Abstract
Background
People with HIV (HIV+) may have increased cardiovascular event rates compared with HIV-negative (HIV-) persons. Cross-sectional data from the United States and Switzerland, based on coronary artery calcium scan (CAC) and coronary computed tomography angiography (CCTA), suggest, respectively, increased and similar prevalence of subclinical atherosclerosis in HIV+ vs HIV- persons.
Methods
We repeated CAC/CCTA in 340 HIV+ and 90 HIV- study participants >2 years after baseline CAC/CCTA. We assessed the association of HIV infection, Framingham risk score (FRS), and HIV-related factors with the progression of subclinical atherosclerosis.
Results
HIV+ were younger than HIV- participants (median age, 52 vs 56 years; P < .01) but had similar median 10-year FRS (8.9% vs 9.0%; P = .82); 94% had suppressed HIV viral load. In univariable and multivariable analyses, FRS was associated with the incidence rate ratio (IRR) of new subclinical atherosclerosis at the follow-up CAC/CCTA, but HIV infection was not: any plaque (adjusted IRR for HIV+ vs HIV- participants, 1.21; 95% CI, 0.62–2.35), calcified plaque (adjusted IRR for HIV+ vs HIV- participants, 1.06; 95% CI, 0.56–2), noncalcified/mixed plaque (adjusted IRR for HIV+ vs HIV- participants, 1.24; 95% CI, 0.69–2.21), and high-risk plaque (adjusted IRR for HIV+ vs HIV- participants, 1.46; 95% CI, 0.66–3.20). Progression of CAC score between baseline and follow-up CAC/CCTA was similar in HIV+ (median annualized change [interquartile range {IQR}], 0.41 [0–10.19]) and HIV- participants (median annualized change [IQR], 2.38 [0–16.29]; P = .11), as was progression of coronary segment severity score (HIV+: median annualized change [IQR], 0 [0–0.47]; HIV-: median annualized change [IQR], 0 [0–0.52]; P = .10) and coronary segment involvement score (HIV+: median annualized change [IQR], 0 [0–0.45]; HIV-: median annualized change [IQR], 0 [0–0.41]; P = .25).
Conclusions
In this longitudinal CAC/CCTA study from Switzerland, Framingham risk score was associated with progression of subclinical atherosclerosis, but HIV infection was not.
Keywords: cardiovascular disease, coronary CT angiography, HIV, longitudinal study, subclinical atherosclerosis
A major concern in HIV-positive (HIV+) persons includes accelerated atherosclerosis, as underlined by increased rates of coronary artery disease (CAD), compared with HIV-negative (HIV-) persons. The authors of a recent meta-analysis of the global CAD burden estimated the CAD risk to be 2.16-fold elevated in HIV+ compared with HIV- persons [1], and US guidelines consider that this risk increase persists even with successful antiretroviral therapy (ART) [2]. Accelerated atherosclerosis in HIV may be mediated by pro-inflammatory and pro-coagulant effects in the context of immune activation, deleterious viral effects, dyslipidemia, insulin resistance, and increased platelet reactivity due to certain ART agents, high rates of smoking and other substance use, and genetic factors [3–6].
Considerable interest has therefore been generated by the notion of early diagnosis of subclinical atherosclerosis in HIV+ persons using coronary computed tomography angiography (CCTA), which can accurately and reproducibly detect noncalcified plaque when compared with intravascular ultrasound [7]. Noncalcified plaque more accurately predicts cardiovascular events than coronary artery calcium (CAC) score or carotid intima-media thickness [8]. Three recent, large, cross-sectional CCTA studies, conducted in the United States [9, 10] and by us in Switzerland [11], however, have not uniformly shown more subclinical atherosclerosis in HIV+ compared with HIV- persons, highlighting the need for additional, especially longitudinal, studies. Our aims were therefore to investigate whether subclinical atherosclerosis progresses more rapidly over a follow-up period of ≥2 years in HIV+ compared with HIV- persons in Switzerland using CAC/CCTA and to evaluate associations of atherosclerosis progression with Framingham risk score and HIV infection.
METHODS
Patient Consent Statement
All participants provided written informed consent. The study was approved by the local ethics committees (Kantonale Ethikkommission Zürich, KEK-ZH Nr. 2013-0103; Commission Cantonale d’Éthique de la Recherché sur l’Être Humain, No. de Référence CER 13–194).
Study Design and Study Participants
We investigated the association of HIV infection with subclinical CAD by noncontrast computed tomography (CT) scan for calculating the CAC score and by CCTA. We obtained baseline CAC/CCTA scans from 10/2013 to 7/2016 and published the main results in 2018 [11] and a detailed analysis of ART agents associated with subclinical atherosclerosis in 2019 [12]. Enrollment criteria for baseline CAC/CCTA included no documented CAD/stroke, GFR ≥50 mL/min, no allergy to iodinated contrast agent, and no history of atrial fibrillation or other irregular cardiac rhythm. HIV+ persons were aged ≥45 years and were asymptomatic participants of the Metabolism and Aging Core Project of the Swiss HIV Cohort Study (www.shcs.ch), and HIV- participants were referred for clinically indicated CAC/CCTA. As previously reported [11], we periodically adjusted selection criteria for the HIV- participants for the baseline CCTA/CAC, resulting in HIV+ and HIV- participants having similar median FRS at baseline (8.9% vs 9.0%; P = .82).
Follow-up CAC/CCTA scans were performed from 10/2015 to 04/2019. The minimum interval between CAC/CCTA scans was 2 years based on previous studies that employed interscan intervals of 1–2.2 years [13, 14, 15, 16], which allowed the detection of annual CAC score increases of 24%–38% in HIV- populations using older-generation CT scanners [15, 17, 18, 19]. A 2-year minimal CCTA interval has been successfully applied by others [20, 21].
HIV+ and HIV- participants were similar at baseline with regards to age, gender, and Framingham risk score (FRS) [22]. HIV+ participants were invited at routine HIV clinic visits and HIV- participants were invited by the study nurse via phone call to undergo follow-up CAC/CCTA. Ineligibility for follow-up CAC/CCTA in HIV+ and HIV- participants included coronary stenosis >50% at baseline scan, cardiovascular event in the interval, eGFR <50 mL/min, unwillingness to undergo repeat CAC/CCTA, death, or loss to follow-up.
Data Collection
For HIV+ participants, data were collected within the SHCS, a prospective cohort study that has continuously enrolled HIV+ adults since 1988 [23]. Demographic, clinical, and laboratory data are collected every 6 months using a standardized protocol, including detailed information on cardiovascular events, hypertension, diabetes mellitus, smoking, alcohol and drug use, and antiretroviral and nonantiretroviral medication. For HIV- participants, additional clinical information was obtained by chart review at the baseline CAC/CCTA using a structured questionnaire that included the indication for CAC/CCTA referral at baseline, verification of inclusion criteria, comorbidities, medication, smoking, alcohol, and drug use. Vital signs, fasting total, low-density lipoprotein cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, glucose, and creatinine were measured at the time of each CAC/CCTA in all participants.
Cardiac Imaging
Cardiac imaging was performed at University Hospitals Zurich and Geneva, as previously reported [11, 12]. Because of personal and institutional restructuring events, we were able to recruit HIV- participants only at the Zurich site to undergo follow-up CAC/CCTA.
Statistical Analysis
Co-variables were defined as previously reported [11, 12]. In addition, we calculated the area under the individual HIV viral load curves and determined the maximum HIV viral load between the 2 CAC/CCTA scans for each HIV+ participant. Characteristics of HIV+ and HIV- participants were compared using the chi-square/Fisher exact test for categorical variables and Wilcoxon rank-sum test for continuous variables. Follow-up time was measured between the 2 CAC/CCTA scans. To evaluate the association between HIV infection and subclinical atherosclerosis, we analyzed the incidence per 100 patient-years of subclinical CAD in participants without subclinical CAD at baseline CAC/CCTA using the same 5 separate categorical outcomes as in the baseline analyses [11, 12]; that is, (1) CAC score >0, (2) any CCTA-detected plaque, (3) calcified plaque, (4) noncalcified/mixed plaque, and (5) high-risk plaque, which was defined as plaque with positive remodeling (remodeling index ≥1.1) and/or low-attenuation plaque (≤30 Hounsfield units) [24]. Incidence rate ratios (IRRs) were calculated with univariable Poisson regression and FRS-adjusted Poisson regression. The main study end point was the FRS-adjusted IRR for noncalcified/mixed plaque, when comparing the HIV+ and HIV- participants. FRS adjustment was chosen over multivariable models including individual cardiovascular risk factors to reduce the number of variables in the model and because several individual risk factors were correlated, interfering with correct interpretation. Further, using FRS for cardiocascular risk stratification facilitates comparison of our results with studies from the United States and elsewhere. We also performed power considerations by evaluating whether a virtual increase (ie, duplication) of the limited numbers of HIV- participants would substantially narrow the confidence intervals of the FRS-adjusted estimates. Segment involvement score (SIS) was calculated using 1 point for each coronary segment with any plaque; segment severity score (SSS) was calculated using the total of all segments scored according to lesion severity [11]. The annualized changes of CAC scores, SIS, and SSS for HIV+ and HIV- participants were compared using Wilcoxon rank-sum tests. Statistical analyses were done using Stata/SE 16.0 (StataCorp, College Station, TX, USA).
RESULTS
Study Population
Of the 704 study participants (428 HIV+, 276 HIV-) who underwent baseline CAC/CCTA [11], 430 participants had follow-up CAC/CCTA done: These included 340 HIV+ (278/355 [78%] Zurich participants and 62/73 [85%] Geneva participants) and 90 HIV- (90/187 [48%] Zurich participants and 0/89 Geneva participants) with baseline CAC/CCTA. Participant disposition is shown in Table 1. There was no evidence for a clinically relevant difference in mean (IQR) baseline FRS for HIV- participants who did or did not undergo follow-up CAC/CCTA, that is, 10.2 (5.5–13.4) vs 11.3 (6.7–15.2; P = .11), or for HIV+ participants who did or did not undergo follow-up CAC/CCTA, that is, mean (IQR) baseline FRS, 10.5 (5.9–13.8) vs 11.3 (6.7–15.1; P = .13). Compared with participants who did not have follow-up CAC/CCTA done (n = 274), the study population included in the present report (ie, participants who had follow-up CAC/CCTA done; n = 430) was slightly younger, more likely to smoke, had less diabetes, had less dyslipidemia, and had less high-risk plaque at baseline (Supplementary Table 1).
Table 1.
Study Flowchart
The median interval between baseline and follow-up scans (IQR) was 2.2 (2.1–2.4) years and 3.4 (2.7–3.6) years in HIV+ and HIV- participants, respectively. Therefore, all analyses are based on annualized rates of change between baseline and second CCTA/CAC. Median effective radiation exposure due to follow-up CAC/CCTA (IQR) was 1.3 (1.1–1.5) mSv. Baseline characteristics of the participants are shown in Table 2. HIV+ participants were more likely to be men, were younger, and had lower body mass index. HIV+ participants had a lower prevalence of hypertension and lower HDL cholesterol levels, and they were more likely to smoke and use drugs. However, median FRS was similar among HIV+ and HIV- participants, as were percentages of participants in the low-, intermediate-, and high-risk FRS categories. Among the HIV+ participants, men who had sex with men were the predominant group, 20% had prior AIDS-defining events, and 94% were on ART, of whom 94% had an undetectable viral load.
Table 2.
Characteristics of HIV-Positive and HIV-Negative Study Participants
| Characteristic | All Participants (n = 430) | HIV+ Participants (n = 340) | HIV- Participants (n = 90) | P Value |
|---|---|---|---|---|
| Male sex, No. (%) | 361 (84.0) | 290 (85.3) | 71 (78.9) | .10a |
| Age, y | 53 (49–58) | 52 (49–57) | 56 (50–62) | <.01b |
| Ethnicity | <.01a | |||
| White | 395 (91.9) | 309 (90.9) | 86 (95.6) | |
| Black | 26 (6) | 22 (6.5) | 4 (4.4) | |
| Other | 9 (2.1) | 9 (2.6) | 0 | |
| Body mass index, kg/m2 | 25.1 (23.0–28.0) | 24.9 (22.8–27.8) | 26.1 (23.8–28.6) | .01b |
| <18.5 | 9 (2.1) | 8 (2.4) | 1 (1.1) | .33a |
| ≥18.5–<25 | 203 (47.2) | 167 (49.1) | 36 (40.0) | |
| ≥25–<30 | 169 (39.3) | 130 (38.2) | 39 (43.3) | |
| ≥30 | 49 (11.4) | 35 (10.3) | 14 (15.6) | |
| Hypertension | 171 (39.8) | 113 (33.2) | 58 (64.4) | <.01a |
| Diabetes mellitus | 15 (3.5) | 12 (3.5) | 3 (3.3) | .61a |
| Dyslipidemia | 166 (38.6) | 129 (37.9) | 37 (41.1) | .33a |
| Total cholesterol, mmol/L | 5.2 (4.6–5.9) | 5.2 (4.6–5.8) | 5.3 (4.6–6.0) | .32b |
| HDL cholesterol, mmol/L | 1.3 (1.1–1.7) | 1.3 (1.1–1.6) | 1.4 (1.2–1.8) | <.01b |
| LDL cholesterol, mmol/L | 3.1 (2.5–3.7) | 3.1 (2.5–3.6) | 3.2 (2.5–3.8) | .56b |
| Triglycerides, mmol/L | 1.4 (1.0–2.1) | 1.4 (1.0–2.1) | 1.4 (0.9–1.9) | .27b |
| Lipid-lowering drug use | ||||
| At baseline CCTA/CAC | 34/430 (7.9)c | 18/340 (5.3) | 16/90 (17.8) | <.01a |
| Started thereafter | 70/396 (17.7)d | 52/322 (16.2) | 18/74 (24.3) | .13a |
| Current smoking | 134 (31.2) | 123 (36.2) | 11 (12.2) | <.01a |
| Alcohol consumption | .13a | |||
| None/mild | 330 (78.6) | 269 (80) | 61 (72.6) | |
| Moderate | 85 (20.2) | 62 (18.5) | 23 (27.4) | |
| Severe | 5 (1.2) | 5 (1.5) | 0 | |
| Active illicit drug use | 11 (2.6) | 11 (3.2) | 0 | .08a |
| Framingham risk score (10-y risk) | 8.9 (5.7–13.8) | 8.9 (5.9–13.8) | 9.0 (5.5–13.4) | .82b |
| <10% | 241 (56.1) | 190 (55.9) | 51 (56.7) | 1.00a |
| 10%–20% | 154 (35.8) | 122 (35.9) | 32 (35.6) | |
| >20% | 35 (8.1) | 28 (8.2) | 7 (7.8) | |
| HIV-specific characteristics | ||||
| HIV acquisition mode | ||||
| MSM | 204 (60) | |||
| IDU | 34 (10) | |||
| heterosexual | 94 (27.7) | |||
| Other/unknown | 8 (2.4) | |||
| Years HIV-infected | 15.1 (6.6–21.8) | |||
| Prior AIDS | 69 (20.3) | |||
| CD4 current, cells/µL | 600 (447–752) | |||
| CD4 nadir, cells/µL | 190 (90–282) | |||
| CD4 nadir <50 cells/µL | 55 (16.2) | |||
| HIV viral load max >100 000 copies/mL | 218 (64.1) | |||
| HIV viral load-years >50 copies/mL between baseline and follow-up CAC/CCTA | 18 (5.7) | |||
| Maximum log10 viral load between baseline and follow-up CAC/CCTA among those with viral load >50 copies/mL | 1.67 (1.48–2.08) | |||
| On antiretroviral therapy | 318 (93.5) | |||
| On ART, undetectable HIV viral load | 300 (94.3) | |||
| ART naïve | 4 (1.2) | |||
| ART interrupted | 18 (5.3) | |||
| Total years on ART | 11.6 (5.3–17.7) | |||
| Hepatitis C seropositivity | 48 (14.1) |
All values shown were obtained at the time of baseline cardiac imaging, unless stated otherwise. Data are presented as No. (%) or median (IQR).
Abbreviations: ART, antiretroviral therapy; CAC/CCTA, coronary artery calcium scan/coronary computed tomography angiography; HDL, high-density lipoprotein; IDU, injection drug use; LDL, low-density lipoprotein; MSM, men who have sex with men.
aFisher exact test.
bWilcoxon rank-sum test.
cStatin in all participants except 1 HIV+ participant on a fibrate.
dStatins in all participants.
Progression of Subclinical CAD in Patients With Subclinical CAD at Baseline CAC/CCTA
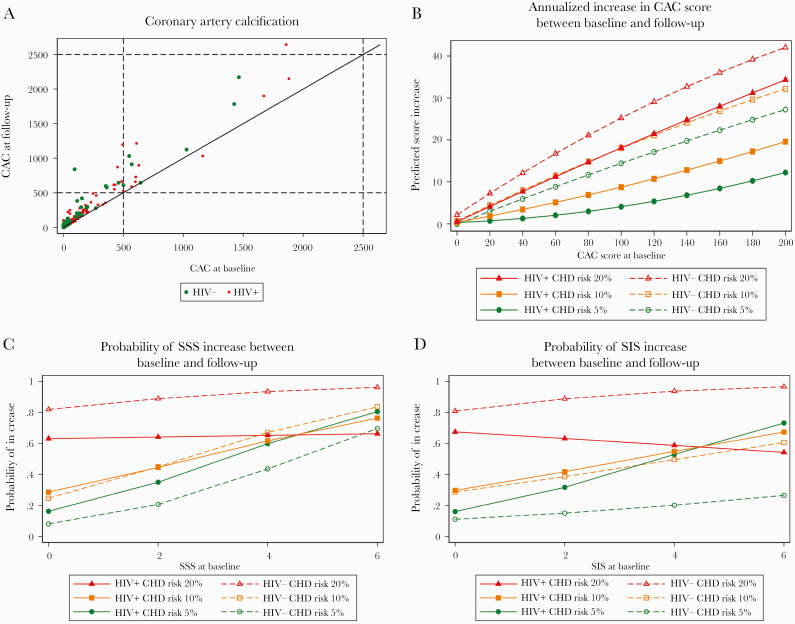
At follow-up scan, CAC increased as a function of baseline CAC (Figure 1A) and baseline FRS category (Figure 1B). Similarly, at follow-up scan, coronary SSS and SIS increased as a function of baseline SSS, baseline SIS, and baseline FRS category (Figure 1C and D; Supplementary Figure 1C and D). Visual inspection of Figure 1B–D suggests no effect of HIV infection on CAC, SSS, or SIS progression.
Figure 1.
Association of baseline CAC score, FRS, SSS, and SIS with measurements at follow-up CAC/CCTA in HIV+ and HIV- study participants. A, Relationship between CAC at baseline and follow-up scans. B, Regression analysis of annualized CAC increase from baseline to follow-up CAC determination. Marginal effects from robust regression analysis with interaction terms of CAC at visit 1, 1-year Framingham risk at visit 1, and HIV status. C, Probability of SSS increase from baseline to follow-up CCTA. Marginal effects from logistic regression analysis with interaction terms of SSS at baseline CCTA, 1-year Framingham risk at baseline CCTA, and HIV status. For visual clarity, no 95% CIs are shown. For figures with 95% CI, see Supplementary Figure 1C. D, Probability of SIS increase from baseline to follow-up CCTA. Marginal effects from logistic regression analysis with interaction terms of SIS at visit 1, 1-year Framingham risk at visit 1, and HIV status. For visual clarity, no 95% confidence intervals are shown. For figures with 95% CIs, see Supplementary Figure 1D. Abbreviations: CAC, coronary artery calcium scan; CCTA, coronary computed tomography angiography; FRS, Framingham risk score; SIS, segment involvement score; SSS, segment severity score.
Progression of Subclinical CAD, Median Annualized Change
HIV infection was not associated with progression of CAC score, SSS, or SIS between baseline and follow-up CAC/CCTA. The median annualized change (IQR) in CAC score was 0.41 (0–10.19) in HIV+ and 2.38 (0–16.29) in HIV- study participants (P = .11). The median annualized changes (IQR) in SSS and SIS were 0 (0–0.47) and 0 (0–0.52; P = .10), and 0 (0–0.45) and 0 (0–0.41), respectively (P = .25).
Annualized Incidence Rate of Subclinical Atherosclerosis at Follow-up CAC/CCTA, Univariable Analysis
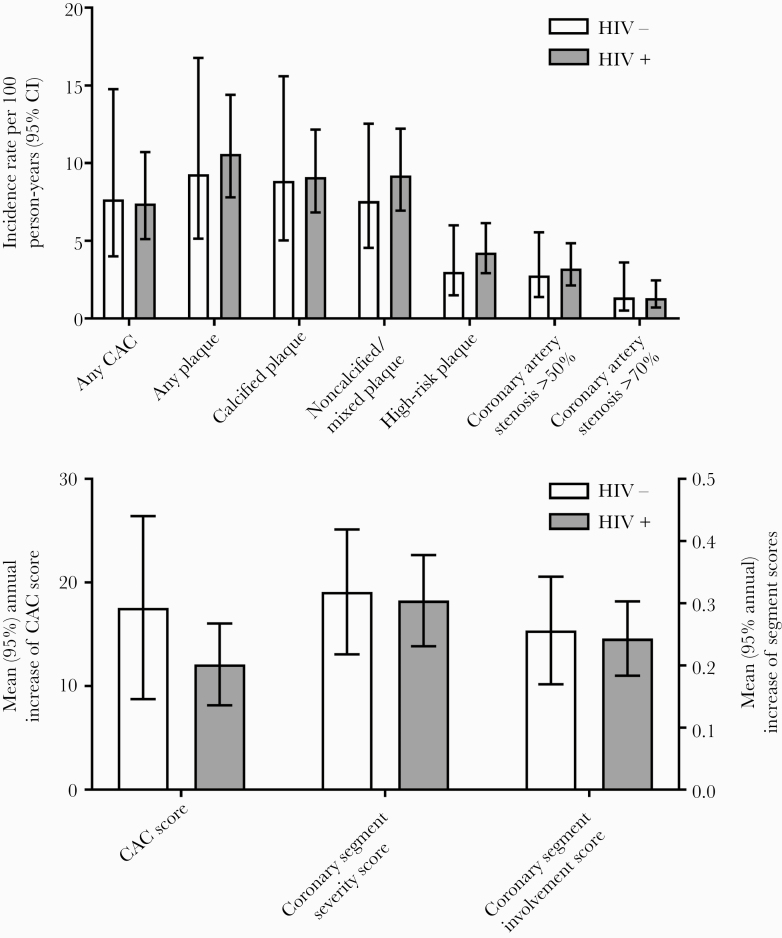
The incidence rates of new subclinical CAD per 100 person-years of follow-up in HIV+ and HIV- participants without any subclinical CAD at baseline CAC/CCTA are shown in Figure 2 (upper panel) and Supplementary Table 2. HIV infection was not associated with any plaque (compared with HIV- participants; annualized IRR, 0.97; 95% CI, 0.46–2.05), calcified plaque (annualized IRR, 1.03; 95% CI, 0.55–1.94), noncalcified/mixed plaque (annualized IRR, 1.22; 95% CI, 0.68–2.17), high-risk plaque (annualized IRR, 1.41; 95% CI, 0.64–3.10), any coronary stenosis (annualized IRR, 1.14; 95% CI, 0.59–2.22), stenosis of ≥50% (annualized IRR, 1.16; 95% CI, 0.52–2.59), and stenosis of ≥70% (annualized IRR, 0.97; 95% CI, 0.30–3.10).
Figure 2.
Incidence rates of new subclinical CAD (upper panel) and mean annual increase of coronary CAC, SSS, and SIS scores (lower panel) in HIV+ and HIV- study participants, univariable analysis. The incidence rates (upper panel) shown here are tabulated in Supplementary Table 1. Abbreviations: CAC, coronary artery calcium scan; CCTA, coronary computed tomography angiography; SIS, segment involvement score; SSS, segment severity score.
Annualized Incidence Rate of New Subclinical Atherosclerosis at Follow-up CAC/CCTA, Multivariable Analysis Adjusted for FRS
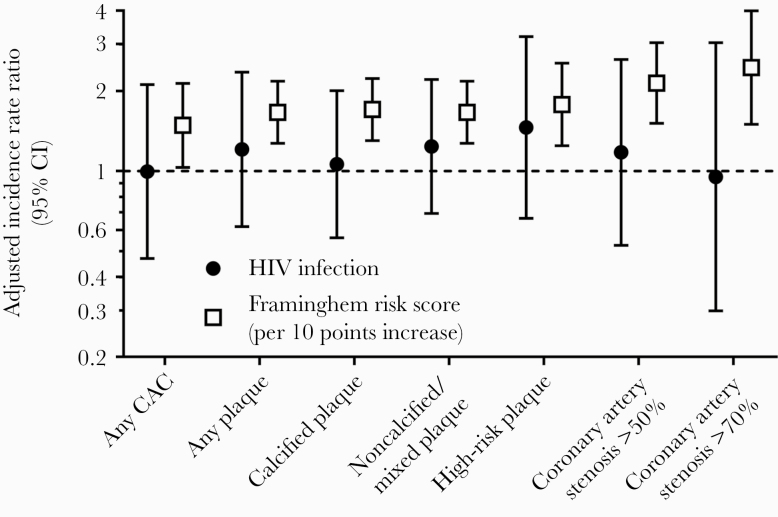
FRS (per point increase) was associated with any plaque (IRR, 1.48; 95% CI, 1.03–2.13), calcified plaque (IRR, 1.70; 95% CI, 1.30–2.23), noncalcified/mixed plaque (IRR, 1.66; 1.27–2.17), high-risk plaque (IRR, 1.78; 95% CI, 1.24–2.54), any coronary stenosis (IRR, 1.66; 95% CI, 1.27–2.17), stenosis of ≥50% (IRR, 2.14; 95% CI, 1.51–3.03), and stenosis of ≥70% (IRR, 2.44; 95% CI, 1.50–3.99) (Figure 3). In contrast, HIV infection was not associated with any plaque (annualized IRR, 1.21; 95% CI, 0.62–2.35), calcified plaque (annualized IRR, 1.06; 0.56–2), noncalcified/mixed plaque (annualized IRR, 1.24; 95% CI, 0.69–2.21), high-risk plaque (annualized IRR, 1.46; 0.66–3.20), stenosis of ≥50% (annualized IRR, 1.17; 95% CI, 0.53–2.62), or stenosis of ≥70% (annualized IRR, 0.95; 95% CI, 0.30–3.03) (Figure 3).
Figure 3.
Adjusted incidence rate ratio of new subclinical CAD in HIV+ and HIV- study participants. Abbreviation: CAC, coronary artery calcium scan.
Associations Between HIV Viral Load and Progression of Subclinical CAD
Because HIV was not associated with subclinical atherosclerosis progression, we did not conduct any subanalyses of HIV-related variables. However, because accelerated plaque progression was seen in HIV+ participants with incomplete HIV suppression in the MACS study [25], we analyzed maximal HIV viral load and area under the HIV viral load curve since baseline CAC/CCTA. These variables were not associated with subclinical atherosclerosis progression in our data set (Supplementary Table 3).
Power Considerations
Because of limited numbers of HIV- participants, we conducted a virtual exercise of duplicating the number of HIV- participants. The conclusion that HIV infection is not associated with progression of any of the subclinical atherosclerosis end points remained unchanged, that is, 95% confidence intervals always included the value 1 even after a virtual, up to 100 000-fold increase in the number of HIV- participants (data not shown).
DISCUSSION
To our knowledge, this is the first longitudinal study to compare subclinical coronary atherosclerosis progression between HIV+ and HIV- persons from Europe. Using CAC and CCTA, and after adjusting for multiple traditional and HIV-related CAD risk factors, we found no evidence of accelerated progression of subclinical CAD in Swiss HIV+ persons, including calcified, noncalcified, and high-risk plaque. As expected, age and FRS were significantly associated with atherosclerosis progression. HIV infection, however, was not. Our findings are reassuring for HIV+ persons, their families, and their treating physicians, as they provide additional evidence that attenuates concerns about accelerated atherosclerosis or even accelerated aging in HIV+ individuals on effective ART. These results extend our recent cross-sectional CAC/CCTA results [11] that prompted editorialists to speculate whether the prevalent notion of increased CAD risk in HIV [1, 2] amounted to “much ado about nothing” [26]. This conclusion may apply particularly to HIV+ persons in Switzerland, considering the regular patient follow-up in the setting of the well-established SHCS, high rates of viral control with modern ART regimens, decreasing smoking rates in recent years [27], and no evidence of increased cardiovascular event incidence compared with the community-based, HIV-negative CoLaus control cohort [28].
Our cross-sectional CAC/CCTA results were consistent with a large CCTA study in African American patients from Baltimore, Maryland [10], whereas the US Multicenter AIDS Cohort Study (MACS) suggested a higher prevalence of noncalcified plaque in HIV+ vs HIV- men who have sex with men [9]. In their longitudinal CAC/CCTA follow-up report, the MACS recently noticed no overall difference in subclinical atherosclerosis progression in HIV+ vs HIV- persons [25], consistent with our longitudinal CAC/CCTA findings from Switzerland presented here. Evidence of accelerated plaque progression in MACS was restricted to the HIV+ participants with incomplete HIV suppression [25]. Insufficient viral control is associated with immune activation and consequent deleterious pro-inflammatory and pro-coagulatory, atherosclerosis-promoting mechanisms [6, 29, 30]. In our Swiss HIV+ persons, incomplete HIV suppression was uncommon: only 6% of our HIV+ participants had a detectable HIV viral load during follow-up, compared with 30% in MACS [25]. Therefore, successful HIV treatment in our study may have prevented us from detecting any atherosclerosis-promoting effects of suboptimal HIV control.
Our study has strengths and limitations. The strengths include this being the first large-scale, longitudinal study comparing subclinical atherosclerosis by CAC/CCTA in HIV+ and HIV- persons in Europe. Our HIV+ participants were followed in the well-established SHCS [23], which allowed us to exploit all relevant clinical, laboratory, and HIV-related data. Our study was limited by the number of HIV- participants available for follow-up CAC/CCTA. However, FRS were similar in the HIV+ and HIV- participants at baseline, and we calculated that even enrolling 100 000 times more HIV- participants with similar FRS as the HIV+ participants would not result in statistically significant associations between HIV infection and atherosclerosis progression. Because we and the investigators of the 2 US CCTA studies in PLWH [9, 10] did not formally match HIV+ and HIV- participants on cardiovascular risk factors, there were differences in the prevalence of cardiovascular risk factors in HIV+ and HIV- participants in all 3 studies [9–11]. However, the 10-year median FRS of our Swiss HIV+ and HIV- participants were comparable. In 2 other studies, HIV- participants were extensively matched on CAD risk factors to the HIV+ participants, and no difference in CIMT progression [31] and a similar prevalence of noncalcified coronary plaque were documented [32]. Few of our participants were >65 years old, and only 16% were women; therefore, our results should be interpreted with caution in these populations.
Although we compared asymptomatic HIV+ with symptomatic HIV- referral patients, we recorded a similar prevalence of >50% coronary stenosis at the baseline scan [11], strongly suggesting that the symptoms that prompted CCTA referral of the HIV- persons were mostly of noncoronary origin [33]. This is also consistent with clinical experience in Switzerland, where family doctors have a low threshold for referring patients with typical chest pain and those with comorbitities and at least moderate cardiovascular risk for invasive coronary angiography; that is, Swiss doctors seem to refer mainly patients with atypical chest pain and low cardiovascular risk for CCTA or other noninvasive testing [34, 35]. Finally, when we designed the study, the funding agency (Swiss National Science Foundation) agreed with us that recruitment of asymptomatic HIV- participants would be problematic, for 3 reasons: (i) complexity of recruiting asymptomatic HIV- participants with a risk factor profile sufficiently similar to HIV+ participants; (ii) among asymptomatic HIV- participants volunteering to undergo 2 CCTA/CACs 2 years apart, the following individuals are likely to be overrepresented: those who have, for example, symptoms that they feel are unexplained or inadequately addressed by their providers, persons who perceive themselves to have a family history of cardiovascular disease, those who are extremely health conscious, the “worried well” etc.; (iii) limited motivation of asymptomatic participants to attend the 2-year follow-up CCTA/CAC (risk of an unacceptably high dropout rate). Our best assessment was therefore that the most appropriate method to recruit HIV- participants was considering the consecutive HIV-negative patients referred for CCTA. The validity of this approach was confirmed by the lack of enrichment for CAD in our HIV- participants.
In conclusion, our longitudinal CAC/CCTA study reassuringly finds no significant differences in coronary plaque progression in HIV+ persons compared with HIV- persons in Switzerland. The similar myocardial infarction incidence rates in HIV+ and HIV- persons in Switzerland [28] are consistent with reports from California [36] and Denmark [37]. In aggregate, these data serve to further attenuate concerns about accelerated atherosclerosis in persons with well-controlled HIV infection.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors acknowledge the effort and commitment of investigators, study nurses, laboratory personnel, and participants. SHCS data are collected by 5 Swiss University Hospitals, 2 Cantonal Hospitals, 15 affiliated hospitals, and 36 private physicians (listed in shcs.ch/180-health-care-providers).
Swiss HIV Cohort Study (SHCS) members. Anagnostopoulos A, Battegay M, Bernasconi E, Böni J, Braun DL, Bucher HC, Calmy A, Cavassini M, Ciuffi A, Dollenmaier G, Egger M, Elzi L, Fehr J, Fellay J, Furrer H (Chairman of the Clinical and Laboratory Committee), Fux CA, Günthard HF (President of the SHCS), Haerry D (deputy of “Positive Council”), Hasse B, Hirsch HH, Hoffmann M, Hösli I, Huber M, Kahlert CR, Kaiser L, Keiser O, Klimkait T, Kouyos RD, Kovari H, Ledergerber B, Martinetti G, Martinez de Tejada B, Marzolini C, Metzner KJ, Müller N, Nicca D, Paioni P, Pantaleo G, Perreau M, Rauch A (Chairman of the Scientific Board), Rudin C (Chairman of the Mother & Child Substudy), Scherrer AU (Head of Data Centre), Schmid P, Speck R, Stöckle M, Tarr P, Trkola A, Vernazza P, Wandeler G, Weber R, Yerly S.
Financial support. This work was supported by the Swiss National Science Foundation (SNF; grant 324730_144209/1) and the SHCS, which is funded by the SNF (grant 177499). Additional funds were obtained from ViiV Healthcare and Gilead Sciences. The funders had no role in the study design, data collection, analysis, or interpretation, or manuscript writing.
Potential conflicts of interest. A.C.’s institution has received financial support from AbbVie, Merck Sharp & Dohme, Gilead, and ViiV and unrestricted educational grants from Gilead, Bristol-Myers Squibb, and ViiV. R.R.B.’s institution holds a research agreement with GE Healthcare. B.L. has received personal fees from Janssen for board membership, personal fees from ViiV for consultancies, and personal fees from Gilead for lectures. P.E.T.’s institution received grants from Gilead and ViiV during the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Swiss HIV Cohort Study:
A Anagnostopoulos, M Battegay, E Bernasconi, J Böni, D L Braun, H C Bucher, A Calmy, M Cavassini, A Ciuffi, G Dollenmaier, M Egger, L Elzi, J Fehr, J Fellay, H Furrer, C A Fux, H F Günthard, D Haerry, B Hasse, H H Hirsch, M Hoffmann, I Hösli, M Huber, C R Kahlert, L Kaiser, O Keiser, T Klimkait, R D Kouyos, H Kovari, B Ledergerber, G Martinetti, B Martinez de Tejada, C Marzolini, K J Metzner, N Müller, D Nicca, P Paioni, G Pantaleo, M Perreau, A Rauch, C Rudin, A U Scherrer, P Schmid, R Speck, M Stöckle, P Tarr, A Trkola, P Vernazza, G Wandeler, R Weber, and S Yerly
References
- 1. Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation 2018; 138:1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation 2019; 140:e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis 2010; 201:318–30. [DOI] [PubMed] [Google Scholar]
- 4. Rotger M, Glass TR, Junier T, et al. ; MAGNIFICENT Consortium; INSIGHT; Swiss HIV Cohort Study Contribution of genetic background, traditional risk factors, and HIV-related factors to coronary artery disease events in HIV-positive persons. Clin Infect Dis 2013; 57:112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker JV, Hullsiek KH, Singh A, et al. ; CDC SUN Study Investigators Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS 2014; 28:831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baker JV, Sharma S, Grund B, et al. Systemic inflammation, coagulation, and clinical risk in the START trial. Open Forum Infect Dis 2017; 4:ofx262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Velzen JE, Schuijf JD, de Graaf FR, et al. Diagnostic performance of non-invasive multidetector computed tomography coronary angiography to detect coronary artery disease using different endpoints: detection of significant stenosis vs detection of atherosclerosis. Eur Heart J 2011; 32:637–45. [DOI] [PubMed] [Google Scholar]
- 8. Hadamitzky M, Distler R, Meyer T, et al. Prognostic value of coronary computed tomographic angiography in comparison with calcium scoring and clinical risk scores. Circ Cardiovasc Imaging 2011; 4:16–23. [DOI] [PubMed] [Google Scholar]
- 9. Post WS, Budoff M, Kingsley L, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med 2014; 160:458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lai H, Moore R, Celentano DD, et al. HIV infection itself may not be associated with subclinical coronary artery disease among African Americans without cardiovascular symptoms. J Am Heart Assoc 2016; 5:e002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tarr PE, Ledergerber B, Calmy A, et al. ; Swiss HIV Cohort Study Subclinical coronary artery disease in Swiss HIV-positive and HIV-negative persons. Eur Heart J 2018; 39:2147–54. [DOI] [PubMed] [Google Scholar]
- 12. Kovari H, Calmy A, Doco-Lecompte T, et al. Antiretroviral drugs associated with subclinical coronary artery disease in the swiss HIV cohort study. Clin Infect Dis 2020; 70(5):884–9. [DOI] [PubMed] [Google Scholar]
- 13. Budoff MJ, Lane KL, Bakhsheshi H, et al. Rates of progression of coronary calcium by electron beam tomography. Am J Cardiol 2000; 86:8–11. [DOI] [PubMed] [Google Scholar]
- 14. Yoon HC, Emerick AM, Hill JA, et al. Calcium begets calcium: progression of coronary artery calcification in asymptomatic subjects. Radiology 2002; 224:236–41. [DOI] [PubMed] [Google Scholar]
- 15. Arad Y, Goodman KJ, Roth M, et al. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol 2005; 46:158–65. [DOI] [PubMed] [Google Scholar]
- 16. Raggi P, Davidson M, Callister TQ, et al. Aggressive versus moderate lipid-lowering therapy in hypercholesterolemic postmenopausal women: Beyond Endorsed Lipid Lowering with EBT Scanning (BELLES). Circulation 2005; 112:563–71. [DOI] [PubMed] [Google Scholar]
- 17. Maher JE, Bielak LF, Raz JA, et al. Progression of coronary artery calcification: a pilot study. Mayo Clin Proc 1999; 74:347–55. [DOI] [PubMed] [Google Scholar]
- 18. Achenbach S, Ropers D, Pohle K, et al. Influence of lipid-lowering therapy on the progression of coronary artery calcification: a prospective evaluation. Circulation 2002; 106:1077–82. [DOI] [PubMed] [Google Scholar]
- 19. Schmermund A, Baumgart D, Möhlenkamp S, et al. Natural history and topographic pattern of progression of coronary calcification in symptomatic patients: an electron-beam CT study. Arterioscler Thromb Vasc Biol 2001; 21:421–6. [DOI] [PubMed] [Google Scholar]
- 20. Zeb I, Li D, Nasir K, et al. Effect of statin treatment on coronary plaque progression—a serial coronary CT angiography study. Atherosclerosis 2013; 231:198–204. [DOI] [PubMed] [Google Scholar]
- 21. Won KB, Lee SE, Lee BK, et al. Longitudinal quantitative assessment of coronary plaque progression related to body mass index using serial coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging 2019; 20:591–9. [DOI] [PubMed] [Google Scholar]
- 22. Piepoli MF, Hoes AW, Agewall S, et al. ; ESC Scientific Document Group 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016; 37:2315–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schoeni-Affolter F, Ledergerber B, Rickenbach M, et al. ; Swiss HIV Cohort Study. Cohort profile: the Swiss HIV Cohort Study. Int J Epidemiol 2010; 39:1179–89. [DOI] [PubMed] [Google Scholar]
- 24. Motoyama S, Ito H, Sarai M, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol 2015; 66:337–46. [DOI] [PubMed] [Google Scholar]
- 25. Post W, Haberlen SA, Zhang L, et al. HIV infection is associated with progression of high risk coronary plaque in the MACS [abstract 77]. Paper presented at: Conference on Retroviruses and Opportunistic Infections; 4–7 March 2019; Seattle, Washington. Available at: http://www.croiconference.org/sessions/hiv-infection-associated-progression-high-risk-coronary-plaque-macs. Accessed 13 October 2019.
- 26. Ma GS, Cotter BR. HIV and cardiovascular disease: much ado about nothing? Eur Heart J 2018; 39:2155–7. [DOI] [PubMed] [Google Scholar]
- 27. Huber M, Ledergerber B, Sauter R, et al. ; Swiss HIV Cohort Study Group Outcome of smoking cessation counselling of HIV-positive persons by HIV care physicians. HIV Med 2012; 13:387–97. [DOI] [PubMed] [Google Scholar]
- 28. Hasse B, Tarr PE, Marques-Vidal P, et al. Strong impact of smoking on multimorbidity and cardiovascular risk among human immunodeficiency virus-infected individuals in comparison with the general population. Open Forum Infect Dis 2015; 2:ofv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuller LH, Tracy R, Belloso W, et al. ; INSIGHT SMART Study Group Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duprez DA, Neuhaus J, Kuller LH, et al. ; INSIGHT SMART Study Group Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 2012; 7:e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Currier JS, Kendall MA, Henry WK, et al. Progression of carotid artery intima-media thickening in HIV-infected and uninfected adults. AIDS 2007; 21:1137–45. [DOI] [PubMed] [Google Scholar]
- 32. Duarte H, Matta JR, Muldoon N, et al. Non-calcified coronary plaque volume inversely related to CD4(+) T-cell count in HIV infection. Antivir Ther 2012; 17:763–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldstein JA, Chinnaiyan KM, Abidov A, et al. ; CT-STAT Investigators The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol 2011; 58:1414–22. [DOI] [PubMed] [Google Scholar]
- 34. Chmiel C, Reich O, Signorell A, et al. Appropriateness of diagnostic coronary angiography as a measure of cardiac ischemia testing in non-emergency patients—a retrospective cross-sectional analysis. PLoS One 2015; 10:e0117172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buechel RR, Kaufmann BA, Tobler D, et al. Non-invasive nuclear myocardial perfusion imaging improves the diagnostic yield of invasive coronary angiography. Eur Heart J Cardiovasc Imaging 2015; 16:842–7. [DOI] [PubMed] [Google Scholar]
- 36. Klein DB, Leyden WA, Xu L, et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis 2015; 60:1278–80. [DOI] [PubMed] [Google Scholar]
- 37. Rasmussen LD, Helleberg M, May MT, et al. Myocardial infarction among Danish HIV-infected individuals: population-attributable fractions associated with smoking. Clin Infect Dis 2015; 60:1415–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.