ABSTRACT
A 65-year-old woman died of congestive heart failure and diabetes mellitus. She had a history of mild anemia since adolescence, but received neither iron supplementation nor transfusion. The cirrhotic liver obtained at autopsy contained a large amount of iron. The heart and pancreas also had excess iron. Her iron overload may be due to excess iron absorption in the gut because of the absence of an iatrogenic background such as transfusion or iron supplementation.
Key Words: anemia, iron overload, cirrhosis, congestive heart failure, diabetes mellitus
INTRODUCTION
Anemia is not always iron-deficient but rarely iron-loading. Refractory anemias are usually complicated with secondary iron-loading after repeated transfusions.1,2 Clinical introduction of the iron regulatory hormone hepcidin led to a new paradigm of iron overloading under various disease conditions.3 Hereditary hemochromatosis (HH) is a proto-type of iron overload syndromes associated with impaired hepcidin synthesis in the liver.4 Some anemic patients with ineffective erythropoiesis are also affected by low levels of serum hepcidin25 as in HH. The primary iron overload in such patients may be camouflaged by repeated transfusions and long-term iron supplementation. Based on an impaired hepcidin system, Camaschella, et al proposed a new disease entity of iron loading anemia (ILA) for refractory anemias associated with a primary iron overload.5
Iron overload syndromes differ between Caucasians and Japanese, mainly due to the major genotype of HFE-HH, especially homozygote of C282Y, in Caucasians. Major genotypes in Japanese are aceruloplasminemia, TFR2-HH, and HJV-HH.6,7 In addition, ILA has not been reported in Japan in spite of a large number of patients participating in oral iron chelation studies for transfusion-dependent refractory anemias.1,2
CASE REPORT
A 65-year-old Japanese woman died from iron-loaded multi-organ damage including congestive heart failure and diabetes mellitus. She had a history of mild anemia since adolescence, but received neither iron supplementation nor transfusion. Blood tests revealed anemia of unknown etiology, iron overload, and mild glucose intolerance (Table 1). Abdominal CT showed a high-dense liver, and a large but nearly normal-dense spleen (Fig. 1). Autopsy confirmed that she had multi-organ damage associated with iron overloading in the liver, spleen, heart, pancreas, and bone marrow (Table 2). The liver structures showed F4 in the Laennec cirrhosis classification with parenchymal cell-dominant iron deposition.
Table 1.
Laboratory data of the female patient aged 61 years old
| Blood test | Reference | Patient |
| WBC | 3,500–9,000/μL | 2,900/μL |
| RBC | (360–480) × 104/μL | 294 × 104/μL |
| Hb | 11.4–14.6 g/dL | 10.7 g/dL |
| Platelets | (15.0–35.9) × 104/μL | 15.0 × 104/μL |
| BUN | 9–22 mg/dL | 17 mg/dL |
| t-Bilirubin | 0.2–1.2 mg/dL | 0.5 mg/dL |
| t-Protein | 6.5–8.0 g/dL | 6.7 g/dL |
| Albumin | 4.0–5.0 g/dL | 4.3 g/dL |
| AST | 10–35 IU/L | 29 IU/L |
| ALT | 5–30 IU/L | 38 IU/L |
| ALP | 100–350 IU/L | 544 IU/L |
| V. B12 | 250–950 pg/mL | 502 pg/mL |
| Folic acid | 3.6–12.9 ng/mL | 6.0 ng/mL |
| Cu | 78–131 μg/dL | 76 μg/dL |
| Ceruloplasmin | 21–37 mg/dL | 18 mg/dL |
| Fe | 70–160 μg/dL | 212 μg/dL |
| Ferritin | 13–58 ng/mL | 1940 ng/mL |
| Transferrin | 200–340 mg/dL | 149 mg/dL |
| TF saturation | 44–79% | 96% |
| PBS | <200 mg/dL | 217 mg/dL |
| HbA1C | <5.6% | 8.0% |
| Urinalysis | Reference | Patient |
| Protein | Negative | Negative |
| Glucose | Negative | Negative |
| Bone Marrow | Reference | Patient |
| r-Sideroblasts | <1.0% | 3% |
ALP: alkaline phosphatase, ALT: alanine aminotransferase, AST: aspartate aminotransferase, BUN: blood urea nitrogen, Hb: hemoglobin, PBS: postprandial blood sugar, r: ringed, RBC: red blood cells, t: total, TF: transferrin, V: vitamin, WBC: white blood cells.
Anemia was one aspect of pancytopenia in the patient. Her liver functions were maintained fairly well in spite of a cirrhotic liver with heavy iron deposition. Biochemical iron parameters were compatible with hemochromatosis. Note that congestive heart failure was followed by symptomatic diabetes in the aged patient.
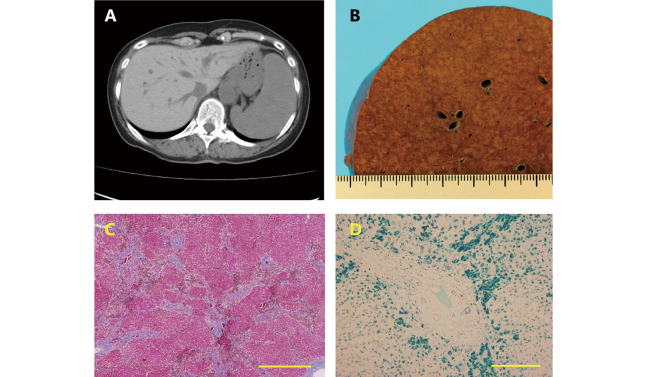
Fig. 1.
Liver images of patient
Fig. 1A: Abdominal CT. Liver parenchyma with a high density indicates iron deposition, and marked splenomegaly has an unknown etiology.
Fig. 1B: The autopsied liver: Macroscopy of a cross-section of the liver. The cross section of the liver is representative of cirrhosis, showing nodular parenchyma among wide fibrous tissues.
Fig. 1C: The autopsied liver: Masson trichrome stain. The liver consists of parenchyma surrounded by wide fibrous septa, indicating F4 of Laennec cirrhosis. Bar indicates 1.0 mm.
Fig. 1D: The autopsy liver: Berlin blue stain. A large amount of iron is seen in the peri-fibrous parenchymal cells. Bar indicates 300 μm.
Table 2.
Structures and functions of iron-loaded organs of the patient
| Liver | Spleen | Heart | Bone Marrow | |
| Structure Change | High-dense liver under CT
Cirrhosis |
Splenomegaly Portal hypertension |
Cardiomegaly | Low cellularity
Relative erythroblast hyperplasia |
| Functional Change | Compensated cirrhosis | Hypersplenism | Congestive heart failure | Mild anemia |
| Iron Deposit | Hepatocyte-dominant | Macrophages | Myocardial cells | Macrophages |
CT, computed tomography.
Iron accumulation and organ dysfunction differ from organ to organ in the patient. Her abdominal CT shows marked deposition in the liver, but not in the spleen or heart. The structure and function of the liver are compatible with those of hemochromatosis with hepatocyte-dominant iron loading. Splenomegaly associated with portal hypertension may result in hypersplenism. Iron overload of the myocardium may be partially responsible for congestive heart failure associated with cardiomegaly. Mild anemia that is unusual in hemochromatosis may be related to iron overload in macrophages of the bone marrow.
DISCUSSION
In Japan, TFR2-HH involves iron-induced multi-organ damage in the liver and pancreas around the age of 50 years, while HJV-HH patients present with heart disease before the age of 30 years.6,7 Because there was no iatrogenic background such as iron supplementation or transfusion, we speculate that the iron loading involving the heart, liver, pancreas, and bone marrow of our patient was primarily due to excess iron absorption in the gut. Recently, Camaschella, et al proposed a new disease entity of ILA, which is characterized by hypohepcidinemia associated with refractory anemias.5 Multi-factorial suppressers may be supplied from the bone marrow to regulate HAMP in the liver, resulting in reduced hepcidin production and leading to excess iron absorption in the gut. The clinical features of our patient with diabetes mellitus, heart failure, cirrhosis, and anemia were compatible with non-transfusion-dependent ILA, but no definitive etiologies were identified because of the lack of determining either the genetic background of anemia or serum levels of hepcidin25.8
Some participants in oral iron chelation studies for post-transfusion iron overload syndromes1,2 show partial improvement in managing anemias. Erythropoiesis may be relieved from an iron-induced oxidative stress via reduced iron storage.9 Considering a large number of Japanese patients with refractory anemias, ILA and its related diseases may be more prevalent than classical HH in Japan.6 It is also important for patients with ILA to be treated with oral iron chelation before transfusion.
ACKNOWLEDGEMENT
None
CONFLICT OF INTEREST STATEMENT
All authors declare that they have no conflict of interest.
Abbreviations
- HH
hereditary hemochromatosis
- ILA
iron loading anemia
REFERENCES
- 1.Takatoku M, Uchiyama T, Okamoto S, et al. Retrospective nationwide survey of Japanese patients with transfusion-dependent MDS and aplastic anemia highlights the negative impact of iron overload on morbidity/mortality. Eur J Haematol. 2007;78(6):487–494. doi: 10.1111/j.1600-0609.2007.00842.x. [DOI] [PubMed]
- 2.Kohgo Y, Urabe A, Kilinç Y, et al. Deferasirox decreases liver iron concentration in iron-overloaded patients with myelodysplastic syndromes, aplastic anemia and other rare anemias. Acta Haematol. 2015;134(4):233–242. doi: 10.1159/000381893. [DOI] [PubMed]
- 3.Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823(9):1434–1443. doi: 10.1016/j.bbamcr.2012.01.014. [DOI] [PMC free article] [PubMed]
- 4.Bridle KR, Frazer DM, Wilkins SJ, et al. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet. 2003;361(9358):669–673. doi: 10.1016/S0140-6736(03)12602-5. [DOI] [PubMed]
- 5.Camaschella C, Nai A. Ineffective erythropoiesis and regulation of iron status in iron loading anaemias. Br J Haematol. 2016;172(4):512–523. doi: 10.1111/bjh.13820. [DOI] [PubMed]
- 6.Hattori A, Miyajima H, Tomosugi N, et al. Clinicopathological study of Japanese patients with genetic iron overload syndromes. Pathol Int. 2012;62(9):612–618. doi: 10.1111/j.1440-1827.2012.02848.x. [DOI] [PubMed]
- 7.Kawabata H. The mechanisms of systemic iron homeostasis and etiology, diagnosis, and treatment of hereditary hemochromatosis. Int J Hematol. 2018;107(1):31–43. doi: 10.1007/s12185-017-2365-3. [DOI] [PubMed]
- 8.Kaneko Y, Miyajima H, Piperno A, et al. Measurement of serum hepcidin-25 levels as a potential test for diagnosing hemochromatosis and related disorders. J Gastroenterol. 2010;45(11):1163–1171. doi: 10.1007/s00535-010-0259-8. [DOI] [PubMed]
- 9.Kikuchi S, Kobune M, Iyama S, et al. Improvement of iron-mediated oxidative DNA damage in patients with transfusion-dependent myelodysplastic syndrome by treatment with deferasirox. Free Radic Biol Med. 2012;53(4):643–648. doi: 10.1016/j.freeradbiomed.2012.06.006. [DOI] [PubMed]