Abstract
Nocardiosis is an infectious disease caused by a group of organisms that are often found in soil and has a very rare incidence of infecting immunocompromised patients. Granulomatosis with polyangiitis patients are often susceptible to being infected with many atypical organisms such as Nocardia cyriacigeorgica. We present a case of a 35-year-old male who is a known case of granulomatosis with polyangiitis and has a repeated history of farm visits. The patient presented with progressive early morning right eye secretions followed by dryness throughout the day with no history of trauma or allergy of seven months duration. An eye swab for culture and sensitivity showed an isolated Nocardia cyriacigeorgica and was treated by trimethoprim/sulfamethoxazole (Bactrim) for one year but was lost to follow-up. Early detection of Nocardia cyriacigeorgica is crucial in those groups of patients, as it can prevent further complicated outcomes while proper hygiene education is important.
Keywords: farms, nocardia cyriacigeorgica, granulomatosis with polyangiitis, eye infection, animals
Introduction
Nocardia are a group of branching, hyphae-like, aerobic, gram-positive, and weak, filamentous acid-fast bacillus with over 50 species being described in the literature. Furthermore, they are members of the Nocardiaceae family and belongs to the suborder of aerobic actinomycetes [1-2]. They are considered opportunistic organisms that are found extensively in soil with the ability to infect individuals in extremely rare incidents, with various subtypes under it, which include but are not limited to Nocardia abscessus,Nocardia cyriacigeorgica, Nocardia brasiliensis, and Nocardia asteroids[3]. Even though opportunistic organisms have a general tendency to infect immunocompromised individuals more frequently, only one-third of Nocardiosis patients have impaired immunity [4]. Despite the fact that infection in granulomatosis with polyangiitis (GPA) remains one of the most common hospitalization reasons in that group, in the last two decades, the mortality rate of infection in GPA greatly decreased [5].
Case presentation
A 35-year-old male, working at an administrative job, was a known case of GPA, which was diagnosed in 2012. During the same year, he presented to our hospital with recurrent relentless hemoptysis, cough, and epistaxis and received a second dose of rituximab with no indication of relapse as antineutrophil cytoplasmic antibodies (ANCA) were not elevated. Further investigation was elicited, including chest computed tomography (CT), which showed a large area of consolidation involvement mainly in the upper left lobe of the lung and the lingua, as well as a paranasal sinuses (PNS) CT, which showed mucosa thickening of the maxillary sinus on the left side. The treatment plan included rituximab every six to nine months, along with prednisone 5 mg oral daily and azathioprine 100 mg daily. In 2013, azathioprine and rituximab were replaced by cyclophosphamide 1500 mg due to recurrent relapse, as he received only four doses and then returned to rituximab as the maintenance dose. In December 2015, the patient was complaining of excessive tearing and underwent bilateral Grommet insertion with right endoscopic dacryocystorhinostomy (DCR) without complications arising.
In 2019, the patient presented to the clinic, complaining of progressive early morning right eye secretions followed by dryness throughout the day, with no history of trauma or allergy of seven months duration. On further questioning, the patient confirmed close contact with various animals when he was at his farm, which included camels, cattle, birds, dogs, and horses. Furthermore, there was a history of multiple travels to multiple Asian, European, and Middle Eastern countries, as well as a history of drinking unpasteurized milk. In addition, the patient was a long-time user of traditional eyeliner (i.e., ithmid). Upon physical examination, there was only mild tearing mainly in the right eye and no signs of redness, itching, thickening, scleral discoloration, corneal abrasions, irritation to light, or any discharge from the eye, and a swab was taken. Regarding the swab, the culture showed an isolated Nocardia cyriacigeorgica organism, which was confirmed with a second swab that was sent to Mayo Clinic, and the patient was admitted to the ward. During the hospital course, a multi-investigational approach was done from blood work, which included complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), serology, renal and bone profile, as well as imaging, which included chest CT, PNS CT, and brain CT.
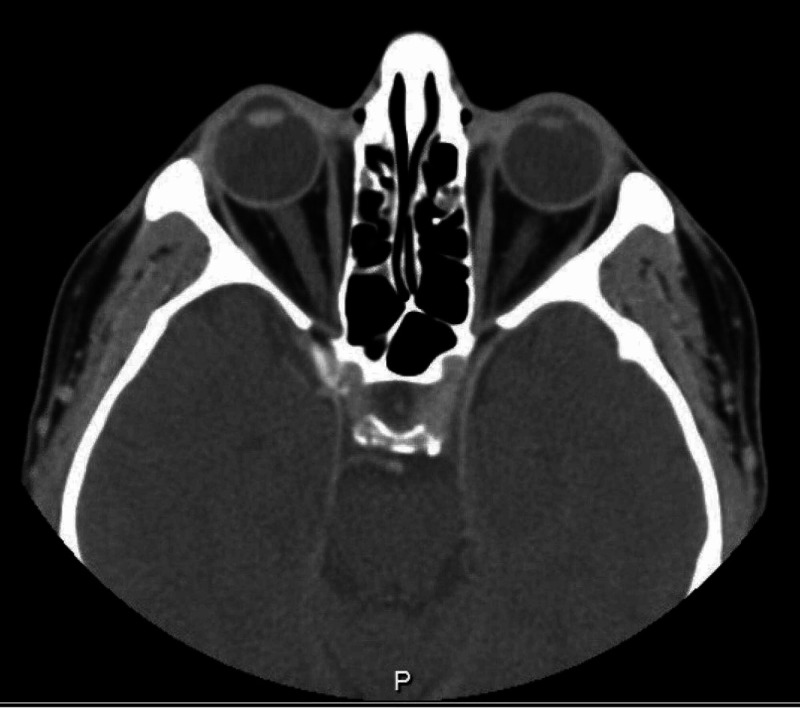
Regarding his blood work, CBC showed lymphopenia, which was regular due to the nature of the immunosuppression drugs, normal range of CRP, ESR, and renal and bone profile. On the other hand, brain and orbit CT scan showed complete opacification of the bilateral mastoid air cells and middle ear cavities, new mucosal disease of the paranasal sinuses suggestive of active GPA, and no reported abnormalities in the orbital region (Figure 1).
Figure 1. Paranasal sinuses computed tomography demonstrated bilateral otomastoiditis with interval development of severe left maxillary sinus mucosal disease.
Meanwhile, his chest CT scan reported stable left upper lobe fibrotic changes in association with volume loss and traction bronchiectasis with small atelectatic bands in both lower lobes. In addition, there were stable pulmonary micronodules in the right lower lobe with no obvious cavitary lesions or lung masses (Figure 2). Lastly, his PNS CT demonstrated bilateral otomastoiditis with interval development of severe left maxillary sinus mucosal disease and mild bilateral mucosal disease in the ethmoidal air cells bilaterally (Figure 3).
Figure 2. Computed tomography of the lungs showing micronodules.
Figure 3. Brain and orbit computed tomography scan showing no abnormality in the orbital regions.
The initial plan for the patient was to start on 480 mg q8 hour trimethoprim/sulfamethoxazole (Bactrim) for one year for the Nocardiosis and to continue on a rituximab dose of 1 gram. His hospital course was uneventful, and the patient was advised to continue the treatment for Nocardia for a complete year with the recommendation to remove the dacryocystorhinostomy stent, CT follow-up, and regular culturing to confirm the eradication of the organism. Lastly, his last visit was to the rheumatology clinic in May 2020 and was in stable condition, with no more eye discharges, and, apparently, he improved with trimethoprim/sulfamethoxazole. From the rheumatology point of view, he was advised to continue on rituximab 1 gram every nine months. Meanwhile, he neglected all his visits to the ophthalmology and infectious disease clinics for swab and monitoring; after six months of continuous compliance with Bactrim, he stopped the medication by himself.
Discussion
Nocardia is considered a very rare infectious opportunistic organism that is found in various sources of soil, with higher rates of organisms present in South Asia [6]. However, due to the continuous rise in infections, particularly in immunocompromised as well as chemotherapy patients, more organism species of Nocardia, which were not known to be infectious, are being reported in the literature. Nocardia cyriacigeorgica has been first described in 2001 by Yassin et al. [7] and is considered one of the emerging infections. In 2005, Thailand and Japan reported various incidences of Nocardia cyriacigeorgica as cases of brain abscess [8]. Meanwhile, in 2010, a sample of oil‐polluted sand samples collected from the Saudi Arabian desert showed contamination with Nocardia cyriacigeorgica [9]. A retrospective chart review in Saudi Arabia over a period of 16 years reported 19 patients with nocardiosis, with the main causative culprit being post-transplantation immunosuppression [10]. In addition, three types of Nocardia infections were reported: Nocardia asteroids, Nocardia brasiliensis, and Nocardia otitidiscaviarum, with the lung being the most common site of infection [10].
Around 52% of GPA patients can present with ophthalmic manifestation, such as scleral, episcleral, nasolacrimal, and corneal [11]. Early recognition of the cause of the ophthalmic manifestation and possible pathologies can be lifesaving and will help preserve eyesight from being lost in GPA patients. GPA can have a long list of various infectious manifestations, including but not limited to dermatological [12], cardiac [13], and nasal [14]. Not only that, the Nocardia infectious course of treatment are often exhaustive, with a long treatment duration that can reach up to a year, with the possibility of resistance to many antimicrobial agents such as trimethoprim-sulfamethoxazole [4] while long-term antimicrobial therapy is necessary to prevent a relapse infection of nocardiosis [15].
Conclusions
GPA can be extremely unpredictable, with the possibility of being infected with various species of organisms that are not known to infect humans. This should elicit educating patients with the disease to always be cautious of their surroundings, avoid animal contact as much as possible, and carry on a more hygienic lifestyle than the normal individual. High suspicion of rare infections should be kept in mind while interacting with immunocompromised patients presenting with mild symptoms of infections.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study. King Faisal Specialist Hospital and Research Center Ethical Committee issued approval NA
References
- 1.Nocardiosis: updates and clinical overview. Wilson JW. Mayo Clin Proc. 2012;87:403–407. doi: 10.1016/j.mayocp.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manifestations and outcomes of Nocardia infections. Comparison of immunocompromised and nonimmunocompromised adult patients. Steinbrink J, Leavens J, Kauffman CA, Miceli MH. Medicine. 2018;97:12436. doi: 10.1097/MD.0000000000012436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Southwick FS. Goldman's Cecil Medicine (Twenty Fourth Edition) Philadelphia: W.B. Saunders; 2012. 338 - Nocardiosis; pp. 1970–1971. [Google Scholar]
- 4.Pathogenic Nocardia cyriacigeorgica and Nocardia nova evolve to resist trimethoprim-sulfamethoxazole by both expected and unexpected pathways. Mehta H, Weng J, Prater A, Elworth RAL, Han X, Shamoo Y. Antimicrob Agents Chemother. 2018;62:364–318. doi: 10.1128/AAC.00364-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nationwide trends in hospitalizations and in-hospital mortality in granulomatosis with polyangiitis (Wegener's) Wallace ZS, Lu N, Miloslavsky E, Unizony S, Stone JH, Choi HK. https://pubmed.ncbi.nlm.nih.gov/27389595/ Arthritis Care Res. 2017;69:915–921. doi: 10.1002/acr.22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Optimizing diagnosis and management of Nocardia keratitis, scleritis, and endophthalmitis: 11-year microbial and clinical overview. DeCroos FC, Garg P, Reddy AK, et al. Ophthalmology. 2011;118:1193–1200. doi: 10.1016/j.ophtha.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 7.Nocardia cyriacigeorgici sp nov. Yassin AF, Rainey FA, Steiner U. Int J Syst Evol Microbiol. 2001;51:1419–1423. doi: 10.1099/00207713-51-4-1419. [DOI] [PubMed] [Google Scholar]
- 8.Nocardia cyriacigeorgica is a significant pathogen responsible for nocardiosis in Japan and Thailand. Kageyama A, Hoshino Y, Yazawa K, Poonwan N, Takeshita N, Maki S, Mikami Y. Mycopathologia. 2005;160:15–19. doi: 10.1007/s11046-005-3050-2. [DOI] [PubMed] [Google Scholar]
- 9.Oxidation of aliphatic, branched chain, and aromatic hydrocarbons by Nocardia cyriacigeorgica isolated from oil-polluted sand samples collected in the Saudi Arabian desert. Nhi-Cong LT, Mikolasch A, Awe S, Sheikhany H, Klenk H-P, Schauer F. https://pubmed.ncbi.nlm.nih.gov/20143352/ J Basic Microbiol. 2010;50:241–253. doi: 10.1002/jobm.200900358. [DOI] [PubMed] [Google Scholar]
- 10.Clinical pattern of nocardiosis in Saudi Arabia: a case series. Hakawi AM, Al Rabiah FA. https://apps.who.int/iris/handle/10665/117516. East Mediterr Health J. 2008;14:966–971. [PubMed] [Google Scholar]
- 11.Central retinal artery occlusion in Wegener's granulomatosis: a diagnostic dilemma. Lozano-López V, Rodríguez-Lozano B, Losada-Castillo MJ, Delgado-Frías E, Dopazo-Luque D, Serrano-García M. J Ophthalmic Inflamm Infect. 2011;1:71–75. doi: 10.1007/s12348-010-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wegener's granulomatosis. Dermatological manifestations in 75 cases with clinicopathologic correlation. Francès C, Piette J-C, Saada V, et al. Arch Dermatol. 1994;130:861–867. [PubMed] [Google Scholar]
- 13.Wegener granulomatosis simulating bacterial endocarditis. Anthony DD, Askari AD, Wolpaw T, McComsey G. https://jamanetwork.com/journals/jamainternalmedicine/article-abstract/1105620. Arch Intern Med. 1999;159:1807–1810. doi: 10.1001/archinte.159.15.1807. [DOI] [PubMed] [Google Scholar]
- 14.Nasal carriage of staphylococcal infection in Wegener granulomatosis. Lawyer C, Henkle J, Bakir H. Ann Intern Med. 1994;121:74–75. doi: 10.7326/0003-4819-121-1-199407010-00023. [DOI] [PubMed] [Google Scholar]
- 15.Nocardial brain abscess in a renal transplant recipient successfully treated with triple antimicrobials. Sabeel A, Alrabiah F, Alfurayh O, Hassounah M. https://pubmed.ncbi.nlm.nih.gov/9725786/ Clin Nephrol. 1998;50:128–130. [PubMed] [Google Scholar]