Graphical abstract
Keywords: Meta-analysis, In vitro test methods, Range of motion, Total disc replacement
Highlights
-
•
Data from two different in vitro test methods for the same evaluation subjects were compared. It was investigated whether spine simulators with real human cadavers (SSCs) and finite element models (FEMs) provide the same data exemplarily for range of motion (ROM) before and after insertion of motion-retaining devices.
-
•
Only fifty-nine percent of SSC meta-analyses show restored ROM after insertion of the device compared to the intact spinal segment. In FEM meta-analyses, ROM is restored in ninety percent.
-
•
Ten percent of ROM analyses show significantly different data between SSCs and FEMs.
-
•
With regard to the included studies, data generated by SSCs and FEMs cannot be used unrestricted as alternative and complementary data.
-
•
Our analysis provides a new approach to compare data from associated test methods.
Abstract
Background
Range-of-motion (ROM) data generated by the in vitro test methods of spine simulators with cadavers (SSCs) and finite element models (FEMs) are used alternatively and complementarily for in vitro evaluations.
Aim of Review
Our purpose is to compare exemplary segmental ROM data from SSCs and FEMs before and after ball-and-socket total disc replacement (bsTDR) to determine whether the two test methods provide the same data for the same evaluation subjects.
Key Scientific Concepts of Review
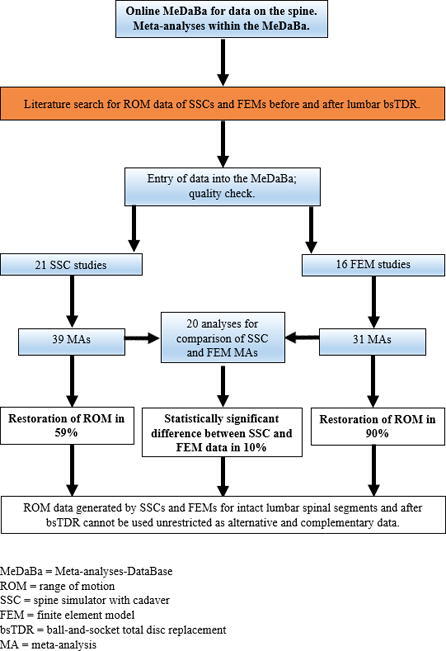
We performed 70 meta-analyses (MAs) and 20 additional comparative analyses based on data from 21 SSC studies used for 39 MAs and 16 FEM studies used for 31 MAs. Only fifty-nine percent (n = 23/39) of SSC MAs show a restored ROM after bsTDR, whereas in FEM MAs, the ROM is restored in ninety percent (n = 28/31). Among the analyses comparing data from the same spinal segments, motion directions and bsTDR, SSC and FEM data are significantly different in ten percent (n = 2/20). According to our results, data generated by SSCs and FEMs cannot be used as alternative and complementary data without restriction. The quality of the evaluation methods itself as well as potential technical reasons for the discrepant results were not our evaluation target. Further SSC and FEM data should be compared using the same approach.
Introduction
In the approval process for motion-retaining devices to be applied to the human spine, animal studies are not suitable. The reason is the different mechanics of quadrupedal animals compared to bipedal humans during locomotion. Thus, approvals of devices for the spine depend on in vitro test results, although even mandatory tests do not provide data reflecting the complete function of spinal segments and the entire spine.
In order to acquire as much information as possible about a motion-retaining device, it is necessary to have detailed knowledge about the function of the device. One of the main functions of a spinal segment is motion, measured as range of motion (ROM), e.g., by the in vitro test methods of spine simulators with real human cadavers (SSCs) and finite element models (FEMs). FEMs are developed on the basis of SSC results; thus, SSC and FEM ROM data are alternatively and complementarily used for further evaluations. However, it is unclear whether SSC and FEM provide the same results for the same evaluation subjects, whether generated data on spinal function motion are reliable. We evaluated exemplary ROM data from SSCs and FEMs before (intact) and after ball-and-socket total disc replacement (bsTDR) to determine whether the same data are provided or to what extent the results differ.
BsTDR was developed as spinal disc substitute to retain motion within the intervertebral space if degenerative disc disease would lead to fusion surgery. Whereas an intervertebral disc is made of fibrocartilage for the performance of motion and further functions, bsTDR consists of at least two articulating prosthetic components to enable motion.
Why this review is important
In our extensive literature research, we identified no analysis comparing ROM data generated by SSCs and FEMs from intact and bsTDR-treated spinal segments to determine whether both in vitro test methods provide the same data for the same test subjects and consequently whether the two methods could be used alternatively and complementarily for further evaluations of the spine.
Material and methods
Study protocol
Our protocol, with a detailed description of our evaluation procedure, is available at https://spinefoundation.info/en/meta-analyses-database [1]: https://spinefoundation.info/medaba/studyprotocols?u=8.
Types of studies
We conducted an initial search to gain an overview, and we found that studies of bsTDR motion-retaining devices in lumbar spinal segments provide the most search results. For this reason, we included single experimental studies using SSCs or FEMs for segments L3/4, L4/5 and L5/S1 in the intact spine and after different bsTDR devices.
Endpoint and measures
The endpoint of our included studies is the segmental ROM, measured in extension, flexion, overall sagittal motion, lateral bending and axial rotation. We chose ROM data of intact spinal segments and after bsTDR because ROM data are provided in most relevant studies, even if not as the primary endpoint.
Key questions
Our first key question was as follows: What are the ROM values measured by SSCs and FEMs for each spinal segment and each direction of motion before and after bsTDR? Our second key question was as follows: Do SSCs and FEMs provide the same ROM data for the same evaluation subjects?
Course of action
First, we performed separate meta-analyses (MAs) for ROM data generated by SSCs and FEMs for intact spinal segments and spinal segments with three specific bsTDR devices known as the Charité artificial disc (CAD), Maverick, and Prodisc-L, as well as all bsTDR (AbsTDR; activL, CAD, InOrbit, Maverick, NewPro, Prodisc-L, prototype of an artificial disc in development [2], Slide-Disc, Triumph) to obtain meta-analytic ROM data. Then, we compared the obtained ROM data from the SSC and FEM MAs to identify whether they are equivalent.
Search strategy
We conducted a comprehensive search in every database from inception to September 2019 for publications related to lumbar ROM before and after bsTDR, measured by either SSCs or FEMs, and published between January 2001 and May 2019.
Inclusion and exclusion criteria
The following inclusion criteria were applied: experimental studies using the test methods of SSCs (with fresh-frozen cadavers) and FEMs; segmental measurements on the human lumbar spine (including L4(6)/S1); single-level bsTDR interventions; and studies for which the full text of the publication was available.
Studies/publications were excluded if any of the following criteria were met:
-
1.Experimental research studies using the following evaluation techniques:
-
•spine simulator without real human cadavers
-
•human in vivo testing
-
•animal study
-
•
-
2.
Measurement of the cervical spine
-
3.
No segmental measurement
-
4.
Total disc replacement of any type other than ball and socket.
Search resources
For our search, we used the databases MEDLINE, Embase, Cochrane Library, Global Health Database (formerly CAB abstracts), World Health Organization regional databases, Google and Google Scholar, as well as doctoral theses and books addressing the topic.
Search terms
We searched for studies with the following terms in the title or abstract: “lumbar”, “spine”, “spinal”, “segment”, “vertebral”, “vertebra”, “biomechanics”, “biomechanical”, “measurements”, “measuring”, “numerical”, “movement”, “motion”, “total disc replacement”, “TDR”, “bsTDR”, “ball and socket”, “ball and cup”, “artificial disc”, “total disc”, “discectomy”, “spinal implant(s)”, “device”, “prosthesis”, “human”, “simulator”, “cadaveric”, “cadaver”, “test”, “testing”, “finite element”, “FE”, “analysis”, “in vitro”, “range of motion”, “ROM”, and “kinematic”.
Selection of studies
Our literature search for potentially eligible studies included title and abstract screening for preselection and full-text retrieval for a second round of selection.
Data extraction, management and assessment for risk of bias
An online system (OS) was created that allows a structured collection of data taken from complete original publications, including quality checks of the studies, evaluations of heterogeneity, and performance of MAs [1]. Basic information on the publications (e.g., title, author(s), date of publication and digital object identifier number) is stored within the OS, whereas duplicates are not stored. The OS provides tools to assess the quality of the studies using a short checklist (eight items) and long checklists (five additional items). Both checklists contain, among others, a question about bias. The short checklist includes a numeric system. When a predetermined score is achieved, the long checklist can be used for a continued quality assessment. Studies passing the long checklist are available for meta-analytic calculations, forest and funnel plots.
Response to missing data
We contacted authors (Cunningham [3], Chen [4], Chung [5], Wang [6]) to provide further information when the data reported in the studies were insufficient. The authors responded and sent the required data in all cases.
Assessment of statistical heterogeneity
Heterogeneity among studies was assessed using forest plots and the chi-squared test, with a statistical significance threshold of p < 0.01; I2 statistics (ranging from 0 to 100%) were used to classify the degree of heterogeneity, with 0% indicating absence of heterogeneity, >0–25% indicating low heterogeneity, >25–50% indicating moderate heterogeneity, >50–75% indicating considerable heterogeneity, and >75–100% indicating high heterogeneity.
Data synthesis
A fixed-effects model was used for the MAs. SSC results were weighted according to the inverse of the variance. Depending on the data, FEM results were weighted equally. All MAs for ROM data from SSCs and FEMs and analyses for the comparison of the two test methods were performed in the OS.
Results
Studies were found for L3/4, L4/5, and L5/S1, with a total number of 21 SSC studies and 16 FEM studies (Table 1). The most evaluated level was L4/5 (n = 18/37). The most evaluated devices were the CAD (n = 12/37), the Maverick (n = 11/37) and the Prodisc-L (n = 17/37). The data of all analyses are given in Table 2 (39 MAs for SSCs), Table 3 (31 MAs for FEMs), and Table 4 (20 analyses for comparison of SSC and FEM). Due to the large number of MAs (n = 70), only one forest plot for each test method is shown (Fig. 1; further forest plots: https://spinefoundation.info/medaba/results?u=8). Table 5 presents an overview of the maintenance of ROM after bsTDR devices with regard to the CAD, Maverick, Prodisc-L and AbsTDR. A significant change in ROM (CROM) (p < 0.01) after bsTDR compared to the intact spinal segment is interpreted as non-restored ROM. Table 6 shows an overview of significant and non-significant differences between SSCs and FEMs with regard to each spinal segment, motion direction and device. As an additional result of our analyses, Table 7 shows the best-performing devices for each test method, spinal segment and direction of motion (best performance defined as the p-value closest to 1 compared to the intact spinal segment).
Table 1.
Studies included in the meta-analyses. SSCs spine simulators with cadavers, FEMs finite element models, segment spinal segment, CAD Charité artificial disc, e extension, f flexion, l lateral bending, a axial rotation, N/A not available, *prototype of an artificial disc in development, displ. contr. displacement controlled ROM with a required moment.
| Study | Segment | Device(s) | Number of specimen | Applied moment (Nm) | Compressive load (N) | |
|---|---|---|---|---|---|---|
| SSCs | Cunningham et al. [3] | L4/5 | CAD | 8 | 8 | N/A |
| Demetropoulos et al. [16] | L4/5 | ProDisc-L | 10 | 10 | 200 | |
| DiAngelo et al. [17] | L5/S1 | CAD, Maverick, ProDisc-L | 20 | 8 | 100 | |
| Erkan et al. [18] | L5/S1 | Maverick | 6 | 7.5 | 100 | |
| Gaffey et al. [19] | L4/5 | ProDisc-L | 7 | 8(f), 6(e) | 400 | |
| Ha et al. [20] | L4/5 | activL | 5 | 8 | 400 | |
| Hitchon et al. [21] | L4/5 | Maverick | 7 | 6 | N/A | |
| Ingalhalikar et al. [22] | L4/5 | Maverick | 10 | displ. contr. | N/A | |
| Kikkawa et al. [23] | L4/5 | Triumph | 7 | 10 | N/A | |
| Kim et al. [24] | L4/5 | CAD | 5 | 8 | 400 | |
| Le Huec et al. [10] | L4/5 | Maverick | 6 | 7 | 400 | |
| Meyers et al. [15] | L5/S1 | ProDisc-L | 12 | 10 | 600/1200 | |
| Moldavsky et al. [25] | L4/5 | InOrbit | 7 | 8 | N/A | |
| O'Leary et al. [13] | L5/S1 | CAD | 5 | 8(f), 6(e) | 400 | |
| Panjabi et al. [26] | L5/S1 | ProDisc-L | 6 | 10 (e,f), 8 (l,a) | 400 | |
| Panjabi et al. [27] | L5/S1 | CAD | 5 | 10 | 400 | |
| Takigawa et al. [28] | L4/5 | Maverick | 7 | 7.5 | 400 | |
| Tsitsopulos et al. [29] | L5/S1 | ProDisc-L | 12 | 8 (f), 6 (e) | 400 | |
| Voronov et al. [30] | L3/4 | CAD | 6 | 8 (f), 6 (e,l), 5 (a) | 400 | |
| Wilke et al. [2] | L3/4 | CAD, ProDisc-L, Prototype* | 6 | 7.5 | 0 | |
| Wong [31] | L5/S1 | Maverick ProDisc-L, | 7 | 7.6 | 100 | |
| FEMs | Chen et al. [4] | L3/4 | Prodisc-L | – | 10 | 150 |
| Chen et al. [32] | L3/4 | Prodisc-L | – | 10 | 400 | |
| Choi et al. [33] | L3/4 | Prodisc-L | – | 10 | 280 | |
| Choi et al. [34] | L3/4 | Prodisc-L, NewPro* | – | 10 | 280 | |
| Chung et al. [5] | L4/5 | CAD, Prodisc-L | – | 6 | 400 | |
| Di Mascio et al. [35] | L4/5 | Maverick | – | 10 | N/A | |
| Dooris et al. [36] | L3/4 | Maverick | – | 6 | 400 | |
| Goel et al. [37] | L5/S1 | CAD | – | 10.6 | 400 | |
| Kim et al. [38] | L4/5 | CAD, Maverick, Prodisc-L | – | 5 | 400 | |
| Knapik et al. [39] | L5/S1 | Prodisc-L | – | N/A | 734* | |
| Le Huec et al. [10] | L4/5 | Maverick | – | 7 | 400 | |
| Rundell et al. [40] | L3/4 | Prodisc-L | – | 7.5 | 500 | |
| Schmidt et al. [41] | L4/5 | CAD, Slide-Disc (mobile/immobile core) | – | 7.5 | 1000 | |
| Wang et al. [6] | L4/5 | Triumph | – | 6 | 400 | |
| Zander et al. [42] | L45 | CAD, Prodisc-L, activL | – | 10(e,f), 7.5(l,a) | 500 | |
| Zhong et al. [43] | L3/4 | Prodisc-L | – | 10 | 150 | |
Table 2.
Results of meta-analyses calculating the difference in range of motion of intact spinal segments and after ball-and-socket total disc replacement of each spinal segment, motion direction and device using spine simulators with cadavers (SSCs). CAD Charité artificial disc, bsTDR ball-and-socket total disc replacement, AbsTDR all bsTDR (activL, CAD, InOrbit, Maverick, NewPro, Prodisc-L, prototype of an artificial disc in development, Slide-Disc, Triumph), CROM change in range of motion in degree, CI confidence interval (l lower and u upper limit), n number of studies, reference no. reference number.
| SSCs | L3/4 |
L4/5 |
L5/S1 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| overall sagittal motion | extension | flexion | lateral bending | axial rotation | overall sagittal motion | extension | flexion | lateral bending | axial rotation | overall sagittal motion | extension | flexion | lateral bending | axial rotation | ||
| CAD | CROM | 5.27 | 1.47 | 2.01 | 0.82 | 2.19 | 1.21 | 1.05 | 0.75 | |||||||
| CI 95% l | 3.22 | 0.32 | 1.49 | −2.04 | 1.2 | 0.7 | −1.43 | −0.06 | ||||||||
| CI 95% u | 7.32 | 2.61 | 2.54 | 3.69 | 3.18 | 1.72 | 3.54 | 1.56 | ||||||||
| p-value | <0.00001 | 0.012 | <0.00001 | 0.57 | 0.000014 | <0.00001 | 0.41 | 0.069 | ||||||||
| Chi2 | 2.22 | 1.58 | 0.26 | 3.57 | 17.63 | 6.54 | 1.37 | 0.71 | ||||||||
| I2 | 55% | 36.8% | 0% | 72% | 94.3% | 84.7% | 0% | 0% | ||||||||
| n | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 2 | ||||||||
| reference no. | [2], [30] | [2], [30] | [2], [30] | [3], [24] | [3], [24] | [3], [24] | [13], [17], [27] | [17], [27] | ||||||||
| Maverick | CROM | 2.06 | 1.26 | −0.38 | −1.31 | −0.03 | −1.07 | 2.7 | −3.86 | −0.22 | 0.36 | |||||
| CI 95% l | 0.09 | 0.42 | −1.81 | −2.29 | −0.59 | −2.27 | 2.04 | −4.41 | −0.61 | 0.1 | ||||||
| CI 95% u | 4.04 | 2.11 | 1.06 | −0.32 | 0.54 | 0.14 | 3.35 | −3.31 | 0.16 | 0.63 | ||||||
| p-value | 0.04 | 0.0033 | 0.6 | 0.0095 | 0.93 | 0.083 | <0.00001 | <0.00001 | 0.25 | 0.0072 | ||||||
| Chi2 | 2.41 | 4.9 | 8.6 | 14.22 | 1.01 | 4.9 | 0.94 | 14.26 | 23.13 | 4.54 | ||||||
| I2 | 0% | 59.1% | 76.7% | 78.9% | 0% | 59.2% | 0% | 86.0% | 91.4% | 56% | ||||||
| n | 4 | 3 | 3 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | ||||||
| reference no. | [10], [21], [22], [28] | [21], [22], [28] | [21], [22], [28] | [10], [21], [22], [28] | [10], [21], [28] | [17], [18], [31] | [17], [18], [31] | [17], [18], [31] | [17], [18], [31] | [17], [18], [31] | ||||||
| Prodisc-L | CROM | −0.8 | −1.58 | 0.17 | −0.64 | 1.37 | −2.52 | 0.07 | 0.08 | |||||||
| CI 95% l | −1.88 | −2.14 | −0.15 | −2.1 | 0.18 | −3.71 | −0.43 | −0.32 | ||||||||
| CI 95% u | 0.28 | −1.02 | 0.49 | 0.81 | 2.55 | −1.33 | 0.57 | 0.48 | ||||||||
| p-value | 0.15 | <0.00001 | 0.31 | 0.39 | 0.024 | 0.000032 | 0.79 | 0.7 | ||||||||
| Chi2 | 0.72 | 6.43 | 12.82 | 0.59 | 0.61 | 0.07 | 10.48 | 5.84 | ||||||||
| I2 | 0% | 68.9% | 84.4% | 0% | 0% | 0% | 71.4% | 48.6% | ||||||||
| n | 3 | 3 | 3 | 4 | 2 | 2 | 4 | 4 | ||||||||
| reference no. | [16], [19], [29] | [16], [19], [29] | [16], [19], [29] | [15], [17], [26], [31] | [17], [31] | [17], [31] | [15], [17], [26], [31] | [15], [17], [26], [31] | ||||||||
| AbsTRD | CROM | 5.28 | 1.01 | 1.62 | −0.8 | −0.24 | 0.69 | −0.35 | 0.36 | −0.76 | 2.49 | −3.61 | −0.23 | 0.2 | ||
| CI 95% l | 3.41 | 0 | 1.18 | −1.54 | −0.67 | −0.19 | −0.72 | 0.14 | −1.57 | 1.91 | −4.12 | −0.53 | −0.02 | |||
| CI 95% u | 7.16 | 2.02 | 2.05 | −0.06 | 0.18 | 1.56 | 0.03 | 0.57 | 0.06 | 3.08 | −3.09 | 0.06 | 0.43 | |||
| p-value | <0.00001 | 0.05 | <0.00001 | 0.034 | 0.26 | 0.12 | 0.07 | 0.0012 | 0.068 | <0.00001 | <0.00001 | 0.12 | 0.078 | |||
| Chi2 | 2.07 | 0.47 | 0 | 51.84 | 27.27 | 14.95 | 83.94 | 29.98 | 7.37 | 1.2 | 19.22 | 55.3 | 13.69 | |||
| I2 | 51.8% | 0% | 0% | 80.7% | 81.7% | 66.5% | 88.1% | 70% | 5% | 0% | 89.6% | 91% | 56.2% | |||
| n | 2 | 2 | 2 | 11 | 6 | 6 | 11 | 10 | 8 | 3 | 3 | 6 | 7 | |||
| reference no. | [2], [30] | [2], [30] | [2], [30] | [3], [10], [16], [19], [20], [21], [22], [23], [24], [25], [28] | [16], [20], [21], [22], [24], [28] | [16], [20], [21], [22], [24], [28] | [3], [10], [16], [19], [20], [21], [22], [23], [24], [25], [28] | [3], [10], [16], [19], [20], [21], [23], [24], [25], [28] | [13], [15], [17], [18], [26], [27], [29], [31] | [17], [18], [31] | [17], [18], [31] | [15], [17], [18], [26], [29], [31] | [15], [17], [18], [26], [27], [29], [31] | |||
Table 3.
Results of meta-analyses calculating the difference in range of motion of intact spinal segments and after ball-and-socket total disc replacement of each spinal segment, motion direction and device using finite element models (FEMs). CAD Charité artificial disc, bsTDR ball-and-socket total disc replacement, AbsTDR all bsTDR (activL, CAD, InOrbit, Maverick, NewPro, Prodisc-L, prototype of an artificial disc in development, Slide-Disc, Triumph), CROM change in range of motion in degree, CI confidence interval (l lower and u upper limit), n number of studies, reference no. reference number.
| FEMs | L3/4 |
L4/5 |
L5/S1 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| overall sagittal motion | extension | flexion | lateral bending | axial rotation | overall sagittal motion | extension | flexion | lateral bending | axial rotation | overall sagittal motion | extension | flexion | lateral bending | axial rotation | ||
| CAD | CROM | 2.27 | 3.32 | −1.03 | 2.85 | 1.16 | ||||||||||
| CI 95% l | 0.16 | −0.33 | −3.1 | 1.73 | −1.03 | |||||||||||
| CI 95% u | 4.38 | 6.96 | 1.05 | 3.97 | 3.34 | |||||||||||
| p-value | 0.035 | 0.074 | 0.33 | <0.00001 | 0.3 | |||||||||||
| Chi2 | 1.95 | 6.22 | 2.17 | 3.95 | 1.87 | |||||||||||
| I2 | 0% | 51.8% | 0% | 49.4% | 0% | |||||||||||
| n | 4 | 4 | 4 | 3 | 3 | |||||||||||
| reference no. | [5], [38], [41], [42] | [5], [38], [41], [42] | [5], [38], [41], [42] | [5], [41], [42] | [5], [41], [42] | |||||||||||
| Maverick | CROM | 1.32 | 1.32 | 0 | 0.29 | −0.05 | ||||||||||
| CI 95% l | −1.41 | −0.9 | −1.58 | −1.08 | −0.36 | |||||||||||
| CI 95% u | 4.05 | 3.54 | 1.58 | 1.66 | 0.25 | |||||||||||
| p-value | 0.34 | 0.24 | >0.99999 | 0.68 | 0.72 | |||||||||||
| Chi2 | 0.61 | 0.83 | 0.3 | 0.21 | 0.05 | |||||||||||
| I2 | 0% | 0% | 0% | 0% | 0% | |||||||||||
| n | 3 | 3 | 3 | 2 | 2 | |||||||||||
| reference no. | [10], [35], [38] | [10], [35], [38] | [10], [35], [38] | [10], [35] | [10], [35] | |||||||||||
| Prodisc | CROM | 3.84 | 2.96 | 0.88 | 1.45 | 1.59 | 2.38 | 3.32 | −0.97 | 2.8 | 1.79 | |||||
| CI 95% l | −4.34 | −0.2 | −4.17 | 0.29 | −0.11 | 1.3 | −0.11 | −3.33 | −0.51 | −0.66 | ||||||
| CI 95% u | 12.01 | 6.12 | 5.92 | 2.61 | 3.28 | 3.46 | 6.76 | 1.39 | 6.11 | 4.24 | ||||||
| p-value | 0.36 | 0.067 | 0.73 | 0.014 | 0.067 | 0.000016 | 0.058 | 0.42 | 0.098 | 0.15 | ||||||
| Chi2 | 10.6 | 8.08 | 5.3 | 2.46 | 3.79 | 1.37 | 4.64 | 1.95 | 2.61 | 1.78 | ||||||
| I2 | 62.3% | 50.5% | 24.5% | 0% | 0% | 0% | 56.9% | 0% | 61.7% | 43.8% | ||||||
| n | 5 | 5 | 5 | 5 | 5 | 3 | 3 | 3 | 2 | 2 | ||||||
| reference no. | [4], [32], [33], [40], [43] | [4], [32], [33], [40], [43] | [4], [32], [33], [40], [43] | [4], [32], [33], [40], [43] | [4], [32], [33], [40], [43] | [5], [38], [42] | [5], [38], [42] | [5], [38], [42] | [5], [42] | [5], [42] | ||||||
| AbsTDR | CROM | 2.88 | 2.42 | 0.46 | 1.09 | 1.1 | 1.31 | 1.84 | −0.53 | 1.36 | 0.67 | 2.55 | ||||
| CI 95% l | −4.79 | −0.74 | −4.19 | −0.79 | −1.52 | −1.35 | −1.74 | −2.74 | −1.74 | −1.29 | 2.45 | |||||
| CI 95% u | 10.55 | 5.58 | 5.1 | 2.97 | 3.72 | 3.97 | 5.42 | 1.68 | 4.45 | 2.64 | 2.65 | |||||
| p-value | 0.46 | 0.13 | 0.85 | 0.25 | 0.41 | 0.34 | 0.31 | 0.64 | 0.39 | 0.5 | <0.00001 | |||||
| Chi2 | 11.08 | 8.66 | 6.63 | 2.62 | 4.8 | 1.96 | 5.26 | 2.47 | 3.39 | 1.96 | 1.45 | |||||
| I2 | 45.8% | 30.7% | 9.5% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 30.9% | |||||
| n | 7 | 7 | 7 | 6 | 6 | 7 | 7 | 7 | 6 | 6 | 2 | |||||
| reference no. | [4], [32], [33], [34], [36], [40], [43] | [4], [32], [33], [34], [36], [40], [43] | [4], [32], [33], [34], [36], [40], [43] | [4], [32], [33], [34], [40], [43] | [4], [32], [33], [34], [40], [43] | [5], [6], [10], [35], [38], [41], [42] | [5], [6], [10], [35], [38], [41], [42] | [5], [6], [10], [35], [38], [41], [42] | [5], [6], [10], [35], [41], [42] | [5], [6], [10], [35], [41], [42] | [37], [39] | |||||
Table 4.
Comparison of results of meta-analyses for each spinal segment, motion direction and device, using spine simulators with cadavers (SSCs) and finite element models (FEMs). CAD Charité artificial disc, bsTDR ball-and-socket total disc replacement, AbsTDR all bsTDR (activL, CAD, InOrbit, Maverick, NewPro, Prodisc-L, prototype of an artificial disc in development, Slide-Disc, Triumph), D ROM difference between SSC and FEM, CROM change in range of motion in degree, CI confidence interval, n number of studies, reference no. reference number, italic not available.
| L3/4 |
L4/5 |
L5/S1 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| overall sagittal motion | extension | flexion | lateral bending | axial rotation | overall sagittal motion | extension | flexion | lateral bending | axial rotation | overall sagittal motion | extension | flexion | lateral bending | axial rotation | ||
| CAD | D in CROM | 1.45 | 0.66 | −0.05 | ||||||||||||
| CI 95% lower | −2.11 | −0.83 | −2.29 | |||||||||||||
| CI 95% upper | 5.01 | 2.15 | 2.19 | |||||||||||||
| p-value | 0.42 | 0.39 | 0.97 | |||||||||||||
| Chi2 | 0 | 0 | 0 | |||||||||||||
| I2 | 0% | 0% | 0% | |||||||||||||
| n | 6 | 5 | 5 | |||||||||||||
| reference no. | [3], [5], [24], [38], [41], [42] | [3], [5], [24], [41], [42] | [3], [5], [24], [41], [42] | |||||||||||||
| Maverick | D in CROM | −0.74 | 0.06 | 0.38 | 1.6 | −0.02 | ||||||||||
| CI 95% lower | −4.11 | −2.31 | −1.76 | −0.09 | −0.66 | |||||||||||
| CI 95% upper | 2.63 | 2.43 | 2.52 | 3.29 | 0.62 | |||||||||||
| p-value | 0.67 | 0.96 | 0.73 | 0.064 | 0.95 | |||||||||||
| Chi2 | 0 | 0 | 0 | 0 | 0 | |||||||||||
| I2 | 0% | 0% | 0% | 0% | 0% | |||||||||||
| n | 7 | 6 | 6 | 6 | 5 | |||||||||||
| reference no. | [10], [21], [22], [28], [35], [38] | [10], [21], [22], [28], [35], [38] | [10], [21], [22], [28], [35], [38] | [10], [21], [22], [28], [35] | [10], [21], [28], [35] | |||||||||||
| Prodisc-L | D in CROM | 3.18 | 4.33 | 1.62 | ||||||||||||
| CI 95% lower | 1.66 | 0.97 | −0.85 | |||||||||||||
| CI 95% upper | 4.7 | 7.69 | 4.09 | |||||||||||||
| p-value | 0.000041 | 0.012 | 0.2 | |||||||||||||
| Chi2 | 0 | 0 | 0 | |||||||||||||
| I2 | 0% | 0% | 0% | |||||||||||||
| n | 6 | 5 | 5 | |||||||||||||
| reference no. | [5], [16], [19], [29], [38], [42] | [5], [16], [19], [29], [42] | [5], [16], [19], [29], [42] | |||||||||||||
| AbsTDR | D in CROM | −2.4 | 0.08 | −0.52 | 2.11 | 2.08 | −1.22 | 1.71 | 0.31 | 3.31 | ||||||
| CI 95% lower | −10.29 | −2.06 | −3.2 | −0.65 | −1.52 | −3.59 | −1.4 | −1.67 | 2.49 | |||||||
| CI 95% upper | 5.49 | 2.22 | 2.16 | 4.87 | 5.68 | 1.15 | 4.82 | 2.29 | 4.13 | |||||||
| p-value | 0.55 | 0.94 | 0.7 | 0.13 | 0.26 | 0.31 | 0.28 | 0.76 | <0.00001 | |||||||
| Chi2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| I2 | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |||||||
| n | 9 | 8 | 8 | 18 | 13 | 13 | 17 | 16 | 10 | |||||||
| reference no. | [2], [4], [30], [32], [33], [34], [36], [40], [43] | [2], [4], [30], [32], [33], [34], [40], [43] | [2], [4], [30], [32], [33], [34], [40], [43] | [3], [5], [6], [10], [16], [19], [20], [21], [22], [23], [24], [25], [28], [35], [38], [41], [42] | [5], [6], [10], [16], [20], [21], [22], [24], [28], [35], [38], [41], [42] | [5], [6], [10], [16], [20], [21], [22], [24], [28], [35], [38], [41], [42] | [3], [5], [6], [10], [16], [19], [20], [21], [22], [23], [24], [25], [28], [35], [41], [42] | [3], [5], [6], [10], [16], [19], [20], [21], [23], [24], [25], [28], [35], [41], [42] | [13], [15], [17], [18], [26], [27], [29], [31], [37], [39] | |||||||
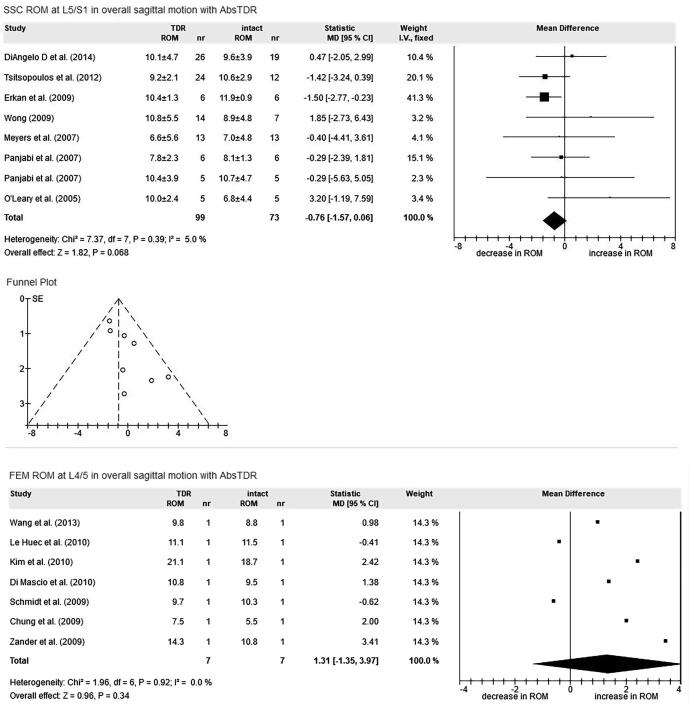
Fig. 1.
Exemplary forest plots of meta-analyses for the evaluation methods SSC spine simulator with cadaver and FEM finite element model. ROM range of motion (in degree). TDR total disc replacement, bsTDR ball-and-socket total disc replacement, AbsTDR all bsTDR (activL, CAD Charité artificial disc, InOrbit, Maverick, NewPro, Prodisc-L, prototype of an artificial disc in development, Slide-Disc, Triumph), intact ROM before TDR, decrease/increase in ROM ROM after TDR compared to intact.
Table 5.
Restoration of range of motion (ROM) after ball-and-socket total disc replacement (bsTDR) for each spinal segment, motion direction and device. SSCs spine simulators with cadavers, FEMs finite element models, + ROM restored, - ROM not restored, CAD Charité artificial disc, bsTDR ball-and-socket total disc replacement, AbsTDR all bsTDR (activL, CAD, InOrbit, Maverick, NewPro, Prodisc-L, prototype of an artificial disc in development, Slide-Disc, Triumph), greyed out not enough studies to perform meta-analyses.
 |
Table 6.
Statistically significant results comparing spine simulators with cadavers and finite element models for each spinal segment, motion direction and device. + significant (p < 0.01), - not significant, CAD Charité artificial disc, bsTDR ball-and-socket total disc replacement, AbsTDR all bsTDR (activL, CAD, InOrbit, Maverick, NewPro, Prodisc-L, prototype of an artificial disc in development, Slide-Disc, Triumph), greyed out not enough studies to perform meta-analyses.
 |
Table 7.
Best performing devices with regard to spinal segments and motion directions. Best performing is defined as the p-value closest to 1 compared to the intact spinal segment. In the case of same p-value, the closest range of motion (ROM) of ball-and-socket total disc replacement to the ROM of the intact spinal segment is defined as best performing. SSCs spine simulators with cadavers, FEMs finite element models, CAD Charité artificial disc, greyed out not enough studies to perform meta-analyses.
 |
SSC results
Table 2 shows the ROM results for the different spinal segments, directions of motion and devices. Furthermore, spinal segments and directions of motion with insufficient numbers of studies to perform MAs are shown. Hereafter, only results with restored ROM after bsTDR are presented, and they are always compared to intact spinal segments.
CAD
The CAD restores motion at L3/4 in lateral bending (CROM = 1.47°). At L4/5, ROM is restored in overall sagittal motion (CROM = 0.82°). At L5/S1, ROM is restored in overall sagittal motion (CROM = 1.05°) and axial rotation (CROM = 0.75°). In all MAs, the CAD increases ROM after insertion, up to 5.27° in overall sagittal motion at L3/4. None of the eight MAs with CAD shows a decrease in ROM after insertion.
Maverick
At L4/5, ROM is restored in overall sagittal motion (CROM = 2.06°), flexion (CROM = −0.38°) and axial rotation (CROM = −0.03°). At L5/S1, ROM is restored in overall sagittal motion (CROM = −1.07) and lateral bending (CROM = −0.22°). The Maverick increases ROM after insertion in 4 out of 10 MAs, up to 2.7° at L5/S1 in extension. In 6 out of 10 MAs, the Maverick decreases ROM after insertion, down to −3.86° at L5/S1 in flexion.
Prodisc-L
At L4/5, ROM is restored in overall sagittal motion (−0.8°) and axial rotation (0.17°). At L5/S1, ROM is restored in overall sagittal motion (CROM = −0.64°), extension (CROM = 1.37°), lateral bending (CROM = 0.07°) and axial rotation (CROM = 0.08°). Prodisc-L increases ROM after insertion in 4 out of 8 MAs, up to 1.37° at L5/S1 in extension. In 4 out of 8 MAs, Prodisc-L decreases ROM, down to −2.52° at L5/S1 in flexion.
AbsTDR
ROM is restored at L3/4 in lateral bending (CROM = 1.01°), at L4/5 in overall sagittal motion (CROM = −0.8°), in extension (CROM = −0.24°), in flexion (CROM = 0.69°), and in lateral bending (CROM = −0.35°), and at L5/S1 in overall sagittal motion (CROM = −0.76°), lateral bending (CROM = −0.23°), and axial rotation (CROM = 0.2°). ROM after bsTDR insertion is increased in 7 out of 13 MAs, up to 5.28° at L3/4 in overall sagittal motion. ROM decreases in 6 out of 13 MAs, down to −3.61° at L5/S1 in flexion.
FEM results
The results for the different spinal segments, motion directions and devices are presented in Table 3, including the results for segments and directions of motion with insufficient numbers of studies to perform MAs. Hereafter, we present only results with restored ROM after bsTDR, which are always compared to intact spinal segments.
CAD
ROM is restored at L4/5 in overall sagittal motion (CROM = 2.27°), extension (CROM = 3.32°), flexion (CROM = −1.03°) and axial rotation (CROM = 1.16°). The CAD increases ROM after insertion in 4 out of 5 MAs, up to 3.32° at L4/5 in extension. In 1 out of 5 MAs, ROM after CAD is decreased, down to −1.03° at L4/5 in flexion.
Maverick
ROM is restored at L4/5 in overall sagittal motion (CROM = 1.32°), extension (CROM = 1.32°), flexion (CROM = 0°), lateral bending (CROM = 0.29°) and axial rotation (CROM = −0.05°). The Maverick increases ROM after insertion in 4 out of 5 MAs, up to 1.32° at L4/5 in overall sagittal motion and extension. In 1 out of 5 MAs, ROM after Maverick is decreased, down to −0.05° at L4/5 in axial rotation.
Prodisc-L
ROM is restored at L3/4 in overall sagittal motion (CROM = 3.84°), extension (CROM = 2.96°), flexion (CROM = 0.88°), lateral bending (CROM = 1.45°) and axial rotation (CROM = 1.59°), as well as at L4/5 in extension (CROM = 3.32°), flexion (CROM = −0.97°), lateral bending (CROM = 2.8°) and axial rotation (1.79°). The Prodisc-L increases ROM after insertion in 9 out of 10 MAs, up to 3.84° at L3/4 in overall sagittal motion. In 1 out of 10 MAs, ROM after Prodisc-L is decreased, down to −0.97° at L4/5 in flexion.
AbsTDR
ROM is restored at L3/4 in overall sagittal motion (CROM = 2.88°), extension (CROM = 2.42°), flexion (CROM = 0.46°), lateral bending (CROM = 1.09°) and axial rotation (CROM = 1.1°), as well as at L4/5 in overall sagittal motion (CROM = 1.31°), extension (CROM = 1.84°), flexion (CROM = −0.53°), lateral bending (CROM = 1.36) and axial rotation (CROM = 0.67). ROM after bsTDR insertion is increased in 10 out of 11 MAs, up to 2.88° at L3/4 in overall sagittal motion. In 1 out of 11 MAs, ROM after insertion is decreased, down to −0.53° at L4/5 in flexion.
Comparison of SSC and FEM results
The results for the different spinal segments, directions of motion and devices are presented in Table 4. Table 6 provides an overview of the statistical significance of the differences between the SSC and FEM results. The detailed results are presented here:
CAD
There was no significant difference between SSC and FEM at L4/5 in overall sagittal motion (p = 0.42), lateral bending (p = 0.39) or axial rotation (p = 0.97).
Maverick
There was no significant difference between SSC and FEM at L4/5 in overall sagittal motion (p = 0.67), extension (p = 0.96), flexion (p = 0.73), lateral bending (p = 0.064) or axial rotation (p = 0.95).
Prodisc-L
There was a significant difference between SSC and FEM at L4/5 in overall sagittal motion (p = 0.000041). There was no significant difference at L4/5 in lateral bending (p = 0.012) or axial rotation (p = 0.2).
AbsTDR
There was a significant difference between SSC and FEM at L5/S1 in overall sagittal motion (p < 0.00001). There was no significant difference at L3/4 in overall sagittal motion (p = 0.55), lateral bending (p = 0.94) or axial rotation (p = 0.7), as well as no significant difference at L4/5 in overall sagittal motion (p = 0.13), extension (p = 0.26), flexion (p = 0.31), lateral bending (p = 0.28), or axial rotation (p = 0.76).
Best performing bsTDR
As shown in Table 7, the CAD performs best in SSCs at L4/5 and L5/S1 in overall sagittal motion. The Maverick performs best in SSC at L4/5 in lateral bending and axial rotation and in FEMs at L4/5 in overall sagittal motion, extension, flexion, lateral bending, and axial rotation. The Prodisc-L performs best in SSCs at L5/S1 in extension, flexion, lateral bending, and axial rotation.
Overall results
Approximately 59% of all SSC MAs show restored ROM (n = 23/39 MAs), whereas in FEM MAs, ROM is restored in 90% (n = 28/31) (Table 5).
The 20 analyses comparing SSCs and FEMs with available data for the same spinal segments, motion directions and bsTDR devices show a statistically significant difference of 10% (n = 2/20) between SSCs and FEMs. For further details, the performance of the Prodisc-L at L4/5 in overall sagittal motion and the performance of AbsTDR at L5/S1 in overall sagittal motion are shown in Table 4.
Results by spinal level
Altogether, the L3/4 segment was tested in 24% of studies (n = 9/37), L4/5 in 49% (n = 18/37), and L5/S1 in 27% (n = 10/37). No suitable studies are available for L1/L2 and L2/L3 MAs (Table 1). Eighty percent (n = 16/20) of analyses for the comparison of SSCs and FEMs are at L4/L5. For the L4/5 segment, the number of studies is sufficient (n = 18/37) to perform MAs for ROM data with inserted CAD, Maverick, Prodisc-L, and AbsTDR devices in all directions of motion, except for extension and flexion for the CAD and Prodisc-L in SSC. Our analyses for the comparison of SSCs and FEMs in this segment show a statistically significant difference of 6% (n = 1/16) (Table 6). For the comparison of SSCs and FEMs with regard to L3/4 and L5/S1 segments, only results for AbsTDR are available.
Results of the bsTDR evaluation
CAD
Comparing the results of the CAD, Maverick and Prodisc-L, the CAD is the best performing bsTDR in 15% (n = 2/13; overall sagittal motion at L4/5 and L5/S1) (Table 7). The CAD restores ROM in 50% (n = 4/8) of SSC MAs and 80% (n = 4/5) of FEM MAs (Table 5). The comparison of SSCs and FEMs shows no significant difference in any of the analyses (n = 3/3) (Table 6).
Maverick
The Maverick is the best performing bsTDR in 54% (n = 7/13) (Table 7). The Maverick restores ROM in 50% of SSC MAs (n = 5/10) and in all of the FEM MAs (n = 5/5) (Table 5). The comparison of SSCs and FEMs shows no significant difference in any of the analyses (n = 5/5) (Table 6).
Prodisc-L
The Prodisc-L is the best performing bsTDR in 31% (n = 4/13) (Table 7). The Prodisc-L restores ROM in 75% (n = 6/8) of SSC MAs and 90% (n = 9/10) of FEM MAs (Table 5). The comparison of SSCs and FEMs shows a significant difference in 33% (n = 1/3) of analyses (Table 6).
AbsTDR
The analyses of AbsTDR allow us to compare evaluations of SSCs and FEMs independently of the specific disc. ROM is restored in 62% (n = 8/13) of SSC MAs and 91% (n = 10/11) of FEM MAs (Table 5). The comparison of SSCs and FEMs shows a significant difference in 11% (n = 1/9) of analyses (Table 6).
Discussion
BsTDR was developed to retain spinal segmental motion in mainly middle-aged patients with degenerative disc disease and at the same time to reduce pain. However, Johnsen et al. [44] found no correlation between lumbar segmental movement and patient reported outcomes.
Another type of disc replacement than bsTDR provides motion by the features of the material between two metal plates, i.e. without articulating prosthetic components. Depending on the number of available studies with segmental ROM data and to avoid data differences from different types of disc replacement, we included bsTDR study data only to our MAs.
Method and evaluation basics
Although more cervical discs than lumbar discs are implanted in humans, our literature search revealed more studies with ROM data for the lumbar spine than for the cervical spine. For this reason, we included single SSC and FEM studies of the lumbar spine in our ROM data MAs.
The cadavers used in the SSC studies of our MAs were confirmed to meet the quality criteria proposed by Wilke et al. for evaluating ROM [7].
According to Jones et al. [8], FEMs were validated by the comparison of data from SSCs and/or from the literature to provide a convincing body of evidence that the calculated results are meaningful.
We evaluated data from SSCs and FEMs to identify whether both evaluation methods provide the same data for the same test subjects, but not the quality of the test methods or issues of the test setups.
For our comparison, we used all available segmental ROM data for extension and flexion as well as overall sagittal motion data. Not all studies provide extension and flexion data separately. A division by two of the overall sagittal motion data is incorrect because the ROM of extension is smaller than the ROM of flexion. Consequently, the provided extension and flexion data were summarized to obtain overall sagittal motion data. In accordance with White and Panjabi [9], lateral bending and axial rotation data were used unilaterally. For this reason, and because some included studies present unilateral data only, provided bilateral motion data were divided by two.
In our analysis, we performed MAs of separately obtained SSC- and FEM-based ROM data of various spinal segments, motion directions and devices to compare SSCs with FEMs. SSC and FEM data from single studies included in our MAs for ROM of intact spinal segments and after bsTDR are independent of each other and are therefore not directly compared. Only one study included in our MAs provides SSC data with dependent FEM data [10], each used as a single dataset for the groups of our meta-analytic calculations.
Results
According to Le Huec et al. [10], SSC and FEM testing are two different but complementary approaches to conduct biomechanical studies of the spine for ROM analyses. Whereas cadaveric tests provide data on motion responses of a spinal segment to varied loading conditions, FEM analyses, using computational models, tend to simulate the biomechanical behaviour of spinal segments.
In order for SSCs and FEMs to be used as complementary test methods, it is necessary to assume that these methods provide the same data for the same evaluated subjects, at least without statistically significant differences.
We compared SSC and FEM ROM data from lumbar spinal segments before and after bsTDR devices to identify whether they are equivalent or to what extent they differ. Our meta-analytic calculations and further analyses with data from the included studies led to unexpected results, demonstrating that SSCs and FEMs do not consistently provide the same results. While 90% (n = 28/31) of FEM MAs show restored ROM, the MAs for SSCs demonstrate restored ROM in only 59% (n = 23/39). A statistically significant difference in the comparison of SSCs and FEMs with data from the same spinal segments, motion directions and bsTDR was found in 10% (n = 2/20).
Recommendations for the standardization of in vitro tests were presented in 1998 [7], i.e., before all studies included in our MAs were published. One reason for the different ROM results in the comparison of SSC and FEM data could be inconsistent validation of the included FEM studies. However, Dreischarf et al. [11] compared and re-validated eight published FEMs and stated that “predicted median segmental intervertebral rotations and disc pressures were in good agreement with measured in vivo values”. Furthermore, technical difficulties in SSC studies and differences in the test setups of included SSC and FEM studies can cause different ROM results. According to Wilke et al. [7], the applied moment should be high enough to achieve normal ROM; 7.5 Nm is suggested for the lumbar spine. In our included studies, the moment varies but remains within the elastic range [7]. The applied moments and compressive loads before (intact) and after bsTDR are consistent in each study. Regarding the compressive load, O’Leary et al. [13] recommend 400 N. Patwardhan et al. [14] describe a decrease in ROM with increasing compressive load. However, according to Meyers et al. [15], the compressive load does not significantly affect ROM in extension, flexion or axial rotation. Volkheimer et al. [12] showed in a review that even with the same testing conditions, the results may vary in some cases.
No further MAs or other analyses with ROM data for the comparison of SSCs and FEMs are available in the literature to discuss our results, which demonstrates the uniqueness of our evaluation.
In summary, SSCs and FEMs do not provide ROM data for unrestricted complementary or alternative evaluations of spinal segments. Otherwise, it is assumed that single SSC and FEM studies can provide high-quality data. Whereas our comparisons of SSC and FEM ROM data are limited to intact spinal segments and inserted bsTDR devices, other motion-retaining devices for the spine could provide ROM results more conveniently.
In order to obtain generally high-quality results from evaluations for, e.g., the approval of motion-retaining devices for the human spine, it will be necessary to further compare data from SSCs and FEMs and to study the origin of the differences for the sake of obtaining more uniform data and thus reliable results. The data and approach of our evaluation can support further evaluations and developments, although the in vivo situation of the spine is complex, not well understood, and variable between individuals [7].
Our evaluation has limitations: The search includes studies in English and German only. For some motion segments and directions of motion, we did not find appropriate studies for further MAs.
Conclusion
Our analyses provide a new approach to compare data from associated in vitro test methods with the same evaluation subjects. Comparing SSC and FEM ROM study data of the same spinal segments, motion directions and devices before and after bsTDR, we detected differences between SSC and FEM data in the endpoint ROM. The conclusion with regard to the included studies is that SSC and FEM ROM data cannot be used unrestricted as alternative and complementary data. We did not evaluate any technical issues as potential reasons for the ROM data differences or whether SSC and FEM test methods can provide high-quality ROM data. Further developments and evaluations should lead to greater consistency of data between SSCs and FEMs.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Biographies

Tobias Bohn is a doctoral student at Charité – Universitätsmedizin Berlin, where he successfully graduated the state examination in human medicine. He has been working together with Prof. Dr. Büttner-Janz and the Spinefoundation. He was part of creating a database for research on the human spine. He is currently doing research on biomechanics of the spine before and after implantation of ball and socket artificial discs as part of his doctoral thesis.
Since 2017 he is working as an intern at the institute of radiology at Ortenau Klinikum Offenburg-Gengenbach as part of his training to become a state certified radiologist.

Susanne Annette Jennifer Lang is Medical Doctor since 2015, after her education at the University of Halle/Saale, Germany, and the Charité - Universitätsmedizin Berlin, Germany. In advance, she became Physiotherapist at the School for Physiotherapy in Stralsund, Germany. She performed several further educations, as e.g. Personal Trainer. She underwent clinical training in surgery in different locations of the world (e.g. Ho Chi Minh City/Vietnam, Cook Islands), Since January 2018, she is living in Los Angeles, CA, USA and preparing for the US medical board exam to apply for Orthopeadic residency. In 2012, she started her scientific work to evaluate lumbar spine stabilizing surgical procedures, in cooperation with the ‘Spinefoundation’, Berlin, Germany.

Dr. Stephanie Roll is currently working as the head statistician at the Institute for Social Medicine, Epidemiology and Health Economics at the Charité University Medical Center in Berlin, Germany. She obtained her MSc in Statistics at the Dortmund University and her MSc in Epidemiology from the London School of Hygiene & Tropical Medicine, UK. In January 2010 she was awarded a PhD from the Charité University Medical Center. Her main working areas are in medical statistics, biometry, and epidemiology.
Dr. Stephanie Roll is a senior statistician and epidemiologist at the Institute for Social Medicine, Epidemiology, and Health Economics. She is experienced in the designing and statistical analysis of clinical and epidemiological studies.

Helene Schrader is a last year medical student at the department of Urology at Charité – Universitätsmedizin Berlin, where she focuses on novel treatment options in the therapy of bone metastases by castration resistant prostate cancer.
She completed her university studies with the second part of the state examination in April 2019 and a good scientific practice course with a retrospective study regarding endovascular aortic repair of abdominal aortic aneurysms.
She has worked for the European Students Conference, one of the biggest student organized biomedical conferences promoting the international scientific exchange between students, clinicians and researchers from different fields and cultural backgrounds worldwide, where she was responsible for the organization of the scientific program dealing with the challenges of increasing antimicrobial resistance.
Another major area of her scientific interest includes clinical-diagnostic and molecular cancer pathology and led her to her work on a Germany-wide database for the collection and analysis of epidemiological and carcinoma-associated data of patients with familial breast- and ovarial cancer.
She has underwent clinical traineeships in the fields of general and thoracic surgery, gynecology and obstetrics, urology, internal medicine, including cardiology, nephrology and family medicine, emergency care and forensic medicine.

Dr. Matthias Pumberger is deputy surgeon in chief of the CMSC. He has extensive experience in basic science and clinical research of spinal surgery. Prior to his position at the Charité, he has been fellow at the Hospital for Special Surgery, New York, USA. His scientific activities focus on outcome related research of spinal fusion surgeries including various surgical strategies, regenerative aspects of surrounding soft tissue and prevention of infection. Matthias Pumberger has authored more than 50 articles in the orthopedic field and is reviewer as well as on the editorial board of renowned Journals as Biomaterials, JSS or PLOS One.

Prof. Dr. Karin Büttner-Janz founded the non-profit ‘Spinefoundation’ to advance scientific work in the field of data analyses in cooperation with the Charité – Universitätsmedizin Berlin, one of the largest university hospitals in Europe. Prior to this, she was for more than twenty years director of clinics and educated surgeons. Together with a colleague, she developed the first artificial disc for the spine, approved by the FDA. After her career as surgeon, she performed a business administration general management study. Her master’s thesis comprised an extensive conception how to reduce surgical site infections. Prof. Dr. Karin Büttner-Janz is honorary member of the “American Orthopaedic Society for Sports Medicine” and past president of the “International Society for the Advancement of Spine Surgery”. Since many years, she is reviewer of international scientific journals.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Büttner-Janz Spinefoundation. https://spinefoundation.info/en/meta-analyses-database.
- 2.Wilke H.J., Schmidt R., Richter M., Schmoelz W., Reichel H., Cakir B. The role of prosthesis design on segmental biomechanics: semi-constrained versus unconstrained prostheses and anterior versus posterior centre of rotation. Eur Spine J. 2012;21:577–584. doi: 10.1007/s00586-010-1552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham B.W., Gordon D., Dimitriev A.E., Hu N., McAfee P.C. Biomechanical evaluation of total disc replacement arthroplasty an in vitro human cadaveric model. Spine J. 2003;28:110–117. doi: 10.1097/01.BRS.0000092209.27573.90. [DOI] [PubMed] [Google Scholar]
- 4.Chen S.H., Zhong Z.C., Chen C.S., Chen W.J., Hung C. Biomechanical comparison between lumbar disc arthroplasty and fusion. Med Eng Phys. 2009;31:244–253. doi: 10.1016/j.medengphy.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Chung S.K., Kim Y.E., Wang K.C. Biomechanical effect of constraint in lumbar total disc replacement - a study with finite element analysis. Spine J. 2009;34:1281–1286. doi: 10.1097/BRS.0b013e3181a4ec2d. [DOI] [PubMed] [Google Scholar]
- 6.Wang W., Zhang H., Sadeghipour K., Baran G. Effect of posterolateral disc replacement on kinematics and stress distribution in the lumbar spine: a finite element study. Med Eng Phys. 2013;35:357–364. doi: 10.1016/j.medengphy.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Wilke H.J., Wenger K., Claes L. Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur Spine J. 1998;7:148–154. doi: 10.1007/s005860050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones A., Wilcox R.K. Finite element analysis of the spine: Towards a framework of verification, validation and sensitivity analysis. Med Eng Phys. 2008;30:1287–1304. doi: 10.1016/j.medengphy.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 9.White A.A., Panjabi M.M. 2nd ed. JB Lippincott; Philadelphia: 1990. Clinical biomechanics of the spine. [Google Scholar]
- 10.Le Huec J., Lafage V., Bonnet X., Lavaste F., Josse Liu M. Validated finite element analysis of the maverick total disc prosthesis. J Spinal Disord Tech. 2010;23:249–257. doi: 10.1097/BSD.0b013e3181a5db24. [DOI] [PubMed] [Google Scholar]
- 11.Dreischarf M., Zander T., Shirazi-Adl A., Puttlitz C.M., Adam C.J., Chen C.S. Comparison of eight published static finite element models of the intact lumbar spine: Predictive power of models improves when combined together. J Biomech. 2014;47:1757–1766. doi: 10.1016/j.jbiomech.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Volkheimer D., Malakoutian M., Oxland T.R., Wilke H.J. Limitations of current in vitro test protocols for investigation of instrumented adjacent segment biomechanics: critical analysis of the literature. Eur Spine J. 2005;24:1882–1892. doi: 10.1007/s00586-015-4040-9. [DOI] [PubMed] [Google Scholar]
- 13.O'Leary P., Nicolakis M., Lorenz M.A., Voronov L.I., Zindrick M.R., Ghanayem A. Response of charite total disc replacement under physiologic loads: prosthesis component motion patterns. Spine J. 2005;5:590–599. doi: 10.1016/j.spinee.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Patwardhan A.G., Havey R.M., Carandang G., Simonds J., Voronov L.I., Ghanayem A.J. Effect ofcompressive follower preload on the flexion-extension response of the human lumbar spine. J Orthop Res. 2003;21:540–546. doi: 10.1016/S0736-0266(02)00202-4. [DOI] [PubMed] [Google Scholar]
- 15.Meyers K.N., Campbell D.A., Lipman J.D., Zhang K., Myers E.R., Girardi F.P. Dynamics of an intervertebral disc prosthesis in human cadaveric spines. HSS J. 2007;3:164–168. doi: 10.1007/s11420-007-9049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demetropoulos C.K., Sengupta D.K., Knaub M.A., Wiater B.P., Abjornson C., Truumees E. Biomechanical evaluation of the kinematics of the cadaver lumbar spine following disc replacement with the ProDisc-L prosthesis. Spine J. 2009;35:26–31. doi: 10.1097/BRS.0b013e3181c4eb9a. [DOI] [PubMed] [Google Scholar]
- 17.DiAngelo D.J., Foley K.T., Morrow B.P., Wong P., Kiehm K., Sin A. In vitro testing of lumbar disc arthroplasty devices. Open Spine J. 2014;6:9–25. [Google Scholar]
- 18.Erkan S., Rivera Y., Wu C., Mehbod A.A., Transfeldt E.E. Biomechanical comparison of a two-level Maverick disc replacement with a hybrid one-level disc replacement and one-level anterior lumbar interbody fusion. Spine J. 2009;9:830–835. doi: 10.1016/j.spinee.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Gaffey J.L., Ghanayem A.J., Voronov M.L., Havey R.M., Carandang G., Abjornson C. Effect of increasing implant height on lumbar spine kinematics and foraminal size using the ProDisc-L prosthesis. Spine J. 2010;35:1777–1782. doi: 10.1097/BRS.0b013e3181ebaa4d. [DOI] [PubMed] [Google Scholar]
- 20.Ha S.K., Kim S.H., Kim D.H., Park J.Y., Lim D.J., Lee S.K. Biomechanical study of lumbar spinal arthroplasty. J Korean Neurosurg Soc. 2009;45:169–175. doi: 10.3340/jkns.2009.45.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hitchon P.W., Eichholz K., Barry C., Rubenbauer P., Ingalhalikar A., Nakamura S. Biomechanical studies of an artificial disc implant in the human cadaveric spine. J Neurosurg Spine. 2005;2:339–343. doi: 10.3171/spi.2005.2.3.0339. [DOI] [PubMed] [Google Scholar]
- 22.Ingalhalikar A.V., Reddy C.G., Lim T.H., Torner J.C., Hitchon P.W. Effect of lumbar total disc arthroplasty on the segmental motion and intradiscal pressure at the adjacent level: an in vitro biomechanical study. J Neurosurg Spine. 2009;11:715–723. doi: 10.3171/2009.7.SPINE094. [DOI] [PubMed] [Google Scholar]
- 23.Kikkawa J., Cunningham B.W., Shirado O., Hu N., McAfee P.C., Oda H. Biomechanical evaluation of a posterolateral lumbar disc arthroplasty device. Spine J. 2010;35:1760–1768. doi: 10.1097/BRS.0b013e3181c87692. [DOI] [PubMed] [Google Scholar]
- 24.Kim S.H., Chang U.K., Chang J.C., Chun K.S., Lim T.J., Kim D.H. The changes in range of motion after a lumbar spinal arthroplasty with Charité in the human cadaveric spine under physiologic compressive follower preload: a comparative study between load control protocol and hybrid protocol. J Korean Neurosurg Soc. 2009;46:144–151. doi: 10.3340/jkns.2009.46.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moldavsky M., Neumann P., Klocke N., Hussain M., Bucklen B.S. In vitro analysis of circumferential joint replacement, including bilateral facet joint replacement with lateral lumber disc prosthesis: a parametric investigation of disc sizing. Eur Spine J. 2017;26:785–793. doi: 10.1007/s00586-016-4793-9. [DOI] [PubMed] [Google Scholar]
- 26.Panjabi M., Henderson G., Abjornson C., Yue J. Multidirectional testing of one- and two-level ProDisc-L versus simulated fusion. Spine J. 2007;32:1311–1319. doi: 10.1097/BRS.0b013e318059af6f. [DOI] [PubMed] [Google Scholar]
- 27.Panjabi M., Malcolmson G., Teng E., Tominaga Y., Henderson G., Serhan H. Hybrid testing of lumbar Charité discs versus fusions. Spine J. 2007;32:959–966. doi: 10.1097/01.brs.0000260792.13893.88. [DOI] [PubMed] [Google Scholar]
- 28.Takigawa T., Espinoza Orías A.A., An H.S., Gohgi S., Udayakumar R.K., Sugisaki K. Spinal kinematics and facet load transmission after total disc replacement. Spine J. 2010;35:1160–1166. doi: 10.1097/BRS.0b013e3181e5352d. [DOI] [PubMed] [Google Scholar]
- 29.Tsitsopoulos P.P., Wojewnik B., Voronov L.I., Havey R.M., Renner S.M., Zelenakova J. Effect of prosthesis endplate lordosis angles on L5–S1 kinematics after disc arthroplasty. Eur Spine J. 2012;21:585–591. doi: 10.1007/s00586-012-2271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voronov L.I., Havey R.M., Sjovold S.G., Funk M., Carandang G., Zindrick D. Kinematics of total facet replacement (TFAS-TL) with total disc replacement. SAS J. 2009;3:85–90. doi: 10.1016/j.esas.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong P. Biomechanical comparison of lumbar disc replacements [Dissertation]. University of Tennessee Health Science Center; 2009.
- 32.Chen W.M., Park C., Lee K., Lee S. In situ contact analysis of the prosthesis components of Prodisc-L in lumbar spine following total disc replacement. Spine J. 2009;34:716–723. doi: 10.1097/BRS.0b013e3181ae23d1. [DOI] [PubMed] [Google Scholar]
- 33.Choi J., Shin D.A., Kim S. Biomechanical effects of the geometry of ball-and-socket artificial disc on lumbar spine: a finite element study. Spine J. 2017;42:332–339. doi: 10.1097/BRS.0000000000001789. [DOI] [PubMed] [Google Scholar]
- 34.Choi J., Shin D.A., Kim S. Finite element analysis of a ball-and-socket artificial disc design to suppress excessive loading on facet joints: a comparative study with ProDisc. Int J Numer Meth Biomed Eng. 2019;35 doi: 10.1002/cnm.3214. [DOI] [PubMed] [Google Scholar]
- 35.DiMascio V., Bellini C.M., Galbusera F., Raimondi M.T., Brayda-Bruno M., Assietti R. Lumbar total disc replacement: a numerical study. J Appl Biomater Biomech. 2010;8:97–101. [PubMed] [Google Scholar]
- 36.Dooris A.P., Goel V.K., Grosland N.M., Gilbertson L.G., Wilder D.G. Load-sharing between anterior and posterior elements in a lumbar motion segment implanted with an artificial disc. Spine J. 2001;26:122–129. doi: 10.1097/00007632-200103150-00004. [DOI] [PubMed] [Google Scholar]
- 37.Goel V.K., Grauer J.N., Patel T.Ch., Biyani A., Sairyo K., Vishnubhotla S. Effects of Charité artificial disc on the implanted and adjacent spinal segments mechanics using a hybrid testing protocol. Spine J. 2005;30:2755–2764. doi: 10.1097/01.brs.0000195897.17277.67. [DOI] [PubMed] [Google Scholar]
- 38.Kim K.T., Lee S.H., Suk K.S., Lee J.H., Jeong B.O. Biomechanical changes of the lumbar segment after total disc replacement: Charite, Prodisc and Maverick using finite element model study. J Korean Neurosurg. 2010;47:446–453. doi: 10.3340/jkns.2010.47.6.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knapik G.G., Mendel E., Marras W.S. Use of a personalized hybrid biomechanical model to assess change in lumbar spine function with a TDR compared to an intact spine. Eur. Spine J. 2012;21:641–652. doi: 10.1007/s00586-011-1743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rundell S.A., Auerbach J.D., Balderston R.A., Kurtz S.M. Total disc replacement positioning affects facet contact forces and vertebral body strains. Spine J. 2008;33:2510–2517. doi: 10.1097/BRS.0b013e318186b258. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt H., Midderhoff S., Adkins K., Wilke H.J. The effect of different design concepts in lumbar total disc arthroplasty on the range of motion, facet joint forces and instantaneous center of rotation of a L4–5 segment. Eur Spine J. 2009;18:1695–1705. doi: 10.1007/s00586-009-1146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zander T., Rohlmann A., Bergmann G. Influence of different artificial disc kinematics on spine biomechanics. Clin Biomech. 2009;24:135–142. doi: 10.1016/j.clinbiomech.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Zhong Z.C., Chen S.H., Hung C.H. Load- and displacement-controlled finite element analyses on fusion and non-fusion spinal implants. Proc Inst Mech Eng H. 2009;223:143–157. doi: 10.1243/09544119JEIM476. [DOI] [PubMed] [Google Scholar]
- 44.Johnsen L.G., Brinckmann P., Hellum C., Rossvoll I., Leivseth G. Segmental mobility, disc height and patient-reported outcomes after surgery for degenerative disc disease – a prospective randomised trial comparing disc replacement and multidisciplinary rehabilitation. Bone Joint J. 2013;95-B:81–89. doi: 10.1302/0301-620X.95B1.29829. [DOI] [PubMed] [Google Scholar]