Abstract
The effect of estrus induction by cabergoline on gonadotropin and steroid hormone responses was examined in anestrous bitches. Eleven beagles were used in the study; seven were included in the estrus induction group and four were included in the spontaneous estrus group. Cabergoline was orally administered to the estrus induction group at 5 µg/kg once daily for four weeks, or until hemorrhagic discharge was detected. The inter-estrus interval in the estrus induction group was significantly shorter than the previous estrus interval. Bitches that showed proestrus within four weeks of treatment showed increased luteinizing hormone (LH) pulse frequency and, subsequently, increased estradiol (E2) levels. Prolactin (PRL) levels declined promptly after treatment, except in one bitch that did not show proestrus during the cabergoline treatment period. There was a significant correlation between the time to proestrus induction and the reduction in PRL levels. A positive correlation was found between the LH levels two weeks after cabergoline administration and PRL reduction. This study demonstrates that an abrupt reduction in PRL is likely to be important for initiation of estrus in bitches. A reduction in PRL indirectly leads to an increase in LH pulse frequency, which regulates follicular development in bitches. However, if the period from the end of the previous estrus to the cabergoline treatment is short, it may take some time to show proestrus without increasing E2 levels, even if the LH level increases after cabergoline administration.
Keywords: bitch, cabergoline, estrus induction, luteinizing hormone pulse, prolactin
Canines have a remarkably long anestrus period. The frequency of estrus is once or twice a year, which is extremely low in comparison with most mammals that ovulate monthly [33]. For this reason, where conception failure has occurred in the facility of a commercial breeder, guide dog breeder, or another facility that needs to supply puppies continuously, they must wait until the next estrus to undertake mating, which could take half a year or longer to occur. Thus, shortening the length of the canine anestrus is clinically desirable. Moreover, canine estrus induction can also be beneficial in studies such as those of canine reproductive disorders and estrous cycle physiology, as it leads to a shortening of the interparturient period of experimental animals and decreases the necessary number of test animals.
Currently, shortening the estrous cycle of bitches has been widely studied, but without satisfactory results [17, 34]. Drug treatments to induce estrus in bitches are classified into three main categories. The first is the stimulation of follicular development by gonadotropins, in which equine chorionic gonadotropin (eCG), follicle-stimulating hormone (FSH), and/or human menopausal gonadotropin are used. eCG is the most widely studied gonadotropin for the purpose of estrus induction in bitches, and has been used in various protocols involving subcutaneous or intramuscular injection, with frequencies of once daily to every week [3, 20, 39]. The second treatment category is the direct stimulation of the pituitary with gonadotropin-releasing hormone (GnRH), in which lutrelin, buserelin, deslorelin, and leuprolide are used [8, 16, 20, 21]. The third category is shortening the length of anestrus by suppressing the synthesis or release of prolactin (PRL) using a dopamine agonist [6, 13, 20, 29, 35].
Cabergoline, a dopamine agonist, is known to suppress PRL to induce estrus in bitches [6, 13, 20, 29, 35]. However, the underlying mechanism has not been fully elucidated. Therefore, this study aimed to examine the effect of cabergoline administration on female anestrus beagles, particularly with respect to the secretion of gonadotropins and steroid hormones in response to the induction of estrus, to obtain a protocol for artificial adjustment of canine breeding as well as to augment basic knowledge of cases in which estrus was not successfully induced.
MATERIALS AND METHODS
Animals
A total of 11 female beagles (aged 4–11 years) in anestrus were used in this study, and some bitches were used repeatedly. Seven bitches were included in the estrus induction group, and four bitches were included in the spontaneous estrus group. Cabergoline was orally administered to the animals in the estrus induction group at a dosage of 5 µg/kg-body weight once daily, starting 106.0 ± 4.9 days (range: 89–119 days) after the end of the previous estrus, for four weeks, or until vulvar swelling and hemorrhagic discharge was detected. The end of the previous estrus was defined as the start of the diestrus based on a vaginal smear assessment. Briefly, vaginal smears were taken daily after hemorrhagic discharge was detected. The first day of diestrus was characterized by an abrupt decrease in cornification cells, the reappearance of intermediate and parabasal cells, and a transient increase in leukocytes. The spontaneous estrus group served as controls and received no treatment or placebo. All experiments were conducted according to the Nihon University Guidelines for Management of Animal Experiments (AP13B024).
Method and timing of sample collection
Individuals in the estrus induction group underwent additional blood collections thrice weekly between the start of cabergoline administration and the detection of hemorrhagic discharge. For the measurement of luteinizing hormone (LH) pulse frequency after the start of cabergoline administration, an indwelling needle was placed in the cephalic vein to collect blood every 15 min in a 6-hr period, once weekly, in the first four weeks of cabergoline treatment.
The first day of proestrus was confirmed by daily observation of vulvar swelling and hemorrhagic discharge. The female beagles in both the spontaneous estrus and estrus induction groups underwent blood collection at a fixed time every day once hemorrhagic discharge was confirmed. Blood was collected in a plain vacuum blood collection tube, allowed to coagulate at room temperature, and then centrifuged at 1,200 × g for 15 min at 4°C. In order to determine the sampling schedule, the serum was measured for progesterone (P4) by means of an enzyme-linked fluorescence assay using an automated fluorescent immunoassay unit (SPOTCHEM™ VIDAS SV-5010; ARKRAY, Kyoto, Japan). A blood P4 level of 2–4 ng/ml was used to indicate Day 0 to calculate the day of LH surge [2, 5, 10, 14, 23]. Daily blood collection was continued until Day 5, after which the frequency was reduced to thrice weekly until Day 60. Blood was collected at a fixed time of day which remained unchanged throughout the sampling period. The serum was stored at −20°C until it was used for hormone measurements. Serum FSH, LH, PRL, estradiol (E2), and P4 levels were analyzed. The first day of estrus was subsequently defined endocrinologically at the preovulatory LH surge measurement.
Hormone assay
Serum E2 was determined using a time-resolved fluorescent immunoassay according to the method of Ono et al. [27]. Intra- and inter-assay coefficients of variation (CVs) were 5.0% and 10.7%, respectively.
Serum P4 was determined using a double-antibody enzyme immunoassay according to the method of Ono et al. [27]. Intra- and inter-assay CVs were 6.0% and 9.5%, respectively.
Serum LH and FSH levels were determined using a heterologous double antibody radioimmunoassay (RIA), as previously described [24] with partial modification. For the LH RIA, rabbit anti-sheep LH serum (YM#18), rat LH for iodination (NIDDK-I-5), and canine LH standard (LER-1685-1) were used. For the FSH RIA, rabbit anti-human FSH serum (M91), rat FSH for iodination (NIDDK-I-5), and canine FSH standard (LER-1685-3-A) were used. As a second antibody, goat anti-rabbit γ-globulin serum was used for the LH and FSH assays. The intra- and inter-assay CVs were 5.8% and 14.3% for LH and 3.1% and 10.8% for FSH, respectively. The determination of whether an alteration in the level of LH was considered as a pulse was judged according to a previous study by Ribadu et al. [30]; LH levels that increased by 40% or more from that of the preceding level and subsequently declined at least twice consecutively, or to the base level, were considered to be an “LH pulse”.
Serum PRL was determined using the homologous double antibody canine PRL RIA, as previously described [32] with partial modification. Guinea pig anti-dog PRL serum (AFP1062091GP), canine PRL (AFP2451B) for iodination and as a reference standard and second antibody, and goat anti-guinea pig serum (HAC-GPA2-01GTP80) were used. Intra- and inter-assay CVs were 7.2% and 10.1%, respectively.
Statistical analysis
All values are expressed as the mean ± standard error. A paired t-test was performed between the last estrus interval and the post-treatment estrus interval. Comparison of means between two groups was performed using a significance test: Student’s t-test for normally distributed parameters or Welch’s t-test for parameters that were not normally distributed. Testing of normality was performed using the Shapiro-Wilk W-test. Pearson’s coefficient of correlation was used to test the correlation between two variables. A logarithmic transformation was performed on data that was not normally distributed to obtain a normal distribution. The difference was considered significant when the probability value (P) was less than the significance level of 5% (P<0.05). Statistical analyses were performed using JMP® 13.2.0 (SAS Institute Inc., Tokyo, Japan).
RESULTS
Days of the estrous cycle in the spontaneous estrus and estrus induction groups
The number of days of the estrous cycle in the spontaneous estrus and estrus induction groups are shown in Table 1. In the estrus induction group, five dogs were in proestrus within four weeks of administration, while the remaining two dogs took longer to reach proestrus (51 and 53 days). Overall, the number of days from cabergoline administration to the estrus period was 27.7 ± 6.5 days. The inter-estrus interval was 150.7 ± 3.5 days in the estrus induction group, which was significantly shorter (P<0.01) than the 191.9 ± 2.3 days in the last estrus interval and the 188.3 ± 1.4 days in the spontaneous estrus group. The period of proestrus was prolonged in the estrus induction group (P<0.01), but there was no difference in the periods of estrus. No side effects from cabergoline were observed in the estrus induction group.
Table 1. Number of days in the estrous cycle of the spontaneous estrus and estrus induction groups.
| Spontaneous estrus groups (n=4) |
Estrus induction groups (n=7) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bitches ID | Last estrus interval (days) | Estrus interval (days) | Duration of proestrus (days) | Duration of estrus (days) | Bitches ID | Last estrus interval (days) | Period from end of previous estrus to cabergoline treatment (days) | Treatment onset to proestrus (days) | Estrus interval after treatment (days) | Duration of proestrus (days) | Duration of estrus (days) |
| B07090 | 188 | 187 | 9 | 9 | B07090 | 187 | 119 | 10 | 147 | 10 | 8 |
| B07055 | 187 | 101 | 16 | 137 | 10 | 10 | |||||
| B07051 | 197 | 185 | 8 | 6 | B07051 | 185 | 119 | 20 | 153 | 8 | 10 |
| B07054 | 201† | 117 | 22 | 155 | 11 | 10 | |||||
| B07049 | 198 | 99 | 22 | 144 | 10 | 13 | |||||
| B07044 | 194 | 89 | 51 | 153 | 10 | 8 | |||||
| B07056 | 191 | 98 | 53 | 166 | 10 | 8 | |||||
| B07038 | 193 | 191 | 9 | 10 | |||||||
| B07083 | 194 | 190 | 7 | 14 | |||||||
| 193.0 ± 1.9 | 188.3 ± 1.4A | 8.3 ± 0.5 | 9.8 ± 1.7 | 191.9 ± 2.3A | 106.0 ± 4.6 | 27.7 ± 6.5 | 150.7 ± 3.5B | 9.9 ± 0.3* | 9.6 ± 0.7 | ||
The data are expressed as the mean ± standard error of the mean. A, B: Values with different superscripts differ significantly (P<0.01). *: Significantly different between the two groups (P<0.05). The dagger indicates the estrus interval two times before, as bitches became pregnant after artificial insemination at the last estrus.
Endocrine changes in the spontaneous estrus and estrus induction groups
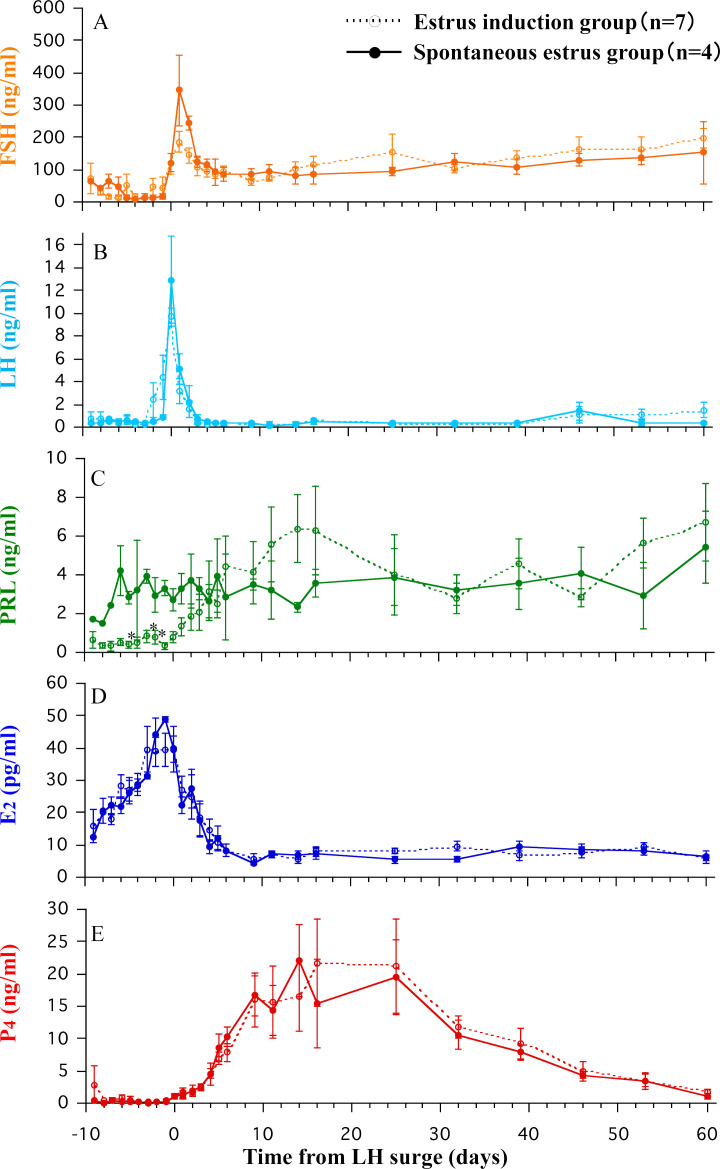
The endocrine changes in the bitches in the spontaneous estrus and estrus induction groups are shown in Fig. 1. The LH surge was 9.7 ± 0.8 ng/ml in the estrus induction group and 12.9 ± 3.8 ng/ml in the spontaneous estrus group and remained low, at the baseline level, after the LH surge. The FSH level peaked 1 day after the LH surge and thereafter remained at the baseline level. E2 levels peaked 1–2 days before the peak of LH and remained at the baseline level thereafter. P4 levels increased after the peak of LH, remained high until 32 days after the LH surge, and then showed a slow decline. No significant difference was observed between the spontaneous estrus and estrus induction groups in the FSH, LH, E2, and P4 levels. However, lower PRL levels were detected 5, 3, and 2 days before the LH surge in the estrus induction group, compared to those in the spontaneous estrus group (P<0.05 on all days).
Fig. 1.
Endocrine changes in the spontaneous estrus and estrus induction groups. Mean ± standard error of the mean serum concentrations of follicle-stimulating hormone (FSH) (A), luteinizing hormone (LH) (B), prolactin (PRL) (C) estradiol (E2) (D), and progesterone (P4) (E) in the spontaneous estrus (n=4; filled circle with solid line) and estrus induction groups (n=7; open circle with dotted line) using cabergoline. Error bar not shown when smaller than symbol size. *: Significantly different between the two groups (P<0.01).
FSH, LH, PRL, E2, and P4 levels as well as LH pulse frequency four weeks after the start of cabergoline administration
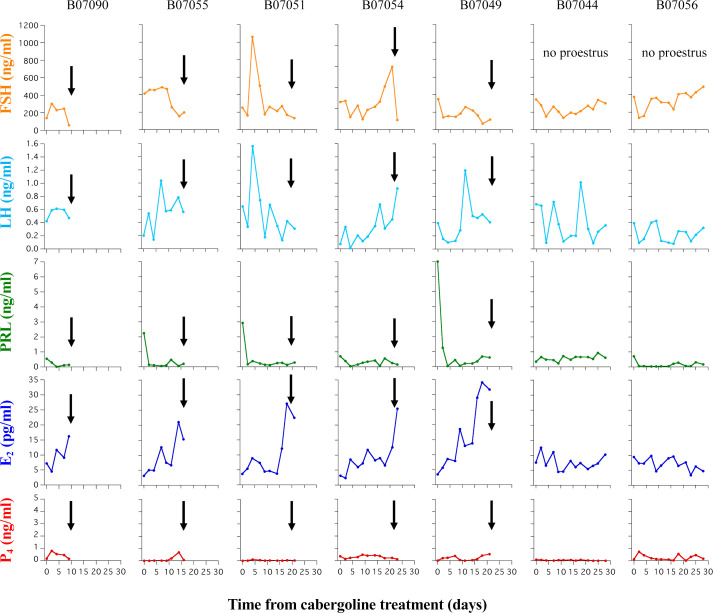
Changes in the FSH, LH, PRL, E2, and P4 levels after cabergoline administration in the seven individuals in the estrus induction group are shown in Fig. 2. The FSH levels after cabergoline treatment increased in two of the five bitches who showed proestrus within four weeks. However, no clear changes were noted in the bitches that did not enter proestrus within four weeks. LH levels increased 1–3 weeks after cabergoline treatment in the four bitches that showed proestrus within four weeks, but one bitch (ID: B07090) showed proestrus with no apparent change. Similarly, there was no clear change in the one bitch (ID: B07056) that did not show proestrus within four weeks. However, B07044 did not show proestrus within 4 weeks after administration, although a clear increase in LH was observed. PRL levels declined promptly after treatment, except in one bitch (ID: B07044) that did not show proestrus during the cabergoline treatment period. E2 levels increased markedly 2–3 weeks after treatment in the four bitches that showed proestrus 16–22 days after the administration of cabergoline. In all bitches, P4 levels remained as low as 1 ng/ml or less during the cabergoline treatment period.
Fig. 2.
Changes in the follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL), estradiol (E2), and progesterone (P4) levels after the start of cabergoline treatment in the estrus induction group. Arrows indicate the start of proestrus.
The LH pulse frequencies after the start of cabergoline administration are shown in Table 2. In the bitches that showed proestrus within four weeks of cabergoline treatment, the LH pulse frequency increased to five at the onset of proestrus, except for in one bitch (B07051). In contrast, among the two bitches that did not show proestrus during the treatment period, one had a low LH pulse frequency and the other had less than four.
Table 2. Luteinizing hormone pulse frequencies after the start of cabergoline treatment in bitches.
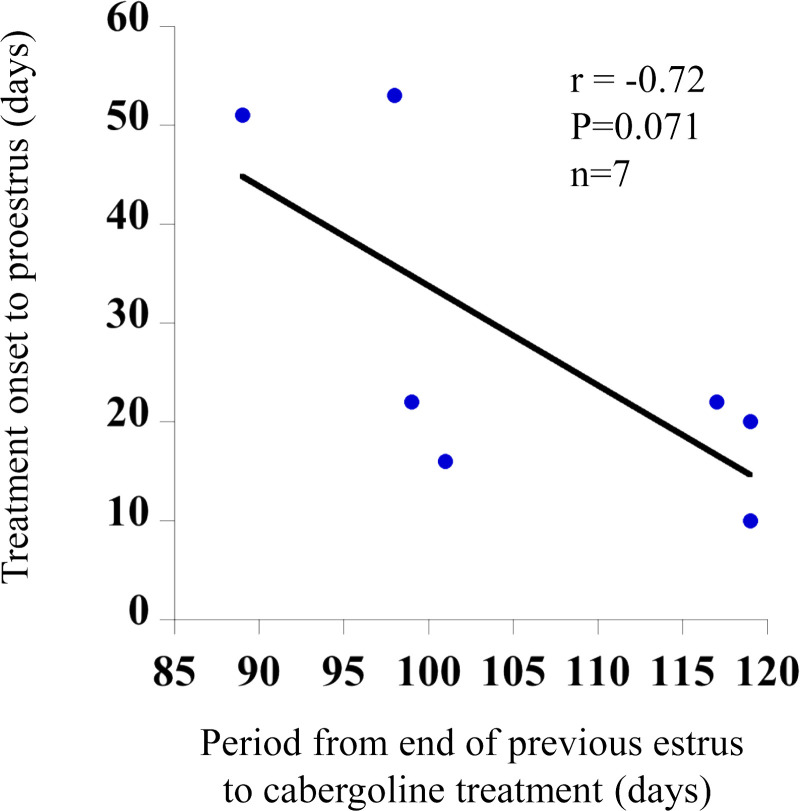
Correlation between the period from the end of the previous estrus to cabergoline administration, and the period from cabergoline administration to proestrus
A negative correlation trend (r= −0.72, P=0.071) was found between the period from the end of the previous estrus to cabergoline administration, and the period from cabergoline administration to proestrus (Fig. 3). This indicated that individuals with a longer period between the previous estrus and the start of cabergoline treatment showed a reduced number of days to proestrus.
Fig. 3.
Correlation between the period from the end of previous estrus to the start of cabergoline treatment, and the period between treatment to onset of proestrus in bitches (n=7).
Correlation between the reduction rate of PRL, treatment onset to proestrus, and FSH, LH, and E2 levels
A negative correlation (r= −0.81, P<0.05) was found between the days from cabergoline administration to proestrus, and the reduction of PRL (logarithmically transformed) from 0 to 1 week of administration (Table 3). This implies that the higher the reduction in PRL, the shorter the days to proestrus.
Table 3. Pearson’s correlation coefficients (r) of the relationships between a reduction in prolactin (PRL), treatment onset to proestrus, and follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (E2) levels.
| Parameters | Reduction in PRL at 0 to 1 weeks (%) (n=7) |
Reduction in PRL at 0 to 2 weeks (%) (n=6) |
|||
|---|---|---|---|---|---|
| r | P | r | P | ||
| Treatment onset to proestrus (days) | −0.81 | 0.03 | −0.08 | 0.88 | |
| One week after treatment | |||||
| FSH | 0.37 | 0.42 | −0.07 | 0.90 | |
| LH | 0.09 | 0.84 | −0.11 | 0.84 | |
| E2 | −0.15 | 0.74 | 0.05 | 0.92 | |
| Two weeks after treatment | |||||
| FSH | −0.12 | 0.83 | −0.20 | 0.70 | |
| LH | 0.78 | 0.07 | 0.10 | 0.85 | |
| E2 | 0.45 | 0.36 | 0.21 | 0.69 | |
There was no clear correlation between the reduction of PRL from 0 to 1 week of administration and the FSH, LH, and E2 levels at 1 week after cabergoline treatment. A positive correlation (r=0.78, P=0.065) trend existed between the LH level 2 weeks after cabergoline treatment and the reduction of PRL from 0 to 1 week of administration (Table 3). This correlation excluded the bitch that showed proestrus 10 days after cabergoline treatment. No correlation was found between the FSH and E2 levels 1 week after cabergoline treatment and the reduction of PRL from 0 to 1 week of administration. No significant correlation was found between the reduction of PRL from 0 to 2 weeks after administration, the days from cabergoline treatment onset to proestrus, and hormone levels.
DISCUSSION
In the estrus induction group, five out of seven bitches showed proestrus within four weeks of cabergoline treatment, and the remaining two dogs showed proestrus approximately 50 days after treatment. The estrus interval with cabergoline was significantly shorter than the previous estrus interval and that of the spontaneous estrus group. The present study corroborated previous data that showed that the administration of a dopamine agonist to anestrus bitches shortens their inter-estrus interval [6, 13, 20, 29, 35].
This study also examined the effect of cabergoline administration to anestrus bitches on the secretion of FSH, LH, PRL, E2, and P4. The changes in FSH, LH, PRL, E2, and P4 in the spontaneous estrus and estrus induction groups were almost congruent with those reported by Concannon [7]. However, in the current study, the PRL concentration in the estrus induction group was lower 2–5 days before the LH surge than that in the spontaneous estrus group. Male and female dogs have distinct seasonal changes in PRL levels, with peaks reported before mid-year and again just before the year’s end [18]. The difference in the PRL levels between the two groups before the LH surge may be due to the fact that estrus in the spontaneous estrus group was concentrated in late May, and that in the estrus induction group was in late October. However, a previous study by Spattini et al. [37], who performed an experiment similar to this study, showed that treatment with cabergoline did not suppress PRL levels. Spattini et al. [37] accounted for the difference by low pre-treatment PRL levels and individual differences. In the current study, the pre-treatment PRL level varied from 0.3 to 7.0 ng/ml. The five bitches that started cabergoline treatment in September showed proestrus within four weeks of dosing, whereas the two bitches that started treatment in July had a delayed onset of proestrus. Seasonal variations in PRL levels may induce seasonal effects in cabergoline treatment for estrus induction.
In the estrus induction group, among the five bitches that showed proestrus within four weeks, the FSH levels of two bitches and LH levels of four bitches increased, but some individuals did not show a clear increase. In the bitch (B07090) who showed proestrus 10 days after cabergoline treatment, no clear increase in LH levels was observed, but the LH pulse frequency increased. However, in one bitch (B07051), the LH level increased significantly, and the LH pulse frequency changed from 1 to 4 times. As the period of increase in LH pulse frequencies is as short as 4 to 8 days and does not continue [9], the increase in LH levels and LH pulse frequencies might not have been detected. However, of the two bitches that did not show signs of proestrus within four weeks, one showed no clear increase in both the FSH and LH levels and one displayed only an increase in LH. Elevated LH and E2 levels were noted in both bitches prior to proestrus. PRL levels declined promptly after treatment, except in one bitch that did not show proestrus during the cabergoline treatment period. E2 levels increased markedly after treatment in the five bitches that showed proestrus 10–22 days after cabergoline administration. This was consistent with previous reports [17, 25, 31, 38], in which administration of dopamine agonists reduced PRL. In addition, Spattini et al. [37] reported that a significant increase in LH concentration was observed one week before the onset of proestrus, but cabergoline administration did not change the decrease in PRL levels. The bitches in the current study that showed proestrus 10 days after the start of cabergoline had low PRL levels at the start of administration, and the decrease in PRL was very small. Where proestrus occurred 10–53 days after administration, statistical analysis of FSH, LH, and E2 levels could not be performed due to variations in estrus expression after cabergoline administration. Spattini et al. [37] used dogs showing estrus within four weeks of cabergoline administration, and reported that the number of days from treatment to estrus was concentrated to about two weeks in beagles, so a clear LH increase must have been observed. In addition, Spattini et al. [37] showed an LH pulse frequency increase 2 weeks after cabergoline administration, which was also apparent in the current study. The results showed that E2 clearly increased prior to proestrus when comparing the estrus of the five bitches that displayed estrus within four weeks of treatment. Bitches that received estradiol benzoate one week before the start of cabergoline treatment were reported to present earlier signs and a shorter duration of proestrus compared to those of the control bitches treated with cabergoline alone [22]. In the bitch (B07044) who showed a short period from the end of the previous estrus to cabergoline treatment, an increase in LH level and LH pulse frequency was observed after the administration of cabergoline, but E2 did not increase owing to poor follicle development, and there is a possibility that this bitch did not show early proestrus promptly. Estrus induction by dopamine agonists is not necessarily associated with PRL depletion, but it is thought to stimulate hypothalamic GnRH levels [26]. A decrease in PRL does not have an immediate effect on the pituitary-gonadal axis, resulting in a lag time for changes in the levels of FSH, LH, and E2. This concurred with a previous observation that the membrane excitability of GnRH neurons is not acutely modulated by prolactin [4]. PRL may modulate the reproductive axis by acting on a specific population of hypothalamic neurons that express the Kiss1 gene [1, 36]. Thus, PRL reduction may not act directly on LH secretion, and a reduction in PRL increase may stimulate follicular development through the induction of a kisspeptin-mediated increase in GnRH secretion and a subsequent increase in LH pulse frequency.
Estrus induction by cabergoline is known to depend on cabergoline dosage, treatment duration, and stage of anestrus. Jöchle et al. [19] reported that, in the case of administration of cabergoline (5 µg/kg/day for 14 days orally) to 28 female beagles that were in estrus 4–6 months earlier, no difference was observed in the inter-estrus interval between the estrus induction and control groups, suggesting that this drug treatment method would therefore require a 30-day or longer administration period. In addition, Verstegen et al. [38] revealed that it takes time for the effect of cabergoline administration to be evident in early anestrus as compared to that in mid- and late anestrus. Similarly, Shimatsu [35] reported that the rate of estrus induction in mid-anestrus was higher than that in early anestrus bitches. The present study was consistent with previous reports [35, 38] and showed that estrus induction was delayed if the delay between the end of the previous estrus period and the treatment was short. Cabergoline has been reported to have two major side effects, a change in coat color and vomiting [12, 19, 33, 38], but no side effects were observed in our study.
A significant correlation was detected in this study between the period from administration to proestrus and the reduction in PRL one week after administration. A positive correlation was observed between the LH levels at two weeks after cabergoline administration and PRL reduction. PRL levels are highly variable depending on the individual bitch [11, 31], and it has been proposed that PRL levels are determined genetically [11]. Although the level of PRL in anestrous bitches is normally low [7], the results of the present study indicated that a reduction from the original PRL level is important for the induction of estrus, and that estrus is induced as long as the differential is sufficient, even when the PRL level is low. Ota et al. [28] reported that rat ovarian LH receptors were reduced by small amounts of exogenous ovine PRL, and high amounts of ovine PRL reduced LH receptors. PRL is considered to act as a luteotropic agent in the rat ovary and stimulates LH receptors. It has been shown [15] that administration of ovine PRL and ovine LH to FSH- pretreated immature female rats stimulated ovarian LH receptors. Additional factors, such as the ratio of LH to prolactin, or the duration of the effects of these hormones may influence binding. Although it is not certain that this also applies to canines, it was suggested that a rapid decrease in PRL (from the PRL level that stimulated the LH receptor) may lead to an increase in LH concentration and LH pulse frequency.
In conclusion, there was a significant correlation between the number of days from the start of dosing to estrus, the LH level at two weeks after cabergoline treatment, and the rate of decrease in PRL. An abrupt reduction in the PRL level is likely to be important for the initiation of estrus in bitches. In addition, if the period from the end of the previous estrus to cabergoline treatment is short, it may take some time to show proestrus without increasing E2 levels, even if the LH level increases after cabergoline administration.
POTENTIAL CONFLICTS OF INTEREST
The authors have nothing to disclose.
Acknowledgments
We hereby express our deep appreciation to the following individuals for the provision of reagents and materials: Dr. S. Lynch (Endocrine Services Limited, Warwickshire, UK) for providing anti-human FSH serum (M91); Dr. Yuji Mori (Graduate School of Agricultural and Life Sciences, The University of Tokyo, Tokyo, Japan) for providing anti-sheep LH serum (YM#18); Dr. L. E. Reichert (Department of Biochemistry and Molecular Biology, Albany Medical College, Albany, NY, USA) providing canine FSH (LER-1685-3A) and canine LH (LER-1685-1); Dr. A. F. Parlow (National Hormone and Pituitary Program, Harbor-UCLA Medical Center, Torrance, CA, USA) for providing anti-canine PRL serum (AFP1062091GP), canine PRL (AFP2451B), rat FSH (NIDDK-FSH I-5) and rat LH (NIDDK-LH I-5); and Dr. K. Wakabayashi (the Institute of Endocrinology, Gunma University, Maebashi, Japan) for providing HAC-GPA2-01GTP80.
REFERENCES
- 1.Araujo-Lopes R., Crampton J. R., Aquino N. S., Miranda R. M., Kokay I. C., Reis A. M., Franci C. R., Grattan D. R., Szawka R. E.2014. Prolactin regulates kisspeptin neurons in the arcuate nucleus to suppress LH secretion in female rats. Endocrinology 155: 1010–1020. doi: 10.1210/en.2013-1889 [DOI] [PubMed] [Google Scholar]
- 2.Arbeiter K., Dobretsberger M., Müller E., Holzmann A.1991. [Indirect detection of ovulation and fertilization in the dog by progesterone level testing]. Zentralbl. Veterinärmed. A 38: 696–701 (in German). doi: 10.1111/j.1439-0442.1991.tb01066.x [DOI] [PubMed] [Google Scholar]
- 3.Arnold S., Arnold P., Concannon P. W., Weilenmann R., Hubler M., Casal M., Döbeli, Fairburn A., Eggenberger E., Rüsch P.1989. Effect of duration of PMSG treatment on induction of oestrus, pregnancy rates and the complications of hyper-oestrogenism in dogs. J. Reprod. Fertil. Suppl. 39: 115–122. [PubMed] [Google Scholar]
- 4.Brown R. S., Piet R., Herbison A. E., Grattan D. R.2012. Differential actions of prolactin on electrical activity and intracellular signal transduction in hypothalamic neurons. Endocrinology 153: 2375–2384. doi: 10.1210/en.2011-2005 [DOI] [PubMed] [Google Scholar]
- 5.Chapwanya A., Clegg T., Stanley P., Vaughan L.2008. Comparison of the Immulite and RIA assay methods for measuring peripheral blood progesterone levels in Greyhound bitches. Theriogenology 70: 795–799. doi: 10.1016/j.theriogenology.2008.05.047 [DOI] [PubMed] [Google Scholar]
- 6.Cirit U., Bacinoglu S., Cangul I. T., Kaya H. H., Taş M., Ak K.2007. The effects of a low dose of cabergoline on induction of estrus and pregnancy rates in anestrous bitches. Anim. Reprod. Sci. 101: 134–144. doi: 10.1016/j.anireprosci.2006.09.005 [DOI] [PubMed] [Google Scholar]
- 7.Concannon P. W.2011. Reproductive cycles of the domestic bitch. Anim. Reprod. Sci. 124: 200–210. doi: 10.1016/j.anireprosci.2010.08.028 [DOI] [PubMed] [Google Scholar]
- 8.Concannon P., Lasley B., Vanderlip S.1997. LH release, induction of oestrus and fertile ovulations in response to pulsatile administration of GnRH to anoestrous dogs. J. Reprod. Fertil. Suppl. 51: 41–54. [PubMed] [Google Scholar]
- 9.Concannon P. W., Whaley S., Anderson S. P.1986. Increased LH pulse frequency associated with termination of anestrus during the ovarian cycle of the dog. Biol. Reprod. 34 suppl 1: 109 (abstract 119). [Google Scholar]
- 10.de Gier J., Kooistra H. S., Djajadiningrat-Laanen S. C., Dieleman S. J., Okkens A. C.2006. Temporal relations between plasma concentrations of luteinizing hormone, follicle-stimulating hormone, estradiol-17beta, progesterone, prolactin, and alpha-melanocyte-stimulating hormone during the follicular, ovulatory, and early luteal phase in the bitch. Theriogenology 65: 1346–1359. doi: 10.1016/j.theriogenology.2005.08.010 [DOI] [PubMed] [Google Scholar]
- 11.Gobello C., Bolognani F., de la Sota R. L., Goya R. G.2001. Twenty-four-hour profiles of serum prolactin and luteinizing hormone in anoestrous crossbred bitches. Reprod. Domest. Anim. 36: 41–45. doi: 10.1046/j.1439-0531.2001.00221.x [DOI] [PubMed] [Google Scholar]
- 12.Gobello C., Castex G., Broglia G., Corrada Y.2003. Coat colour changes associated with cabergoline administration in bitches. J. Small Anim. Pract. 44: 352–354. doi: 10.1111/j.1748-5827.2003.tb00166.x [DOI] [PubMed] [Google Scholar]
- 13.Gobello C., Castex G., Dela sota L., Corrada Y.2004. Shortening of interestrous intervals with cabergoline in bitches: a clinical trial. J. Am. Anim. Hosp. Assoc. 40: 115–119. doi: 10.5326/0400115 [DOI] [PubMed] [Google Scholar]
- 14.Hase M., Hori T., Kawakami E., Tsutsui T.2000. Plasma LH and progesterone levels before and after ovulation and observation of ovarian follicles by ultrasonographic diagnosis system in dogs. J. Vet. Med. Sci. 62: 243–248. doi: 10.1292/jvms.62.243 [DOI] [PubMed] [Google Scholar]
- 15.Holt J. A., Richards J. S., Midgley A. R., Jr., Reichert L. E., Jr.1976. Effect of prolactin on LH receptor in rat luteal cells. Endocrinology 98: 1005–1013. doi: 10.1210/endo-98-4-1005 [DOI] [PubMed] [Google Scholar]
- 16.Inaba T., Tani H., Gonda M., Nakagawa A., Ohmura M., Mori J., Torii R., Tamada H., Sawada T.1998. Induction of fertile estrus in bitches using a sustained-release formulation of a GnRH agonist (leuprolide acetate). Theriogenology 49: 975–982. doi: 10.1016/S0093-691X(98)00046-6 [DOI] [PubMed] [Google Scholar]
- 17.Jeukenne P., Verstegen J.1997. Termination of dioestrus and induction of oestrus in dioestrous nonpregnant bitches by the prolactin antagonist cabergoline. J. Reprod. Fertil. Suppl. 51: 59–66. [PubMed] [Google Scholar]
- 18.Jöchle W.1997. Prolactin in canine and feline reproduction. Reprod. Domest. Anim. 32: 183–193. doi: 10.1111/j.1439-0531.1997.tb01280.x [DOI] [Google Scholar]
- 19.Jöchle W., Arbeiter K., Post K., Ballabio R., D’Ver A. S.1989. Effects on pseudopregnancy, pregnancy and interoestrous intervals of pharmacological suppression of prolactin secretion in female dogs and cats. J. Reprod. Fertil. Suppl. 39: 199–207. [PubMed] [Google Scholar]
- 20.Kutzler M. A.2007. Estrus induction and synchronization in canids and felids. Theriogenology 68: 354–374. doi: 10.1016/j.theriogenology.2007.04.014 [DOI] [PubMed] [Google Scholar]
- 21.Kutzler M. A., Wheeler R., Volkmann D. H.2001. Canine oestrus induction using the GnRH agonist, deslorelin. Proc. Ann. Symp. Eur. Vet. Soc. Small Anim. Reprod.: 147–148.
- 22.Mogheiseh A., Mosavi Ghiri M. J., Bandarian E.2017. The clinical follow-up of estradiol benzoate priming during induction of estrus with cabergoline in dogs. Top. Companion Anim. Med. 32: 16–19. doi: 10.1053/j.tcam.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 23.Moxon R., Copley D., England G. C.2010. Technical and financial evaluation of assays for progesterone in canine practice in the UK. Vet. Rec. 167: 528–531. doi: 10.1136/vr.c5082 [DOI] [PubMed] [Google Scholar]
- 24.Nakada K., Tsuchiya E., Moriyoshi M., Nakao T., Watanabe G., Taya K.1998. Radioimmunoassays of follicle stimulating hormone and luteinzing hormone in the dog. J. Reprod. Dev. 44: 39–45. doi: 10.1262/jrd.98-446j39 [DOI] [Google Scholar]
- 25.Okkens A. C., Bevers M. M., Dieleman S. J., Willems A. H.1985. Shortening of the interoestrous interval and the lifespan of the corpus luteum of the cyclic dog by bromocryptine treatment. Vet. Q. 7: 173–176. doi: 10.1080/01652176.1985.9693979 [DOI] [PubMed] [Google Scholar]
- 26.Okkens A. C., Kooistra H. S.2006. Anoestrus in the dog: a fascinating story. Reprod. Domest. Anim. 41: 291–296. doi: 10.1111/j.1439-0531.2006.00702.x [DOI] [PubMed] [Google Scholar]
- 27.Ono M., Ohtaki T., Tanemura K., Ishii M., Watanabe G., Taya K., Tsumagari S.2011. Effect of short-term fasting on hepatic steroid hormone metabolism in cows. J. Vet. Med. Sci. 73: 1145–1149. doi: 10.1292/jvms.10-0578 [DOI] [PubMed] [Google Scholar]
- 28.Ota H., Wakizaka A., Fukushima M., Maki M.1986. Enhanced ovarian gonadotropin receptors in the testosterone-induced polycystic ovary in rats. Tohoku J. Exp. Med. 148: 313–325. doi: 10.1620/tjem.148.313 [DOI] [PubMed] [Google Scholar]
- 29.Phillips T. C., Larsen R. E., Hernandez J., Strachan L., Samuelson D., Shille V. M., Archbald L. F.2003. Selective control of the estrous cycle of the dog through suppression of estrus and reduction of the length of anestrus. Theriogenology 59: 1441–1448. doi: 10.1016/S0093-691X(02)01183-4 [DOI] [PubMed] [Google Scholar]
- 30.Ribadu A. Y., Nakada K., Moriyoshi M., Zhang W. C., Tanaka Y., Nakao T.2000. The role of LH pulse frequency in ACTH-induced ovarian follicular cysts in heifers. Anim. Reprod. Sci. 64: 21–31. doi: 10.1016/S0378-4320(00)00196-2 [DOI] [PubMed] [Google Scholar]
- 31.Rota A., Mollo A., Marinelli L., Gabai G., Vincenti L.2003. Evaluation of cabergoline and buserelin efficacy for oestrous induction in the bitch. Reprod. Domest. Anim. 38: 440–443. doi: 10.1046/j.0936-6768.2003.00460.x [DOI] [PubMed] [Google Scholar]
- 32.Sato M., Tsubota T., Komatsu T., Watanabe G., Taya K., Murase T., Kita I., Kudo T.2001. Changes in sex steroids, gonadotropins, prolactin, and inhibin in pregnant and nonpregnant Japanese black bears (Ursus thibetanus japonicus). Biol. Reprod. 65: 1006–1013. doi: 10.1095/biolreprod65.4.1006 [DOI] [PubMed] [Google Scholar]
- 33.Schaefers-Okkens A. C.1996. Ovaries pp. 131–156. In: Clinical Endocrinology of Dogs and Cats (Rijnberk, A. ed.), Kluwer Academic Publishers, Dordrecht. [Google Scholar]
- 34.Shille V. M., Thatcher M. J., Simmons K. J.1984. Efforts to induce estrus in the bitch, using pituitary gonadotropins. J. Am. Vet. Med. Assoc. 184: 1469–1473. [PubMed] [Google Scholar]
- 35.Shimatsu Y.2017. Use of cabergoline for oestrus induction in multiparous anoestrous Beagle bitches. Aust. Vet. J. 95: 350–352. doi: 10.1111/avj.12622 [DOI] [PubMed] [Google Scholar]
- 36.Sonigo C., Bouilly J., Carré N., Tolle V., Caraty A., Tello J., Simony-Conesa F. J., Millar R., Young J., Binart N.2012. Hyperprolactinemia-induced ovarian acyclicity is reversed by kisspeptin administration. J. Clin. Invest. 122: 3791–3795. doi: 10.1172/JCI63937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spattini G., Borghi V., Thuróczy J., Balogh L., Scaramuzzi R. J., De Rensis F.2007. Follicular development and plasma concentrations of LH and prolactin in anestrous female dogs treated with the dopamine agonist cabergoline. Theriogenology 68: 826–833. doi: 10.1016/j.theriogenology.2007.06.007 [DOI] [PubMed] [Google Scholar]
- 38.Verstegen J. P., Onclin K., Silva L. D., Concannon P. W.1999. Effect of stage of anestrus on the induction of estrus by the dopamine agonist cabergoline in dogs. Theriogenology 51: 597–611. doi: 10.1016/S0093-691X(99)00013-8 [DOI] [PubMed] [Google Scholar]
- 39.Weilenmann R., Arnold S., Döbeli M., Rüsch P., Zerobin K.1993. [Estrus induction in bitches by the administration of PMSG and HCG]. Schweiz. Arch. Tierheilkd. 135: 236–241 (in German). [PubMed] [Google Scholar]