Abstract
Objective To hierarchize the anterior inferior cerebellar artery (AICA)–subarcuate artery (SAA) complex's variations in the surgical field.
Background The AICA's “subarcuate loop” (SL) presents multiple variations, closely related to the SAA. AICA-SAA complex's variations may represent major issues in cerebellopontine angle (CPA) surgery. As the spectrum of configurations is originated during the development, a systematized classification was proposed based on the interaction between the petrosal bone and the AICA in the embryonic period.
Methods The variations were defined as follow: Grade 0: free, purely cisternal AICA, unidentifiable or absent SAA; Grade 1: purely cisternal AICA, loose SL, SAA > 3 mm; Grade 2: AICA near the subarcuate fossa, pronounced SL, SAA <3 mm; Grade 3: “duralized” AICA, unidentifiable SAA, or included in the petromastoid canal (PMC); and Grade 4: intraosseous AICA, unidentifiable SAA, or included in the PMC. The classification was applied to a series of patients assessed by magnetic resonance constructive interference in steady state sequence. Surgical examples were also provided.
Results Eighty-four patients were evaluated, including 161 CPA. The proportions found in the gradation remained within the range of previous publications (Grade 0: 42.2%; Grade 1: 11.2%; Grade 2: 35.4%; Grade 3: 10.6%; and Grade 4: 0.6%). Moreover, the degrees of the classification were related to the complexity of the anatomical relationships and, therefore, to the difficulty of the maneuvers required to overcome them.
Conclusion The proposed AICA-SAA complex classification allowed to distinguish and objectify pre- and intraoperatively the spectrum of variations, to thoroughly plan the required actions and instrumentation.
Keywords: anterior inferior cerebellar artery, cerebellopontine angle, petromastoid canal, subarcuate artery, subarcuate fossa, subarcuate loop
Introduction
The cerebellopontine angle (CPA) cistern is the main surgical corridor to reach pathologies arising in the lateral portion of the posterior fossa. 1 2 3 In this anatomical region, however, multiple eloquent neurovascular structures take place; among them, the anterior inferior cerebellar artery (AICA) 4 5 which presents a highly variable path. 6 7 8 9
This work is focused on the description of a portion referred as “subarcuate loop” (SL), corresponding to the lateral pontine segment of the AICA. It is closely related to an area of the petrous region of the temporal bone, posterior–superior to the internal auditory canal (IAC), the subarcuate fossa (SF). 10 This arterial loop usually gives rise to the subarcuate artery (SAA). 5 11 12 Its flow permeates the petromastoid region through the petromastoid canal (PMC), or “subarcuate canal,” 13 14 although it can exceptionally irrigates neural structures. 15 16 17 The patency and length of the SAA present significant variability. 8 10 18 19 20 21 22 Indeed, also AICA variants have been reported, as covered by dura mater 23 24 25 and even by petrous bone. 25 26 27 28 29 These situations involve complex maneuvers for the identification and eventual mobilization of the AICA-SAA complex. Despite the surgical implications, the anatomical variations regarding the SL and its branches have been barely reported.
Embryology of the Temporal Bone and the Subarcuate Region
To categorize the nature of these variations, we appeal to their embryological development. The constitution of the subarcuate region is closely linked to the development of the superior semicircular canal (SCC). Initially formed as a common vesicle, the SCCs then flatten on each of their respective axes, forming disks, and finally cavitate in the center, forming the annular structures that characterize them. 30 The arch generated by the superior SCC is gradually filled by connective tissue, thus forming the PMC. 13 31
On the contrary, the AICA is formed since the dominant vessel of the embryonic plexus generated from the basilar artery, as the requirements of the infratentorial neural structures increase. 15 32 After arising from the AICA, the SAA travels a variable length on the CPA cistern from the cranial cavity to the SF and continues, surrounded by an invagination of dura mater, through the connective matrix of the PMC, to supply the petrous and mastoid portions of the temporal bone.
As the temporal bone develops, the PMC thins and elongates, while the perilabyrinthic connective tissue gives rise to the cartilaginous matrix. The cartilage will then allow the seat for mature bone tissue formation. This last stage does not take place until the development of the SCC ends, between the weeks 20–22 of the fetal period. 30 33 In this period, the SF can be deepened until reaching the mastoid region, barely separated by a few layers of connective tissue. 31
In the postnatal period, the pneumatization of the mastoid cells takes place. Together with the ossification of the petrous bone, both processes continue to fill the interior of the superior SCC arch from lateral to medial, thus obliterating the PMC. 34 35 36 37 The petrosal intracranial surface of the adult then presents the vestige of this communication (i.e., the SF), penetrated by the SAA and, eventually, a variable amount of the AICA.
Embryological–Anatomical Correlation
It is possible to deduce that the spectrum of the anatomical variations in the configuration of the subarcuate region is due to the combination of the proximity of the AICA-SAA complex, with the proliferation of the PMC mesenchymal tissue in early periods of the fetal development. In this way, the proliferating mesenchymal tissue near the arterial vessels can coat them, thus being able to fuse the petrous dura mater with the perivascular connective tissue, or even be covered by bone in the succeeding petrosal osteoid maturation.
Considering the provided descriptions, we propose a grading system to characterize the variations of the AICA-SAA complex under a comprehensive and hierarchical concept that allows the surgeon to take accurate actions when facing them.
Material and Methods
Methodological Design
This is an observational, descriptive, retrospective cross-sectional study (Institutional Review Board and/or informed consent not required). The study was conducted in accordance with the Anatomical Quality Assurance 38 research reporting guidelines.
Population
Adult patients older than 18 years, of both sexes, followed up in our center due to trigeminal neuralgia (TN).
Sample
The CPA cisterns and their content were evaluated by magnetic resonance imaging (MRI) obtained by the Diagnostic Imaging Department between January 2008 and April 2018 (Avanto 1.5T or Vision 1.5T, Siemens Inc., Munich, Germany); Achieva 1.5T or Ingenia 3.0T (Royal Philips, Amsterdam, The Netherlands) and processed by neuroimaging specialists for diagnostic interpretation.
Inclusion Criteria
The inclusion criteria are as follows:
MRI T2 constructive interference in steady state (CISS) sequences, submillimetric axial cuts, posterior fossa (from the tentorium to the foramen magnum) scanning.
MRI acquired in equipment of field power 1.5T or greater.
AICA identifiable uni- or bilateral, single or multiple.
Exclusion Criteria
The exclusion criteria are as follows:
Surgery in the CPA prior to the MRI assessment.
Lesions in the homolateral CPA or with contralateral mass effect.
AICA absence or agenesis.
Incomplete studies or with cuts more than 1 mm CISS sequence axial thickness.
Data Acquisition
The Alma Workstation V4.2.0.2 software (Alma IT Systems) was used to visualize and measure the images.
The anatomical variations of the AICA-SAA complex were documented emphasizing the relationship between the SL and the subarcuate regions of the petrosal bone. For this purpose, the following anatomical definitions were established:
SF : Region of the petrous surface of the temporal bone, immediately posterior and cephalic to the IAC, in axial and coronal sections, respectively. In addition to IAC, the apparent origin of the facial and vestibulocochlear nerves in the pontomedulary sulcus, the superior SCC, and the PMC was taken into account.
AICA : Vascular structure, with hypointense signal in CISS sequence, identified from its origin in the basilar artery, distal to the confluence of the vertebral arteries and proximal to the superior cerebellar arteries. In the cisternal path, the lateral pontine segment was identified, with special detail in the nearness of the SF (i.e., the SL), previously identified.
SAA : Vascular structure, of hypointense signal in CISS sequence, originated in the lateral pontine segment of the AICA and directed to the SF.
PMC : Intrapetrosal pathway originated in the depth of the SF, in perpendicular direction to the posterior SCC. If identifiable, it usually delivers an iso/hyperintense signal in CISS sequence.
SCC : Arciform tubular formation with a superior projection, in the petrosal bone, filled with endolymph emitting a clearly hyperintense signal in CISS sequence.
IAC : Depression in the petrous surface of the temporal bone, forming a canal through which the acoustic-facial bundle runs together with variable portions of the “meatal loop” and eventually the labyrinthine artery (LA, internal auditory artery) of the AICA; all of them presenting hypointense signal, contrasted by the hyperintense signal generated by intracanalicular cerebrospinal fluid (CSF), in the CISS sequence.
The findings were classified by a gradation proposed by the authors, reflecting the interaction between the components of the AICA during the period of embryological development, as described below ( Fig. 1 ):
Fig. 1.
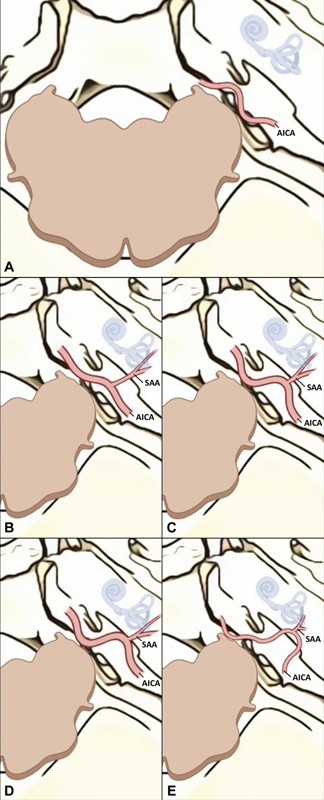
Schematic digital art interpretation of the classification, right CPA superior view. (A) Grade 0 (cisternal AICA, unidentifiable SAA); (B) Grade 1 (SAA >3 mm); (C) Grade 2 (SAA <3 mm); (D) Grade 3 (AICA adhered to the dura mater); (E) Grade 4 (AICA with intraosseous path). AICA, anterior inferior cerebellar artery; CPA, cerebellopontine angle; SAA, subarcuate artery.
Grade 0 : AICA with a purely cisternal path, no adhesions to the petrosal bone, SAA absent or unidentifiable ( Fig. 1A ).
Grade 1 : AICA separated from petrosal bone, loose SL, and elongated SAA (>3 mm) ( Fig. 1B ).
Grade 2 : AICA near the SF, pronounced SL, short SAA (<3 mm) ( Fig. 1C ).
Grade 3 : AICA “duralized,” adhered or covered by dural tissue; no SAA identifiable in the CPA cistern or completely included in the PMC path ( Fig. 1D ).
Grade 4 : AICA included in the bone of the SF, forming a distinctly intraosseous canal; no SAA identifiable in the CPA cistern or completely included in the PMC path ( Fig. 1E ).
In cases Grade 1 or 2, the length and laterality of the SAA were also recorded.
Statistical Analysis
For statistical purposes, each CPA and its contents were considered separately. The variable “grade” was taken as an ordinal qualitative variable, expressed in increasing order of complexity, in units from 0 to 4 (excluding). The sample size was estimated taken into account the expected proportion of the “anomalous” SL (6% 21 ), a width of the confidence interval (CI) of 0.1 and confidence level at 95%. 39 The distribution of frequencies was expressed as a percentage of the total CPA included. The length of the SAA was considered as a continuous quantitative variable, expressed in millimeters with a decimal digit. The average (mean) was reported as a measure of central tendency, and the 95% CI and standard deviation as measures of statistical dispersion. The SAA length averages were then compared between both sides and with the total values obtained (categorical qualitative variable: right, left, total, nonexcluding) by one-way analysis of variance (ANOVA). It was considered statistically difference means a value of p < 0.05. The laterality of the SAA was considered as a dichotomous categorical qualitative variable, right versus left; as for bilaterality, yes versus not. Both variables were reported as percentages of the total SAA identified. The relationship between the variables length and laterality were expressed as absolute (number of SAA identified) and relative (percentage of total SAA evaluated) frequencies.
Results
Data from 84 patients were evaluated in this study, 47 (56%) women and 37 (44%) men, average age 67.6 years (range: 37–97). A total of 161 CPAs were included in the study. Seven CPAs were excluded from the evaluation (five neurovascular conflict microsurgical decompression, one CPA tumor excision, and one incomplete MRI CISS sequence). There were 41 MRI studies on 1.5T resonators and 44 on the 3.0T equipment. Trigeminal neurovascular conflict was evidenced in 24 (14.9%) of the CPA studied.
All the proposed grades were identified in the evaluated series ( Fig. 2 ) and also identified in our surgical video library recordings ( Fig. 3 ). The SAA could be recognized with a cisternal path in 44% of the CPA evaluated by MRI. The distribution of the AICA-SAA complex according to the proposed scale is summarized in Table 1 .
Fig. 2.
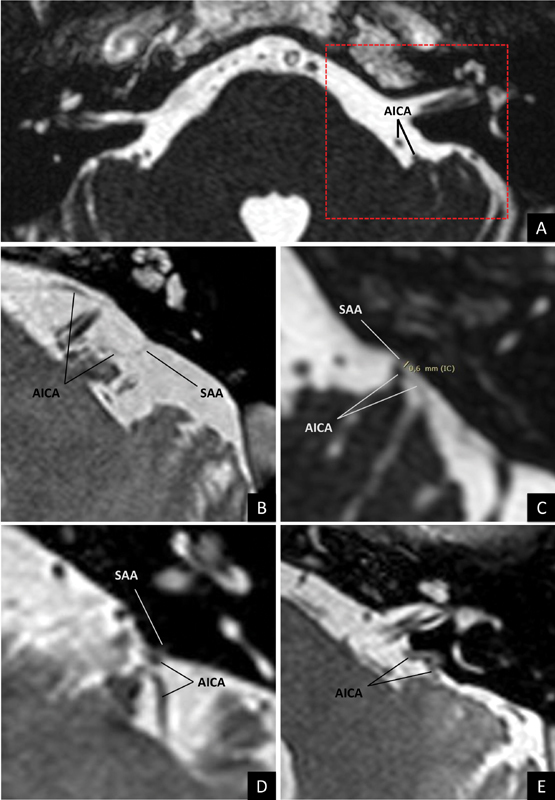
Identification of each degree of the proposed classification, MRI CISS sequence. (A) Grade 0; (B) (mirrored): Grade 1; (C) Grade 2; (D) Grade 3; and (E) (mirrored): Grade 4. The dotted area in (A) symbolizes the region focused in each example. AICA, anterior inferior cerebellar artery; CISS, constructive interference in steady state; MRI, magnetic resonance imaging; SAA, subarcuate artery.
Fig. 3.
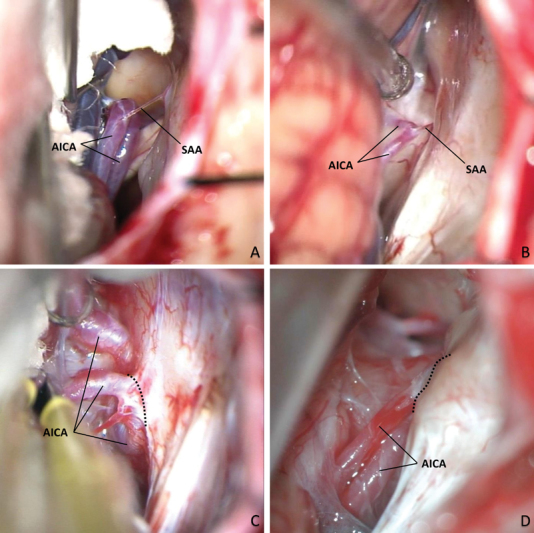
Identification of each degree of the proposed classification, intraoperative recordings. (A) (Right park bench position, 90-degree right rotation): Grade 1; (B) (left park bench position, 90-degree left rotation, mirrored): Grade 2; (C) (left park bench position, 90-degree left rotation, mirrored): Grade 3; and (D) (right semisitting position): Grade 4. Dashed lines indicate where the AICA is attached to the dura mater (C) or encased by bone (D). AICA, anterior inferior cerebellar artery; SAA, subarcuate artery.
Table 1. Classification distribution by grades.
| Grade | 0 | 1 | 2 | 3 | 4 | Total |
|---|---|---|---|---|---|---|
| n (CPA) | 68 | 18 | 57 | 17 | 1 | 161 |
| Percentage | 42.2% | 11.2% | 35.4% | 10.6% | 0.6% | 100% |
Abbreviation: CPA, cerebellopontine angle.
The distribution of the SAA is summarized in Table 2 . In the cases where the SAA could be identified, the average length was 2.1 mm (95% CI: 1.7–2.5 mm), which showed no statistically significant difference with respect to those obtained on each side separately ( p = 0.96, n = 75, one-way ANOVA, Fig. 4 ). No statistical significance was achieved by the multiple comparison test between particular groups (left vs. right: p = 0.95, total vs. right: p = 0.98, total vs. left: p = 0.98). As detailed in Table 1 , the SAA less than 3 mm was the most frequent presentation (Grade 1 vs. Grade 2).
Table 2. Subarcuate artery cisternal configuration.
| Left | Right | Total | |
|---|---|---|---|
| n (identified SAA) | 38 | 37 | 75 |
| Percentage | 50.6% | 49.4% | 100% |
| Range (mm) | 0.3–6.3 | 0.4–7.3 | 0.3–7.3 |
| Average | 2.10 | 2.20 | 2.15 |
| SD | 1.50 | 1.84 | 1.66 |
| 95% CI | 1.60–2.59 | 1.59–2.81 | 1.76–2.53 |
Abbreviations: CI, confidence interval; SAA, subarcuate artery; SD, standard deviation.
Fig. 4.
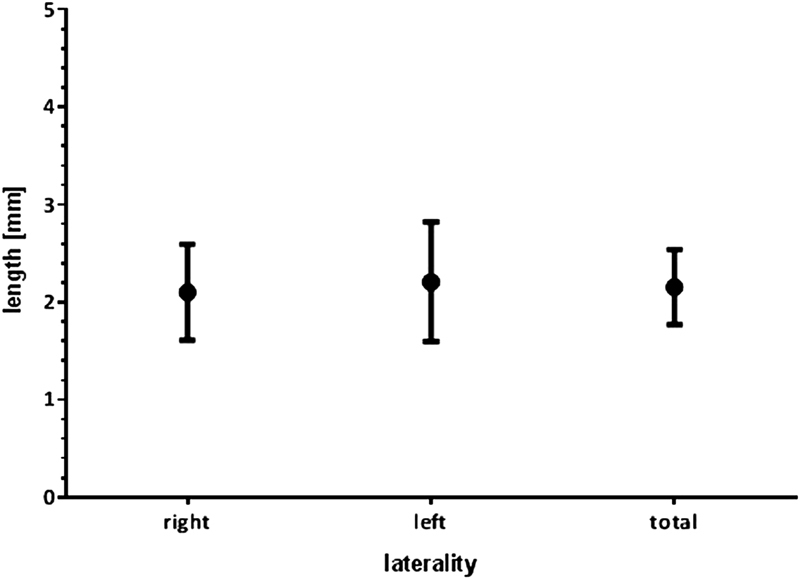
Length of the SAA according to laterality (average ± 95% CI). There were no significant differences ( p = 0.9634, n = 75, one-way ANOVA). ANOVA, analysis of variance; CI, confidence interval; SAA, subarcuate artery.
When crossing the data of length and laterality, it was observed that the relative proportions and the total frequency of the SAA length remained similar between both sides ( Figs. 5 and 6 ). It is worth mentioning that a correlation between both sides in the same patient was found in 46.8% of the cases, taken into account all the proposed grades, and 70% when SAA was present bilaterally (Grades 1 and 2). Additionally, a duplication of the left SAA was identified in one patient (1.3 and 3.7 mm); only the shortest one was considered for the study given its surgical implication, as detailed in the “Discussion” section. In one case, SAA was identified forming a common trunk with LA.
Fig. 5.
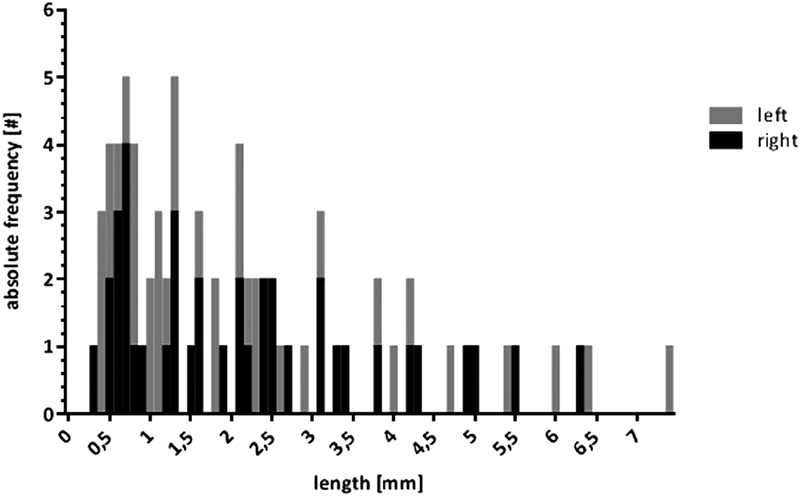
Absolute frequencies of the SAA according to length and laterality. A Gaussian distribution with a peak incidence of 1 mm was evident. SAA, subarcuate artery.
Fig. 6.
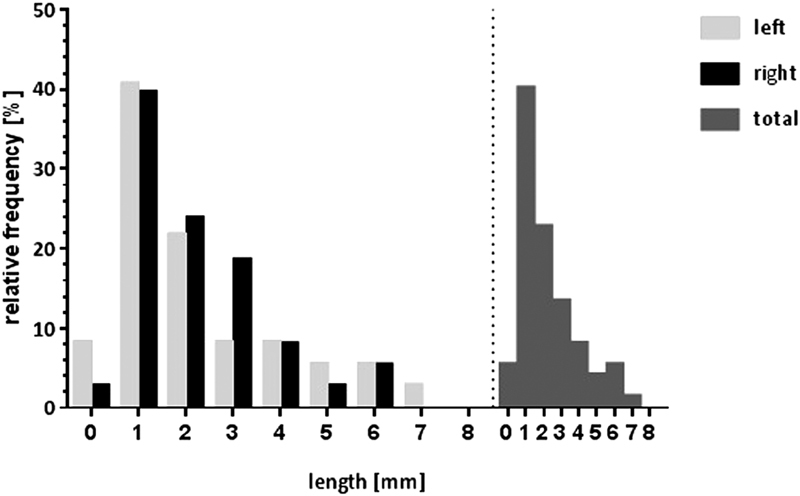
Histogram of relative frequencies of the SAA according to length and laterality. A concordant unimodal distribution was found on both sides, which also was reflected in the global distribution. SAA, subarcuate artery.
Discussion
Anatomical variations are particularly frequent in the vasculature of the posterior fossa. 7 8 15 The descriptions of the lateral pontine segment of the AICA are mainly focused on the meatal loop and the LA in relation to the IAC and the cranial nerves VII and VIII. 1 4 5 9 21 40 41 Contrarily, the SL and the SAA have been referred to in a succinct and isolated way in relation to the SF. 10 11 18 19 20 21 22 23 24 25 26 28 29 However, a gradual spectrum of interaction between the AICA-SAA complex and the petrosal bone can be inferred.
To identify the anatomical disposition of the AICA-SAA complex in adults, the proposed classification was applied to a series of patients studied through MRI. Although AICA is clearly identifiable by this method, 42 the SAA requires special sequences due to its small size. 43 44 In this case, the submillimetric thickness section by CISS sequence allowed identifying it in almost half of the CPA (46.6%), taking into account the inconstancy of this vessel (30–80% according to cadaver studies). 4 8 12 Other diagnostic imaging protocols, such as magnetic resonance angiography with triple contrast, 45 could be useful if a greater degree of precision is required for the interpretation of its route in the preoperative planning. However, the development of high-field resonator systems offers ever greater graphic resolution. Further, studies relating preoperative neuroimages and intraoperative findings are required to better address this issue. Although the comparison was not the aim of our study, we identified the SAA in both 1.5 and 3.0T equipment.
In this work, we take as reference the process of embryogenesis involved in the conformation of the petromastoid region of the temporal bone to propose a gradual, systematized, and hierarchical classification of the AICA-SAA complex. In this way, we describe a more comprehensive perspective of the findings that the neurosurgeon can identify pre- or intraoperatively in the vicinity of the SF, allowing to optimize presurgical planning and thus avoid unnecessary risks, ensuring the therapeutic success ( Table 3 ).
Table 3. Proposed grades, anatomical features, and preferred surgical actions.
| Grade | AICA | SL | SAA | Required maneuvers | Literature frequency | MRI CISS frequency | |
|---|---|---|---|---|---|---|---|
| 0 | Purely cisternal | Unidentifiable | Coagulation and division of the SAA | – | 42.2% | ||
| 1 | Separated from the SF | Loose | Elongated (>3 mm) | 72%–80% | 11.2% | 46.6% | |
| 2 | Near the SF | Pronounced | Short (<3 mm) | 35.4% | |||
| 3 | Adhered to the SF dura mater | “Anomalous” | Unidentifiable or completely included in the PMC | Dissection of the AICA with a dural flap Drilling to release the SAA from the PMC |
1–6% | 10.6% | |
| 4 | Included in the SF petrous bone | Dissection of the AICA with a dural flap Drilling to release the AICA from the SF and/or the SAA from the PMC |
0.6% | ||||
Abbreviations: AICA, anterior inferior cerebellar artery; CISS, constructive interference in steady state; MRI, magnetic resonance imaging; PMC, petromastoid canal; SAA, subarcuate artery; SF, subarcuate fossa; SL, subarcuate loop.
The proposed classification also implies graduality in anatomical complexity. Starting from a classical anatomy, Grade 1 is probably the most favorable for surgery because if a relatively long SAA is identified ( Figs. 1B and 2B ), the cerebellar retraction will have a greater margin of mobilization ( Fig. 3A ), allowing to early identify and divide the SAA if necessary, with space enough to protect the AICA.
Although in Grade 2, the SAA is identifiable ( Figs. 1C and 2C ), the short cisternal trajectory may represent a limitation to cerebellar retraction 1 ( Fig. 3B ), while its coagulation and division also presupposes additional risk of AICA injury.
In our MRI series, Grades 1 and 2 reached together 46.6% of the total, which is between the values published in cadaveric studies 4 8 11 12 and by images. 43 46 Probably, the differences with respect to the reported incidences are due to the resolution of the used technique, as mentioned earlier. In this sense, Grade 0 represented 42.2% of the CPA evaluated by MRI, despite being favorable for the CPA cistern dissection, its surgical implication was uncertain in our study, since it was not possible to confirm the absence of the SAA by other methods. For this reason, even identifying a Grade 0 AICA-SAA complex in the preoperative imaging studies ( Figs. 1A and 2A ), a gentle cerebellar retraction should be the rule in all the cases.
From Grade 3, the anatomical complexity became marked. This pattern, characterized by the adherence or coating of the AICA by dura mater of the petrosal bone ( Figs. 1D , 2D , and 3C ), has been described with a frequency ranging between 1 23 25 and 6%, 21 compared with 10.6% of the cases identified in our MRI results. In this case, although the AICA can be mobilized with the dura mater flap that covers the posterior edge of the acoustic meatus, 2 23 24 the risk of AICA tearing persists. Even achieving a meticulous dissection of the dura mater, the AICA can continue adhering to the petrosal bone by means of a SAA firmly included in the PMC. This situation may require drilling of the temporal bone even though the AICA does not be directly attached to it, to allow a safe release of the intraosseous route of the SAA, which can be obliterated with bone wax after its section. 23
Finally, the situation of greater surgical complexity, Grade 4, when the AICA is totally included in the SF bone, forming an intraosseous trajectory ( Figs. 1E , 2E , and 3D ); these cases require special skills to be able to skeletonize the AICA through meticulous drilling, to release and mobilize it. 23 25 26 28 29
From the embryological perspective, by means of a similar SAA “anchoring” phenomenon that brings the SL closer to the SF in a variable amount, an analogous LA “pulling” mechanism can explain the different positions described for the meatal loop in relation to the IAC: extrameatal, meatal, and intrameatal. However, once the IAC is formed in the embryonic week 18, its patency persists the rest of life, since it is not invaded by mesenchymal tissue because it is not part of the osseous labyrinth, 47 unlike the SF. Therefore, despite it can have an intrameatal course, the dural and/or osseous encasement of the meatal loop within the IAC is exceptional.
Other variations reported in the course of the SAA, such as single SAA-LA 12 40 or SAA-cerebellosubarcuate artery trunks, 4 12 21 were not included expressly in the classification, since they were interpreted as particular situations of Grades 1 and 2. However, the surgeon should be extremely careful when facing them because intentional split or unintentional damage of the common trunks could cause neurological and/or sensory deterioration. 48 49 50 51 Although the sacrifice of the SAA when presented as a single branch has less risk than the common trunks, it can also generate permanent deficits, mainly hearing loss. 12 15 16 17 The presence of a SL arising from the posterior inferior cerebellar artery 25 in the analyzed MRI sequences was not evidenced.
The incidence of the cases corresponding to Grades 3 and 4, referred to in other works such as “anomalous SL,” presented in our study an incidence of 11.2%. Although in these cases, the detection of the AICA, unlike the SAA, offers no problem, it should be mentioned that the differentiation between soft tissues (blood vessels, dura mater, arachnoid) can become difficult in the CISS sequence, 46 particularly in Grade 3.
The application of the concepts and recommendations here described are particularly useful in vestibular schwannoma (VS) surgery because a total resection can only be secured by a transmeatal technique, to fully remove the intracanalicular extension of the tumor. 2 Therefore, several grades of the AICA-SAA complex could prevent the access to the posterior lip of the internal auditory meatus, due to the proximity between the IAC and the SF. Leaving aside VS surgery, the maneuvers aimed to release and mobilize the AICA-SAA complex can be applied discretionally by the surgeon in other any pathology. Often, posterior petrous meningiomas invade the IAC, also requiring IAC posterior wall drilling. 52 On the contrary, petroclival meningiomas surgery also involves the AICA-SAA complex despite the approach selected: if retrosigmoid, large CPA dissection is needed to remove the tumor through the dissected space between neurovascular structures and, eventually, suprameatal tubercle drilling 53 54 ; if transpetrosal, deep temporal bone drilling can injury intrapetrosal arterial courses. Regarding CPA tumors, especially meningiomas, the SAA can be enlarged providing vascular feeding to the tumor, 4 due to anastomotic connections with dural branches of the posterior meningeal artery. 13 In case of microvascular decompression surgery for patients suffering some kind of neuralgia, the mobilization of the AICA-SAA complex is frequently required to recognize and properly decompress neurovascular conflicts despite being produced 55 or not 56 by itself. Regardless of its low incidence, the AICA-SAA complex vascular pathology represents a challenging issue, mainly by distal AICA aneurysms, 57 58 involving both the meatal loop 59 and SL. 60 Anatomic variations in the AICA and the posterior circulation seem to contribute to the development of distal AICA aneurysms, 61 62 as well as high-flow situations, as vascularized tumors, 62 63 arteriovenous fistula, 64 and malformations. 65 66 Also, a case of ruptured aneurysm of a SAA feeding a dural arteriovenous fistula was reported. 64 Isolated AICA aneurysms have been interpreted in two major groups: pontine (proximal, premeatal) and cerebellar (distal, postmeatal) 67 68 ; parent vessel occlusion may lead to life-threatening consequences in the former group 68 ; therefore, these patients are candidates to arterial bypass. 69 Indeed, proximal aneurysm exposure and dissection also require detailed knowledge along the entire AICA and its branches, 67 as well as others posterior circulation aneurysms.
In addition to the specific risks identified for each situation, it is important to mention that also other maneuvers aimed to the dissection of the CPA can be a source of morbidity. For example, the dissection of the petrous dura mater may cause CSF leak and/or meningitis through a persistent PMC, 70 communicated to the mastoid antrum, 35 vestibular aqueduct, or endolymphatic sac. 1 13 The risk of opening the SCC by drilling the posterior wall of the IAC in VS surgery 2 also applies to the petrosal bone drilling for releasing the AICA-SAA complex. In certain approaches (e.g., transpetrosal, translabyrinthine), the preoperative identification is even more important, since the unnoticed course of the intrapetrosal AICA can generate hemorrhages and distal ischemic complications. In these cases, to perform the releasing of the involved vessel under direct vision through a suboccipital craniotomy is highly recommended.
As a limitation of the study, we detected that, as previously mentioned, although the CISS sequence allows to identify submillimetric structures, the differentiation between soft tissues or within bone tissue may not be conclusive in all cases. In addition, the lack of data from intraoperative or postmortem sources prevented corroborating the findings found by MRI, particularly in Grades 0 to 2 regarding the cisternal path of the SAA. To mitigate these limitations, we also consider others anatomical landmarks, fully described in the “Material and Methods” section, to add confidence to our observations; the data collection was performed tracking the path of every structure present on the entire CPA CISS sequence scans.
The low incidence of reported neurovascular conflict (14.9%) in a series of patient followed by TN can lead to infer that the MRI methodology applied is not able to distinguish the neurovascular structures properly. Instead, this value seems to denote the presence of selection bias in the studied sample. As the data come from the Clinical Neurology Department records, the reported incidence refers mainly “surgically unsolved” neurovascular conflicts, and does not reflect the complete population with TN. Besides this explanation, the incidence rises to ∼35% if we consider the number of patients ( n = 84, trigeminal conflict 24, plus 5 patients surgically decompressed) instead the number of evaluated CPA ( n = 161), as reported in the “Results” section.
Finally, since it is a retrospective study based on imaging studies, the determination of the implied risk by each grade of the proposed scale is subject to its application in surgical series; probably, the experience of the neurosurgeon, the volume of patients treated in the center, and the pathology to be treated are major conditioning factors.
Conclusion
The proposed classification allowed to consistently objectify, distinguish, and hierarchize the variations spectrum of the AICA-SAA complex, providing clear directions to a safe management of unfavorable anatomical dispositions through exhaustive preoperative planning and accurately surgical execution.
Footnotes
Conflict of Interest None.
References
- 1.Rhoton A L., JrThe cerebellopontine angle and posterior fossa cranial nerves by the retrosigmoid approach Neurosurgery 200047(3, Suppl):S93–S129. [DOI] [PubMed] [Google Scholar]
- 2.Rhoton A L, Jr, Tedeschi H.Microsurgical anatomy of acoustic neuroma. 2002 Neurosurg Clin N Am 20081902145–174., v [DOI] [PubMed] [Google Scholar]
- 3.Tatagiba M. Stuttgart, Germany: Springer; 2014. Retrosigmoid approach to the posterior and middle fossa; pp. 217–235. [Google Scholar]
- 4.Martin R G, Grant J L, Peace D, Theiss C, Rhoton A L., Jr Microsurgical relationships of the anterior inferior cerebellar artery and the facial-vestibulocochlear nerve complex. Neurosurgery. 1980;6(05):483–507. doi: 10.1227/00006123-198005000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Rhoton A L., JrThe cerebellar arteries Neurosurgery 200047(3, Suppl):S29–S68. [DOI] [PubMed] [Google Scholar]
- 6.Akgun V, Battal B, Bozkurt Y. Normal anatomical features and variations of the vertebrobasilar circulation and its branches: an analysis with 64-detector row CT and 3T MR angiographies. ScientificWorldJournal. 2013;2013:620162. doi: 10.1155/2013/620162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kazawa N, Togashi K, Ito J. The anatomical classification of AICA/PICA branching and configurations in the cerebellopontine angle area on 3D-drive thin slice T2WI MRI. Clin Imaging. 2013;37(05):865–870. doi: 10.1016/j.clinimag.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 8.Kim H N, Kim Y H, Park I Y, Kim G R, Chung I H.Variability of the surgical anatomy of the neurovascular complex of the cerebellopontine angle Ann Otol Rhinol Laryngol 199099(4 Pt 1):288–296. [DOI] [PubMed] [Google Scholar]
- 9.Sampath P, Rini D, Long D M. Microanatomical variations in the cerebellopontine angle associated with vestibular schwannomas (acoustic neuromas): a retrospective study of 1006 consecutive cases. J Neurosurg. 2000;92(01):70–78. doi: 10.3171/jns.2000.92.1.0070. [DOI] [PubMed] [Google Scholar]
- 10.Tekdemir I, Aslan A, Elhan A. The subarcuate canaliculus and its artery--a radioanatomical study. Ann Anat. 1999;181(02):207–211. doi: 10.1016/S0940-9602(99)80009-0. [DOI] [PubMed] [Google Scholar]
- 11.Mazzoni A. The subarcuate artery in man. Laryngoscope. 1970;80(01):69–79. doi: 10.1288/00005537-197001000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Matsushima T. Tokyo: Springer; 2015. The subarcuate artery; pp. 178–180. [Google Scholar]
- 13.Proctor B.The petromastoid canal Ann Otol Rhinol Laryngol 198392(6 Pt 1):640–644. [DOI] [PubMed] [Google Scholar]
- 14.Skrzat J, Leszczyński B, Kozerska M, Wróbel A. Topography and morphometry of the subarcuate canal. Folia Morphol (Warsz) 2013;72(04):357–361. doi: 10.5603/fm.2013.0059. [DOI] [PubMed] [Google Scholar]
- 15.Chen M M, Chen S R, Diaz-Marchan P, Schomer D, Kumar V A. Anterior inferior cerebellar artery strokes based on variant vascular anatomy of the posterior circulation: clinical deficits and imaging territories. J Stroke Cerebrovasc Dis. 2018;27(04):e59–e64. doi: 10.1016/j.jstrokecerebrovasdis.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Lescanne E, Velut S, Lefrancq T, Destrieux C. The internal acoustic meatus and its meningeal layers: a microanatomical study. J Neurosurg. 2002;97(05):1191–1197. doi: 10.3171/jns.2002.97.5.1191. [DOI] [PubMed] [Google Scholar]
- 17.Mom T, Chazal J, Gabrillargues J, Gilain L, Avan P. Cochlear blood supply: an update on anatomy and function. Fr ORL. 2005;88:81–88. [Google Scholar]
- 18.Akyol Y, Galheigo D, Massimore M, Fatterpekar G. Subarcuate artery and canal: an important anatomic variant. J Comput Assist Tomogr. 2011;35(06):688–689. doi: 10.1097/RCT.0b013e318234232a. [DOI] [PubMed] [Google Scholar]
- 19.Chen K, Lyu H, Yang L, Zhang T, Dai P. Morphological variation of subarcuate artery and canal in atresia. ORL J Otorhinolaryngol Relat Spec. 2016;78(05):276–280. doi: 10.1159/000450651. [DOI] [PubMed] [Google Scholar]
- 20.Grammatica A, Alicandri-Ciufelli M, Molteni G, Marchioni D, Presutti L. Subarcuate canal and artery: a case report. Surg Radiol Anat. 2010;32(02):171–174. doi: 10.1007/s00276-009-0527-6. [DOI] [PubMed] [Google Scholar]
- 21.Mazzoni A, Hansen C C. Surgical anatomy of the arteries of the internal auditory canal. Arch Otolaryngol. 1970;91(02):128–135. doi: 10.1001/archotol.1970.00770040198005. [DOI] [PubMed] [Google Scholar]
- 22.Nager G T. Origins and relations of the internal auditory artery and the subarcuate artery. Ann Otol Rhinol Laryngol. 1954;63(01):51–61. doi: 10.1177/000348945406300104. [DOI] [PubMed] [Google Scholar]
- 23.Goel A, Sekhar L N. Anomalous subarcuate loop. Technical note. J Neurosurg. 1991;75(06):985–986. doi: 10.3171/jns.1991.75.6.0985. [DOI] [PubMed] [Google Scholar]
- 24.Tatagiba M S, Evangelista-Zamora R, Lieber S. Mobilization of the anterior inferior cerebellar artery when firmly adherent to the petrous dura mater-a technical nuance in retromastoid transmeatal vestibular schwannoma surgery: 3-dimensional operative video. Oper Neurosurg (Hagerstown) 2018;15(05):E58–E59. doi: 10.1093/ons/opy052. [DOI] [PubMed] [Google Scholar]
- 25.Warren D T, Warren M D, Malfair D, Akagami R.An incidence of anteroinferior cerebellar artery/posteroinferior cerebellar artery anatomic variants penetrating the subarcuate fossa dura: operative technique and identification with 3-dimensional fast imaging employing steady-state acquisition magnetic resonance imagingNeurosurgery 2010;66(6, Suppl Operative):199–203, discussion 204 [DOI] [PubMed]
- 26.Campero Á, Rasmussen J, Diloné J, Ajler P, Elizalde R L. [Drilling of the subarcuate fossa to release the anterior inferior cerebellar artery in a surgery of a vestibular Schwannoma] Surg Neurol Int. 2018;9 03:S66–S72. doi: 10.4103/sni.sni_219_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erdogan N, Altay C, Akay E. MRI assessment of internal acoustic canal variations using 3D-FIESTA sequences. Eur Arch Otorhinolaryngol. 2013;270(02):469–475. doi: 10.1007/s00405-012-1994-7. [DOI] [PubMed] [Google Scholar]
- 28.Ovenden C, Barker O, Bramwell J. Bilateral aberrant infratentorial vasculature : a rare cadaveric encounter. Eur J Anat. 2015;19(03):295–298. [Google Scholar]
- 29.Tanriover N, Rhoton A L., JrThe anteroinferior cerebellar artery embedded in the subarcuate fossa: a rare anomaly and its clinical significance Neurosurgery 20055702314–319., discussion 314–319 [DOI] [PubMed] [Google Scholar]
- 30.Som P M, Curtin H D, Liu K, Mafee M F. Current embryology of the temporal bone, part I: the inner ear. Neurographics. 2016;6(04):250–265. [Google Scholar]
- 31.Hilding D A. Petrous apex and subarcuate fossa maturation. Laryngoscope. 1987;97(10):1129–1135. doi: 10.1288/00005537-198710000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Menshawi K, Mohr J P, Gutierrez J. A functional perspective on the embryology and anatomy of the cerebral blood supply. J Stroke. 2015;17(02):144–158. doi: 10.5853/jos.2015.17.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemzek W R, Brodie H A, Chong B W. Imaging findings of the developing temporal bone in fetal specimens. AJNR Am J Neuroradiol. 1996;17(08):1467–1477. [PMC free article] [PubMed] [Google Scholar]
- 34.Kenis C, Ditchfield M, Paul E, Parizel P M, Stuckey S. The petromastoid canal in the young child: appearance on computed tomography. Int J Pediatr Otorhinolaryngol. 2013;77(05):803–807. doi: 10.1016/j.ijporl.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Koral K, Vachha B, Gimi B. MRI of the petromastoid canal in children. J Magn Reson Imaging. 2014;39(04):966–971. doi: 10.1002/jmri.24236. [DOI] [PubMed] [Google Scholar]
- 36.Maślanka M, Skadorwa T, Ciszek B. Postnatal development of the subarcuate fossa and subarcuate canaliculus-a computed tomographic study. Surg Radiol Anat. 2018;40(10):1111–1117. doi: 10.1007/s00276-018-2045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Migirov L, Kronenberg J. Radiology of the petromastoid canal. Otol Neurotol. 2006;27(03):410–413. doi: 10.1097/00129492-200604000-00019. [DOI] [PubMed] [Google Scholar]
- 38.Tomaszewski K A, Henry B M, Kumar Ramakrishnan P. Development of the Anatomical Quality Assurance (AQUA) checklist: guidelines for reporting original anatomical studies. Clin Anat. 2017;30(01):14–20. doi: 10.1002/ca.22800. [DOI] [PubMed] [Google Scholar]
- 39.Peacock J L, Peacock P J. New York, USA: Oxford University Press, Inc.; 2011. Sample size for estimation studies: proportions; pp. 60–61. [Google Scholar]
- 40.Haidara A, Peltier J, Zunon-Kipré Y, N'da H A, Drogba L, Gars D L. Microsurgical anatomy of the labyrinthine artery and clinical relevance. Turk Neurosurg. 2015;25(04):539–543. doi: 10.5137/1019-5149.JTN.9136-13.0. [DOI] [PubMed] [Google Scholar]
- 41.Rodríguez-Hernández A, Rhoton A L, Jr, Lawton M T. Segmental anatomy of cerebellar arteries: a proposed nomenclature. Laboratory investigation. J Neurosurg. 2011;115(02):387–397. doi: 10.3171/2011.3.JNS101413. [DOI] [PubMed] [Google Scholar]
- 42.Held P, Fellner C, Fellner F, Seitz J, Strutz J. MRI of inner ear anatomy using 3D MP-RAGE and 3D CISS sequences. Br J Radiol. 1997;70(833):465–472. doi: 10.1259/bjr.70.833.9227227. [DOI] [PubMed] [Google Scholar]
- 43.Casselman J W, Kuhweide R, Deimling M, Ampe W, Dehaene I, Meeus L. Constructive interference in steady state-3DFT MR imaging of the inner ear and cerebellopontine angle. AJNR Am J Neuroradiol. 1993;14(01):47–57. [PMC free article] [PubMed] [Google Scholar]
- 44.Graf H, Schick F, Claussen C D, Seemann M D. MR visualization of the inner ear structures: comparison of 1.5 Tesla and 3 Tesla images. RoFo Fortschr Geb Rontgenstr Nuklearmed. 2004;176(01):17–20. doi: 10.1055/s-2004-814672. [DOI] [PubMed] [Google Scholar]
- 45.Naganawa S, Koshikawa T, Nakamura T, Fukatsu H, Ishigaki T, Aoki I. High-resolution T1-weighted 3D real IR imaging of the temporal bone using triple-dose contrast material. Eur Radiol. 2003;13(12):2650–2658. doi: 10.1007/s00330-003-1922-8. [DOI] [PubMed] [Google Scholar]
- 46.Chung E C, Choi H Y, Lee J S, Ko E J, Lee M S. Constructive interference in steady state(CISS) 3DFT MR imaging of the inner ear and adjacent structures. J Korean Radiol Soc. 1997;36(03):385–391. [Google Scholar]
- 47.Som P M, Curtin H D, Liu K, Mafee M F. Current embryology of the temporal bone, part II: the middle and external ears, the statoacoustic and facial nerves, and when things go developmentally wrong. Neurographics. 2016;6(05):332–349. [Google Scholar]
- 48.Kim J S, Lopez I, Liu F, DiPatre P L, Baloh R W, Ishiyama A. Internal auditory artery infarction: clinicopathologic correlation. Neurology. 2012;52(01):40–40. doi: 10.1212/wnl.52.1.40. [DOI] [PubMed] [Google Scholar]
- 49.Kim J S, Cho K H, Lee H.Isolated labyrinthine infarction as a harbinger of anterior inferior cerebellar artery territory infarction with normal diffusion-weighted brain MRI J Neurol Sci 2009278(1-2):82–84. [DOI] [PubMed] [Google Scholar]
- 50.Mom T, Telischi F F, Martin G K, Stagner B B, Lonsbury-Martin B L. Vasospasm of the internal auditory artery: significance in cerebellopontine angle surgery. Am J Otol. 2000;21(05):735–742. [PubMed] [Google Scholar]
- 51.Sando I, Ogawa A, Jafek B W.Inner ear pathology following injury to the eighth cranial nerve and the labyrinthine artery Ann Otol Rhinol Laryngol 198291(2 Pt 1):136–141. [DOI] [PubMed] [Google Scholar]
- 52.Mehdorn H M, Buhl R M. London: Springer London; 2009. Petrous meningiomas I: an overview; pp. 433–441. [Google Scholar]
- 53.Samii M, Tatagiba M, Carvalho G A. Resection of large petroclival meningiomas by the simple retrosigmoid route. J Clin Neurosci. 1999;6(01):27–30. doi: 10.1054/jocn.1997.0201. [DOI] [PubMed] [Google Scholar]
- 54.Singh N, Singh D K, Ahmad F, Kumar R. The retrosigmoid approach: workhorse for petroclival meningioma surgery. Asian J Neurosurg. 2019;14(01):188–192. doi: 10.4103/ajns.AJNS_192_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferroli P, Messina G, Franzini A, Broggi G.VII-VIII nerve complex hung up by the subarcuate artery: a cause of hemifacial spasm Acta Neurochir (Wien) 200714906633–635., discussion 635 [DOI] [PubMed] [Google Scholar]
- 56.Cheng C-Y, Shetty R, Martinez V, Sekhar L N. Microvascular decompression of facial nerve and pexy of the left vertebral artery for left hemifacial spasm: 3-dimensional operative video. Oper Neurosurg (Hagerstown) 2019;16(01):E2–E3. doi: 10.1093/ons/opy058. [DOI] [PubMed] [Google Scholar]
- 57.Rodríguez-Hernández A, Zador Z, Rodríguez-Mena R, Lawton M T.Distal aneurysms of intracranial arteries: application of numerical nomenclature, predilection for cerebellar arteries, and results of surgical management World Neurosurg 201380(1-2):103–112. [DOI] [PubMed] [Google Scholar]
- 58.Andaluz N, Pensak M L, Zuccarello M.Multiple, peripheral aneurysms of the anterior inferior cerebellar artery Acta Neurochir (Wien) 200514704419–422., discussion 422 [DOI] [PubMed] [Google Scholar]
- 59.Sun Y, Wrede K H, Chen Z, Bao Y, Ling F. Ruptured intrameatal AICA aneurysms--a report of two cases and review of the literature. Acta Neurochir (Wien) 2009;151(11):1525–1530. doi: 10.1007/s00701-009-0269-6. [DOI] [PubMed] [Google Scholar]
- 60.Takeuchi S, Takasato Y, Masaoka H. [Trapping of ruptured dissecting aneurysm of distal anterior inferior cerebellar artery--case report] Brain Nerve. 2009;61(02):203–207. [PubMed] [Google Scholar]
- 61.Tokimura H, Ishigami T, Yamahata H.Clinical presentation and treatment of distal anterior inferior cerebellar artery aneurysms Neurosurg Rev 20123504497–503., discussion 503–504 [DOI] [PubMed] [Google Scholar]
- 62.Saito A, Nishino A, Suzuki I. Subarachnoid hemorrhage caused by rupture of a distal anterior inferior cerebellar artery aneurysm--three case reports. Neurol Med Chir (Tokyo) 2008;48(11):506–511. doi: 10.2176/nmc.48.506. [DOI] [PubMed] [Google Scholar]
- 63.Guzman R, Grady M S. An intracranial aneurysm on the feeding artery of a cerebellar hemangioblastoma. Case report. J Neurosurg. 1999;91(01):136–138. doi: 10.3171/jns.1999.91.1.0136. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki T, Okamoto K, Genkai N, Ito Y, Abe H. Multiple aneurysms on the subarcuate artery arising from the anterior inferior cerebellar artery in a patient with a Borden type I transverse-sigmoid dural arteriovenous fistula manifesting as subarachnoid hemorrhage: a case report. Interv Neuroradiol. 2019;25(01):90–96. doi: 10.1177/1591019918799299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menovsky T, André Grotenhuis J, Bartels R HMA. Aneurysm of the anterior inferior cerebellar artery (AICA) associated with high-flow lesion: report of two cases and review of literature. J Clin Neurosci. 2002;9(02):207–211. doi: 10.1054/jocn.2001.0945. [DOI] [PubMed] [Google Scholar]
- 66.Lee S J, Koh J S, Ryu C W, Lee S H. Ruptured intrameatal aneurysm of the anterior inferior cerebellar artery accompanying an arteriovenous malformation: a case report. Cerebellum. 2012;11(03):808–812. doi: 10.1007/s12311-011-0349-z. [DOI] [PubMed] [Google Scholar]
- 67.Bambakidis N C, Manjila S, Dashti S, Tarr R, Megerian C A. Management of anterior inferior cerebellar artery aneurysms: an illustrative case and review of literature. Neurosurg Focus. 2009;26(05):E6. doi: 10.3171/2009.1.FOCUS0915. [DOI] [PubMed] [Google Scholar]
- 68.Gi H, Inoha S, Uno J. [Four cases of direct surgery for anterior inferior cerebellar artery aneurysms] No Shinkei Geka. 2007;35(06):571–578. [PubMed] [Google Scholar]
- 69.Fujimura M, Inoue T, Shimizu H, Tominaga T. Occipital artery-anterior inferior cerebellar artery bypass with microsurgical trapping for exclusively intra-meatal anterior inferior cerebellar artery aneurysm manifesting as subarachnoid hemorrhage. Case report. Neurol Med Chir (Tokyo) 2012;52(06):435–438. doi: 10.2176/nmc.52.435. [DOI] [PubMed] [Google Scholar]
- 70.Remenschneider A K, Kozin E D, Curtin H, Santos F. Histopathology of idiopathic lateral skull base defects. Laryngoscope. 2015;125(08):1798–1806. doi: 10.1002/lary.25366. [DOI] [PubMed] [Google Scholar]


