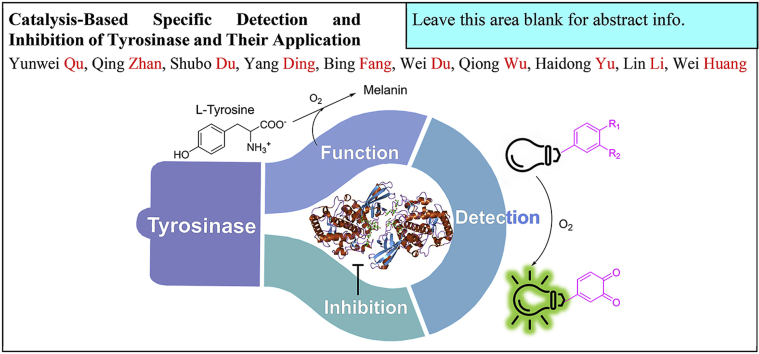
Abstract
Tyrosinase is an important enzyme in controlling the formation of melanin in melanosome, and plays a key role in the pigmentation of hair and skin. The abnormal expression or activation of tyrosinase is associated with several diseases such as albinism, vitiligo, melanoma and Parkinson disease. Excessive deposition of melanin could cause diseases such as freckles and brown spots in the human body, and it is also closely related to browning of fruits and vegetables and insect molting. Detecting and inhibiting the activity of tyrosinase is of extraordinary value in the progress of diagnosis and treatment of these diseases. Therefore, many selective optical detection probes and small molecular inhibitors have been developed, and have made significant contributions to the basic and clinical research on these diseases. In this paper, the detection and inhibition of tyrosinase and their application in whitening products are reviewed, with special emphasis on development of fluorescent probes and inhibitors. Hopefully, this review will help design more efficient and sensitive tyrosinase probes and inhibitors, as well as shed light on novel treatment of diseases such as melanoma.
Keywords: Tyrosinase, Melanin, Detection probe, Inhibitors, Melanoma
Graphical abstract
Highlights
-
•
The abnormal expression or activation of tyrosinase is the pathogenesis of several diseases such as albinism, vitiligo, and melanoma.
-
•
Detecting and inhibiting tyrosinase activity is of great value in the diagnosis and treatment of these diseases.
-
•
The detection/inhibition of tyrosinase and its application in whitening products are reviewed, with special emphasis on probes/inhibitors.
1. Introduction
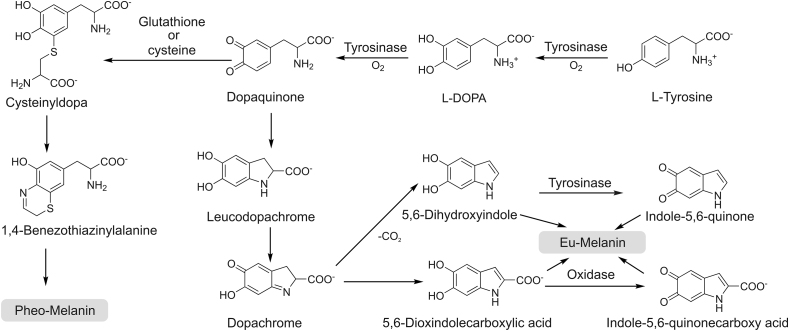
Tyrosinase (EC 1.14.18.1; catecholoxidase; polyphenol oxidase [1] or diphenolase) is considered as the most important copper-containing enzyme in melanin formation. Tyrosinase can catalyze the hydroxylation and subsequent oxidation of monophenol unit to orthoquinone under the action of molecular oxygen. It is found in melanosomes, the site for synthesis, storage and transport of melanin. The pH values of mildly and severely melanized melanosomes are about 4.5 and 3, respectively. The optimum pH for tyrosinase activity is 6.8 [2]. Raper [3] and Mason [4] were the first to clarify the biosynthetic pathways of melanin formation in various organisms, which were recently embellished by Schallreuter et al. [5] and Cooksey et al. (Fig. 1) [6].
Fig. 1.
The process of melanin biosynthesis catalyzed by tyrosinase.
Tyrosinase is widely found in plants, animals and microorganisms. Given the involvement of tyrosinase in the pathogenesis in melanoma, monitoring and pharmacologically regulating of its activity will help the diagnosis and treatment of the disease [7]. Probes were developed to specifically detect tyrosinase activity in physiological environment. Inhibitors that could make tyrosinase inactive were also developed, and some of them were used clinically to treat diseases. For example, kojic acid and arbutin [8], as specific inhibitors of tyrosinase, were clinically utilized as a whitening product. With advances in drug discovery, an increasing number of effective compounds that can detect/inhibit tyrosinase activity have been designed and synthesized recently. This would greatly facilitate the progress of diagnosing and treating those diseases resulted from aberrant melanin production. In this review, we will discuss the progress in the development of small-molecule based probes and inhibitors for endogenous tyrosinase activity. Hopefully, it will help us learn more about tyrosinase and find more functional compounds targeting/regulating tyrosinase.
2. Structure of tyrosinase
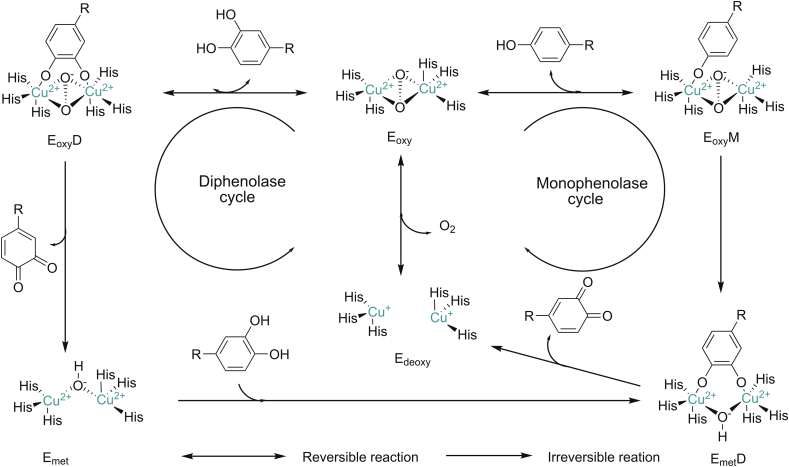
The tyrosinase active site presents a dual-core copper center structure composed of two copper ions (Fig. 2), which bind to histidine residues in the protein. The two copper ion centers are connected by an endogenous coordination bridge. Tyrosine and other substances are complexed with the enzyme, through the bond between the active center of the enzyme and the hydroxyl group. In the process of the catalytic reaction of melanin, the catalytic site is categorized into three forms: oxidation state (Eoxy), reduction state (Emet) and deoxygenation state (Edeoxy), the difference lies in the structure of binuclear copper ion active center (Fig. 2).
Fig. 2.
Eoxy/Emet/Edeoxy forms of oxidation state of tyrosinase. Monophenol cycle and bisphenol cycle catalytic mechanism. EoxyM: monophenolase Eoxy complex, EoxyD: diphenolase Eoxy complex, EmetD: diphenolase Emet complex.
Eoxy is composed of two square copper (II) atoms; each atom is made up of two strong equators and the ligand is one weaker axial NH [9]. The exogenous oxygen molecule binds and bridges the two copper centers in the form of peroxides. The Cu–Cu bond length is about 0.35 nm. The combination of oxygen molecules leads to the formation of (μ-η2:η2-peroxo) structure [10], so the Eoxy active center can be written as [Cu(II)–O2–Cu(II)]. The electronic structure of peroxides plays a crucial role in the biological functions of Eoxy. Due to the strong σ∗ acceptor action, the peroxide has less negative charge, while the π electron acceptor interacts with the electrons in the σ∗ orbital of the peroxide, which greatly weakens the strength of the oxygen-oxygen bond, rendering the nucleus of tyrosinase active center easily breakable [9]. Mettyrosinase (Emet) is similar to Eoxy, and also contains two tetragonal copper (II) atoms coupled through an endogenous bridge. The difference is that the bridging ligand between copper ions is hydroxide instead of peroxide [2]. In terms of oxidative properties, Emet and Eoxy are also slightly different. Emet is not able to oxidize monophenolic compounds. In the absence of substrates, Emet exists as the main form in organisms. Deoxytyrosinase (Edeoxy), similar to deoxyhemocyanin, has a symmetrical structure [(Cu(I)–Cu(I)]. There is no bridging ligand such as peroxide or hydroxide between binuclear copper; thus hydroxide in water is an essential bridging ligand.
3. Mechanism of the action of tyrosinase
Although researchers have conducted in-depth studies on tyrosinase and its related proteins, the mechanism of its catalytic reaction is still controversial. For example, the catalytic activity at the same active site of tyrosinase is different. The catalytic center of tyrosinase contains a binuclear copper center, named Cu(A) and Cu(B), respectively. Each copper ion in the active center is coordinated with three different histidine residues. There is a large difference between monophenolase activity and diphenolase activity, and reduced tyrosinase obviously has a lag phase when reacting with monophenol. These are important topics that need to be continuously explored and studied [11].
The reaction between tyrosinase and related substrates is mainly through the formation of an effective coordination bond between the hydroxyl group on the substrate and tyrosinase active center. Olivares et al. [12] proposed that Eoxy and suitable substrates in mammals trigger monophenolase activity and diphenolase activity. During monophenolase activity, monophenols (l-Tyrosine) are oxidized to form o-quinones (o-dopaquinone), an important precursor of melanin, and Edeoxy. During diphenolase activity, Eoxy and Emet can also oxidize o-diphenols (l-DOPA) to produce o-dopaquinone [13]. This mechanism is generally accepted by researchers as it can most accurately reflect the kinetic characteristics of tyrosinase, in which the rate-limiting step in melanin production is monophenol cycle [14].
3.1. Mechanism of monophenolase activity
During melanin synthesis, the main function of the enzyme is to oxidize monophenol substrates to o-quinone by Eoxy. This process is an important feature that distinguishes tyrosinase from other oxidoreductases such as catechol oxidase. During the monophenol cycle (Fig. 2), the oxygen atom on the deprotonated phenol is coordinated with the copper ion of the oxidized tyrosinase active center to form monophenolase Eoxy complex (EoxyM), and then the phenol is ortho-electrophilically substituted to diphenolase Emet complex (EmetD). EmetD undergoes a cleavage process to directly generate o-quinone and Edeoxy. Edeoxy directly combines with oxygen molecules to re-form Eoxy. This is the cyclic process of monophenolase activity. This process does not end until the substrate reaction is complete. In the reaction process of the monophenol cycle, if the Emet in the natural state meets a monophenol substrate, it will undergo an extremely slow oxidation reaction and hinder the normal progress of the monophenol reaction. Therefore, this period is called ‘lag period’ because Emet itself does not have the ability to bind oxygen molecules [12].
3.2. Mechanism of diphenolase activity
During the diphenolase activity, both Eoxy and Emet can react with o-diphenols to complete the bisphenol cycle. Eoxy can also oxidize bisphenol while oxidizing monophenol, and even shows a higher activity than the corresponding monophenol oxidation process, which may reflect the difficulty of direct binding of different substrates to the active site. The difference is that monophenol is more likely to complex with Cu(A), while bisphenol preferably binds to Cu(B) first [15]. There are two reaction processes in the oxidation process of bisphenol (Fig. 2). They can be deprotonated by adjacent hydroxyl groups so that oxygen can be connected to two copper atoms, thereby completing coordination with the active site. During the reaction between Eoxy and catechol (Fig. 2), the enzyme is reduced to produce Emet, which still maintains the state of divalent copper ions. During the reaction between Emet and catechol (Fig. 2), the active site copper ions of the enzyme change from divalent to monovalent. As a result of the bisphenol cycle, the catechol/enzyme complex is decomposed from the bound oxygen molecules to generate the corresponding o-quinone and water oxygen atoms to dissolve the peroxy conformation [16].
4. Function of tyrosinase
Tyrosinase is part of the type 3 copper family and exists in the early process of melanin formation. It mainly participates in the following two reaction processes [17]: (1) hydroxylation of l-tyrosine to l-DOPA; (2) oxidation of l-DOPA to form dopaquinone. Dopaquinone will eventually form melanin through a series of reactions. The other two family members of the type 3 copper family are catechol oxidase and hemocyanin. Catechol oxidase only exhibits diphenolase activity and hemocyanin is on the lymph of many mollusks and arthropods oxygen carrier. Although the active centers of the type 3 copper family proteins are conserved in terms of total structure and ability to connect oxygen molecules, their potential activities of enzymatic are slightly distinct due to the variability of the substrate’s attachment to the enzyme center or the uncontrollability that the substrate can reach.
In addition to participating in the process of melanin production, tyrosinase also has other important physiological functions. In sponges, plants and some invertebrates, tyrosinase is mainly involved in the process of wound healing and primary immune response [18]. In arthropods, tyrosinase can promote the hardening process of the stratum corneum of animals after molting. Bacterial tyrosinase can be secreted in soil and participate in the process of random coupling of different aromatic compounds to form humus. And, it was discovered that tyrosinase can also be a potential antidote for benzene toxic substances [13,19]. In addition, it plays an indispensable role in killing parasitic plants against phenolic symbiotic bacteria, the production of natural pigments and the synthesis of amino acid antibiotics such as lincomycin. The common point of these effects is inseparable from the tyrosinase using redox reactions with oxygen molecules [11].
5. Tyrosinase-related diseases
The distribution of tyrosinase is closely connected with the physiological functions of plants and animals. It is generally believed that the colors of feathers, hair, eyes, insect epidermis, seeds and other pigments are the results of tyrosinase [10]. Tyrosinase has varying but important functions in different organisms. In most insects under normal physiological conditions, tyrosinase exists in the form of zymogen, and different types of tyrosinase exist in specific parts of insects in order to complete specific physiological functions [20]. In addition to participating in the production of melanin, insect tyrosinase is the only enzyme involved in keratinosis. Insect-hardened keratin can block the invasion of microorganisms and foreign bodies and provide protection for the soft invertebrate body. In arthropods, tyrosinase also participates in two other important physiological processes, namely, defense response and wound healing. The melanin produced by tyrosinase in mammals is secreted into the keratinocytes of the epidermis and hair, discoloring the body surface, thereby protecting the skin and eyes, resisting ultraviolet radiation and preventing internal tissues from overheating [10]. Tyrosinase found in mammalian is commonly discovered in melanocytes, which are highly specific cells that exist in the skin, hair follicles, and eyes to produce pigments [4,21]. When tyrosinase function is reduced or missing, it will affect the melanin metabolism and cause diseases such as epilepsy and albinism. Autosomal recessive diseases in animals and humans are also related to the loss of tyrosinase or decreased activity [22].
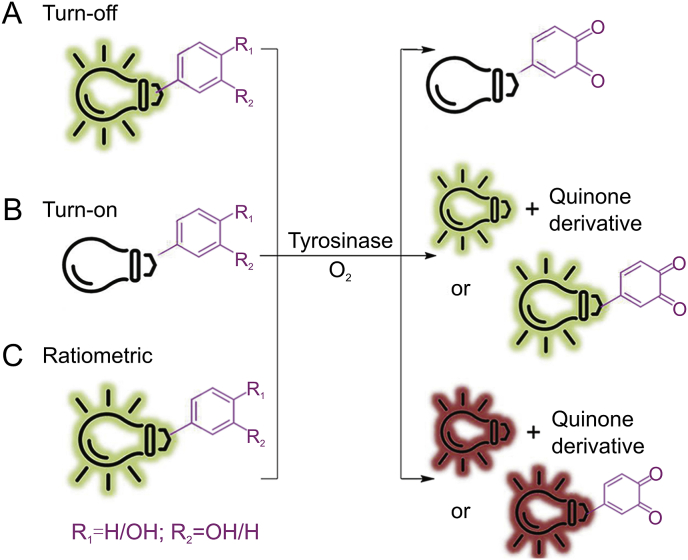
6. Probes of tyrosinase
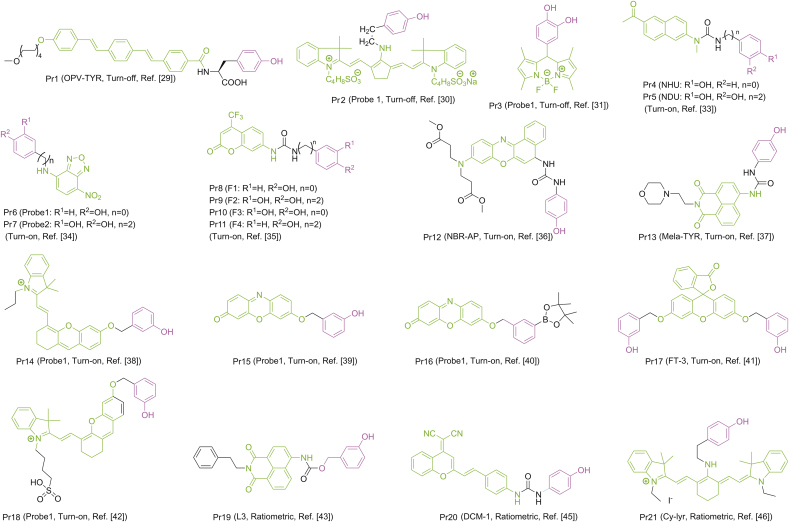
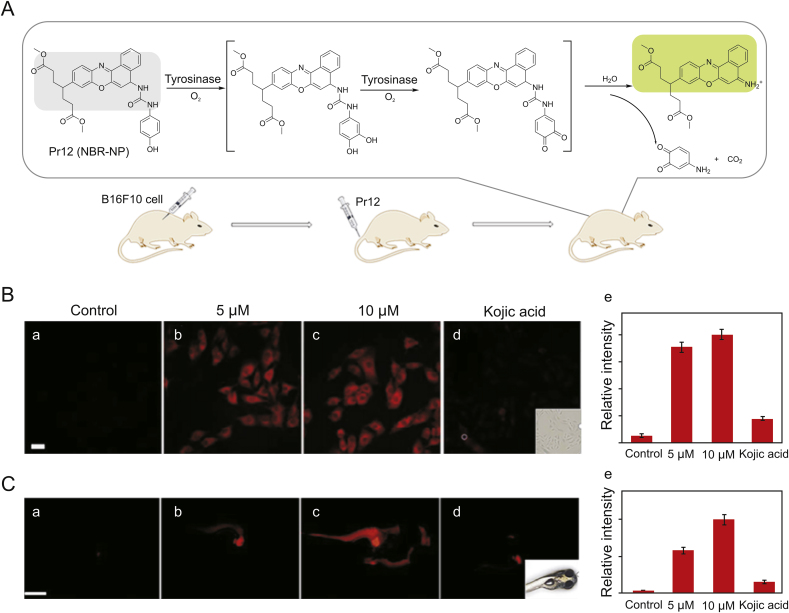
Probes are substances that specifically recognize the target and release detectable signals that reflect the presence and activity of the target. The traditional colorimetric method for tyrosinase analysis has been limited due to their low sensitivity [23]. At first, several other detection methods based on electrochemistry and gold nanoparticles were reported by Willner’s group [[24], [25], [26], [27]], which not only increases the versatility of detection, but also greatly updates the colorimetry in terms of sensitivity. Fluorescent strategy has also been introduced to design highly sensitive tyrosinase probes as shown in Fig. 3. Initially developed quantum dots and conjugated polymer fluorescent probes may be applied to monitor activity of tyrosinase [28]. Nevertheless, small-molecule fluorescent probes are particularly attractive because of their special advantages such as sensitivity, specificity and compatibility. In 2008, Zhu’s team synthesized a new water-soluble oligo (phenylenevinylene) (Pr1) as a fluorophore containing tyrosine war head (WH) as a fluorescent probe for tyrosinase. It was demonstrated that Pr1 is suitable for detecting activity of tyrosinase so far in aqueous buffer solution even in agarose gel [29]. First a cyanine-based near-infrared (NIR) fluorescent probe (Pr2) was used to monitor activity of tyrosinase in 2010 by Ma et al. [30]. The significant color change before and after the reaction could be detected by the naked eyes. However, these probes also show a turn-off mode caused by the quinone moiety generated by tyrosinase-catalyzed oxidation. For functional use, however, the best approach is to implement a bioassay in the turn-on mode because of its sensitivity and more suitability for bioimaging of tyrosinase in living systems. In 2010, Kim et al. [31] proposed a BODIPY-based turn-on fluorogenic probe to detect endogenous tyrosinase activity in live melanoma cells (Pr3, Fig. 4). Based on previous research of tyrosinase applied to remove the amines protecting groups [32], Yan et al. [33] prepared tyrosinase probes, Pr4 and Pr5, in which a phenol group and a naphthylamine group were connected through a urea linkage in 2012. Importantly, Pr4 was the first two-photon turn-on fluorogenic probe designed to detect the activity of tyrosinase in aqueous buffer and living cells. In 2013, Wang et al. [34] showed that the NBD-NH2 based fluorescent probe (Pr6 and Pr7) containing phenol moieties (WH) can be used to detect the activity of tyrosinase and screen for potential tyrosinase inhibitors with a “turn-on” strategy. However, there is no biological experiment related to cell imaging in this work. In 2016, Li and colleagues designed a group of new probes based on 7-amino-4-(trifluoromethyl)-coumarin as a fluorophore and synthesized them, Pr8-11, with varying distance between fluorophore and phenols. Pr9 was found to be a highly sensitive and selective “turn on” fluorogenic probe for imaging live melanoma cells [35]. In 2018, Wu’s team was the first to make use of a fluorescent probe (Pr12) to diagnose early melanoma in rodent mouse models (Fig. 4, Fig. 5A). The probe can be activated by tyrosinase-mediated oxidation and then hydrolyze urea bonds to produce fluorescence signal. At the same time, it can also monitor the level of endogenous tyrosinase in living cells and zebrafish sensitively and selectively (Fig. 5 B/C) [36].
Fig. 3.
Schematic representation of three main types of probes for tyrosinase activity detection.
Fig. 4.
Structures of tyrosinase fluorescent probes; fluorophore and WH are in green and purple, respectively.
Fig. 5.
(A) Schematic diagram for detection of tyrosinase by probe Pr12 and melanoma imaging in the mouse model. (B) Images of B16F10 cells with different treatments (a-d); (e) relative intensity values obtained from panels (a-d) in B. (C) Images for 3-day-old zebrafishes with different treatments (a-d); (e) relative intensity values obtained from panels (a-d) in C. Reproduced with permission from Ref. [36]. Copyright 2018 American Chemical Society.
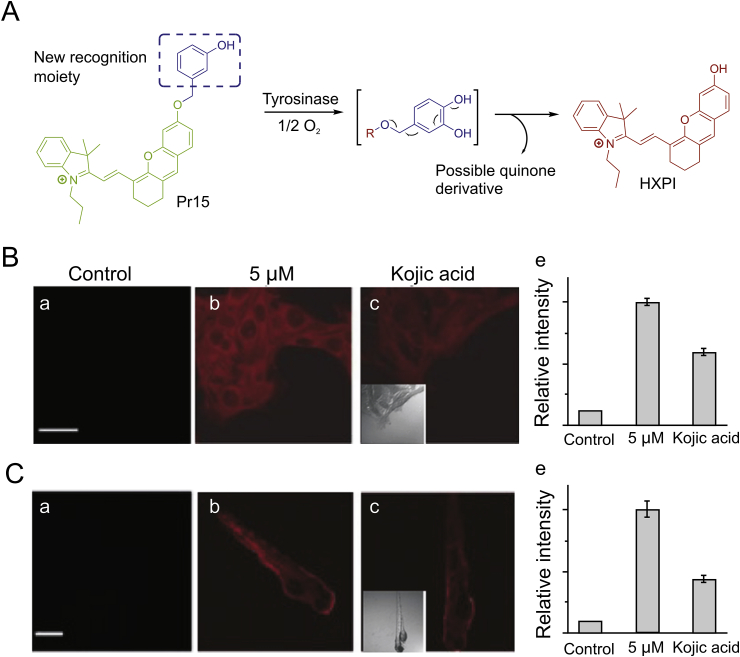
In 2016, Ma’s group [37] developed a novel fluorogenic probe named Mela-TYR (Pr13, Fig. 4) to target melanosome for detecting activity of tyrosinase. Pr13 was designed by incorporating naphthalimide with morpholine and 4-amino-phenol-derived urea. The probe shows a highly sensitive and selective turn-on response to tyrosinase through an oxidization-cleavage reaction. The fluorescence probes described above mainly contained 4-hydroxyphenyl group as recognition moiety (WH) and showed parallel fluorescence response to a number of reactive oxygen species (ROS) and tyrosinase, thus interfered by ROS. Ma’s group discovered a new tyrosinase-recognition moiety, 3-hydroxybenzyloxy (WH), which showed diverse reaction mechanisms for tyrosinase and ROS [38]. An NIR fluorescence probe (Pr14, Fig. 4) was developed by installing 3- hydroxybenzyloxy into an NIR fluorophore (HXPI), and showed a highly specific off-on response to tyrosinase instead of ROS, thus overcoming the interference. The presence of the 3-hydroxy group facilitates the hydroxylation by tyrosinase at the 4-position vacancy but not by ROS, and the intermediate would undergo spontaneous 1,6-rearrangement–elimination, releasing free fluorophore. The high specificity of the developed probe was proved by imaging and detection of endogenous tyrosinase activity in living cells and zebrafishes, and the high specificity of the probe was further verified by an enzyme-linked immunosorbent assay (Fig. 4, Fig. 6). Ma’s group subsequently developed another turn-on fluorogenic probe (Pr15, Fig. 4) based on resorufin incorporated with a 3-hydroxyphenyl group [39]. It was used to detect and image the activity of endogenous tyrosinase in a variety of living cells. Inspired by the above design, Zhang and co-workers [40] proposed a fluorogenic tyrosinase probe (Pr16, Fig. 4) with resorufin as fluorophore, and the m-tolylboronic acid pinacol ester (WH) as a new tyrosinase-recognition moiety. The probe showed high selectivity for tyrosinase over other biological substances, including ROS. However, it was severely interfered by H2O2. In 2019, Hu et al. [41] reported a new fluorogenic probe with high chemical selectivity based on fluorescein (Pr17, Fig. 4), which can track tyrosinase in vitro and in vivo, and realize the high chemoselective detection of tyrosinase. In addition, the probe reacted in aqueous solution and exhibited a fluorescence enhancement of more than 24 times in the presence of tyrosinase. In addition, Pr17 showed great cell membrane and tissue permeability characteristics, which helped its success in following endogenous tyrosinase activity in distinct helped living cells and zebrafish models. Ding’s group constructed a novel water-soluble NIR fluorescence probe (Pr18, Fig. 4) which can specifically recognize tyrosinase, which is highly stable within physiological temperature and pH, and can accurately detect tyrosinase in biological systems without being disturbed by ubiquitous entities. It can be used for imaging tyrosinase in living cells, zebrafishes and xenogeneic mouse model [42].
Fig. 6.
(A) The proposed reaction mechanisms of Pr14 with tyrosinase. (B) Confocal fluorescence images of B16 cells with different treatments (a-c); (d) relative intensity values obtained from panels (a-c) in B. (C) Fluorescence images of 3-day-old zebrafishes with different treatments (a-c); (d) relative intensity values obtained from panels (a-c) in C. Reproduced with permission from Ref. [38]. Copyright 2016 John Wiley and Sons.
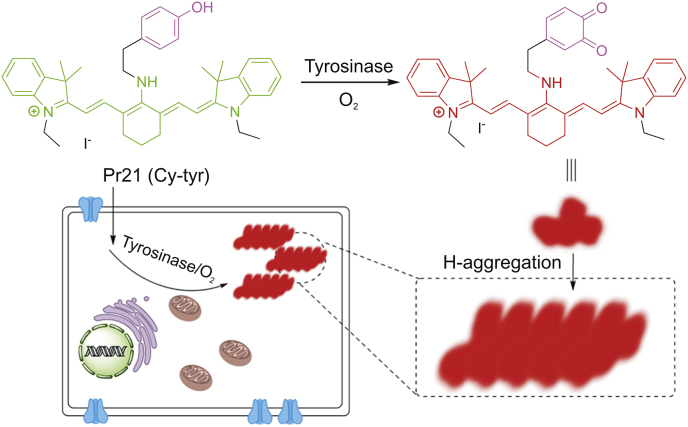
Sidhu et al. devised and synthesized a ratiometric fluorescent probe (Pr19, Fig. 4) based on naphthalimide. Pr19 has high selectivity and sensitivity for tyrosinase, and the limit of detection (LOD) is quite low [43]. The excitation or emission spectrum shift occurs after the probe is combined with reactants. It can be recorded using the ratio of the fluorescence intensity measured at two different wavelengths, which is called ratio measurement. Ratiometric fluorescent probes based on this principle show their sensitivity and selectivity, and can be used for the study of enzyme function in live system [44]. Guo’s team proposed a new ratiometric and turn-on NIR fluorescent probe (Pr20, Fig. 4) for real-time detection of endogenous tyrosinase activity. These special characteristics of Pr20, combined with rare cytotoxicity, superior photophysical characters and cell membrane permeability, make it ideal for quantitative detection of endogenous tyrosinase activity [45]. Cyanine derivatives, as typical NIR fluorescent dyes, can be controllably aggregated in aqueous solutions and thus exhibit many significantly different spectral properties. The chlorine atom in the center of the cyanine skeleton is easily substituted by other functional groups. Based on this function, Zhang et al. [46] developed a new cyanine-based fluorescent probe (Pr21, Fig. 4) for the ratiometric fluorescence detection of tyrosinase activity (Fig. 7). The ratio determination obtained a well signal-to-noise ratio, and the LOD value of tyrosinase activity was 0.02 U/mL. In addition, Pr21 was successfully employed in imaging the endogenous tyrosinase activity in B16 cells and qualitatively distinguishing it from other cancer/normal cells in the absence of tyrosinase (Fig. 7).
Fig. 7.
The proposed sensing mechanism for the tyrosinase induced enzymatic activation of Pr21.
7. Inhibitors of tyrosinase
Inhibitors are generally divided into reversible inhibitors and irreversible inhibitors based on whether the inhibitors interacting with enzymes cause permanent inactivation of the enzyme. The inhibition characteristic of tyrosinase is reversible inhibition. For the inhibition characterized by reversible inhibition, the combination of inhibitor and enzyme is a reversible dynamic equilibrium process [[47], [48], [49]]. Increasing the concentration of the inhibitor will cause the enzyme activity to decrease but the inhibitor only inhibits the enzyme activity rather than permanently inactivates the enzyme. When the inhibitor concentration decreases, the tyrosinase activity will increase. Meanwhile, the irreversible inhibition will be permanent inactivation of tyrosinase. According to the different sites and methods of tyrosinase inhibitors interacting with the enzyme, they can be divided into four forms: competitive, noncompetitive, mixed and slow binding.
Tyrosinase inhibitors extracted from natural resources have attracted wide attention from researchers because of their lower toxicity and better bioavailability. Natural substances including bacteria, plants and fungi have gradually become a research hotspot to extract tyrosinase inhibitors. Phenolic chemical compounds are the most abundant compounds that can be extracted from plants, so many plants have become sources of tyrosinase inhibitors [50]. Researchers have reported the tyrosinase inhibitory activity of the following plant extracts: Morus nigra L [51], Artemisia aucheri Boiss [52], Vitis vinifera leaf extracts [53], Mangifera indica [54], Cassia tora [51], and Arctostaphylos uva-ursi [55].
Flavonoids are a set of compounds composed of two benzene rings connected by a three-carbon chain. Because the hydroxyl, methoxy and glycoside side-chain groups are in the benzene rings, the arrangement can be subdivided into flavanols, chalcones, dihydroflavones, and orangeones (Fig. 8) [56]. Flavonoids are extensively distributed in the leaves, seeds, skins and flowers of plants, and researchers have verified more than 4000 flavonoids. For some plants, flavonoids and their derivatives have protection against UV rays, pathogens, and herbivores [57]. Analysis of the structure of licorice root extract shows that the tyrosinase inhibitory ability of neoglycyrrhizin, glycyrrhetin, isoliquiritigenin and glycyrrhizin is related to their lipophilicity. Among them, the inhibition of monophenol is more effective than that of diphenol, indicating that it is a rate-limiting reaction in the first step of the oxidation reaction [58].
Fig. 8.
Structure of the main classes of flavonoids.
Some flavones containing a 3-hydroxy-4-ketone structure can competitively inhibit enzyme activity by chelating copper at the active site of tyrosinase, resulting in irreversible inactivation of tyrosinase. After chelating tyrosinase, the molecule theoretically loses its planar structure and becomes distorted. Competitive inhibitors are usually parallel in structure to the substrate, so the molecule easily enters the tyrosinase active site and prevents the entry of l-DOPA [59,60]. Jeong et al. extracted two flavonols from the leaves of Zanthoxylum piperitum. The flavonols can inhibit the tyrosinase activity of mushrooms, which is a competitive inhibition, but it cannot inhibit the melanin production of Streptomyces bikiniensis. Later, it was found that the flavonoid compound isolated from the Philippine Formosa can also inhibit tyrosinase, and the inhibitory effect is better than that of kojic acid [62]. Liang et al. found that safflower yellow pigment can also inhibit mushroom tyrosinase activity, with a half-maximal inhibitory concentration (IC50) value of 1.01 mg/mL. This inhibition relationship appears to be dose-dependent [63]. (2R, 3R)-(+)-purpurin extracted from Shuiliao inhibits 70% of tyrosinase activity and the concentration is 0.50 mM. The inhibitory ability is better than that of kojic acid and arbutin [64].
7,8,4′-trihydroxyflavone is a flavonoid derivative that inhibits tyrosinase diphenolase activity in a noncompetitive way with an IC50 value of 10.31 ± 0.41 μM and a Ki of 9.50 ± 0.40 μM. The mechanism of action of this compound with tyrosinase is a static mechanism and shows a single binding site with a binding constant of (7.05 ± 1.20) × 104 M−1 at 298 K. Thermodynamic parameters show that the bonding process is related to hydrogen bonding and van der Waals forces [51].
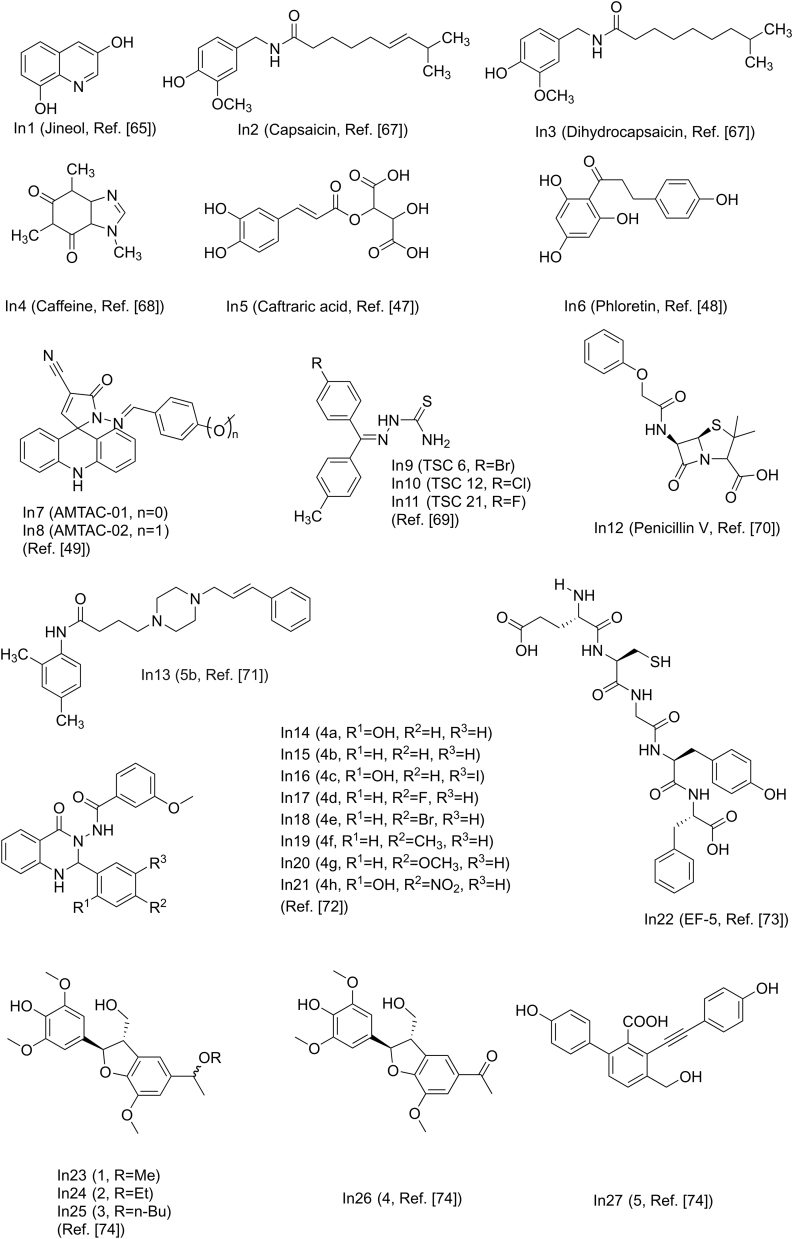
3,8-dihydroxyquinoline (In1, Fig. 9 [65]) separated from Scolopendra subspinipes mutilans can inhibit the production and oxidation of melanin in Melan-a cells. In1 shows a concentration-developed antioxidant effect and significantly inhibits mushroom tyrosinase activity through noncompetitive inhibition. At the same time, In1 is found to be non-cytotoxic in the study as shown in Table 1 [65]. The ethyl acetate fraction of Nymphaea nouchali flower extract (NNFE) (100 μg/mL) can effectively reduce melanin production and inhibit mushroom tyrosinase activity. The underlying mechanism involves interfering with transcription factors and universal signaling pathways in melanin synthesis [66]. Capsaicin (In2, Fig. 9 [67]) and dihydrocapsaicin (In3, Fig. 9) extracted from pepper can inhibit tyrosinase activity. The result show that the IC50 value of In2 is 1.73 times smaller than that of In3. The inhibition constant (Ki) also supports that the inhibitory activity of In3 (0.39 mM, Table 1) on tyrosinase is lower than that of In2 (0.30 mM, Table 1) [67]. It was found that caffeine (In4, Fig. 9 [68]) extracted from camellia pollen shows strong inhibitory activity towards mushroom tyrosinase in a non-competitive model as shown in Table 1. In4 changes the binding site of l-tyrosine and the ring conformation adjacent to the active center by binding to tyrosinase. The experimental results show that In4 has an obvious inhibitory effect on the tyrosinase activity in the cells and melanin generation of B16F10 melanoma cells is related to the concentration [68]. The caftaric acid (In5, Fig. 9) extracted from grapes can competitively inhibit tyrosinase, and the IC50 value (Table 1) is lower than that of the relational compounds, caffeic and chlorogenic acids [47]. Phloretin (In6, Fig. 9) can bind to tyrosinase through a static process, which causes the conformation of tyrosinase to change, thereby inhibiting its activity. At the same time, In6 has a strong antioxidant capacity and the ability to reduce o-dopaquinone to l-DOPA [48].
Fig. 9.
Structure of the main inhibitors of tyrosinase.
Table 1.
IC50 value and inhibition type of some inhibitors. NA = Not available.
| Ref. | Inhibitor | IC50 | Type |
|---|---|---|---|
| [65] | In1(jineol) | 39.46 ± 0.01 μM L-Tyrosine |
Noncompetitive |
| 50.35 ± 0.05 μM L-DOPA |
Noncompetitive | ||
| [67] | In2(Capsaicin) | 0.79 ± 0.011 mM | Competitive |
| In3(Dihydrocapsaicin) | 1.37 ± 0.16 mM | Mixed type | |
| [68] | In4(Caffeine) | 18.5 ± 23.1 μg/mL | Noncompetitive |
| [47] | In5(Caftaric acid) | 30 μM | Competitive |
| [48] | In6(Phloretin) | 169.36 μM | Mixed type |
| [49] | In7(AMTAC-01) | 189.40 μM | Noncompetitive |
| In8(AMTAC-02) | 96.29 μM | Mixed type | |
| [69] | In9(TSC-6) | 0.5 μM | Competitive |
| In10(TSC-12) | 0.9 μM | Competitive | |
| In11(TSC-21) | 0.8 μM | Competitive | |
| [70] | In12(Penicillin V) | 16.6 ± 0.5 mM monophenolase | Mixed type |
| 11.0 ± 0.2 mM diphenolase | Mixed type | ||
| [71] | In13(5b) | 0.013 ± 0.001 μM | Noncompetitive |
| [72] | In14(4a) | 1.071 ± 0.881 μM | NA |
| In15(4b) | 0.411 ± 0.012 μM | NA | |
| In16(4c) | 1.419 ± 0.962 μM | NA | |
| In17(4d) | 0.006 ± 0.074 μM | Noncompetitive | |
| In18(4e) | 1.609 ± 0.324 μM | NA | |
| In19(4f) | 0.057 ± 0.096 μM | NA | |
| In20(4g) | 0.239 ± 0.054 μM | NA | |
| In21(4h) | 0.347 ± 0.051 μM | NA | |
| [73] | In22(EF-5) | 0.46 mM | NA |
| [74] | In23(1) | 5.75 ± 0.16 μM | NA |
| In24(2) | 11.86 ± 0.09 μM | NA | |
| In25(3) | 30.37 ± 0.21 μM | NA | |
| In26(4) | 67.76 ± 0.13 μM | NA | |
| In27(5) | 23.79 ± 0.11 μM | NA |
Researchers have evaluated two spiroacridines (AMTAC-01, In7, Fig. 9) and (AMTAC-02, In8, Fig. 9) as inhibitors of tyrosinase. The results show that acridine derivatives interact strongly with mushroom tyrosinase. In8 is more effective in inhibiting enzyme activity than In7 as shown in Table 1, which indicates that the methoxy group of In8 is highly correlated with the inhibitory activity [49]. A number of 21 halogenated thiosemicarbazones (TSCs) were synthesized and investigated. It was found that TSCs 6, 12, and 21 (In9/10/11, Fig. 9 [69]) exhibit the potent inhibitory properties with different IC50, respectively (Table 1). They demonstrate a mutually reversible and competitive mechanism to inhibit tyrosinase. Among the compounds studied, para-substituted acetophenone derivatives of thiosemicarbazones have the highest affinity to the enzyme [69]. Penicillin V (In12, Fig. 9) is a bacteriolytic β-lactam antibiotic drug. Studies have found that In12 can inhibit monophenolase and diphenolase activity. Fluorescence quenching and molecular docking studies have shown that In12 can form a static interaction near the catalytic pocket of the enzyme, thereby hindering substrate transport to the active site and reducing the plasticity of copper for catalysis [70]. Recently, Raza et al. studied the inhibitory potential of N-(substituted-phenyl)-4-{(4-[(E)-3-phenyl-2-propenyl]-1-piperazinyl) butanamides (5a-e) on tyrosinase. All compounds were found to be biologically active, with 5b (In13, Fig. 9) showing the highest inhibitory potential [71]. Mahajan et al. designed and synthesized quinazolinone ben zamides 4a-h (In14–21, Fig. 9). Through the study of the inhibitory effect of compounds on the activity of tyrosinase, it was found that all compounds show lower IC50 values than standard kojic acid as shown in Table 1 [72]. Shen et al. found a new tyrosinase inhibitor, the peptide ECGYF (EF-5, In22) as shown in Fig. 9. The binding between In22 and tyrosinase mainly depends on hydrogen bonding and hydrophobic interactions, and the effect of inhibiting tyrosinase is stronger than that of arbutin and glutathione [73]. He et al. isolated three tamariscinols 1/2/3 (In23/24/25) and two phenolics 4 & 5 (In26 & 27) as shown in Fig. 9. Experiments have shown that all isolates have inhibitory effects on mushroom tyrosinase, with In23 being the most effective one (Table 1) [74].
8. Application of tyrosinase
As an important biological resource, tyrosinase has a wide range of uses in the field of environmental engineering and many important physiological functions in the body. In addition, in combination with immobilization [75], biosensor and other technologies, the use of tyrosinase for catalytic oxidation, treatment of industrial wastewater and detection of compounds has gradually become a focus of research in the fields of environmental protection and biological detection.
8.1. Environmental protection
Tyrosinase can catalyze the oxidation of monophenolic compounds. Wada et al. [76] revealed that the rate of tyrosinase removal of substituted phenols in aqueous solutions follows the order of catechol > pcresol > p-chlorophenol > phenol > p-methoxyphenol. Tyrosinase can remove not only phenols but also various organic substances such as organic amines, which eventually form a precipitate and can be easily processed. Therefore, tyrosinase in microorganisms can be used in environmental engineering fields such as factories and hospitals to degrade and treat wastewater containing phenol and amine [77]. With the continuous exploration of the treatment process, the reaction conditions have gradually been optimized. Yamada et al. found that the combination of tyrosinase and chitosan has a better effect on removing phenolic compounds in artificial wastewater. Tyrosinase catalyzes the oxidation of phenolic compounds into quinone derivatives, which are subsequently chemisorbed onto the chitosan membrane. Some alkyl-substituted phenols, such as p-methylphenol, p-propylphenol, p-butylphenol and p-chlorophenol, have removal rates of up to 93% [78]. If the amino group of tyrosinase was fixed on the cation exchange resin, it could completely remove phenol after 2 h with hardly weakened activity for 10 cycle reuses [71]. Fixed on modified sodium aluminosilicate (NaA) and calcium aluminosilicate (CaA), tyrosinase can also be used multiple times without any decrease in activity [79]. Furthermore, the complex formed by nanomaterials and polyphenol oxidase can effectively reduce the disadvantages of traditional enzymes in treating wastewater [80].
8.2. Biological detection
Biosensor, as an emerging technology for biological detection, is an analytical device that immobilizes enzymes, DNA, antibodies, cells, etc. as molecular recognition substances on a conductor and converts chemical or thermal changes, etc. into electrical signals. It is widely used in the fields such as food industry, environmental engineering, fermentation engineering and medicine, because of its sensitivity, specificity, traceless, speediness and accuracy. Wu et al. [81] quickly detected bisphenol A using nano-scale graphene as the basic tyrosinase biosensor. Yang et al. [82] developed a new tyrosine biosensor based on a chitosan carbon-coated nickel composite film, which was used to detect catechol due to the characteristics of fast, reusable and good stability. Jiang et al. [83] by using layer-by-layer assembly technology, created an immobilized capillary tyrosinase reactor to screen for tyrosinase inhibitors. Singh et al. [84] proposed a fiber-optic biosensor based on surface plasmon resonance to detect phenolic compounds in aqueous solutions.
9. Conclusion
Because tyrosinase is involved in the process of food browning and depigmentation disorders in humans, specific probes and inhibitors have been extensively studied by researchers. Effective compounds in natural sources such as plants have the potential to inhibit tyrosinase. Using probes to detect the tyrosinase activity mechanism provides an effective means for studying the tyrosinase activity mechanism and the screening tyrosinase inhibitors. However, the currently developed probes need to be optimized due to the poor biocompatibility and stability. This article summarizes many natural, semisynthetic and synthetic inhibitors and discusses the inhibitory effects of these compounds on the activity of tyrosinase. Based on the review, despite the wide variety of natural inhibitors, phenol-unit is still a major part of many tyrosinase inhibitors. Suitable scaffolds have been designed by many researchers based on those structures of natural compounds, but newly developed inhibitors need more efforts in the future. With the development of chemical biology, more and more probes and inhibitors have better biological characteristics, which will promote our research on tyrosinase.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (81672508, 21675085), Jiangsu Provincial Foundation for Distinguished Young Scholars (BK20170041, BK20170042), the Joint Research Funds of Department of Science & Technology of Shaanxi Province and Northwestern Polytechnical University (2020GXLH-Z-008, 2020GXLH-Z-023), the Natural Science Basic Research Program of Shaanxi (Program No. 2019JM-016), Key Research and Development Program of Shaanxi (2020ZDLGY13-04), Open Research Fund of Anhui Key Laboratory of Tobacco Chemistry (20181140), China-Sweden Joint Mobility Project (51811530018) and Fundamental Research Funds for the Central Universities.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
Shubo Du, Email: chmshu@nus.edu.sg.
Qiong Wu, Email: iamqwu@njtech.edu.cn.
Lin Li, Email: iamlli@njtech.edu.cn.
References
- 1.Seo S.Y., Sharma V.K., Sharma N. Mushroom tyrosinase: recent prospects. J. Agric. Food Chem. 2003;51:2837–2853. doi: 10.1021/jf020826f. [DOI] [PubMed] [Google Scholar]
- 2.Halaban R., Patton R.S., Cheng E. Abnormal acidification of melanoma cells induces tyrosinase retention in the early secretory pathway. J. Biol. Chem. 2002;277:14821–14828. doi: 10.1074/jbc.M111497200. [DOI] [PubMed] [Google Scholar]
- 3.Raper H.S. The aerobic oxidases. Physiol. Rev. 1928;8:245–282. [Google Scholar]
- 4.Mason H.S. Oxidases, Annu. Rev. 1965;34:595–634. doi: 10.1146/annurev.bi.34.070165.003115. [DOI] [PubMed] [Google Scholar]
- 5.Schallreuter K.U., Kothari S., Chavan B. Regulation of melanogenesis–controversies and new concepts. Exp. Dermatol. 2008;17:395–404. doi: 10.1111/j.1600-0625.2007.00675.x. [DOI] [PubMed] [Google Scholar]
- 6.Cooksey C.J., Garratt P.J., Land E.J. Evidence of the indirect formation of the catecholic intermediate substrate responsible for the autoactivation kinetics of tyrosinase. J. Biol. Chem. 1997;272:26226–26235. doi: 10.1074/jbc.272.42.26226. [DOI] [PubMed] [Google Scholar]
- 7.Chang T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funayama M., Arakawa H., Yamamoto R. Effects of α– and β–arbutin on activity of tyrosinases from mushroom and mouse melanom. Biosci. Biotechnol. Biochem. 1995;59:143–144. doi: 10.1271/bbb.59.143. [DOI] [PubMed] [Google Scholar]
- 9.Van Gastel M., Bubacco L., Groenen E.J.J. EPR study of the dinuclear active copper site of tyrosinase from Streptomyces antibioticus. FEBS Lett. 2000;474:228–232. doi: 10.1016/s0014-5793(00)01609-4. [DOI] [PubMed] [Google Scholar]
- 10.Matoba Y., Bando N., Oda K. A molecular mechanism for copper transportation to tyrosinase that is assisted by a metallochaperone, caddie protein. J. Biol. Chem. 2011;286:30219–30231. doi: 10.1074/jbc.M111.256818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Washington, Maxwell J., Stevenson J. Mechanistic studies of the tyrosinase–catalyzed oxidative cyclocondensation of 2–aminophenol to 2–aminophenoxazin–3–one. Arch. Biochem. Biophys. 2015;557-578:24–34. doi: 10.1016/j.abb.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olivares C., Solano F. New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigment Cell Melanoma Res. 2009;22:750–760. doi: 10.1111/j.1755-148X.2009.00636.x. [DOI] [PubMed] [Google Scholar]
- 13.Fenoll L.G., Rodríguez–López J.N., García–Sevilla F. Analysis and interpretation of the action mechanism of mushroom tyrosinase on monophenols and diphenols generating highly unstable o–quinones. Biochim. Biophys. Acta. 2001;1548:1–22. doi: 10.1016/s0167-4838(01)00207-2. [DOI] [PubMed] [Google Scholar]
- 14.Fairhead M., Thöny–Meyer L. Bacterial tyrosinases: old enzymes with new relevance to biotechnology. N. Biotech. 2012;29:183–191. doi: 10.1016/j.nbt.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 15.McMahon A.M., Doyle E.M., Brooks S. Biochemical characterisation of the coexisting tyrosinase and laccase in the soil bacterium Pseudomonas putida F6. Enzym. Microb. Technol. 2007;40:1435–1441. [Google Scholar]
- 16.Min K., Park G.W., Yoo Y.J. A perspective on the biotechnological applications of the versatile tyrosinase. Bioresour. Technol. 2019;289:121730. doi: 10.1016/j.biortech.2019.121730. [DOI] [PubMed] [Google Scholar]
- 17.Rolff M., Schottenheim J., Decker H. Copper–O2 reactivity of tyrosinase models towards external monophenolic substrates: molecular mechanism and comparison with the enzyme. Chem. Soc. Rev. 2011;40:4077–4098. doi: 10.1039/c0cs00202j. [DOI] [PubMed] [Google Scholar]
- 18.Marino S.M., Fogal S., Bisaglia M. Investigation of Streptomyces antibioticus tyrosinase reactivity toward chlorophenols. Arch. Biochem. Biophys. 2011;505:67–74. doi: 10.1016/j.abb.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Park J.W., Dec J., Kim J.E. Dehalogenation of xenobiotics as a consequence of binding to humic materials. Arch. Environ. Contam. Toxicol. 2000;38:405–410. doi: 10.1007/s002449910054. [DOI] [PubMed] [Google Scholar]
- 20.Funatsu M., Inaba T. Studies on tyrosinase in housefly. Agric. Biol. Chem. 1962;26:535–540. [Google Scholar]
- 21.Zimmerman W.C., Blanchette R.A., Burnes T.A. Melanin and perithecial development in ophiostoma piliferum. Mycologia. 1995;87:857–863. [Google Scholar]
- 22.Goto M., Sato K.C., Matsumura, Sawamura D. Tyrosinase gene analysis in Japanese patients with oculocutaneous albinism. J. Dermatol. Sci. 2004;35:215–220. doi: 10.1016/j.jdermsci.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Gauillard F.N.J., Richard F., Gauillard Forget. New sprectrophotometric assay for polyphenol oxidase activity. Anal. Biochem. 1993;215:59–65. doi: 10.1006/abio.1993.1554. [DOI] [PubMed] [Google Scholar]
- 24.Li D., Gill R., Freeman R. Probing of enzyme reactions by the biocatalyst–induced association or dissociation of redox labels linked to monolayer–functionalized electrodes. Chem. Commun. 2006:5027–5029. doi: 10.1039/b614141b. [DOI] [PubMed] [Google Scholar]
- 25.Baron R., Zayats M., Willner I. Dopamine–, L-DOPA–, adrenaline–, and noradrenaline–induced growth of Au nanoparticles: assays for the detection of neurotransmitters and of tyrosinase activity. Anal. Chem. 2005;77:1566–1571. doi: 10.1021/ac048691v. [DOI] [PubMed] [Google Scholar]
- 26.Freeman R., Elbaz J., Gill R. Analysis of dopamine and tyrosinase activity on ion–sensitive field-effect transistor (ISFET) devices. Chemistry. 2007;13:7288–7293. doi: 10.1002/chem.200700734. [DOI] [PubMed] [Google Scholar]
- 27.Yildiz H.B., Freeman R., Gill R. Electrochemical, photoelectrochemical, and piezoelectric analysis of tyrosinase activity by functionalized nanoparticles. Anal. Chem. 2008;80:2811–2816. doi: 10.1021/ac702401v. [DOI] [PubMed] [Google Scholar]
- 28.Gill R., Freeman R., Xu J.P. Probing biocatalytic transformations with CdSe–ZnS QDs. J. Am. Chem. Soc. 2006;128:15376–15377. doi: 10.1021/ja066636t. [DOI] [PubMed] [Google Scholar]
- 29.Feng X., Feng F., Yu M. Synthesis of a new water–soluble oligo (phenylenevinylene) containing a tyrosine moiety for tyrosinase activity detection. Org. Lett. 2008;10:5369–5372. doi: 10.1021/ol802210s. [DOI] [PubMed] [Google Scholar]
- 30.Li X., Shi W., Chen S. A near–infrared fluorescent probe for monitoring tyrosinase activity. Chem. Commun. 2010;46:2560–2562. doi: 10.1039/c001225d. [DOI] [PubMed] [Google Scholar]
- 31.Kim T.-I., Park J., Park S. Visualization of tyrosinase activity in melanoma cells by a BODIPY–based fluorescent probe. Chem. Commun. 2011;47:12640–12642. doi: 10.1039/c1cc15061h. [DOI] [PubMed] [Google Scholar]
- 32.Osborn H.M.I., Williams N.A.O. Development of tyrosinase labile protecting groups for amines. Org. Lett. 2004;6:3111–3113. doi: 10.1021/ol040042i. [DOI] [PubMed] [Google Scholar]
- 33.Yan S., Huang R., Wang C. A two–photon fluorescent probe for intracellular detection of tyrosinase activity. Chem. Asian J. 2012;7:2782–2785. doi: 10.1002/asia.201200762. [DOI] [PubMed] [Google Scholar]
- 34.Wang C., Yan S., Huang R. A turn–on fluorescent probe for detection of tyrosinase activity. Analyst. 2013;138:2825–2828. doi: 10.1039/c3an00272a. [DOI] [PubMed] [Google Scholar]
- 35.Li Z., Wang Y.F., Zhang X. A tyrosinase–triggered oxidative reaction–based “Turn–on” fluorescent probe for imaging in living melanoma cells. Sensor. Actuator. B Chem. 2017;242:189–194. [Google Scholar]
- 36.Zhan C., Cheng J., Li B. A fluorescent probe for early detection of melanoma and its metastasis by specifically imaging tyrosinase activity in a mouse model. Anal. Chem. 2018;90:8807–8815. doi: 10.1021/acs.analchem.8b00594. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J., Shi W., Li L. Detection of misdistribution of tyrosinase from melanosomes to lysosomes and its upregulation under psoralen/ultraviolet a with a melanosome–targeting tyrosinase fluorescent probe. Anal. Chem. 2016;88:4557–4564. doi: 10.1021/acs.analchem.6b00742. [DOI] [PubMed] [Google Scholar]
- 38.Wu X., Li L., Shi W. Near–Infrared fluorescent probe with new recognition moiety for specific detection of tyrosinase activity: design, synthesis, and application in living cells and zebrafish. Angew. Chem. Int. Ed. Engl. 2016;55:14728–14732. doi: 10.1002/anie.201609895. [DOI] [PubMed] [Google Scholar]
- 39.Wu X., Li X., Li H. A highly sensitive and selective fluorescence off–on probe for the detection of intracellular endogenous tyrosinase activity. Chem. Commun. 2017;53:2443–2446. doi: 10.1039/c6cc09679d. [DOI] [PubMed] [Google Scholar]
- 40.Li H., Liu W., Zhang F. Highly selective fluorescent probe based on hydroxylation of phenylboronic acid pinacol ester for detection of tyrosinase in cells. Anal. Chem. 2018;90:855–858. doi: 10.1021/acs.analchem.7b03681. [DOI] [PubMed] [Google Scholar]
- 41.Hu S., Wang T., Zou J. Highly chemoselective fluorescent probe for the detection of tyrosinase in living cells and zebrafish model. Sensor. Actuator. B Chem. 2019;283:873–880. [Google Scholar]
- 42.Zhang J., Li Z., Tian X. A novel hydrosoluble near–infrared fluorescent probe for specifically monitoring tyrosinase and application in a mouse model. Chem. Commun. 2019;55:9463–9466. doi: 10.1039/c9cc04714j. [DOI] [PubMed] [Google Scholar]
- 43.Singh Sidhu J., Singh A., Garg N. A highly selective naphthalimide–based ratiometric fluorescent probe for the recognition of tyrosinase and cellular imaging. Analyst. 2018;143:4476–4483. doi: 10.1039/c8an01136b. [DOI] [PubMed] [Google Scholar]
- 44.Lee M.H., Kim J.S., Sessler J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015;44:4185–4191. doi: 10.1039/c4cs00280f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Q., Yan C., Zhang J. Ratiometric and light–up near–infrared fluorescent DCM–based probe for real–time monitoring endogenous tyrosinase activity. Dyes Pigments. 2019;162:802–807. [Google Scholar]
- 46.Zhang P., Li S., Fu C. A colorimetric and near–infrared ratiometric fluorescent probe for the determination of endogenous tyrosinase activity based on cyanine aggregation. Analyst. 2019;144:5472–5478. doi: 10.1039/c9an01045a. [DOI] [PubMed] [Google Scholar]
- 47.Honisch C., Osto A., Dupas de Matos A. Isolation of a tyrosinase inhibitor from unripe grapes juice: a spectrophotometric study. Food Chem. 2020;305:125506. doi: 10.1016/j.foodchem.2019.125506. [DOI] [PubMed] [Google Scholar]
- 48.Chen J., Li Q., Ye Y. Phloretin as both a substrate and inhibitor of tyrosinase: inhibitory activity and mechanism. Spectrochim. Acta Mol. Biomol. Spectrosc. 2020;226:117642. doi: 10.1016/j.saa.2019.117642. [DOI] [PubMed] [Google Scholar]
- 49.Menezes T.M., de Almeida S.M.V., de Moura R.O. Spiro–acridine inhibiting tyrosinase enzyme: kinetic, protein–ligand interaction and molecular docking studies. Int. J. Biol. Macromol. 2019;122:289–297. doi: 10.1016/j.ijbiomac.2018.10.175. [DOI] [PubMed] [Google Scholar]
- 50.Ye L., Liu Y., Ju X. Research progress of tyrosinase inhibitors. Chem. Bioeng. 2013;30:14–20. [Google Scholar]
- 51.Zolghadri S., Bahrami A., Hassan Khan M.T. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019;34:279–309. doi: 10.1080/14756366.2018.1545767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Şenol F.S., Orhan I., Yilmaz G. Acetylcholinesterase, butyrylcholinesterase, and tyrosinase inhibition studies and antioxidant activities of 33 Scutellaria L. taxa from Turkey. Food Chem. Toxicol. 2010;48:781–788. doi: 10.1016/j.fct.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Chiari M.E., Joray M.B., Ruiz G. Tyrosinase inhibitory activity of native plants from central Argentina: isolation of an active principle from Lithrea molleoides. Food Chem. 2010;120:10–14. [Google Scholar]
- 54.Saeio K., Yotsawimonwat S., Anuchapreeda S. Development of microemulsion of a potent antityrosinase essential oil of an edible plant. Drug Discov. Ther. 2011;5:246–252. doi: 10.5582/ddt.2011.v5.5.246. [DOI] [PubMed] [Google Scholar]
- 55.Kim N.Y., Kwon H.S., Lee H.Y. Effect of inhibition on tyrosinase and melanogenesis of Agastache rugosa Kuntze by lactic acid bacteria fermentation. J. Cosmet. Dermatol. 2017;16:407–415. doi: 10.1111/jocd.12264. [DOI] [PubMed] [Google Scholar]
- 56.Bi Y., Song F., Liu Z. Research progress on types of natural tyrosinase inhibitors and its inhibitory effects on tyrosinase. Journal of Jilin University (Medicine Edition) 2014;40:454–459. [Google Scholar]
- 57.Harborne J.B., Williams C.A. Advances in avonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 58.Nerya O., Vaya J., Musa R. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J. Agric. Food Chem. 2003;51:1201–1207. doi: 10.1021/jf020935u. [DOI] [PubMed] [Google Scholar]
- 59.Kubo I., Ikuyo K.H. Flavonols from saffron flower: tyrosinase inhibitory activity and inhibition mechanism. J. Agric. Food Chem. 1999;47:4121–4125. doi: 10.1021/jf990201q. [DOI] [PubMed] [Google Scholar]
- 60.Kubo I., Kinst–Hori I., Chaudhuri S.K. Flavonols from Heterotheca inuloides: tyrosinase inhibitory activity and structural criteria. Bioorg. Med. Chem. 2000;8:1749–1755. doi: 10.1016/s0968-0896(00)00102-4. [DOI] [PubMed] [Google Scholar]
- 62.Masuda T., Yamashita D., Takeda Y. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005;69:197–201. doi: 10.1271/bbb.69.197. [DOI] [PubMed] [Google Scholar]
- 63.Liang C., Lim J.H., Kim S.H. Dioscin: a synergistic tyrosinase inhibitor from the roots of Smilax China. Food Chem. 2012;134:1146–1148. doi: 10.1016/j.foodchem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Miyazawa M., Tamura N. Inhibitory compound of tyrosinase activity from the sprout of Polygonum hydropiper L. (Benitade) Biol. Pharm. Bull. 2007;30:595–597. doi: 10.1248/bpb.30.595. [DOI] [PubMed] [Google Scholar]
- 65.Alam M.B., Bajpai V.K., Lee J.I. Inhibition of melanogenesis by jineol from Scolopendra subspinipes mutilans via MAP–Kinase mediated MITF downregulation and the proteasomal degradation of tyrosinase. Sci. Rep. 2017;7:45858. doi: 10.1038/srep45858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alam M.B., Ahmed A., Motin M.A. Attenuation of melanogenesis by Nymphaea nouchali (Burm. f) flower extract through the regulation of cAMP/CREB/MAPKs/MITF and proteasomal degradation of tyrosinase. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-32303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nanok K., Sansenya S. α–Glucosidase, α–amylase, and tyrosinase inhibitory potential of capsaicin and dihydrocapsaicin. J. Food Biochem. 2020;44:1–10. doi: 10.1111/jfbc.13099. [DOI] [PubMed] [Google Scholar]
- 68.Yang Y., Sun X., Ni H. Identification and characterization of the tyrosinase inhibitory activity of caffeine from camellia pollen. J. Agric. Food Chem. 2019;67:12741–12751. doi: 10.1021/acs.jafc.9b04929. [DOI] [PubMed] [Google Scholar]
- 69.Hałdys K., Goldeman W., Jewgiński M. Halogenated aromatic thiosemicarbazones as potent inhibitors of tyrosinase and melanogenesis. Bioorg. Chem. 2019;94:103419. doi: 10.1016/j.bioorg.2019.103419. [DOI] [PubMed] [Google Scholar]
- 70.Dong X., Wang S., Xu L. Inhibitory mechanism of Penicillin V on mushroom tyrosinase. Mol. Biol. Rep. 2020;47 doi: 10.1007/s11033-019-05188-6. [DOI] [PubMed] [Google Scholar]
- 71.Raza H., Abbasi M.A. Aziz–ur–Rehman, et al., Synthesis, molecular docking, dynamic simulations, kinetic mechanism, cytotoxicity evaluation of N–(substituted–phenyl)–4–{(4–[(E)–3–phenyl–2–propenyl]–1–piperazinyl} butanamides as tyrosinase and melanin inhibitors: in vitro, in vivo and in silico approaches. Bioorg. Chem. 2020;47:103445. doi: 10.1016/j.bioorg.2019.103445. [DOI] [PubMed] [Google Scholar]
- 72.Mahajan P.G., Dige N.C., Vanjare B.D. Facile synthesis of new quinazolinone benzamides as potent tyrosinase inhibitors: comparative spectroscopic and molecular docking studies. J. Mol. Struct. 2019;1198:126915. [Google Scholar]
- 73.Shen Z., Wang Y., Guo Z. Novel tyrosinase inhibitory peptide with free radical scavenging ability. J. Enzym. Inhib. Med. Chem. 2019;34:1633–1640. doi: 10.1080/14756366.2019.1661401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He X.R., Xu L.Y., Jin C. Tamariscinols U–W, new dihydrobenzofuran–type norneolignans with tyrosinase inhibitory activity from Selaginella tamariscina. Phytochem. Lett. 2019;34:79–83. [Google Scholar]
- 75.Qu J. Development of researches on immobilized microorganism technique and its application to wastewater treatment. Ind. water Treat. 2010;30:14–16. [Google Scholar]
- 76.Wada S., Ichikawa H., Tatsumi K. Removal of phenols from wastewater by soluble and immobilized tyrosinase. Biotechnol. Bioeng. 1993;42:854–858. doi: 10.1002/bit.260420710. [DOI] [PubMed] [Google Scholar]
- 77.Duan H., Wan X. Treatment of hospital sewage by immobilization of tyrosinase. China Trop. Med. 2010;10 513–314. [Google Scholar]
- 78.Yamada K., Akiba Y., Shibuya T. Water purification through bioconversion of phenol compounds by tyrosinase and chemical adsorption by chitosan beads. Biotechnol. Prog. 2005;21:823–829. doi: 10.1021/bp0495668. [DOI] [PubMed] [Google Scholar]
- 79.Seetharam G.B., Saville B.A. Degradation of phenol using tyrosinase immobilized on siliceous supports. Water Res. 2003;37:436–440. doi: 10.1016/s0043-1354(02)00290-7. [DOI] [PubMed] [Google Scholar]
- 80.Mukherjee S., Basak B., Bhunia B. Potential use of polyphenol oxidases (PPO) in the bioremediation of phenolic contaminants containing industrial wastewater. Rev. Environ. Sci. Biotechnol. 2013;12:61–73. [Google Scholar]
- 81.Wu L., Deng D., Jin J. Nanographene–based tyrosinase biosensor for rapid detection of bisphenol A. Biosens. Bioelectron. 2012;35:193–199. doi: 10.1016/j.bios.2012.02.045. [DOI] [PubMed] [Google Scholar]
- 82.Yang L., Xiong H., Zhang X. A novel tyrosinase biosensor based on chitosan–carbon–coated nickel nanocomposite film. Bioelectrochemistry. 2012;84:44–48. doi: 10.1016/j.bioelechem.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 83.Jiang T.F., Liang T.T., Wang Y.H. Immobilized capillary tyrosinase microreactor for inhibitor screening in natural extracts by capillary electrophoresis. J. Pharmaceut. Biomed. Anal. 2013;84:36–40. doi: 10.1016/j.jpba.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 84.Singh S., Mishra S.K., Gupta B.D. SPR based fibre optic biosensor for phenolic compounds using immobilization of tyrosinase in polyacrylamide gel. Sensor. Actuator. B Chem. 2013;186:388–395. [Google Scholar]