Second, a left-to-right carotid-carotid bypass was performed with a saphenous vein graft (SVG). The right great saphenous vein was taken out and infused with heparin saline to full expansion. A bypass with two end-to-side anastomoses was successfully performed by using the expanded SVG and avoiding the infected area to maintain cerebral perfusion (Figure 3a). The intraoperative cross-clamp time of the carotid artery was 15 minutes.
Figure 3.
Surgical views indicating (a) the saphenous vein graft (SVG) from the left-to-right carotid-carotid artery (yellow arrow), and (b) removal of the covered stent from the root of the innominate artery (yellow arrow).
Last, a median thoracotomy was performed to remove the pseudoaneurysm and the implanted covered stent (Figure 3b). After thoracotomy, the light yellow fluid in the pseudoaneurysm cavity was extracted for bacterial culture. A large number of thrombus components were found and removed, and the implanted covered stent was removed after cutting the pseudoaneurysm sac. However, it was difficult to separate and suture the root of the innominate artery because of heavy tissue adhesion. Therefore, the root was sutured and covered with the peripheral pericardial tissue following cross-clamp of the ascending aorta within 1 minute. Intraoperative blood loss was 4000 mL, and 3200 mL of red blood cells was infused during the operation.
Tracheal intubation was removed on the first day after surgery. Anticoagulant therapy was started on the second day. Two weeks later, repeat thoracic contrast-CT angiography revealed no evidence of the pseudoaneurysm or compression of the main trachea (Figure 4a, b). Good patency of the SVG was observed, as well as occlusion in the innominate and right subclavian arteries (Figure 4c). However, the pulse of the right brachial and radial arteries weakened and right upper limb muscle strength decreased to a classification of IV on postoperative day 2. Postoperative pathological results illustrated degenerative muscular vessels and thrombosis components. Postoperative anti-infection therapy was maintained for 6 months, and antiplatelet combined with anticoagulant therapy was maintained for at least 1 year. Another CT angiography at the 1-year follow-up revealed a patent SVG and recanalization of the right subclavian artery (Figure 5a, b). Fortunately, pulsation of the right brachial and radial arteries and upper limb muscle strength recovered to normal, and the great saphenous vein of the neck was palpable. Postoperative fever did not return.
Figure 4.
Computed tomography angiography showing (a, b) normal trachea diameter, good patency of the saphenous vein graft, and disappearance of the pseudoaneurysm on postoperative day 14 (yellow arrow); and (c) occlusion of the innominate and right subclavian artery (yellow arrow).
Figure 5.
Computed tomography angiography at the 1-year postoperative follow-up showing (a) good patency of the saphenous vein graft (yellow arrow), and (b) the occluded innominate artery but recanalized right subclavian artery (yellow arrow).
Discussion
Innominate aneurysm is associated with 3% morbidity and the rupture rate is 11%.7 Pseudoaneurysm of the innominate artery has lower morbidity but the rupture rate can reach 60% because of blunt chest trauma, infection, and iatrogenesis.8 Considering the normal immunological markers, the patient described here presented with persistent fever for 40 days, with underlying infection despite multiple negative blood and bacterial cultures. Based on the abnormal inflammatory markers, the patient’s pseudoaneurysm was thought to be related to infection. Bacterial infection is the most common cause, including typhoid, Staphylococcus aureus, and busheliae. Antibiotic therapy was therefore recommended until 6 months after surgery.9 After the patient had been hospitalized and on imipenem for 1 week, his temperature and infectious markers had returned to near-normal levels, and thus surgery could proceed (Figure 6). Although the markers of infection increased again postoperatively, the patient’s temperature remained normal with continuous antibiotic therapy. When the patient was discharged 2 weeks after the operation, 6 months of oral anti-infection treatment was recommended because of the high procalcitonin level. In this case, anti-infection therapy played an important role in determining the appropriate time for surgery and in reducing the risk of reinfection.
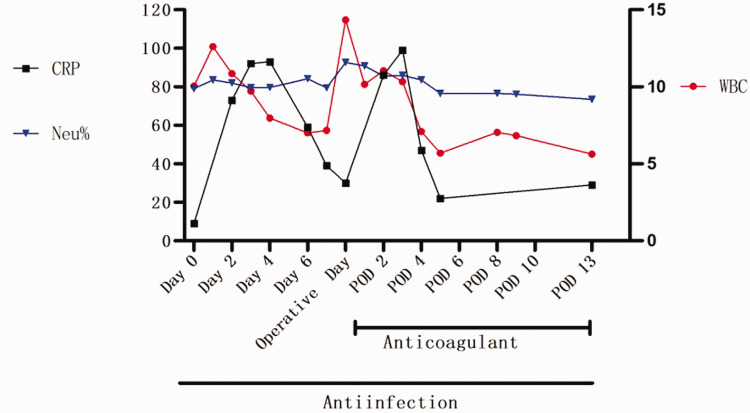
Figure 6.
Changes in the infectious markers white blood cell count (WBC, 109/L), neutrophils (Neu%), and C-reactive protein (CRP, mg/L) on pre-, peri-, and post-operative days. POD, post-operative day.
Generally, the classical surgical approach to infectious pseudoaneurysm is local debridement plus extra-anatomic bypass, the advantage of which is that resecting the infected area can prevent rupture caused by reinfection of the local bridging vessel. However, the postoperative infection rate can reach 21% to 32%. If patients suffer persistent infection, including uncontrollable fever and intestinal and esophageal fistula, the survival rate is only 39%.10 In our case, the purpose of surgery was mainly to prevent the pseudoaneurysm from rupturing and to relieve compression of the trachea. A key detail in left-to-right carotid–carotid SVG bypass is not only to minimize risks of cerebral ischemia or infarction caused by occlusion of the innominate artery, but also to keep away from the local infective site. Fortunately, although the right vertebral artery was occluded under ligation of the innominate artery, we observed no clinical evidence of cerebrovascular ischemic event due to the cerebral Willis loop. However, the unexpected ischemia of the right upper limb could have resulted from sudden malperfusion associated with occlusion of the right subclavian artery, where the slow process of formation of collateral arteries was not able to meet requirements. Therefore, the patient lost strength of the right upper limb muscle and radial pulse on postoperative day 2, but no pallor or necrosis was observed. The right upper limb muscle and radial pulse strength recovered to normal following 3 months of antiplatelet and anticoagulant treatment. Another operative detail was that the root of the innominate artery and the surrounding pericardial tissue were sutured together to cover the infective local site and minimize the risk of suture dehiscence due to reinfection.
At present, a covered stent can be implanted in the pseudoaneurysm based on effective anti-infection therapy, including normal temperature and infectious markers.11,12 Of course, for a pseudoaneurysm that has already ruptured, the covered stent must be implanted first.13 Unfortunately, if patients have aorto-enteral or aorto-esophageal fistula caused by infection of the covered stent, resulting in the severe hemorrhage and septic shock, the covered stent must be urgently removed and an extra-anatomic bypass created.14 Considering the severe infectious condition, it was not optimal for this patient to receive traditional open surgery under deep hypothermic circulatory arrest (DHCA), because of risks such as cerebrovascular accident, heart failure, prolonged respiratory failure, and reinfection. The surgical mortality under DHCA is 6.8%.2 Although not all patients are candidates, the endovascular approach offers several advantages, including lower overall mortality.15 First, compared with an open surgery under DHCA, the advantage of the hybrid option used here was that endovascular implantation of the covered stent not only protected against rupture, but its concurrent removal reduced the reinfection risk. Second, compared with a pure endovascular procedure, removal of the huge pseudoaneurysm relieved the severe compression of the trachea. Third, the left-to-right carotid-carotid SVG bypass prevented cerebral infarction. The only disadvantage of our approach was the massive hemorrhage caused by severe tissue adhesion when separating and suturing the root of the innominate artery. Therefore, hybrid surgery may represent a more practical solution and offers an alternative to individualize treatment based on comorbidities, disease, and location of infection. Therefore, it is important to evaluate the perioperative condition of each patient to choose the optimal surgical procedure, including endovascular therapy, open surgery, and hybrid surgery.
In conclusion, infectious innominate artery pseudoaneurysm has low morbidity but high mortality. Once infectious innominate artery pseudoaneurysm is diagnosed according to clinical evidence, it is important not only to consider the condition of the patient, but also to choose the best surgical method under effective anti-infection therapy.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Ethics statement: Publication of this study was approved by the local ethics committee of the Beijing Friendship Hospital. The patient consented in writing to the publication.
Funding: This study was supported by the Beijing Natural Science Foundation (No. 7184197).
ORCID iDs
Li-Shan Lian https://orcid.org/0000-0003-4256-2522
Zhe Zhang https://orcid.org/0000-0001-6965-3622
References
- 1.Wang XL, Guan XL, Jiang WJ, et al. Innominate artery aneurysm, how to solve it. J Int Med Res 2017; 45: 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee CW, Song S, Choi SU, et al. Hybrid repair for anastomotic pseudoaneurysm on the innominate artery following blunt chest trauma. J Card Surg 2015; 30: 836–838. [DOI] [PubMed] [Google Scholar]
- 3.Rispoli P, Varetto G, Savia FM, et al. Large post-stenting innominate artery pseudoaneurysm. Interact Cardiovasc Thorac Surg 2008; 7: 444–446. [DOI] [PubMed] [Google Scholar]
- 4.Sibille JA, Harding JP, Ballast JK, et al. Endovascular repair of an innominate artery pseudoaneurysm using the Valiant Mona LSA branched graft device. J Vasc Surg Cases Innov Tech 2017; 3: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu MY, Jiao Y, Yang Y, et al. Open surgery and endovascular repair for mycotic aortic aneurysms: benefits beyond survival. J Thorac Cardiovasc Surg 2019: 1–10. [DOI] [PubMed] [Google Scholar]
- 6.Kan CD, Lee HL, Yang YJ. Outcome after endovascular stent graft treatment for mycotic aortic aneurysm: a systematic review. J Vasc Surg 2007; 46: 906–912. [DOI] [PubMed] [Google Scholar]
- 7.Qureshi AZ. Fatal innominate artery hemorrhage in a patient with tetraplegia: case report and literature review. J Spinal Cord Med 2018; 41: 731–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang H, Liu Y, Moro A, et al. Intraoperative rupture of tuberculous pseudoaneurysm associated with spinal tuberculosis: a case report and literature review. J Infect Dev Ctries 2019; 13: 174–178. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Yuan D, Zhao J, et al. Hybrid repair for a complex infection aortic pseudoaneurysm with continued antibiotic therapy. Medicine (Baltimore) 2019; 98: e14330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cevasco M, Menard MT, Bafford R, et al. Acute infectious pseudoaneurysm of the descending thoracic aorta and review of infectious aortitis. Vasc Endovascular Surg 2010; 44: 697–700. [DOI] [PubMed] [Google Scholar]
- 11.Roussel A, Fabre D, Fadel E, et al. Hybrid treatment of an aortic pseudoaneurysm arising at the innominate artery junction secondary to superior vena cava stenting. J Vasc Surg Cases 2015; 1: 127–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhry AJ, Shaw P, Gonzalez L, et al. Hybrid endovascular exclusion of a bleeding innominate artery pseudoaneurysm in a patient with no open surgical options. J Vasc Surg Cases Innov Tech 2019; 5: 132–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asai M, Van Houtte O, Sullivan TR, et al. Endovascular repair of three concurrent mycotic pseudoaneurysms. Vasc Endovascular Surg 2018; 52: 473–477. [DOI] [PubMed] [Google Scholar]
- 14.Mukaihara K, Yamamoto H, Arata K, et al. Emergent rescue operation for expanding mycotic pseudoaneurysm causing hemoptysis, originating from right subclavian artery. Ann Vasc Dis 2015; 8: 62–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arima D, Hisagi M, Nonaka T, et al. Staged hybrid treatment for a coronary artery pseudoaneurysm after percutaneous coronary intervention; report of a case. Kyobu Geka 2018; 71: 693–695. [PubMed] [Google Scholar]