Summary:
The authors performed a transumbilical, single-port robotically assisted, nipple-sparing mastectomy on a cadaveric model to assess technical feasibility. Surgeon-controlled, robotic-wristed instrumentation, as well as 3-dimensional high definition (HD) vision allowed the entire dissection to be performed through a single incision placed in the umbilicus. The technique warrants further exploration and development before any application in clinical applied research.
INTRODUCTION
Nipple-sparing mastectomy (NSM) for the treatment of breast cancer and prophylaxis is associated with improved cosmetic outcomes over conventional mastectomy techniques.1,2 The benefits include the option for immediate reconstruction and the preservation of the nipple and breast skin envelope, while maintaining acceptable oncological outcomes.3,4 Despite the potential for improved cosmetic outcome offered to patients over conventional mastectomy, open NSM still requires an incision to be placed on the breast5 or inframammary fold (IMF).
Robotically assisted technology has spread to multiple surgical specialties over the past 2 decades since the introduction of the da Vinci Surgical System by Intuitive (Sunnyvale, CA) in 1999. The enhanced visualization, dexterity, precision, and ergonomics offered by the system have led to the expansion of minimally invasive surgery available to patients while improving postoperative outcomes.6–9 Toesca and Sarfati have used a robotically assisted surgical system (da Vinci Xi, Intuitive) to perform robotically assisted NSM (RNSM) and immediate breast reconstruction with implant.10–18 We should note that at this present time, the US Food and Drug Administration (FDA) has not cleared this procedure for use with the da Vinci Surgical System. One potential benefit of RNSM over open NSM could be the ability to reduce the length of the incision and move it off the breast and onto the lateral thoracic wall, where it can be hidden in the bra footprint or axilla.
Recently, the FDA cleared Intuitive’s da Vinci SP system in the United States. This next generation of robotically assisted system enables surgeon control of 3 multijointed instruments and a fully wristed 3-dimensional high definition (HD) endoscope through a single 2.5-cm port. In February 2019, the authors collaborated on a cadaver laboratory to investigate if the da Vinci SP system could enable any novel RNSM techniques. One technique that was explored and showed initial success was RNSM through transumbilical access.
METHODS
The subject, a fresh frozen Caucasian female cadaver, weighing approximately 53 kg, had been used previously for robotically assisted colorectal surgery technique exploration, and thus, had 4 subcostal incisions. These incisions were closed at the skin and did not interfere with the RNSM.
The subject was placed in the supine position and moved to the contralateral edge of the table to avoid conflict between the table and the da Vinci SP system. A 2.5-cm superior periumbilical incision was made. Sharp and blunt dissection with Metzenbaum scissors was used to create the initial subcutaneous cavity to insert the da Vinci SP port. Tumescence infiltration with epinephrine was not used due to the lack of vascular perfusion in the cadaveric model. The cavity was extended toward the left breast as far as possible to reduce the amount of robotic dissection required to reach the breast. The robotic port was then inserted into the incision, insufflation was started at 10 mm Hg to create subcutaneous pneumocavity, and the da Vinci SP system was docked. After docking, the remote center was repositioned anteriorly to provide a trajectory for the robotic arms over the rib cage. A marker was used to draw lines on the subject from the umbilicus to the medial and lateral borders of the breast to map out the correct trajectory and delineate the extents of the required dissection. A bipolar grasper and monopolar scissors were inserted and controlled from the surgeon console to continue the subcutaneous dissection cranially toward the left breast (Fig. 1). Using guidance from the patient-side assist, the cavity was widened as necessary to reach the medial and lateral borders of the breast.
Fig. 1.

Robotic subcutaneous dissection toward the left breast.
Once the dissection reached the IMF, as noted by the patient-side assist, the surgeon released the IMF from the chest wall and performed the anterior mammary gland dissection, following the plane where the subcutaneous tissue and breast parenchyma intersected. This dissection progressed cranially until the entire anterior dissection had been completed to the limits of the breast. To reach the axillary tail of the gland, the port had to be temporarily inserted further into the umbilicus (Fig. 2). After completion of the anterior dissection, the instruments and endoscope were retracted and the patient-side assist pressed down on the IMF, allowing the inferior border of the breast to be identified. Once identified, the posterior dissection was performed (Fig. 3) until the mammary gland was completely released from the chest wall.
Fig. 2.

Anterior dissection near completion.
Fig. 3.
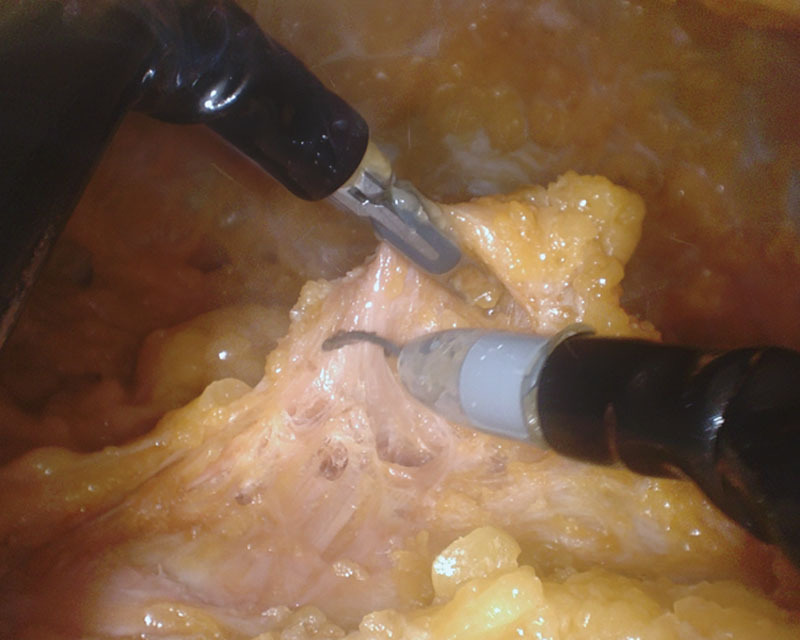
Initiation of posterior dissection to release the mammary gland from the chest wall.
After complete mobilization of the mammary gland, the robotic instruments were removed, the system undocked, and the port removed. A laparoscopic grasper was used to pull the gland to the umbilicus. The gland was removed en bloc from the umbilicus using a towel clamp (Fig. 4). Reconstruction was not attempted.
Fig. 4.

Mammary gland removed through the umbilicus. Retained subcutaneous gas from insufflation is visible.
DISCUSSION
To our knowledge, this is the first successful transumbilical RNSM performed in a cadaveric model. The entire mammary gland was mobilized and removed en bloc through a single incision in the umbilicus.
Due to there being no landmarks when viewing the subcutaneous cavity endoscopically, we found it necessary to have the patient-side assist direct the dissection to ensure that the subcutaneous dissection was not performed more than necessary to access the breast and remove the mammary gland. We assume that the subcutaneous emphysema as a result of this technique will naturally dissipate, similar to Toesca and Sarfati’s experience with RNSM. In addition to the unilateral mastectomy described here, this method may enable a bilateral NSM to be performed through a single incision hidden in the umbilicus. This technique has the potential to further improve upon the cosmetic benefits of NSM and RNSM over conventional mastectomy.
Reconstruction would likely be performed with a direct-to-implant technique following a transumbilical NSM. However, silicone implants are unlikely to be compatible with this procedure due to the small and remote incision. It may be possible to perform the reconstruction in a manner similar to transumbilical breast augmentation using saline implants.19 We recognize that the IMF is obliterated using this approach; however, after the placement of an unfilled, saline implant, it may be possible to re-dock the SP system and reapproximate the IMF to the chest wall using interrupted or running sutures, followed by the filling of the implants. The size of the gland should be considered when selecting patients because the incision may need to be enlarged to accommodate the removal and insertion of larger glands and implants, respectively.
An additional indication for this procedure may be for the treatment of gynecomastia. With conventional mastectomy to treat gynecomastia, it is impossible to hide the scar in the IMF because of the lack of ptosis, resulting in a visible scar, which can reduce the quality of life for the patient. The transumbilical approach could eliminate this concern by enabling removal of the gland without placing an incision on the chest.
It is important to note again that, at the time of writing this article, the FDA has not cleared any robotically assisted surgical system for NSM, or any mastectomy. This means that any RNSM should be considered an off-label procedure in the United States. The procedure should only be performed under specific clinical studies approved by the relevant hospital investigational review board and the applicable regulatory agency, such as the US FDA.
CONCLUSIONS
We have shown transumbilical RNSM to be feasible in a cadaveric model. The wristed instrumentation as well as 3-dimensional HD magnified vision of the SP system allowed the entire dissection to be performed through a single port placed in the umbilicus. Besides the treatment of breast cancer or prophylaxis in women, the technique may be an appropriate therapy for gynecomastia in men.
Cadaveric research is one appropriate preclinical method for exploration of this and other new surgical techniques. This particular technique warrants further exploration and development before being attempted in a clinical setting.
ACKNOWLEDGMENT
The authors would like to acknowledge Intuitive, especially Glenn Stante and Markus Rheinwald, PhD, for funding this project, providing access to laboratory resources, and additional general support.
Footnotes
Published online 27 May 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Ueda S, Tamaki Y, Yano K, et al. Cosmetic outcome and patient satisfaction after skin-sparing mastectomy for breast cancer with immediate reconstruction of the breast. Surgery. 2008;143:414–425. [DOI] [PubMed] [Google Scholar]
- 2.Salgarello M, Visconti G, Barone-Adesi L.Nipple-sparing mastectomy with immediate implant reconstruction: cosmetic outcomes and technical refinements. Plast Reconstr Surg. 2010;126:1460–1471. [DOI] [PubMed] [Google Scholar]
- 3.Headon HL, Kasem A, Mokbel K.The oncological safety of nipple-sparing mastectomy: a systematic review of the literature with a pooled analysis of 12,358 procedures. Arch Plast Surg. 2016;43:328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galimberti V, Morigi C, Bagnardi V, et al. Oncological outcomes of nipple-sparing mastectomy: a single-center experience of 1989 patients. Ann Surg Oncol. 2018;25:3849–3857. [DOI] [PubMed] [Google Scholar]
- 5.Corso G, De Lorenzi F, Vicini E, et al. Nipple-sparing mastectomy with different approaches: surgical incisions, complications, and cosmetic results. Preliminary results of 100 consecutive patients at a single center. J Plast Reconstr Aesthet Surg. 2018;71:1751–1760. [DOI] [PubMed] [Google Scholar]
- 6.Gamagami R, Dickens E, Gonzalez A, et al. Open versus robotic-assisted transabdominal preperitoneal (R-TAPP) inguinal hernia repair: a multicenter matched analysis of clinical outcomes. Hernia. 2018;22:827–836. [DOI] [PubMed] [Google Scholar]
- 7.Kent M.Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg. 2014;97:236–244. [DOI] [PubMed] [Google Scholar]
- 8.Lim P.Multicenter analysis comparing robotic, open, laparoscopic, and vaginal hysterectomies performed by high-volume surgeons for benign indications. Int J Gynaecol Obstet. 2016;133359–364. [DOI] [PubMed] [Google Scholar]
- 9.Tewari A, Sooriakumaran P, Bloch DA, et al. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: a systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur Urol. 2012;62:1–15. [DOI] [PubMed] [Google Scholar]
- 10.Toesca A, Peradze N, Galimberti V, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with implant: first report of surgical technique. Ann Surg. 2017;266:e28–e30. [DOI] [PubMed] [Google Scholar]
- 11.Toesca A.Reply to the letter to the editor “Robotic-assisted Nipple Sparing Mastectomy: a feasibility study on cadaveric models” by Sarfati B. et al. J Plast Reconstr Aesthet Surg. 2017;70558–560. [DOI] [PubMed] [Google Scholar]
- 12.Toesca A, Peradze N, Manconi A, et al. Robotic nipple-sparing mastectomy for the treatment of breast cancer: feasibility and safety study. Breast. 2017;31:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarfati B, Honart JF, Leymarie N, et al. Robotic-assisted nipple sparing mastectomy: a feasibility study on cadaveric models. J Plast Reconstr Aesthet Surg. 2016;69:1571–1572. [DOI] [PubMed] [Google Scholar]
- 14.Honart JF.Response to the reply to the letter to the editor “Robotic-assisted Nipple Sparing Mastectomy: a feasibility study on cadaveric models”. J Plast Reconstr Aesthet Surg. 2017;70:560–561. [DOI] [PubMed] [Google Scholar]
- 15.Sarfati B, Honart JF, Leymarie N, et al. Robotic da Vinci Xi-assisted nipple-sparing mastectomy: first clinical report. Breast J. 2018;24:373–376. [DOI] [PubMed] [Google Scholar]
- 16.Sarfati B, Struk S, Leymarie N, et al. Robotic prophylactic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: a prospective study. Ann Surg Oncol. 2018;25:2579–2586. [DOI] [PubMed] [Google Scholar]
- 17.Sarfati B, Struk S, Leymarie N, et al. Robotic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: surgical technique. Plast Reconstr Surg. 2018;142:624–627. [DOI] [PubMed] [Google Scholar]
- 18.Toesca A, Invento A, Massari G, et al. Update on the feasibility and progress on robotic breast surgery. Ann Surg Oncol. 2019;26:3046–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handel N.Transumbilical breast augmentation. Clin Plast Surg. 2009;36:63, vi–74, vi. [DOI] [PubMed] [Google Scholar]


