Abstract
Coronavirus disease 2019 (COVID-19) is the disease caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Genome sequencing of the virus revealed that it is a new zoonotic virus that might have evolved by jumping from bats to humans with one or more intermediate hosts. The immediate availability of the sequence information in the public domain has accelerated the development of quantitative RT-PCR-based diagnostics. Numerous clinical trials have been prioritized globally for testing new vaccines and treatments against this disease. This review provides a broad insight into different aspects of COVID-19, an introduction to SARS-CoV-2 mitigation strategies and the present status of diagnostics and therapeutics.
Keywords: Cov-2, COVID-19, coronavirus, diagnostics, disease, global, pandemic, SARS, ssRNA, virus
Introduction
In December 2019, a cluster of patients linked to a local seafood market in Wuhan, Hubei Province, China, were diagnosed with a viral pneumonia having severe acute respiratory syndrome (SARS) -like symptoms of unknown aetiology [1]. Initial denials of human-to-human transmission of the virus, which were later refuted in January 2020, led to a rapid spread of the disease throughout China within a short span of time. Following this, the virus quickly spread both within China and across the globe with more than 210 countries affected.
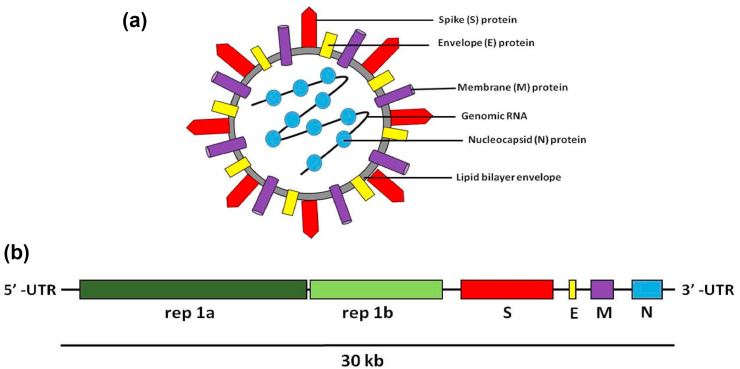
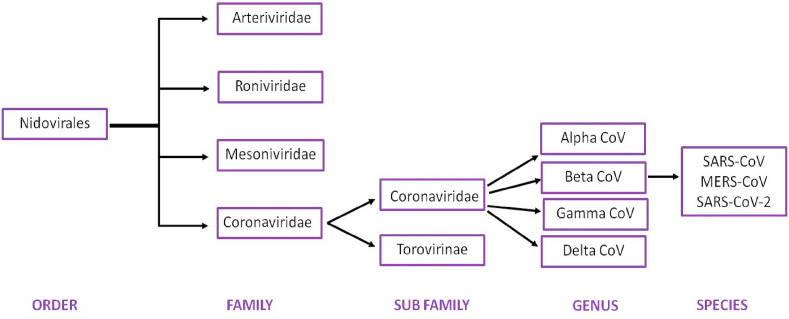
In January 2020, the first genome sequence of the virus was released by Prof. Yong-Zhen Zhang (GenBank accession no. MN908947). Based on its genome sequence similarity to SARS coronaviruses, it was identified as a novel coronavirus and was named SARS-CoV-2. Coronaviruses refer to the largest group of viruses characterized by a spherical morphology with a single-stranded RNA surrounded by an outer envelope (Fig. 1). SARS-CoV-2 belongs to the genus Betacoronavirus, one of four genera of coronaviruses, which also includes SARS-CoV-1 and Middle East respiratory syndrome (MERS) -CoV (Fig. 2) [1,2]. The genome sequence of the novel coronavirus revealed that, similar to other coronaviruses, SARS-CoV-2 has a zoonotic origin and is thought to have jumped from bats to humans with one or more intermediate hosts (including pangolin) [3]. Similar to the present scenario, jumping of coronaviruses (SARS-CoV-1 and MERS-CoV) from animals to humans has wreaked havoc in the past, mainly as a result of the inability of our immune system to fight against the new enemy.
Fig. 1.
(a) Schematic structure of virion of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with genomic RNA and its major structural proteins. (b) Schematic diagram of genomic organization of coronaviruses, rep1a (replicase polyprotein orf1a), rep1b (replicase polyprotein orf1b), S (spike protein), E (envelope protein), M (membrane protein), N (nucleocapsid protein), UTR (untranslated region), 30 kb refers to the length of the single-stranded RNA genetic material [14].
Fig. 2.
Classification of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2].
This review provides information regarding the disease caused by SARS-CoV-2, the life cycle of the virus in the human body, information about the genetic variants and the different types of diagnostic methods currently available or under development. It also describes the present status of therapeutics and vaccine development, and different ways in which the disease is being managed at the global level.
The disease COVID-19
The disease caused by the novel coronavirus SARS-CoV-2 is referred to as coronavirus disease 2019 (COVID-19). It is primarily transmitted by infected people while coughing, sneezing or even talking to healthy individuals through inhalation of droplets or aerosols. As the virus can survive on inanimate surfaces for some time (a few hours to a few days), it can also spread by touching contaminated surfaces with bare hands through hand to nasal, oral or even eye contact [4]. WHO declared COVID-19 as a pandemic on 11 March 2020, as it had spread to more than 120 countries by that time [5]. In any given region of the world, the disease first originates as a small number of imported cases (Stage 1) with subsequent clusters of cases defined by common exposure (Stage 2) which, if not quarantined properly, leads to community transmission (Stage 3) followed by a widespread outbreak in public (Stage 4). Community transmission refers to a stage when the source of infection is not known for most cases. The spread and severity of any infectious disease is quantified by the transmission rate (R0) and the case fatality rate (CFR), which refer to the number of newly infected people from a single case and the per cent of cases resulting in death, respectively [5]. The R0 and CFR % values for COVID-19 are 2.0–2.5 and 2.3%, respectively, as estimated by WHO in February 2020. The current statistics claims that these values could be ∼1.5–3.5 and ∼3.4%. However, these data are being revised continuously because of differences in the global scenario, rapidly growing new cases and unaccounted asymptomatic cases [6]. The most common symptom of COVID-19 is fever or chills, along with other symptoms such as cough, fatigue, breathing difficulties, muscle or body aches, headache, new loss of smell or taste, sore throat, congestion, runny nose, nausea or vomiting, diarrhoea, sputum production and loss of appetite (Table 1) [7,8]. However, these symptoms overlap with common flu or other viral fevers, making it difficult to diagnose the disease on the basis of symptoms alone. To add to this complication, most cases (>80%) remain asymptomatic or with mild symptoms. Around 20% of infected individuals progress to more serious or critical conditions like severe pneumonia accompanied by multi-organ failure, among which some may succumb to death (3%–4%) [5]. The existence of COVID-19-associated coagulopathy has also gained significant prominence, following the exhibition of various neurological manifestations by a significant proportion of patients. Ischaemic stroke, cerebral haemorrhage and thrombosis are some of the more common symptoms [9]. However, mortality to a larger extent is also determined by access to quality health care. According to the global data, as well as the early estimates from China, both old age and co-morbidities may render individuals at higher risk of developing severe disease or dying from COVID-19, perhaps because of weaker immune function [8,10].
Table 1.
A rough time-line of progression of coronavirus disease 2019 (COVID-19) symptoms [8]
| Day 1a | Fever in 88% of infected cases, often accompanied by fatigue, nausea or diarrhoea |
| Day 2–4 | Fever with dry cough and muscle pain |
| Day 5–6 | + Breathing difficulties |
| Day 7–8 | Recovery for some patients; however, hospitalization may be needed for patients with severe conditions (∼15%), may develop acute respiratory distress syndrome due to fluid accumulation in the lungs |
| Day 9–11 | Some cases may worsen needing admission to the intensive care unit |
| Day 12–14 | For most cases fever ends but coughing may persist |
| Day 13–14 | For patients headed towards recovery, breathing problems may end at this stage |
| Day 17–18 | Full recovery; however, in some cases (5%) it may lead to death |
‘Day 1’ refers to the appearance of the first symptom.
The time between the onset of infection and appearance of the first symptom of the disease in an individual is defined as the incubation period. SARS-CoV-2 incubation period is typically around 5–6 days (ranging from 0–14 days) [5,11], which is similar to its distant cousin, SARS-CoV (4–6 days and up to 10 days in some cases) [7]. Apart from the high mortality rate (11%) of SARS, one of the factors contributing to the limited transmissibility in the SARS outbreak of 2002 could be the lack of transmission during the incubation period [7]. This made it possible to isolate the infected individuals at an early stage, so significantly fewer people were infected globally. In contrast, although SARS-CoV-2 is most contagious during the first 3 days after the onset of symptoms, transmission may take place even during presymptomatic or asymptomatic stages, making it an ‘invisible enemy’, allowing each infected person to potentially infect several others [12].
Life cycle of SARS-CoV-2
A virus is a non-living obligate parasite that multiplies only after its entry into host cells. The life cycle of SARS-CoV-2 begins in the lungs, where it infects alveolar type II cells. The SARS-CoV-2 attaches to cells by binding to the cellular angiotensin-converting enzyme 2 (ACE2) receptor present on the cell membrane through its spike (S) protein, which is expressed on the viral surface [13]. The conformational change in S protein as a result of binding promotes entry of SARS-CoV-2 into the cell, largely by endocytosis. The S protein acts like a key, locking on to the ACE2 receptor; this makes it entry into the cell possible. Once inside the cell, the virus releases its genetic material, a single-stranded RNA, and starts synthesis of viral replicase polyproteins using the host machinery. These polyproteins are cleaved to smaller fragments by viral proteinases and then assemble in to the RNA-dependent RNA replicase complex. The replicase complex synthesizes more copies of viral RNA and a series of smaller sub-genomic RNAs, which are translated into the viral proteins on the endoplasmic reticulum membrane using host translation machinery. The fresh copies of the genomic RNA and the SARS-CoV-2 viral proteins synthesized are finally packed into new viral particles or virions, which are then released outside the cell by exocytosis or pyroptosis [14].
The newly formed SARS-CoV-2 virions can infect a large number of healthy lung cells, thereby continuing the cycle. The virions are recognized by alveolar epithelial and endothelial cells and by macrophages. The cellular interactions of this virus with these cells trigger the release of cytokines, which then signal certain types of immune cells, such as T cells, monocytes and macrophages, and promote inflammation. In most cases, the immune system responds by producing T cells, which kill SARS-CoV-2 while the neutralizing antibodies produced by B cells block the S protein–ACE2 receptor interaction, thereby inhibiting further spread to the other organs, leading to patient recovery. In contrast, in a weak immune response, there is accumulation of immune cells in the lungs leading to production of a disproportionate level of pro-inflammatory cytokines resulting in cytokine storm. In parallel, the uncontrolled rapid spread of SARS-CoV-2 to other organs triggers multi-organ failure and even death [15]. An analysis of 326 individuals with COVID-19 from China showed that the elderly and patients with low levels of T cells and high levels of the cytokine interleukin-6 were found to have more severe symptoms [16].
Global variants and disease severity
A virus genome may accumulate mutations while it replicates inside the host cell, thereby generating genomic variants across the globe. SARS-CoV-2 mutates, albeit at a slow rate [17]. Characterization of these variants may be helpful in understanding the disease mechanism, how the virus evades the immune system or how it develops resistance to drugs. However, it would be premature to infer from the currently available data that a particular mutation makes the virus more virulent. A recent study found two major versions of SARS-CoV-2, called clade I and clade II, based on the genome sequence analysis of more than 100 sequences [16]. The two clades were not found to be any different in terms of contagiousness or disease severity. The disease severity largely depended on host factors such as age, lymphocytopenia, and its associated cytokine storm, not so much on the viral genetic variation [16]. In an independent study, researchers found three blood-based biomarkers that can predict disease severity at least 10 days in advance with more than 90% accuracy, based on a database of 485 infected patients from Wuhan, China. Based on these results, the disease severity was found to be associated with a high level of lactate dehydrogenase, low lymphocyte level and increased amount of high-sensitivity C-reactive protein [18].
Phylogenetic analysis of different variants could also help in tracing the route of the virus across the globe. A large-scale analysis based on the sequences of 3067 SARS-CoV-2 genomes revealed a clonal geo-distribution and several mutation hotspots on the genome [19]. Information about the mutation hotspots could be critical in both therapeutics and vaccine design so that the genomic regions showing higher mutation rates could be avoided as drug targets or in vaccine design.
Diagnostic strategies
According to the WHO, as the symptoms of COVID-19 overlap with those of the common cold and influenza, laboratory testing is the only way for confirmation of COVID-19. For this reason, testing or diagnosis is an essential step in patient screening for COVID-19 and achieving control over the global pandemic. All the testing strategies have a common aim, which is to identify the presence of SARS-CoV-2 in an individual. The testing strategies can be broadly classified into two types: (a) detection of the virus itself either by RNA/DNA-based approaches or viral antigen detection, and (b) detection of the antibodies produced in response to the viral infection.
Among DNA/RNA-based approaches, quantitative RT-PCR is the reference standard method that detects the presence of viral RNA using RT-PCR. This approach is highly sensitive because it involves amplification of signal by exponentially increasing the target molecules. The specificity is controlled by designing primer probes specific to SARS-CoV-2 RNA. It is being used worldwide for confirmation of virus presence in patients' nasal/throat swabs. An alternative approach combines isothermal amplification of target with a CRISPR-based detection method [20]. However, these approaches involve amplification of signal, which may give rise to non-specific off-target signal, thereby increasing the possibility of false positives. Also, these are costly, time-consuming and need technically trained staff and special infrastructure, such as clean room and expensive real-time PCR machines. Hence, the immediate priority would be to develop highly sensitive, specific and rapid, point-of-care COVID-19 detection strategies.
At present, several research groups are trying to develop accurate, fast, field-compatible and reliable detection methods. Antibody-based antigen detection is one such rapid and cost-effective method. This is a lateral flow assay where monoclonal antibodies against the virus are used for rapid detection of the viral antigen in the patient samples. However, lower sensitivity of the assay remains a concern [21]. Another commonly used rapid assay is the serological test, which detects the presence of antibodies in the patient's blood using labelled viral proteins [22]. Although serology tests are rapid, comparatively easier to perform and require fewer laboratory skills, they can be only used from about 5 days after infection because the antibody titre produced by the body in response to infection develops only at a later stage (1 week for IgM and 2–3 weeks for IgG). Therefore, it can be used for initial screening of the population followed by a more confirmatory test like quantitative RT-PCR. Apart from its use for rapid detection of infection during the symptomatic period, the serological test could be used after the infection has receded, because the antibodies may remain in the blood for a longer period. This makes it a perfect tool for population studies to obtain more accurate transmission data worldwide, at a later stage of infection or recovery, to obtain a clear picture of the CFR of COVID-19. Though some of the presently available commercial serological tests have low specificity, it is possible to design highly specific tests by using peptides that are unique to SARS-CoV-2 with no cross-reactivity with other coronaviruses.
Several other novel approaches that are still under development and validation, are chip-based detection of viral RNA–DNA probe hybridization using electrical detection or immunoassays, and laser-based surface-enhanced Raman spectra. These are rapid, sensitive and appear promising; however, the major challenge would be to make them available at point of care. More details about these methods can be found in a recent review by Li et al. [22]. To expedite the rapid diagnostics, the National Institutes of Health, USA, have launched an online competition named ‘Rising to the COVID-19 Challenge: Rapid Acceleration of Diagnostics (RADx)’ to provide both technical and financial support to the potential developers of rapid methods for COVID-19 detection (www.poctrn.org/radx).
Therapeutics and vaccines
The availability of the genome sequence of SARS-CoV-2 has accelerated research into therapeutic interventions against the virus; however, we do not yet have a treatment or a vaccine against COVID-19. In general, viruses use the host machinery for multiplication, so it is difficult to specifically target its survival in the host cells. Nevertheless, some of the steps where SARS-CoV-2 infection could be intercepted are: inhibition of S protein interaction with the ACE2 receptor and processing or functioning of polyprotein replicase [23]. Also, several generic antiviral agents are being tested for their effectiveness against COVID-19. An alternative strategy could be to decrease the severity of the symptoms by inhibiting the cytokine storm generated by the body's immune response.
Drugs that are under clinical trials include remdesivir, a nucleotide analogue that inhibits SARS-CoV-2 replication, and tocilizumab, a monoclonal anti-interleukin-6 antibody that inhibits cytokine storm by blocking the inflammatory protein interleukin-6 [24]. Only remdesivir has shown a positive outcome, with a modest increase in speed of recovery of treated patients, in the National Institutes of Health-funded randomized placebo control clinical trials [25]. Remdesivir will also be tested in combination with monoclonal antibodies in the subsequent trials. In the tocilizumab clinical trials, the end point will be to evaluate if the antibody treatment is able to reduce time on ventilators and in the intensive care unit for individuals with COVID-19 [26]. Treatment with a combination of the protease inhibitor drugs lopinavir and ritonavir did not show any benefits beyond standard care in hospitalized adults with severe COVID-19 [27]. Most of the therapeutic strategies are targeting the repurposing of drugs already approved by the US Food and Drug Administration, but there are certain novel candidates like lenzilumab, which is a humanized monoclonal antibody for alleviating pneumonia [28]. Clinical trials of plasma therapy using convalescent plasma from recovered patients for transfer of passive immunity have also been initiated both in India and the USA [24]. The malaria drugs chloroquine or hydroxychloroquine, which created a stir in the initial stages of the pandemic, failed to live up to expectations during clinical trials [29,30].
Vaccines teach the immune system to fight against future infections, thereby conferring immunity. For most viral infections, prevention is considered better than cure. A good vaccine should be effective, safe and durable. During the 2002 SARS outbreak, attempts were made to develop vaccines against SARS-CoV; however, the rapid disappearance of the epidemic because of the low transmissibility of the virus decelerated the research and commercial interests in this direction, so we still do not have an effective vaccine for SARS-CoV [31]. At present, several strategies are being considered for development of a vaccine against SARS-CoV-2. The vaccines could be based on DNA, RNA or protein, or could use a more conventional attenuated/killed virus based approach. More than 100 different vaccines are in the preclinical phase with at least five candidates being tested in clinical evaluation. Among the vaccines that are undergoing Phase II trials for clinical evaluation to assess safety, immunogenicity and efficacy are the non-replicating viral-vector-based vaccine (ChiCTR2000031781) developed by the Beijing Institute of Biotechnology and mass produced by CanSino Biologicals (Tianjin, China) [32] and RNA-based vaccine (NCT04405076) developed by the National Institute of Allergy and Infectious Diseases (NIAID) in association with Moderna (Cambridge, MA, USA) [33]. But the candidate vaccine that has garnered a lot of attention because of its rapid progress, is the experimental vaccine (ChAdOx1-S) developed by Oxford University in association with AstraZeneca (Cambridge, UK). This vaccine is a non-replicating viral-vector-based vaccine and is the first to enter Phase III of clinical evaluation [34]. Another candidate vaccine (NCT04456595) developed by Sinovac (Beijing, China) has also recently entered Phase III clinical trials. It is based on the conventional inactivated virus approach using alum as an adjuvant [35].
Disease management strategies
As there are no vaccines or approved effective treatments at present against COVID-19, different mitigation strategies have been employed by governments to curb the spread of and eventual deaths from the virus. Continuous attempts are being made to educate people with the correct information about the virus and the disease, as well as about maintaining good personal hygiene, such as frequent hand washing. Several myths related to COVID-19 have appeared on social media that need to be fact-checked. Some of them have been compiled here for better awareness among readers (Table 2).
Table 2.
Myth busters [37]
| Myths | Facts |
|---|---|
| Warmer climates adversely affect SARS-CoV-2 survival and transmission | No reports of the temperature sensitivity of the virus, it spreads equally rapidly in warmer tropical countries like India and Pakistan |
| Young and healthy individuals are immune and need not take strict precautionary measures | Although fewer in number, there have been instances of serious illness and even deaths in young and healthy individuals. Therefore, it is essential for every individual to take precaution irrespective of their age and health |
| COVID-19 can be detected by thermal scanners | Thermal scanners CANNOT detect COVID-19 |
| The prolonged use of medical masks may cause CO2 intoxication or oxygen deficiency | The prolonged use of medical masks when properly worn, DOES NOT cause CO2 intoxication nor oxygen deficiency |
| Exposure of self to UV light may kill the virus | UV radiation is extremely harmful for eyes and skin; it should NOT be used to disinfect hands or other areas of your skin |
| Consumption or injection of disinfectants will kill the virus in the body | Disinfectants are effective in killing the virus on inanimate surfaces. However, they are extremely toxic for ingestion |
Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; UV, ultraviolet.
Furthermore, as COVID-19 is highly transmissible and spreads mainly through human-to-human transmission, physical distancing at an early stage of the outbreak could be an essential strategy to break the chain of infection. Emphasis is also given to quarantine of infected individuals during early infection as well as to screening and isolating asymptomatic people, who can unknowingly spread the virus to others, using diagnostic testing. This is in parallel supported by contact tracing to isolate people who might have come in contact with the infected individuals. According to the WHO, the only way for confirmation of COVID-19 is testing. Continuous attempts are being made to improve the testing capabilities by increasing the numbers of testing centres/laboratories and training more people, some of the major bottle-necks are the lack of appropriate biosafety facilities to handle the viral samples, limited supply of the reagents and the high cost associated with the available testing reagents/kits/specialized equipment. Attempts are also being made to develop cost-effective and easy-to-use point-of-care tests.
Though most scientists support the idea of physical distancing for flattening the curve, there is a parallel school of thought that says developing ‘herd immunity’ against SARS-CoV-2 could be a way of controlling further infections. Herd immunity develops in a population when most individuals (at least ∼70%) acquire adaptive immunity against an infectious agent, either through vaccination or previous exposure, and will be able to protect others who are not yet immune to the virus [36]. This is an indirect way of developing immunity for an entire population, as the required number of hosts for the infectious agent would no longer be available, thereby reducing the spread. However, COVID-19 has a higher risk of manifestation into severe disease and death, with even higher probability of mortality in vulnerable groups. As a result, it is advised to space out the number of infections over time by physical distancing to avoid overburdening health-care workers and hospitals.
Conclusion and future perspectives
The COVID-19 pandemic has affected countries worldwide causing irreparable damage to both human lives and the economy. One lesson we have learned from this pandemic is that a higher commitment to investment in research & development, health-care infrastructure and diagnostics is needed for better preparedness in the future. This pandemic demands the combined action of research groups across the globe. It is equally important to evolve strong collaboration between the research sector, universities and the pharmaceutical industry in unprecedented ways to bring their collective strengths to a unified platform to fight this disease or similar future outbreaks. At present, the immediate priority is expediting the testing of vaccines and treatments; developing low cost, rapid, point-of-care diagnostics; and cheaper, reusable PPE.
The biology of SARS-CoV-2 is evolving with each passing day, and we are far from fully understanding the disease mechanisms and SARS-CoV-2's interactions with our immune system. Biologists need to address basic fundamental questions about COVID-19, such as why patients respond differently to the disease, for how long the immune system of a recovered patient will give protection against re-infection, what are the chances of recurrence in recovered people, the effect of genomic mutations on virulence, rate of mutation and many more. Answers to these questions will be instrumental in the advancement of the therapeutics. The best case scenario for getting these solutions will take at least 12–18 months; sadly, we may have to live with our ‘invisible enemy’ until then. At present, increasing testing and maintaining physical distancing, good hygiene and a healthy immune system could be only weapon for resuming our normal lives.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgements
The research was supported by Bhabha Atomic Research Centre, Mumbai, India.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rehman S., Shafique L., Ihsan A., Liu Q. Evolutionary trajectory for the emergence of novel coronavirus SARS-CoV-2. Pathogens. 2020;9:240. doi: 10.3390/pathogens9030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlos W.G., Dela-Cruz C.S., Cao B., Pasnick S., Jamil S. COVID-19 disease due to SARS-CoV-2 (novel coronavirus) Am J Resp Crit Care Med. 2020;201:7–8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization https://www.who.int/emergencies/diseases/novel-coronavirus-2019/ Available at:
- 6.Worldometers https://www.worldometers.info/coronavirus/ Available at:
- 7.Centers for Disease Control https://www.cdc.gov/coronavirus/2019-ncov/ Available at:
- 8.Sharma R., Agarwal M., Gupta M., Somendra S., Saxena S.K. Clinical characteristics and differential clinical diagnosis of novel coronavirus disease 2019 (COVID-19) In: Saxena S., editor. Coronavirus Disease 2019 (COVID-19). Medical Virology: From Pathogenesis to Disease Control. Springer; Singapore: 2020. pp. 55–70. [Google Scholar]
- 9.Divani A.A., Andalib S., Di Napoli M.D., Lattanzi S., Hussain M.S., Biller J. Coronavirus disease 2019 and stroke: clinical manifestations and pathophysiological insights. J Stroke Cerebrovasc Dis. 2020;29:104941. doi: 10.1016/j.jstrokecerebrovasdis.2020.104941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furukawa N.W., Brooks J.T., Sobel J. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis. 2020;26(7):e1–e6. doi: 10.3201/eid2607.201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. e00127–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X., Tan Y., Ling Y., Lu G., Liu F., Yi Z. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583:437–440. doi: 10.1038/s41586-020-2355-0. [DOI] [PubMed] [Google Scholar]
- 17.Mercatelli D., Giorgi F.M. Geographic and genomic distribution of SARS-CoV-2 mutations. Front Microbiol. 2020:11. doi: 10.3389/fmicb.2020.01800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan L., Zhang H., Goncalves J., Xiao Y., Wang M., Guo Y. An interpretable mortality prediction model for COVID-19 patients. Nat Mach Intell. 2020;2:283–288. [Google Scholar]
- 19.Laamarti M., Alouane T., Kartti S., Chemao M.W., Hakmi M., Essabbar A. Large scale genomic analysis of 3067 SARS-CoV-2 genomes reveals a clonal geo-distribution and a rich genetic variations of hotspots mutations. BioRxiv. 2020 doi: 10.1371/journal.pone.0240345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J. CRISPR–Cas12-based detection of SARS-CoV-2. Nat Biotechnol. 2020;38:870–874. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y.-W., Schmitz J.E., Persing D.H., Stratton C.W. Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol. 2020;58:e00512–e00520. doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z., Yi Y., Luo X., Xiong N., Liu L., Li S. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020;92:1518–1524. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prajapat M., Sarma P., Shekhar N., Avti P., Sinha S., Kaur H. Drug targets for corona virus: a systematic review. Indian J Pharmacol. 2020;52:56–65. doi: 10.4103/ijp.IJP_115_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NIH ClinicalTrials.gov https://clinicaltrials.gov/ct2/results/ Available at:
- 25.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMc2022236. NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 26.Biran N., Ip A., Ahn J., Go R.C., Wang S., Mathura S. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2:e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NIH ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04351152 Available at:
- 29.Das S., Bhowmick S., Tiwari S., Sen S. An updated systematic review of the therapeutic role of hydroxychloroquine in coronavirus disease-19 (COVID-19) Clin Drug Invest. 2020;40:591–601. doi: 10.1007/s40261-020-00927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.https://www.ox.ac.uk/news/2020-06-05-no-clinical-benefit-use-hydroxychloroquine-hospitalised-patients-covid-19 Available at:
- 31.Regalado E.P. Vaccines for SARS-CoV-2: lessons from other coronavirus strains. Infect Dis Ther. 2020;9:255–274. doi: 10.1007/s40121-020-00300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chinese Clinical Trial Registry http://www.chictr.org.cn/showprojen.aspx?proj=52006 Available at:
- 33.NIH ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04405076 Available at:
- 34.ISRCTN Registry Available at: [DOI]
- 35.NIH ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04456595 Available at:
- 36.Randolph H.E., Barreiro L.B. Herd immunity: understanding COVID-19. Immunity. 2020;52:737–741. doi: 10.1016/j.immuni.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/myth-busters Available at: