Abstract
Disorders of thyroid function are common in pregnancy and have implications for foetal and maternal health. Thyroid autoimmunity, as evidenced by the presence of elevated levels of anti-thyroid antibodies (anti-TPO and anti-Tg antibodies) is associated with an increased risk of miscarriage, though the mechanism remains poorly understood. There has been considerable focus on the implications and optimal management of pregnant women with thyroid disease, especially those undergoing assisted reproduction. Pregnancy results in significant changes in thyroid physiology and these need to be understood by clinicians involved in the care of pregnant women. Guidelines for the use of thyroxine and target thyroid function tests have been produced by international bodies but it is recognised that these predominantly reflect expert opinion rather than established evidence-based practice. Importantly a number of key clinical trials have been performed to aid understanding, particularly of the consequences of hypothyroidism for mother and baby, and the effectiveness of thyroid hormone use in autoimmune and subclinical hypothyroidism. This review summarises the current knowledge base and guidance for practice relating to thyroid disorders in pregnancy and subfertility.
Keywords: autoimmune disease, hyperthyroidism, hypothyroidism, pregnancy, subclinical hyperthyroidism, subclinical hypothyroidism, thyroid auto-antibody
Introduction
Approximately 2–3% of all pregnancies are complicated by thyroid disease.1 After diabetes, it is the second most common significant pathology affecting women during pregnancy and detailed understanding of the pathophysiology, diagnosis and treatment is required to limit the effects on both maternal and foetal health.2 Diagnosis of thyroid dysfunction during pregnancy is complicated by the hormonal changes that take place, posing specific challenges for both detection and management. The Endocrine Society recommends that screening for thyroid conditions in pregnancy is performed in women >30 years, those with previous personal or family history of thyroid disease, women with issues with conception and existing autoimmune conditions.3 Thyroid hormones are essential for foetal brain development and other aspects of foetal growth and it is therefore necessary that healthcare professionals involved in antenatal care recognise thyroid dysfunction and involve expert specialists when clinically required.4 There is emerging new data seeking to provide clarity on areas of controversy and uncertainty. This review summarises the recent developments in the understanding of consequences and management of thyroid disorders during pregnancy and assisted conception.
Data collection of material used in the review
We performed a detailed literature search for articles covering thyroid disorders in pregnancy and subfertility using PubMed and Medline as the key resources. The search terms included ‘thyroid, hypothyroidism, subclinical hypothyroidism, hyperthyroidism, subclinical hyperthyroidism, Graves’ disease, thyroid nodule, and thyroid cancer’, and combined them with ‘pregnancy, fertility, subfertility, and foetal outcome’. Filters were applied to include articles in English, human studies and to contain reviews, meta-analysis, and original articles. Duplicate articles and those covering thyroid dysfunction postpartum and lactation were excluded. The search was undertaken by two authors (SA and MJ) and 114 articles were selected to inform the final review.
Thyroid physiology and gonadal function
Thyroid dysfunction results in menstrual irregularities reflecting ovulatory disturbance. Thyroid status influences folliculogenesis via effects of triiodothyronine (T3) on the P13K/Akt pathway.5 These actions both enhance proliferation and inhibit apoptosis of the granulosa cells. Hypothyroidism is associated with reduced rate of fertilisation, and despite the recognition of endometrial expression of deiodinase type 3 and placental increases in T3, the exact mechanism behind reduced fertility in hypothyroidism remains unknown.6,7 Although it is known that thyroid disorders lead to menstrual disturbances, the association with subfertility has not been explored in detail. Possible aetiologies impacting fertility are changes in oestrogen metabolism, variations in Gonadotrophin releasing hormone (GnRH) pulsatility, and elevations in prolactin.8 Data on prevalence of subfertility in hyperthyroidism are equally scarce.
Male fertility studies have shown that both hyperthyroidism and hypothyroidism in males can affect sperm count, motility, and morphology; however, the mechanisms remain unclear and further research in male factor issues is required to improve understanding.6
Thyroid physiology during pregnancy
There are a number of important physiological changes that occur during pregnancy that need to be considered when interpreting thyroid status, testing thyroid function, and managing thyroid conditions.
Maternal thyroid physiology
The maternal thyroid gland increases in vascularity and undergoes mild hyperplasia during pregnancy, typically increasing in size between 10–40% of volume. This enlargement can be more pronounced if there is underlying iodine deficiency.9 Thyroxine-binding globulin (TBG) along with maternal total thyroxine (T4) starts rising from week 4 of pregnancy.10 TBG half-life increases from a nonpregnancy time of 15 min to up to 3 days in pregnancy;11,12 there is also oestrogen-mediated glycosylation, which increases the TBG concentration by up to 75% in the second trimester.11,13 Free thyroid hormones and not total hormone concentrations are best measured, as total hormone levels are less interpretable, and require gestation-specific reference ranges. Increasing human chorionic gonadotropin (hCG) levels cause a significant decrease in thyroid-stimulating hormone (TSH) levels due to thyrotropic activity. TSH and hCG have identical alpha subunits and comparable beta subunits and therefore, there can be both related stimulation of receptor and negative feedback, which can cause a measurable reduction of the TSH level.8 This effect is proportional to the level of hCG and therefore, in conditions that increase hCG concentration, such as multiple pregnancies and trophoblastic disease, symptoms of hyperthyroidism may become more pronounced. In these clinical situations, thyroid function tests (TFTs) need cautious interpretation. It is therefore important to have trimester-specific ranges for maternal TSH; usually significantly lower (than in the nongravid state) in the first trimester.1,14
Total T4 rises secondary to the increasing TBG, which peaks at approximately 16 weeks of gestation and remains elevated until the postpartum period.8 Variations in thyroid function within normal ranges in otherwise euthyroid women with no autoimmunity is considered to have no impact on pregnancy outcome.15 In a recent Swedish study, body mass index (BMI) and gestational age were identified to be key determinants of maternal TSH results (Table 1).16
Table 1.
Generalised trimester-specific reference ranges for TSH levels without adjusting for variables such as maternal age, maternal race, iodine status and laboratory assay (for free T4 and free T3 values the ATA guidelines suggest population and assay specific references).17.
| TSH range | |
|---|---|
| First trimester | >0.1 mIU/L and <2.5 mIU/L |
| Second trimester | >0.2 mIU/L and <3.0 mIU/L |
| Third trimester | >0.3 mIU/L and <3.0 mIU/L |
ATA, American Thyroid Association; TSH, thyroid-stimulating hormone.
There is increased uptake of iodine into the thyroid gland and also enhanced excretion of renal iodine. These changes reduce the amount of available iodine, which may become clinically significant if deficiency is present prior to conception. Iodine is also transferred to the foetus via the placenta and further depletes the maternal iodine availability. In extreme iodine deficiency there is lack of transplacental iodine and the foetus can develop neurological developmental compromise (historically termed cretinism).14 Dietary demand of iodine is increased during pregnancy via several mechanisms:
Increased hormone production and therefore maternal thyroid gland uptake of iodine.
Increased renal excretion of iodine.
Increased foetal demand of iodine.18
Research has demonstrated that if iodine sufficiency is achieved prior to conception there is adequate maternal adaptation2 and it is for this reason that both the World Health Organization (WHO)19 and American Thyroid Association (ATA) recommend a daily iodine intake of 250 µg during pregnancy and breast feeding.18
Thyroid hormones are converted to active forms (T4 to T3) by combining with deiodinase hormones of which there are three main forms. Deiodinase 3 concentration and activity is upregulated in the placenta and continues to rise with increasing gestation.14
Foetal thyroid physiology
From week four of gestation the thyroid begins its formation, initially with endodermal thickening from the midline floor between the first and second pharyngeal pouch. It then descends to its final anatomical location by gestational week seven and its track regresses giving rise to the thyroglossal duct. While foetal thyroid starts to demonstrate iodine concentrating ability, it is not until the end of the third month of gestation (weeks 12–14) that the foetal thyroid gland is able to function independently.20 Foetal TSH can be detected in serum from around 11 weeks; however, full maturation of the foetal axis is much later, until which time the baby relies on placental transfer of thyroxine.6,21,22
Thyroid hormones play a vital role in the early embryogenesis. They are essential for neurodevelopment, somatic growth, and tissue differentiation.23,24 Deiodinase 2 has been identified to be expressed in much higher quantities in the first trimester, compared with the later stages. This suggests the importance of local T3 conversion for the development of foetal tissues.25 Studies have highlighted the association of thyroid dysfunction and neurocognitive development.26–28
Screening for thyroid dysfunction
There is currently no consensus as to whether all women or which specific groups should be routinely screened for thyroid dysfunction in pregnancy29 and therefore a targeted approach to screening of women considered at ‘higher risk’ has been formulated. The most recent ATA guidance suggests that screening should be considered in these at-risk groups:1,18,30,31
Personal or family history of thyroid dysfunction.
Symptoms of thyroid dysfunction.
Presence of goitre or known elevation of anti-thyroid antibody.
Personal or family history of autoimmune conditions (e.g. type 1 diabetes).
Maternal age >30 years.
Infertility investigations (including past history of pregnancy loss, preterm delivery and recurrent miscarriage).
Recurrent miscarriage.
Morbid obesity (BMI >40 kg/m2).
Previous head/neck irradiation or thyroid surgery.
Use of amiodarone, lithium or recent administration of iodinated contrast.
Residing in an area of known moderate or severe iodine insufficiency.
Multiple prior pregnancies (⩾2).
Sitoris et al. have suggested that screening for subclinical hypothyroidism could be improved further by including obesity (BMI >30 kg/m2) and white ethnicity as additional risk factors.32 While they commented on the possibilities of screening being extended to women with iron deficiency and history of smoking, the study did not explore these associations in detail.
Thyroid disorders in pregnancy
Hypothyroidism
Hypothyroidism in pregnancy is defined as a TSH concentration above the trimester-specific ranges, if specific values are unavailable, conventionally a TSH >4.0 mIU/L may be used.18 If the free T4 level remains within normal range this is referred to as subclinical, and if below the reference range this is termed overt hypothyroidism.18
The prevalence of hypothyroidism in pregnancy is difficult to establish as a large proportion of the disease is undiagnosed; however, is estimated at 3% with the majority being subclinical.33,34 Overt hypothyroidism is estimated to have a prevalence of 0.2–1% with the greatest risk factors being iodine deficiency and autoimmune thyroiditis.6
Other risk factors include previous treated thyroid disease (thyroidectomy, radioiodine therapy, and pituitary disease) and medications (namely amiodarone, anti-thyroid medications, and lithium).1,2,35
Overt hypothyroidism
Overt hypothyroidism and its association with a reduction in foetal intelligence quotient (IQ) was first described in 1999 by Haddow et al.36 Since then, multiple obstetric complications have also been described. The most frequently documented complications include an increased risk of miscarriage, perinatal death, and preterm delivery with coexisting foetal distress. Limited evidence suggests that maternal hypertension, placental abruption, and postpartum haemorrhage may be a result of overt hypothyroidism.1,2,35
In women with overt hypothyroidism, the recommended pre-conception TSH is between 0.4–2.5 mIU/L and this range should be applied throughout the first trimester.18 In order to achieve this the established pre-pregnancy dose of levothyroxine may need to be increased by 30–50% when pregnancy is achieved.37,38 TFTs should be measured every 6–8 weeks in pregnancy and 30 days after a dose titration.39
It is essential that overt hypothyroidism, once identified, is treated promptly. Current literature suggests commencing women on a weight-based dose of levothyroxine at 2.33 µg/kg/day40 with repeat TFTs every 14 days in the first trimester and then every 30 days,39,41 until normal thyroid function is established. The dose should be adjusted according to serum TSH and FT4 concentrations aiming for the aforementioned trimester-specific TSH levels.1,2 The dose requirement of levothyroxine is often increased during pregnancy and practitioners should consider reducing the dose following delivery to the pre-conception level, with blood values rechecked 6 weeks postpartum.1
The ATA guidelines from 2017 recommend that the serum TSH target is maintained within trimester-specific ranges for management of hypothyroidism. Taking into account the ethnic variations and iodine status, the ATA recognised that the upper reference range for women may be more liberal than the earlier guidelines. Additional information such as BMI and antibody status need to be considered when setting pregnancy targets for previously undiagnosed pregnant women. It is recommended that these decisions be based on tests undertaken in the late first trimester (7–12 weeks).18
Subclinical hypothyroidism in pregnancy
Limited evidence is available about the effects of subclinical hypothyroidism (SCH) in pregnancy and clinical trials that have investigated SCH have employed a variety of study designs. Therefore, there is no clear evidence base on which to treat with levothyroxine; however, there is also no significant harm reported when treatment is initiated. When treatment is commenced, the same reference ranges apply as for the management of overt hypothyroidism. It is also important for these women to have adequate iodine supplementation.18,42
A meta-analysis of three studies by Zhang et al. involved the comparison of euthyroid women and those with isolated SCH and demonstrated a higher risk of miscarriage in SCH (Relative risk (RR) = 1.45, 95% CI 1.07–1.96, p = 0.02)43; however, a meta-analysis of four studies comparing treatment showed no statistically significant difference when SCH is treated (RR = 1.14, 95% CI 0.82–1.58, p = 0.43).43
A large study by Carty et al. recruiting 4643 pregnant women showed that there was no difference in the rates of miscarriage [OR = 0.73 (95% CI 0.2–3.1, p = 1.0)], preterm delivery <37 weeks of gestation [OR = 0.83 (95% CI 0.46–1.47, p = 0.584)] or corrected birthweight (p = 0.536) when comparing a TSH 2.5–5.0 mU/L with TSH <2.5 mU/L.44 Similar findings were also demonstrated by Plowden et al. in a prospective cohort randomised control study.45
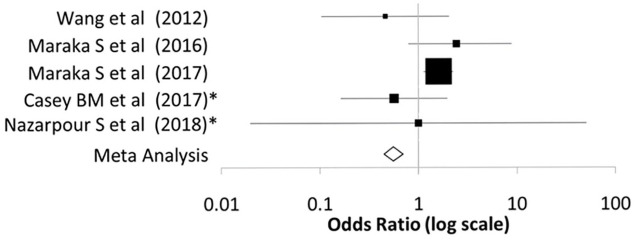
Figure 1.
Forest plot illustrating the comparison of spontaneous abortion rates between treated and nontreated subclinical hypothyroid women from the controlled clinical trials described in Tables 2 and 3.
The meta-analysis indicates no benefit on rate of spontaneous abortions with treatment.
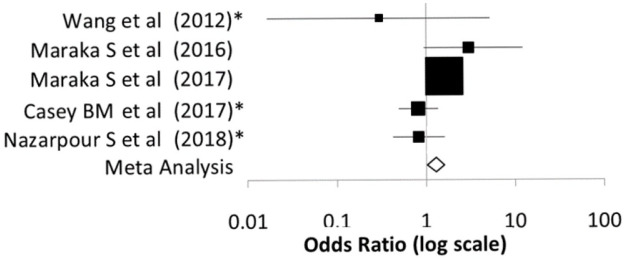
Figure 2.
Forest plot illustrating the comparison of preterm delivery rates between treated and nontreated subclinical hypothyroid women from the controlled clinical trials described in Tables 2 and 3
The meta-analysis indicates a reduction of preterm delivery rates in treated subclinical hypothyroidism.
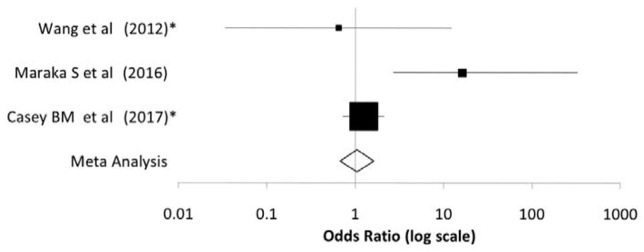
Figure 3.
Forest plot illustrating the comparison of low birth weights between treated and nontreated subclinical hypothyroid women from the clinical trials described in Tables 2 and 3.
The meta-analysis indicates no impact of treatment on low birth weight.
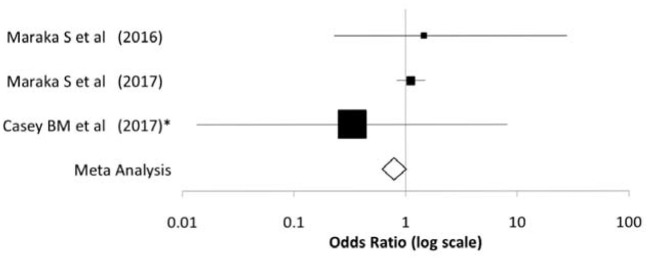
Figure 4.
Forest plot illustration the comparative rates of IUGR between treated and nontreated subclinical hypothyroid women from the clinical trials described in Tables 2 and 3.
The meta-analysis indicates no impact on rate of IUGR with treatment in women with subclinical hypothyroidism.
IUGR, intrauterine growth restriction.
Table 2.
The main clinical trials in subclinical hypothyroidism (excluding those focused on antibody titre and assisted conception) and their key features (Figure 1, Figure 2, Figure 3 and Figure 4).
| Study | Country | Design | Number of patients | Criteria | Number treated with levothyroxine |
|---|---|---|---|---|---|
| Wang et al.46 | China | Multicentre retrospective cohort study | 196 (aged 19–45 years) | TSH >2.5 mIU/L | 28 |
| Maraka S et al.47 | United States | Single centre retrospective cohort study | 366 (aged 18-45 years) | TSH >2.5 mIU/L in first trimester, >3.0 mIU/L thereafter (<10 mIU/L throughout) | 82 |
| Maraka S et al.48 | United States | Multicentre retrospective cohort study | 5405 (aged 18–55 years) | TSH concentration 2.5–10 mIU/L | 843 |
| Casey BM et al.49 | United States | Multicentre randomised, placebo-controlled trial | 677 (<20 weeks’ gestation) | TSH >4.0 mIU/L | 339 |
| Nazarpour S et al.50 | Tehran | Single centre, single-blind randomized clinical trial | 366 | TSH >2.5 mIU/L | 183 |
TSH, thyroid-stimulating hormone.
Table 3.
Pregnancy outcomes in women with subclinical hypothyroidism in the studies described in Table 2 comparing those treated with levothyroxine with nontreated women and their statistical significance in terms of odds ratios, 95% confidence intervals and p values.
| Study | Spontaneous abortion/miscarriage | Preterm delivery (<37 weeks) | Low birth weight | IUGR |
|---|---|---|---|---|
| Wang et al.46 | OR = 0.462 95% CI 0.104–2.054 p = 0.299 |
OR = 0.295*
95% CI 0.017–5.204* p = 0.404* |
OR = 0.641*
95% CI 0.034–12.238* p = 0.768* |
|
| Maraka S et al.47 | OR = 2.44 95% CI 0.8–8.87 p = 0.12 |
OR = 3.06 95% CI 0.96–12.28 p = 0.06 |
OR = 16.4
95% CI 2.7–326.9 p = <0.001 |
OR = 1.45 95% CI 0.23–28.1 p = 0.99 |
| Maraka S et al.48 |
OR = 1.6
95% CI 1.14–2.24 p = 0.01 |
OR = 1.61
95% CI 0.1–2.37 p = 0.01 |
OR = 1.12 95% CI 0.84–1.5 p = 0.45 |
|
| Casey BM et al.49 | OR = 0.565*
95% CI 0.164–1.964* p = 0.37* |
OR = 0.819*
95% CI 0.729–2.116* p = 0.425* |
OR = 1.242*
95% CI 0.729–2.116* p = 0.425* |
OR = 0.331*
95% CI 0.014–8.136* p = 0.499* |
| Nazarpour S et al.50 | OR = 1*
95% CI 0.02–50.67* p = 1.0* |
OR = 0.841*
95% CI 0.432–1.638* p = 0.612* |
unadjusted values calculated using medcal.net.
CI, confidence interval; IUGR, intrauterine growth restriction; OR, odds ratio.
It is important to note that Maraka et al. found that there was no observed difference in pregnancy loss when comparing those treated with levothyroxine whose TSH was between 2.5 and 4.0 mIU/L compared with those with a TSH <2.5 mIU/L; however, a difference was observed when the TSH was above 4.1 mIU/L.48
The first randomised controlled trial investigating thyroid dysfunction in pregnancy was the Controlled Antenatal Thyroid Screening Study (CATS), which recruited patients from the UK and Italy. It was designed to establish the effect of treating suboptimal thyroid function during pregnancy on the cognitive outcomes of the foetus. Patients were screened at 12 weeks’ gestation and those identified with a low free T4 (<2.5th percentile) or elevated TSH (>97.5th percentile) were randomly allocated to placebo or levothyroxine treatment at 150 µg daily. There was a documented difference in IQ of 0.8 in the offspring at the age of 3 years (95% CI −1.1–2.6), which was deemed statistically insignificant with a p value of 0.40.51 The CATS II trialled looked specifically at the UK proportion of the CATS I population and again proved no statistical significance in the IQ of the offspring at 9 years (adjusted odds ratio 1.15 (95% CI 0.52–2.51), p = 0.731).51
In addition to foetal effects, there are significant maternal consequences of subclinical hypothyroidism that are important to recognise during pregnancy. Through unclear mechanisms, thought to be an interaction with angiogenic factors including nitric oxide, there is a significant increased prevalence of hypertensive disorders in pregnancy (p = 0.01 in first trimester and p = 0.03 in second trimester) when SCH persists through first and second trimester.52
Thyroid autoantibodies and euthyroidism
Euthyroidism is the term used to describe the status and function of the thyroid being within normal limits. Thyroid antibodies (anti-thyroid peroxidase antibodies and/or anti-thyroglobulin antibodies) can lead to changes within both the structure and function of the thyroid gland. Despite a normal functioning thyroid gland, the presence of the antibodies, especially thyroid peroxidase (anti-TPO), can have direct impacts on fertility and reproduction. In individuals with anti-TPO-Ab there are no proposed mechanisms for the changes seen in follicular fluid, spermatogenesis or embryogenesis.53 Overall, 10% of women will have detectable thyroid antibodies (anti-thyroglobulin (anti-Tg-Ab) or anti-thyroid peroxidase (anti-TPO-Ab)). Elevated antibody titres are associated with an increase in miscarriage rate and infertility and the documented rate from various studies ranges between 1.8 and 4-fold.54 Interestingly, a similar association of thyroid antibody levels in early pregnancy loss in women undergoing assisted conception techniques has not been demonstrated.55,56 Plowden et al. concluded that thyroid antibody (presence of anti-TPO or anti-TG Abs) did not increase risk of pregnancy related complications including subclinical hypothyroidism.57 However a recent meta-analysis by Busnelli et al. adds the possibility of the antibody status impacting on the rate of live birth.58 Wang et al. did not identify any changes to offspring neurological development in isolated positive maternal anti-Tg antibodies.59
Currently no treatments have been shown to reduce thyroid autoimmunity and this aspect continues to be studied.60 The use of glucocorticoids evaluated by Turi et al.61 and Litwicka et al.62 in patients with thyroid autoimmunity, demonstrated an increase in pregnancy rates with treatment compared with placebo (p = 0.03). Both authors used different protocols; Turi et al. described using prednisolone 4 weeks prior to IUI and Litwicka et al. used 5 mg prednisolone from oocyte retrieval until end of first trimester. There is therefore a lack of clinical evidence to advocate its use routinely. There is very limited evidence for intravenous immunoglobulin and the American guidelines do not routinely advocate its use, recommending further research in the form of randomised controlled trials to aid understanding of this treatment.18 The SERENA study investigated the impact on natural selenium supplementation and found that there was a reduction in antibody concentration during pregnancy (p < 0.01); however, there was no benefit on foetal or maternal outcomes.63
Numerous meta-analyses have evaluated the role of levothyroxine supplementation in euthyroid and/or subclinical autoimmune thyroid disease, to improve both the foetal and maternal outcome of in vitro fertilisation (IVF) treatment with no statistically proven benefit [pregnancy rate RR = 1.46 (95% CI 0.86–2.48)].64,65 Sullivan et al. reported that a strategy to empirically increase levothyroxine dose at diagnosis of pregnancy was more likely to result in a suppressed TSH in the first trimester. TSH results during pregnancy were not significantly different in either of the groups with TSH guided dose monitoring versus empiric dose increase.66
One of the most recent and comprehensive trials undertaken investigating thyroid pathology in pregnancy is the Thyroid AntiBodies and LEvoThyroxine (TABLET) trial. This study was designed to investigate whether the supplementation of levothyroxine in patients with normal thyroid function with positive antibodies would reduce the known risk of miscarriage and early pregnancy loss. The study was based in the UK and in a double-blind protocol allocated 952 women with positive antibodies in the pre-conception period to either levothyroxine treatment or placebo and compared live birth rates between the two groups after 34 weeks’ gestation. Despite previously documented smaller trials demonstrating benefit of levothyroxine treatment the TABLET trial concluded that there were no statistically significant differences in birth rates between the two groups [live birth rate RR = 0.97 (95% CI 0.83–1.14, p = 0.74)] indicating absence of benefit of treatment.56
Although there is a lack of evidence currently to support treatment in those with elevated levels of antibody (anti-TPO or anti-Tg antibodies) who remain euthyroid, the ATA guidelines advise rechecking thyroid function every 4 weeks from conception to mid-pregnancy to ensure that progression towards hypothyroidism is detected and managed appropriately.18
Hyperthyroidism
Hyperthyroidism is defined as a TSH concentration below the lower limit of the trimester-specific reference range. If the free T4 levels remains within the normal range this is referred to as subclinical and if above reference range this is termed overt hyperthyroidism.67
Overt hyperthyroidism affects 0.1–0.4% of all pregnancies.34 It can either be secondary to an underlying pre-existing condition such as Graves’ disease or nonautoimmune conditions (e.g. toxic nodular disease) or related to pregnancy itself. As previously described; in the first trimester there can often be a suppression of TSH and in the presence of hyperemesis gravidarum (HG) there may be biochemical similarities to overt hyperthyroidism.4,20
Gestational transient thyrotoxicosis is often seen to present by the middle of the first trimester and usually resolves before the end of second trimester.68 It is differentiated from Graves’ disease by the absence of anti-TSH-receptor antibody (TRAb). However, it can present with symptoms similar to thyrotoxicosis.69 Despite the abnormal thyroid status there is generally no indication for anti-thyroid medication administration in this condition, which is self-limiting.18,60,61
HG often presents in the first trimester of pregnancy with severe nausea and vomiting.70 It may result in dehydration and ketonuria. Thyroid biochemistry will begin to normalise to trimester specific reference range; initially with FT4 resolving with the cessation of vomiting however the TSH can often remain suppressed for several weeks.18 Differentiating this from the autoimmune condition of Graves’ disease is important and there can often be a goitre, thyroid eye disease (Graves’ orbitopathy) or TRAbs in the latter.69 Yoshihara et al. suggested that using a free T3/free T4 ratio could be discriminatory, as patients with gestational thyrotoxicosis have significantly lower T3 values, frequently in the normal range.71
Graves’ disease has a prevalence in pregnancy of 0.4% and can either be detected prior to pregnancy, during pregnancy or postpartum (e.g. detected due to the infant having thyroid disturbance).72 If diagnosed prior to conception it is treated with anti-thyroid medications, thyroidectomy or radioiodine treatment. The symptoms of thyrotoxicosis can often be taken for expected pregnancy-related effects (shortness of breath on exertion, mild tachycardia, and heat intolerance).73 Patients should be examined for thyroid eye signs as these can often worsen in the first trimester. Unlike hypothyroidism, where there is often an increased requirement of treatment as pregnancy progresses, with Graves’ disease there is often a reduction in the amount of medication required with each trimester, which may be related to the immunological changes in pregnancy. It is important to remain vigilant of symptoms in the postpartum period as thyroiditis can often exacerbate disease activity.1,4,20
It is standard practice to use the lowest dose of anti-thyroid medications to control hyperthyroidism. The aim of treatment being a FT4 in the upper third of normal nonpregnancy range.74 Over treatment will lead to foetal hypothyroidism and foetal goitre formation.73 The medications should be adjusted every 4 weeks to achieve control and avoid over-treatment. For symptom control beta-blocking therapy (e.g. propranolol 20–40 mg every 6 h) can be used. It is advised that in the first trimester propylthiouracil (PTU) is commenced at a dose of 100–450 mg/day as the preferred anti-thyroid drug. All anti-thyroid medications have proved equivalent in achieving biochemical control of the thyrotoxicosis however specific side effects have been reported. Propylthiouracil has demonstrated a higher incidence of hepatotoxicity. Methimazole and carbimazole have an increased risk of foetal malformations such as aplasia cutis, oesophageal atresia, and dysmorphic features. It is therefore recommended that PTU is used in the first trimester and preconception with a change to carbimazole or methimazole from the second trimester.4,18,20
Pregnancy outcome is correlated with biochemical control of hyperthyroidism. Untreated thyrotoxicosis can lead to spontaneous abortion, premature delivery, intrauterine growth restriction (IUGR), placental abruption, and maternal cardiac failure.69,75 Foetal outcomes are affected by the TRAb titre and control of hyperthyroidism. TRAbs can cross the placenta and can lead to foetal hyperthyroidism, which may manifest as foetal tachycardia, IUGR, and foetal goitre formation.70 Women suspected of autoimmune hyperthyroidism or previously treated disease should have TRAb titres measured by week 24 of gestation to guide foetal management.76 Overtreatment with anti-thyroid medications can lead to polyhydramnios and foetal hypothyroidism, where the treatment options include cessation of maternal anti-thyroid medications and rarely, intra-amniotic levothyroxine administration.4,18,20
Thyroidectomy should be considered if extremely high doses of anti-thyroid drugs are being used or a patient is unable to tolerate the medications. Iodine therapy is avoided due to foetal goitre and hypothyroidism developing. Radioactive iodine (RAI) is contraindicated in pregnancy, breast feeding, and for at least 6 months prior to conception.18 Due to the association of TRAb titres with foetal outcomes, women with high titres seeking conception may benefit from considering thyroidectomy over RAI therapy.70 TRAb titres are known to reduce significantly after surgery, in contrast to post-RAI, where they remain elevated.69
Assisted conception and thyroid disease
A study conducted by Karmon et al. involving 1477 women who underwent intrauterine insemination observed no adverse outcomes or changes to fertility rate if the pre-conception TSH was between 0.4–4.99 mU/L, a finding which has been since reproduced in multiple centres using smaller cohorts.77–79 However, smaller trials have reported minor implications of TSH variability and therefore the ATA guidelines still recommend that should a patient be undergoing IVF or intracytoplasmic sperm injection (ICSI) the TSH should be kept <2.5 mU/L.18 If using ovarian hyperstimulation for assisted conception, the TFTs may be affected by the hCG administered for oocyte maturation or endometrial support by mechanisms described previously and it is therefore recommended that TFTs are taken 1–2 weeks before starting treatment.18 Negro et al. suggested that ovarian stimulation leads to expected physiological alterations in thyroid status, thereby predisposing to lower free thyroid hormones. Using a pre-IVF target of 2.5 mIU/L is likely to allow a margin of change facilitating safe effects of gonadotrophin therapy without significant thyroid consequence.80
Studies performed looking into the impact of thyroid antibodies and pregnancy outcomes, of both prospective and retrospective design, with assisted conception have demonstrated that there was no difference in birth rate [OR 1.0 (95% CI 0.66–1.52, p = 0.9880)] or miscarriage rate [OR 0.46 (95% CI 0.11–1.96, p = 0.29410)] in those with antibody positivity versus those without.81,82 The ATA 2017 guidance does not recommend the routine use of levothyroxine in patients who are antibody positive with thyroid function within normal range undergoing assisted conception;18 but this area requires further research for more complete understanding and guidance.
Hyperthyroidism can lead to changes within the ovulation cycle and irregular menses have been reported in over double the proportion of women compared with age and weight-matched individuals.8 However, there has been no substantial research to date looking into the changes and outcomes of patients who are actively thyrotoxic undergoing assisted fertility.
Thyroid nodules and cancer
The widespread availability of ultrasound has resulted in the increasing identification of thyroid nodules both in symptomatic and healthy women. Nodules are present in up to 65% of the adult female population of which about 10% will be malignant.83 Ultrasound investigation should be undertaken to stratify risk of a nodule even in pregnancy however the use of nuclear medicine imaging techniques is generally avoided in pregnancy.84 Oh et al. undertook serial ultrasound scans in pregnant women with papillary thyroid cancer and reassuringly did not demonstrate any significant growth during pregnancy.85
The same criteria as in the nonpregnant state should be applied for indications for fine-needle aspiration (FNA); however, some authors suggest that if pregnancy is near term, then FNA can be delayed to the postpartum period. If FNA demonstrates malignancy, then surgery can be offered in the second trimester; however, the decision should be undertaken by a multidisciplinary team. If there is any compromise to airways from the nodule then immediate surgical intervention may be required.86 Additional indications to consider surgery during pregnancy may include rapid growth rate, nodal metastasis or features such as extracapsular invasion.87
Conclusion
The relationships between thyroid function, autoimmunity, fertility, and pregnancy outcomes have been the focus of considerable attention in recent years. The need for consistent approaches in clinical practice has resulted in the development of guidelines for practice. These have reflected expert opinion and have recognised that there is a lack of an evidence base in many of the controversial areas, including targets for TSH in hypothyroid woman and use of levothyroxine in women with autoimmune thyroid disease. Thyroid physiology changes considerably in pregnancy, complicating the assessment and management of thyroid disorders. Multiple trials have demonstrated that thyroid dysfunction can be associated with subfertility, early pregnancy, and obstetric complications. There is agreement that patients with established thyroid dysfunction should normalise thyroid status in the pre-conception period to prevent possible complications. Identified high-risk groups for hypothyroidism should be screened and all patients should have the recommended iodine supplementation. Due to the assay implications and pregnancy changes, free hormone assays should be used to guide treatment. In patients diagnosed with overt hypothyroidism there is clear evidence that treatment helps prevent adverse outcomes. The evidence for subclinical hypothyroidism remains less clear; however, the higher the TSH, the greater the evidence and therefore a TSH >4.0 mU/L indicates a requirement for treatment with levothyroxine. Hyperthyroidism requires an understanding of the aetiology to establish treatment; those with autoimmune hyperthyroidism (Graves’ disease) should be initiated on the lowest possible dose of anti-thyroid drug to reach normal levels of free thyroid hormones and trimester-specific TSH reference ranges. In the first trimester research indicates that there is fewer side effects with PTU; however, this can be changed to carbimazole/methimazole for later pregnancy. At present there is no evidence to suggest that treatment with levothyroxine in the presence of anti-thyroid antibodies (but normal thyroid function) alters reproductive outcomes. Research is required in women undergoing assisted conception with a history of hyperthyroidism as there are currently no trials to guide optimal treatment. Investigation of thyroid nodules in pregnancy needs to be undertaken on an individualised approach, ensuring risks are discussed and if possible FNA can be deferred until the postpartum period.
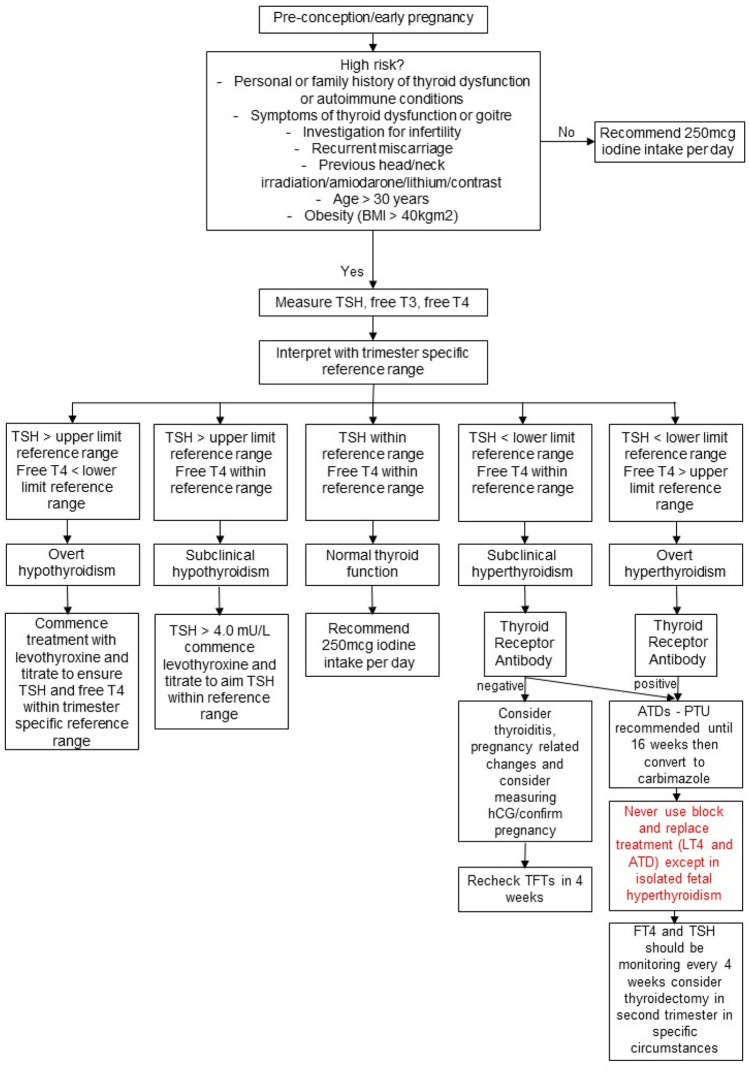
Proposed algorithm for assessment and management of thyroid disease in the pre-conception period or early pregnancy based on the information gathered in this review article.
Key learning points
- Screening for thyroid dysfunction should be considered in all those who have a previous personal or family history of thyroid dysfunction, if there are ongoing symptoms of thyroid dysfunction, in those with a past medical history of other autoimmune conditions (e.g. type 1 diabetes mellitus), as part of infertility investigations and recurrent miscarriage history and those with previous head and neck irradiation.
- Ensure free thyroid hormone levels are measured and in cases of multiple pregnancy, hyperemesis, and trophoblastic disease interpret results with caution.
- Ensure trimester-specific reference ranges are used.
- Consider the need for iodine supplementation in all pregnant patients.
- Thyroid antibodies may indicate a risk of miscarriage; however, if thyroid function is within trimester-specific reference ranges there are currently no data suggesting benefit of levothyroxine supplementation.
Footnotes
Author contribution(s): Samantha Anandappa: Conceptualization; Data curation; Formal analysis; Writing-review & editing.
Mamta Joshi: Conceptualization; Data curation; Formal analysis; Methodology; Writing-review & editing.
Lukasz Polanski: Formal analysis; Writing-review & editing.
Paul V. Carroll: Conceptualization; Formal analysis; Supervision; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Paul V. Carroll  https://orcid.org/0000-0002-3864-8721
https://orcid.org/0000-0002-3864-8721
Contributor Information
Samantha Anandappa, Department of Endocrinology, Guy’s & St. Thomas’ NHS Foundation Trust, London, UK.
Mamta Joshi, Department of Endocrinology, Guy’s & St. Thomas’ NHS Foundation Trust, London, UK.
Lukasz Polanski, Assisted Conception, Guy’s & St. Thomas’ NHS Foundation Trust, London, UK.
Paul V. Carroll, Department of Endocrinology, Guy’s & St. Thomas’ NHS Foundation Trust, DEDC 3rd Floor Lambeth Wing, St. Thomas’ Hospital, London, SE1 7EH, UK.
References
- 1. Negro R, Mestman JH. Thyroid disease in pregnancy. Best Pract Res Clin Endocrinol Metab 2011; 25: 927–943. [DOI] [PubMed] [Google Scholar]
- 2. Klein RZ, Haddow JE, Falx JD, et al. Prevalence of thyroid deficiency in pregnant women. Clin Endocrinol 1991; 35: 41–46. [DOI] [PubMed] [Google Scholar]
- 3. Casey B, De Veciana M. Thyroid screening in pregnancy. Am J Obstet Gynecol 2014; 211: 351–353.e1. [DOI] [PubMed] [Google Scholar]
- 4. Andersen SL, Laurberg P. Managing hyperthyroidism in pregnancy: current perspectives. Int J Womens Health 2016; 8: 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang C, Guo L, Zhu B, et al. Effects of 3,5,3′-triiodothyronine (T3) and follicle stimulating hormone on apoptosis and proliferation of rat ovarian granulosa cells. Chin J Physiol 2013; 56: 298–305. [DOI] [PubMed] [Google Scholar]
- 6. Korevaar TIM, Medici M, Visser TJ, et al. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat Rev Endocrinol 2017; 13: 610–622. [DOI] [PubMed] [Google Scholar]
- 7. Huang SA, Dorfman DM, Genest DR, et al. Type 3 iodothyronine deiodinase is highly expressed in the human uteroplacental unit and in fetal epithelium. J Clin Endocrinol Metab 2003; 88: 1384–1388. [DOI] [PubMed] [Google Scholar]
- 8. Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev 2010; 31: 702–755. [DOI] [PubMed] [Google Scholar]
- 9. Soldin OP. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Clin Chem 2011; 21: 1081–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tingi E, Syed AA, Kyriacou A, et al. Benign thyroid disease in pregnancy: a state of the art review. J Clin Transl Endocrinol 2016; 6: 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev 1997; 18: 404–433. [DOI] [PubMed] [Google Scholar]
- 12. Brent GA. Maternal thyroid function: interpretation of thyroid function tests in pregnancy. Clin Obstet Gynecol 1997; 40: 3–15. [DOI] [PubMed] [Google Scholar]
- 13. Ain KB, Mori Y, Refetoff S. Reduced clearance rate of thyroxine-binding globulin (TBG) with increased sialylation: a mechanism for estrogen-induced elevation of serum TBG concentration. J Clin Endocrinol Metab 1987; 65: 689–696. [DOI] [PubMed] [Google Scholar]
- 14. Girling JC. Thyroid disorders in pregnancy. Curr Obstet Gynaecol 2006; 16: 47–53. [Google Scholar]
- 15. Veltri F, Kleynen P, Grabczan L, et al. Pregnancy outcomes are not altered by variation in thyroid function within the normal range in women free of thyroid disease. Eur J Endocrinol 2018; 178: 189–197. [DOI] [PubMed] [Google Scholar]
- 16. Derakhshan A, Shu H, Broeren MAC, et al. Reference ranges and determinants of thyroid function during early pregnancy: the SELMA study. J Clin Endocrinol Metab 2018; 103: 3548–3556 [DOI] [PubMed] [Google Scholar]
- 17. Zhang D, Cai K, Wang G, et al. Trimester-specific reference ranges for thyroid hormones in pregnant women. Medicine 2019; 98: e14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alexander EK. 2017 guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017; 27: 315–389. [DOI] [PubMed] [Google Scholar]
- 19. Harding KB, Peña-Rosas JP, Webster AC, et al. Iodine supplementation for women during the preconception, pregnancy and postpartum period. Cochrane Database Syst Rev 2017; 3: CD011761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2012; 92(8 Suppl): S1–S47. [DOI] [PubMed] [Google Scholar]
- 21. Policeni BA, Smoker WRK, Reede DL. Anatomy and embryology of the thyroid and parathyroid glands. Semin Ultrasound CT MR 2012; 33: 104–114. [DOI] [PubMed] [Google Scholar]
- 22. De Felice M, Di Lauro R. Thyroid development and its disorders: genetics and molecular mechanisms. Endocr Rev 2004; 25: 722–746. [DOI] [PubMed] [Google Scholar]
- 23. Karmisholt J, Carlé A, Andersen SL, et al. Mechanisms in endocrinology: neurodevelopmental disorders in children born to mothers with thyroid dysfunction: evidence of fetal programming? Eur J Endocrinol 2017; 177: R27–R36. [DOI] [PubMed] [Google Scholar]
- 24. Forhead AJ, Fowden AL. Thyroid hormones in fetal growth and prepartum maturation. J Endocrinol 2014; 221: R87–R103. [DOI] [PubMed] [Google Scholar]
- 25. Chan S, Kachilele S, McCabe CJ, et al. Early expression of thyroid hormone deiodinases and receptors in human fetal cerebral cortex. Dev Brain Res 2002; 138: 109–116. [DOI] [PubMed] [Google Scholar]
- 26. Prezioso G, Giannini C, Chiarelli F. Effect of thyroid hormones on neurons and neurodevelopment. Horm Res Paediatr 2018; 90: 73–81. [DOI] [PubMed] [Google Scholar]
- 27. Drover SSM, Villanger GD, Aase H, et al. Maternal thyroid function during pregnancy or neonatal thyroid function and attention deficit hyperactivity disorder: a systematic review. Epidemiology 2019; 30: 130–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andersen SL, Andersen S, Liew Z, et al. Maternal thyroid function in early pregnancy and neuropsychological performance of the child at 5 years of age. J Clin Endocrinol Metab 2018; 103: 660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. FIGO Working Group on Good Clinical Practice in Maternal-Fetal Medicine. Good clinical practice advice: thyroid and pregnancy. Int J Gynecol Obs 2019; 144: 347–351. [Google Scholar]
- 30. Diéguez M, Herrero A, Avello N, et al. Prevalence of thyroid dysfunction in women in early pregnancy: does it increase with maternal age? Clin Endocrinol 2016; 84: 121–126. [DOI] [PubMed] [Google Scholar]
- 31. Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011; 21: 1081–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sitoris G, Veltri F, Kleynen P, et al. Screening for thyroid dysfunction in pregnancy with targeted high-risk case finding: can it be improved? J Clin Endocrinol Metab 2019; 104: 2346–2354. [DOI] [PubMed] [Google Scholar]
- 33. Mendes D, Alves C, Silverio N, et al. Prevalence of undiagnosed hypothyroidism in Europe: a systematic review and meta-analysis. Eur Thyroid J 2019; 8: 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cooper DS, Laurberg P. Hyperthyroidism in pregnancy. Lancet Diabetes Endocrinol 2013; 1: 238–249. [DOI] [PubMed] [Google Scholar]
- 35. Sullivan SA. Hypothyroidism in pregnancy. Clin Obstet Gynecol 2019; 62: 308–319. [DOI] [PubMed] [Google Scholar]
- 36. Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. Obstet Gynecol Surv 2000; 341: 549–555. [DOI] [PubMed] [Google Scholar]
- 37. Hubaveshka J, Michaelsson LF, Nygaard B. The dose of levothyroxine in pregnant women with hypothyroidism should be increased by 20–30% in the first trimester. Dan Med J 2014; 61: A4959. [PubMed] [Google Scholar]
- 38. Yassa L, Marqusee E, Fawcett R, et al. Thyroid hormone early adjustment in pregnancy (The THERAPY) trial. J Clin Endocrinol Metab 2010; 95: 3234–3241. [DOI] [PubMed] [Google Scholar]
- 39. Kashi Z, Bahar A, Akha O, et al. Levothyroxine dosage requirement during pregnancy in well-controlled hypothyroid women: a longitudinal study. Glob J Health Sci 2015; 8: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abalovich M, Vázquez A, Alcaraz G, et al. Adequate levothyroxine doses for the treatment of hypothyroidism newly discovered during pregnancy. Thyroid 2013; 23: 1479–1483. [DOI] [PubMed] [Google Scholar]
- 41. Gaiser R. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. Surv Anesthesiol 2005; 49: 15–16. [DOI] [PubMed] [Google Scholar]
- 42. Korevaar TIM. Evidence-based tightrope walking: the 2017 guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017; 27: 309–311. [DOI] [PubMed] [Google Scholar]
- 43. Zhang Y, Wang H, Pan X, et al. Patients with subclinical hypothyroidism before 20 weeks of pregnancy have a higher risk of miscarriage: a systematic review and meta-analysis. PLoS One 2017; 12: e0175708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carty DM, Doogan F, Welsh P, et al. Thyroid stimulating hormone (TSH) ⩾2.5 mU/l in early pregnancy: prevalence and subsequent outcomes. Eur J Obstet Gynecol Reprod Biol 2017; 210: 366–369. [DOI] [PubMed] [Google Scholar]
- 45. Plowden TC, Schisterman EF, Sjaarda LA, et al. Subclinical hypothyroidism and thyroid autoimmunity are not associated with fecundity, pregnancy loss, or live birth. J Clin Endocrinol Metab 2016; 101: 2358–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang S, Teng WP, Li JX, et al. Effects of maternal subclinical hypothyroidism on obstetrical outcomes during early pregnancy. J Endocrinol Invest 2012; 35: 322–325. [DOI] [PubMed] [Google Scholar]
- 47. Maraka S, Singh Ospina NM, O’Keeffe DT, et al. Effects of levothyroxine therapy on pregnancy outcomes in women with subclinical hypothyroidism. Thyroid 2016; 26: 980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maraka S, Mwangi R, McCoy RG, et al. Thyroid hormone treatment among pregnant women with subclinical hypothyroidism: US national assessment. BMJ 2017; 356: i6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med 2017; 376: 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nazarpour S, Tehrani FR, Simbar M, et al. Effects of levothyroxine on pregnant women with subclinical hypothyroidism, negative for thyroid peroxidase antibodies. J Clin Endocrinol Metab 2018; 103: 926–935. [DOI] [PubMed] [Google Scholar]
- 51. Hales C, Taylor PN, Channon S, et al. Controlled antenatal thyroid screening II: effect of treating maternal suboptimal thyroid function on child cognition. J Clin Endocrinol Metab 2018; 103: 1583–1591. [DOI] [PubMed] [Google Scholar]
- 52. Wu M-Q, Liu J, Wang Y-Q, et al. The impact of subclinical hypothyroidism on adverse perinatal outcomes and the role of thyroid screening in pregnancy. Front Endocrinol 2019; 10: 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vissenberg R, Manders VD, Mastenbroek S, et al. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum Reprod Update 2015; 21: 378–387. [DOI] [PubMed] [Google Scholar]
- 54. Stagnaro-Green A, Roman SH, Cobin RH, et al. Detection of at-risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. JAMA 1990; 264: 1453–1454. [PubMed] [Google Scholar]
- 55. Leiva P, Schwarze JE, Vasquez P, et al. There is no association between the presence of anti-thyroid antibodies and increased reproductive loss in pregnant women after ART: a systematic review and meta-analysis. J Bras Reprod Assist 2017; 21: 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Poppe K, Autin C, Veltri F, et al. Thyroid autoimmunity and intracytoplasmic sperm injection outcome: a systematic review and meta-analysis. J Clin Endocrinol Metab. Epub ahead of print 12 March 2018. DOI: 10.1210/jc.2017-02633. [DOI] [PubMed] [Google Scholar]
- 57. Plowden TC, Schisterman EF, Sjaarda LA, et al. Thyroid-stimulating hormone, anti-thyroid antibodies, and pregnancy outcomes. Am J Obstet Gynecol 2017; 217: 697.e1–697e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Busnelli A, Paffoni A, Fedele L, et al. The impact of thyroid autoimmunity on IVF/ICSI outcome: a systematic review and meta-analysis. Hum Reprod Update 2016; 22: 775–790. [DOI] [PubMed] [Google Scholar]
- 59. Wang X, Liu H, Zhang Y, et al. Effects of isolated positive maternal thyroglobulin antibodies on brain development of offspring in an experimental autoimmune thyroiditis model. Thyroid 2015; 25: 551–558. [DOI] [PubMed] [Google Scholar]
- 60. De Leo S, Pearce EN. Autoimmune thyroid disease during pregnancy. Lancet Diabetes Endocrinol 2018; 6: 575–586. [DOI] [PubMed] [Google Scholar]
- 61. Turi A, Giannubilo SR, Zanconi S, et al. Preconception steroid treatment in infertile women with antithyroid autoimmunity undergoing ovarian stimulation and intrauterine insemination: a double-blind, randomized, prospective cohort study. Clin Ther 2010; 32: 2415–2421. [DOI] [PubMed] [Google Scholar]
- 62. Litwicka K, Arrivi C, Varricchio MT, et al. In women with thyroid autoimmunity, does low-dose prednisolone administration, compared with no adjuvant therapy, improve in vitro fertilization clinical results? J Obstet Gynaecol Res 2015; 41: 722–728. [DOI] [PubMed] [Google Scholar]
- 63. Mantovani G, Isidori AM, Moretti C, et al. Selenium supplementation in the management of thyroid autoimmunity during pregnancy: results of the “SERENA study”, a randomized, double-blind, placebo-controlled trial. Endocrine 2019; 66: 542–550. [DOI] [PubMed] [Google Scholar]
- 64. Rao M, Zeng Z, Zhao S, et al. Effect of levothyroxine supplementation on pregnancy outcomes in women with subclinical hypothyroidism and thyroid autoimmuneity undergoing in vitro fertilization/intracytoplasmic sperm injection: an updated meta-analysis of randomized controlled trials 1. Reprod Biol Endocrinol 2018; 16: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Akhtar MA, Owen DJ, Peitsidis P, et al. Thyroxine replacement for subfertile women with euthyroid autoimmune thyroid disease or subclinical hypothyroidism. Cochrane Database Syst Rev 2014; 6: CD011009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sullivan SD, Downs E, Popoveniuc G, et al. Randomized trial comparing two algorithms for levothyroxine dose adjustment in pregnant women with primary hypothyroidism. J Clin Endocrinol Metab 2017; 102: 3499–3507. [DOI] [PubMed] [Google Scholar]
- 67. Labadzhyan A, Brent GA, Hershman JM, et al. Thyrotoxicosis of pregnancy. J Clin Transl Endocrinol 2014; 1: 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Goldman AM, Mestman JH. Transient non-autoimmune hyperthyroidism of early pregnancy. J Thyroid Res 2011; 2011: 142413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Moleti M, Di Mauro M, Sturniolo G, et al. Hyperthyroidism in the pregnant woman: maternal and fetal aspects. J Clin Transl Endocrinol 2019; 16: 100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nguyen CT, Sasso EB, Barton L, et al. Graves’ hyperthyroidism in pregnancy: a clinical review. Clin Diabetes Endocrinol 2018; 4: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yoshihara A, Noh JY, Mukasa K, et al. Serum human chorionic gonadotropin levels and thyroid hormone levels in gestational transient thyrotoxicosis: is the serum hCG level useful for differentiating between active Graves’ disease and GTT? Endocr J 2015; 62: 557–560. [DOI] [PubMed] [Google Scholar]
- 72. Earl R, Crowther CA, Middleton P. Interventions for hyperthyroidism pre-pregnancy and during pregnancy. Cochrane Database Syst Rev 2013; 11: CD008633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Marx H, Amin P, Lazarus JH. Pregnancy plus: hyperthyroidism and pregnancy. BMJ 2008; 336: 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yim CH. Update on the management of thyroid disease during pregnancy. Endocrinol Metab 2016; 31: 386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang Y, Sun X-L, Wang C-L, et al. Influence of screening and intervention of hyperthyroidism on pregnancy outcome. Eur Rev Med Pharmacol Sci 2017; 21: 1932–1937. [PubMed] [Google Scholar]
- 76. Bucci I, Giuliani C, Napolitano G. Thyroid-stimulating hormone receptor antibodies in pregnancy: clinical relevance. Front Endocrinol 2017; 8: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Karmon AE, Batsis M, Chavarro JE, et al. Preconceptional thyroid-stimulating hormone levels and outcomes of intrauterine insemination among euthyroid infertile women. Fertil Steril 2015; 103: 258–263.e1. [DOI] [PubMed] [Google Scholar]
- 78. Baker VL, Rone HM, Pasta DJ, et al. Correlation of thyroid stimulating hormone (TSH) level with pregnancy outcome in women undergoing in vitro fertilization. Am J Obstet Gynecol 2006; 194: 1668–1674; discussion 1674–1675. [DOI] [PubMed] [Google Scholar]
- 79. Cai YY, Zhong LP, Guan J, et al. Outcome of in vitro fertilization in women with subclinical hypothyroidism. Reprod Biol Endocrinol 2017; 15: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Negro R. Thyroid and assisted reproduction technologies: a brief clinical update with recommendations for practice. Endocr Metab Immune Disord Drug Targets 2018; 18: 194–200. [DOI] [PubMed] [Google Scholar]
- 81. Karacan M, Alwaeely F, Cebi Z, et al. Effect of antithyroid antibodies on ICSI outcome in antiphospholipid antibody-negative euthyroid women. Reprod Biomed Online 2013; 27: 376–380. [DOI] [PubMed] [Google Scholar]
- 82. Tan S, Dieterle S, Pechlavanis S, et al. Thyroid autoantibodies per se do not impair intracytoplasmic sperm injection outcome in euthyroid healthy women. Eur J Endocrinol 2014; 170: 495–500. [DOI] [PubMed] [Google Scholar]
- 83. Durante C, Grani G, Lamartina L, et al. The diagnosis and management of thyroid nodules a review. JAMA 2018; 319: 914–924. [DOI] [PubMed] [Google Scholar]
- 84. The American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Committee Opinion No. 723: guidelines for diagnostic imaging during pregnancy and lactation. Obstet Gynecol 2017; 130: e210–e216. [DOI] [PubMed] [Google Scholar]
- 85. Oh H-S, Kim WG, Park S, et al. Serial neck ultrasonographic evaluation of changes in papillary thyroid carcinoma during pregnancy. Thyroid 2017; 27: 773–777. [DOI] [PubMed] [Google Scholar]
- 86. Smith A, Eccles-Smith J, D’Emden M, et al. Thyroid disorders in pregnancy and postpartum. Aust Prescr 2017; 40: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mazzaferri EL. Approach to the pregnant patient with thyroid cancer. J Clin Endocrinol Metab 2011; 96: 265–272. [DOI] [PubMed] [Google Scholar]