Abstract
Objectives
The pervasiveness of hearing loss and the development of new potential therapeutic approaches have led to increased animal studies of the inner ear. However, translational relevance of such studies depends upon verification of protein localization data in human samples. Cadavers used for anatomical education provide a potential research resource, but are limiting due to difficulties in accessing sensory tissues from the dense temporal bones. This study seeks to reduce the often months‐long process of decalcification and improve immunofluorescent staining of human cadaveric temporal bones for research use.
Methods
Temporal bones were decalcified in either (a) hydrochloric acid‐containing RDO solution for 2 days followed by 0.5 M ethylenediaminetetraacetic acid (EDTA) for 3 to 5 additional days, or (b) 0.5 M EDTA alone for 2 to 4 weeks. Image‐iT FX signal enhancer (ISE) was used to improve immunofluorescent signal‐to‐noise ratios.
Results
The data indicate that both methods speed decalcification and allow for immunolabeling of the extranuclear proteins neurofilament (heavy chain), myosin VIIa, oncomodulin and prestin. However, RDO decalcification was more likely to alter structural morphology of sensory tissues and hindered effective labeling of the nuclear proteins SRY‐box transcription factor 2 and GATA binding protein 3.
Conclusions
Although both approaches allow for rapid decalcification, EDTA appears superior to RDO for preserving cytoarchitecture and immunogenicity.
Level of evidence
NA.
Keywords: cochlea, decalcification, immunocytochemistry, otology, pathology
Immunofluorescent staining of cadaveric human temporal bones is technically challenging due partly to the time needed for proper decalcification and also to the reduced signal‐to‐noise ratio that results from long incubation and variable fixation. Here we report that RDO speeds decalcification dramatically, but may not be suitable for all antibodies while 0.5 M ethylenediaminetetraacetic acid appears less damaging to tissue integrity and antigenicity, though not as rapid as RDO. Finally, Image‐IT FX signal enhancer, but not 0.3 M glycine, was effective at improving signal‐to‐noise in long‐fixed cadaveric temporal bones.

1. INTRODUCTION
Hearing loss is one of the most common sensory deficits, affecting 466 million people worldwide. 1 Various animal models including birds, fish, and mammals have been studied to understand the development, function, and pathology of hearing loss. While these studies are crucial and have contributed greatly to our understanding of the auditory system, the data obtained from animal studies may not always faithfully translate to what occurs in humans. For example, mutations in the human gene for gap junction protein beta 3 (GJB3), result in either high‐frequency hearing loss 2 or nonsyndromic hearing loss. 3 However, no auditory deficits were observed in mice generated with homozygous deletion of the Gjb3 gene. 4 Similarly, mutation of the gene that encodes nonsyndromic hearing impairment protein 5 (DFNA5) results in progressive hearing loss in humans whereas no hearing deficit was found in mice bearing this mutation. 5 Previous studies have also shown that the patterns of immunofluorescent staining of protein products of several known deafness causing genes can differ between primates and rodents. 6 , 7 , 8 , 9 Indeed, more broadly, several recent reports suggest that a sizeable proportion of animal research experiments, most performed in rodent models, fail to be translated into effective human therapies, 10 , 11 , 12 , 13 emphasizing the need to validate data from animal research in human tissues, particularly, if the aim is to devise pharmacological or gene therapies for human inner ear dysfunction.
One potential avenue for human research of the inner ear is the use of tissues obtained from medical cadavers which can be purposed toward a variety of pathological studies including immunofluorescent staining. While immunofluorescence is an affordable and facile technique widely used to determine spatiotemporal expression of proteins of interest, its use is somewhat limited in cadaveric human inner ears for multiple reasons. First, the methods of embalming, the composition of fixative solutions used, and the time between death and embalming can vary substantially across individuals. 14 , 15 , 16 Second, the prolonged immersion of tissues in aldehydes for medical education or celloidin impregnation for tissue structural preservation can adversely affect the outcomes of immunofluorescence. 17 , 18
Finally, and perhaps most importantly, human cochleae are located inside the petrous portions of the temporal bones of the skull, which are the densest bones in the body. 19 Published methods for decalcifying human temporal bones to access the sensory tissues can require as long as 9 months. 20 Inorganic acids, such as hydrochloric acid, are often used to decalcify bone for histopathological studies; however, these can be detrimental to soft tissue morphology and the integrity of nucleic acids and other intracellular molecules making samples unsuitable for some histological or nucleic acid hybridization studies. 21
Here, we tested two different methods to shorten the time required for decalcification and used Image‐iTFX signal enhancer (ISE, ThermoFisher cat# I36933) to improve immunofluorescent labeling. To hasten temporal bone decalcification, we evaluated the commercially available RDO rapid decalcifier solution (RDO, Apex Engineering Products Corp.) and compared this to varying concentrations of the calcium chelator, ethylenediaminetetraacetic acid (EDTA). 22 , 23 , 24 , 25 Subsequent to decalcification, we tested the extent to which proteins in the organ of Corti and spiral ganglion (SG) could be effectively immunostained. The data indicate that RDO significantly reduces decalcification time to as little as 3 to 4 days and that samples can be efficiently labeled with antibodies that recognize cytoplasmic or membrane bound proteins. However, neither DNA (chromatin) nor nuclear proteins could be consistently visualized in RDO samples. EDTA was more reliable than RDO in preserving tissue morphology and allowing for consistent immunolabeling of nuclear and extranuclear proteins. However, decalcification with EDTA was not as rapid as with RDO. Still, at relatively high concentrations of (0.5 M) EDTA could achieve sufficient decalcification in as little as 2 weeks. Finally, we found that preincubating sections from cadaveric temporal bones with ISE significantly reduced background and enhanced immunofluorescent detection.
2. MATERIALS AND METHODS
2.1. Tissue fixation and collection
Human temporal bones were obtained from cadavers that were generously donated to University of Mississippi Medical Center's (UMMC) body donation program for medical education and research. Arterial embalming was performed at local mortuaries with 1.5% formalin containing 10% phenol and 15% glycerin. Only cadavers that were embalmed within 6 hours from the time of death were utilized. Upon receipt at UMMC cadavers were stored at 4°C and then maintained at 20°C in anatomy tables for approximately 3 months where they were either immersed in, or covered with sheets that had been immersed in, additional fixative solution (0.005% phenol in 0.1% formalin). The average age of individuals used in these studies at time of death was 68 ± 3 years (mean ± SEM). Equal numbers of male and female temporal bones were used for each study, and no major difference in age at time of death was apparent (mean age of males = 69 ± 2, mean age of females = 66 ± 3). Temporal bones were resected using autopsy saws (Stryker Corp, model #810).
2.2. Decalcification by EDTA or RDO
Human temporal bones were immersed in 0.5 M EDTA (pH 8.0) or 50% RDO solution (in water) immediately after collection and left on an orbital shaker (100 rpm) at room temperature (RT). For EDTA samples, solution was replaced every 2 to 3 days and extraneous bone was shaved off using a micro rotary tool or a surgical scalpel until the otic capsule was visible. The temporal bone was incubated in EDTA for one additional week until the capsule was transparent enough to visualize the spiral of the cochlea. For RDO samples, the solution was changed daily and extraneous bone shaved away daily with a scalpel. Once the otic capsule could be clearly visualized, the samples were then incubated in 0.5 M EDTA (pH 8.0) for 1 to 4 days to further soften and clear the tissue for sectioning.
2.3. Cryosectioning
Decalcified tissues were immersed in 30% sucrose in phosphate buffered saline (PBS) for 1 week. A 1:1 mixture of optimum cutting temperature (OCT) compound and 30% sucrose was injected through the round window and the tissues were embedded in OCT and frozen on dry ice. Sequential sections (12‐30 μm) were collected on positively charged slides and stored at −80°C.
2.4. Immunostaining
For each antibody used, and for each treatment condition (RDO or EDTA) immunolabeling was carried out on sections from at least four independent samples with an equal number coming from women as from men. The sections were washed with ultrapure water to remove OCT compound and then incubated with ISE for 30 minutes at RT. Slides were washed in PBS then incubated for 1 hour at RT in blocking buffer containing 1% triton‐X, 1% bovine serum albumin (BSA) and 10% normal serum followed by overnight incubation at 4°C in primary antibodies (Table 1). The samples were then incubated with secondary antibodies for 3 hours at RT, washed with PBS then immersed in Hoechst 33342 (1:1000) for 1 hour at RT. Tissues were washed and coverslipped using fluoro‐gel with 1,4‐diazabicyclo[2,2,2]octane (DABCO,Electron Microscopy Sciences) diluted 1:1 with 50% glycerol (in PBS).
TABLE 1.
List of antibodies used in the current study
| Antibody | Host and type | Dilutions | Vendor | Catalog no. | RRID |
|---|---|---|---|---|---|
| MYO7A | Rabbit, polyclonal | 1:100 | Proteus Bioscience | 25‐6790 | AB_10015251 |
| MYO7A | Mouse, monoclonal IgG2a | 1:100 | Santa Cruz | sc‐74 516 | AB_2148626 |
| Prestin | Goat, polyclonal | 1:250 | Santa Cruz | sc‐22 692 | AB_2302038 |
| OCM | Goat, polyclonal | 1:250 | Santa Cruz | sc‐7446 | AB_2267583 |
| NF‐H | Chicken, polyclonal | 1:1000 | Millipore | AB5539 | AB_11212161 |
| GATA3 | Mouse, monoclonal IgG1 | 1:200 | BD Bioscience | 558 686 | AB_2108590 |
| SOX2 | Goat, polyclonal | 1:250 | Santa Cruz | sc‐17 320 | AB_2286684 |
Abbreviations: GATA3, GATA binding protein 3; MYO7A, myosin VIIa; NF‐H, neurofilament heavy chain; OCM, oncomodulin; SOX2, SRY‐box transcription factor 2.
For GATA binding protein 3 (GATA3) staining, the tissues were treated with antigen unmasking solution (H‐3300, Vector Laboratories) for 40 minutes at 98°C in a humidified chamber and washed with PBS prior to incubation with ISE.
2.5. Imaging and image processing
Images were acquired using either a Nikon C2+ confocal microscope or a Zeiss LSM 880 confocal microscope and were imported into Image J Fiji (https://imagej.net/Fiji). For myosin VIIa (MYO7A) and SRY‐box transcription factor 2 (SOX2) immunolabeling which appeared to contain numerous non‐specific puncta throughout and on top of the sections, despeckling was conducted in Image J using the process: contrast > process > noise > remove outlier.
3. RESULTS
3.1. Both RDO and 0.5 M EDTA rapidly decalcify human temporal bones
Consistent with previous reports, 20 our initial efforts to decalcify human temporal bones with 0.1 to 0.2 M EDTA resulted in several months elapsing before sufficient softening could permit cryosectioning. By increasing the concentration of EDTA to 0.5 M and by paring off extraneous bone every 2 days, temporal bone decalcification time was reduced to as little as 7 to 10 days with total time in EDTA for any given sample ranging from 1 to 4 weeks. As an alternative to EDTA, temporal bones were immersed in RDO which reduced decalcification time even further to as little as 2 days and generally never more than 3 to 4 days. The main factor contributing to variability of immersion times in these decalcifying agents was the size of the temporal bone and the amount of extraneous bone initially resected. For some larger samples, RDO immersion longer than 3 to 4 days was attempted; however, RDO, which is opaque, stains tissues black making important structures difficult to identify. Furthermore, overexposure to RDO was observed to make inner ear soft tissues lose much of their characteristic architecture or disintegrate altogether. Therefore, limited RDO treatment of temporal bones to 3 days or less, removing extraneous bone and testing for softness daily. Then decalcification of RDO samples was finished in 0.5 M EDTA for an additional 2 or more days. By using either RDO with EDTA or 0.5 M EDTA alone, human temporal bone samples were decalcified to where they could be successfully cryosectioned as thin as 12 μm. However, 12‐μm sections from RDO‐treated samples generally did not maintain integrity or adhere to charged slides during immunostaining so sections were generally cut to a thickness of 20 μm.
3.2. Both EDTA and RDO allow for consistent immunostaining and visualization of cytoplasmic or membrane‐bound proteins
To test the utility of RDO and EDTA as decalcifying agents for immunofluorescence, we immunolabeled human cochlear sections using antibodies against proteins that are known to be enriched in the cytoplasm, cell membrane, and nuclei of cells in the organ of Corti and SG. These include cell membrane‐bound prestin, cytosolic MYO7A, oncomodulin (OCM), and neurofilament heavy chain (NF‐H), and nuclear GATA3 and SOX2. All of the selected target proteins are highly conserved in terms of cochlear expression across numerous mammalian species. 26 , 27 , 28 , 29 , 30 , 31 , 32 MYO7A is distributed in the cytoplasm and stereocilia of all inner ear hair cells 33 whereas prestin is selectively expressed in the membrane of cochlear outer hair cells (OHCs). OCM is expressed in the cytoplasm of OHCs, 28 and NF‐H is expressed in neuronal soma and neurites. 32 , 34 As shown in Figure 2, regardless of whether EDTA or RDO was used, OHC membranes were labeled by the antibody against prestin (Figure 2B,E), and the cytoplasm of OHCs was stained by the antibody against OCM (Figure 1, 2H,K). Both OHCs and inner hair cells (IHCs) were labeled by the MYO7A (Figure 2A,D,G,J ) antibody and NF‐H immunoreactivity was readily detected in SG neuronal cell bodies and projections (Figure2M,N ). There were no obvious differences in labeling patterns between EDTA and RDO decalcified samples for these cytosolic or membrane‐bound proteins.
FIGURE 1.

Flowchart for the two methods investigated here to decalcify and immunostain cadaveric temporal bones. Option 1 (green) which utilizes RDO solution for decalcification is more rapid, but denatures chromatin and may render labeling of nuclear, DNA‐binding proteins unreliable. Option 2 (orange) utilizes EDTA‐only for decalcification and takes somewhat longer, but preserves chromatin allowing for the use of nuclear dyes and reliable labeling of nuclear proteins. Glycine treatment prior to immunostaining did not exhibit any obvious benefit in either approach. EDTA, ethylenediaminetetraacetic acid
FIGURE 2.

Immunolabeling of cytoplasmic and membrane bound proteins in sections from ethylenediaminetetraacetic acid‐treated (EDTA), A‐C,G‐I,M, and RDO‐treated, D‐F,J‐L,N, temporal bones. A‐I, Both inner and outer hair cells (IHCs and OHCs) were labeled by the antibody against the cytosolic protein myosin VIIA (MYO7A, green) while the membranes of OHCs were labeled with antiprestin antibody (Magenta). G‐L, Sensory hair cells (OHCs and IHCs) were labeled by the anti‐MYO7A antibody (green) and OHCs were labeled with an antibody against cytoplasmic protein oncomodulin (OCM, magenta). M,N, The cytoplasm of spiral ganglion neuron cell bodies were labeled with an antibody against neurofilament heavy chain (NF‐H, green). In all the images above, the scale bar represents 20 μm. In images A‐L, white arrowheads indicate OHCs and IHCs
3.3. RDO hindered the labeling of nuclear components
Both EDTA and RDO decalcified samples were stained using antibodies raised against the nuclear proteins GATA3 and SOX2 and the nuclear dye Hoechst 33342. In adult mice, GATA3 is readily detectable in the nuclei of inner phalangeal and inner border cells as well as Hensen's and Claudius cells. 35 , 36 SOX2 has been shown in multiple mammalian species to be expressed in the nuclei of all of the cochlear supporting cells (SCs) at both young and adult ages. 6 , 37 , 38 , 39 , 40 The nuclei of EDTA‐decalcified samples were clearly labeled by the antibodies against GATA3 (Figure 3B) and SOX2 (Figure 4B). However, GATA3 was undetectable in RDO samples, and SOX2 could not be detected consistently (Figure 3F and 4F) . SOX2 immunolabeling in RDO samples was sporadic, generally absent from some SCs even when laser power and detector gain on the confocal microscope were increased dramatically. Similarly, Hoechst 3342 chromatin staining was visible only in EDTA‐treated sections, not in RDO samples (Figures 3C, 3G, 4C and 4G). These data suggest that EDTA‐mediated decalcification may be a better choice for the labeling of nuclear proteins and nucleic acids. However, as only two nuclear proteins were assessed, more study is needed to determine if immunolabeling of other nuclear proteins may differ between RDO‐ and EDTA‐treated samples or if the sodium citrate unmasking that is required for this particular GATA3 antibody is a factor that, when coupled with RDO treatment, causes a failure of immunodetection.
FIGURE 3.

Immunolabeling of nuclear protein GATA3 in sections from EDTA‐treated, A‐D, and RDO‐treated, E‐H, temporal bones. A,E, Both outer and inner sensory hair cells (OHCs and IHCs) were labeled by an antibody against myosin VIIa (MYO7A, green). B,F, The nuclei of Hensen's and Claudius cells were prominently labeled with anti‐GATA3 (magenta) in EDTA, B, but not RDO, F, sections. C,G, Nuclei of the cells in the organ of Corti were distinctly labeled with Hoechst3342 (gray) in EDTA, C, but not RDO, G sections. D,H, Merged images of MYO7A, GATA3, and Hoechst for EDTA, D, and RDO, H. Scale bar 20 μm. In all the images white arrowheads indicate OHCs and IHCs. EDTA, ethylenediaminetetraacetic acid; GATA3, GATA binding protein 3
3.4. ISE solution improved immunofluorescent signal‐to‐noise ratios
One of the major challenges for investigating cadaveric human samples is the presence of high levels of background. Increased time between death and fixation can lead to lack of preservation of protein tertiary structure or reduced quantities of epitope which can hinder antigen‐antibody reactions. 41 In addition, where anatomy education is the primary mission of cadaveric body donation programs, cadavers generally spend a significant amount of time (usually months) in anatomy classrooms, before being redirected for research use, further contributing to protein degradation. One countermeasure against this is prolonged exposure to or the use of high concentrations of fixative solutions, or celloidin impregnation. However, such overfixation notably increases background fluorescence and reduces antibody efficiency. In the current study, cadavers were embalmed with 10% phenol and 1.5% formalin, then further exposed to 0.1% formalin in the anatomy tables, which largely preserved the tissue architecture during the 2 to 3 months of medical education. However, the long duration and prolonged exposure to fixative between initial embalming and tissue retrieval and staining led to cadaveric sections routinely exhibiting extensive background fluorescence. To address this, cryosections were preincubated for 30 minutes in either 0.3 M glycine (in 1× PBS) or in ISE solution. While the glycine treatment did not appear to have any noticeable effect (Figure 5A), the ISE solution did reduce nonspecific background fluorescence and significantly improved the signal‐to‐noise ratio (Figure 5B).
FIGURE 4.
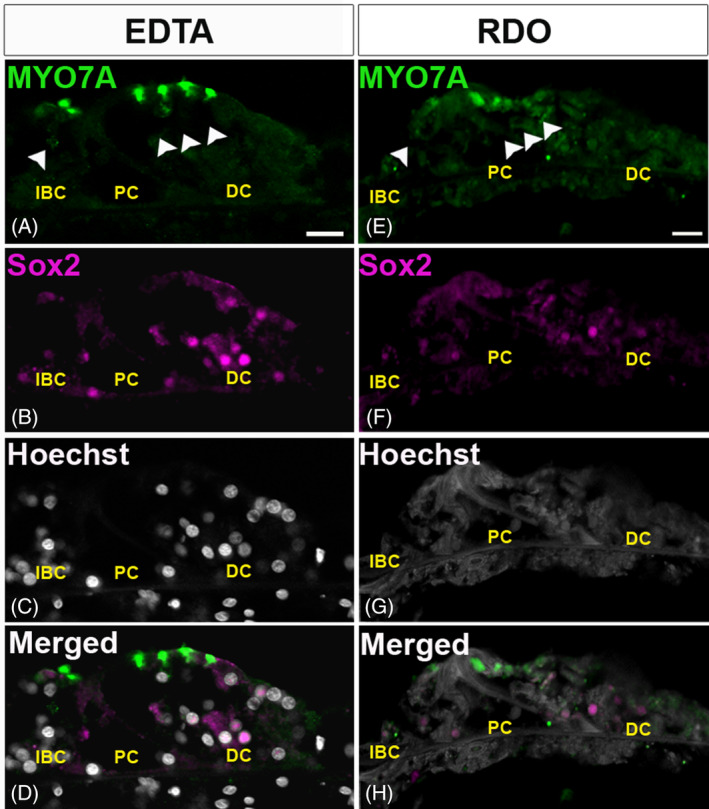
Immunolabeling of nuclear protein SRY‐box transcription factor 2 (SOX2) in sections from ethylenediaminetetraacetic acid (EDTA), A‐D, and RDO, E‐H, treated temporal bones. A,E, Sensory hair cells (outer and inner sensory hair cells [OHCs and IHCs]) were labeled by an antibody against myosin VIIa (MYO7A, green). B,F, Supporting cell (SC) nuclei were immunolabeled with anti‐SOX2 antibody (magenta). SCs including Dieters' cells (DC), outer pillar cells (OPCs), inner pillar cells (IPCs), and inner border cells (IBCs) were prominently labeled in the EDTA sections, B, but not in the RDO sections, F. C,G, Staining of chromatin with Hoechst 3342 (gray) showed clear nuclear labeling in the EDTA samples, C, and no nuclear labeling was observed in RDO samples, G. D,H, The merged images for EDTA and RDO sections, respectively. Scale bar 20 μm. In all the images white arrowheads indicate OHCs and IHCs
4. DISCUSSION
The encapsulation of the human cochlea within the dense temporal bone makes it difficult to access and affects tissue fixation, retrieval, and processing of cadaveric samples. Combined with educational use, these technical challenges lead to extensive delays. These delays present a barrier to research not only in terms of time as its own cost, but also because they can lead to molecular and structural degradation, and hinder the use of many techniques including immunofluorescent detection. Here, we show that by forgoing the use of celloidin, and by paring down extraneous bone at various intervals, either RDO or 0.5 M EDTA can be used to decalcify human temporal bones on the order of a few weeks rather than several months. 20 The findings also suggest that decalcification with 0.5 M EDTA is likely better than RDO for preserving tissue morphology and for more reliable immunodetection, particularly of nuclear proteins. Though RDO may present a suitable alternative for immunolabeling of extranuclear proteins, and may even be preferable if time is a critical factor such as in the case of labile proteins. Alternative approaches, such as microwave radiation decalcification of human temporal bones in EDTA, have been proposed to reduce the time needed for decalcification. 42 , 43 However, microwaves suitable for biological sample preparation can be costly and microwave decalcification carries the potential risk of unmasking antigen epitopes and altering tissue morphology. 42 The current approach using 0.5 M EDTA at room temperature is low cost, should be achievable in most labs, and leads to less manipulation to the samples (Figure 5).
FIGURE 5.

Signal‐to‐noise ratio was significantly improved by Image‐iT FX signal enhancer (ISE). Serial sections from the same adult male temporal bone (RDO decalcified) were treated with either 0.3 M glycine, A, or ISE, B, prior to blocking and immunostaining. In samples that were treated with ISE, B, prestin immunofluorescence shows greater specificity and signal‐to‐noise in the membranes of the outer hair cells (yellow arrowheads), than what can be seen with immunostaining using the exact same antibodies in cochlear sections not treated with ISE, A. Scale bar = 20 μm
Of the proteins immunostained here, MYO7A, prestin, and neurofilament have been previously shown in human cochleae by immunolabeling. 20 , 44 , 45 However this, to the authors' knowledge, is the first report of SOX2, GATA3, and OCM immunostaining in adult human inner ear tissue. The distribution patterns of OCM, GATA3, and SOX2 were consistent with published data in animal models, suggesting that these proteins may function similarly in humans as they do in rodents and other laboratory models. However, there are numerous other proteins which have been shown to be critical to auditory function in animal models that have yet to be validated in humans. As noted, previous studies comparing marmosets to rodents indicated differences in expression patterns of many proteins known to be important in hearing function. 6 , 7 , 8 , 9 Thus there may be some key differences between primate ears and rodent ears. This underscores the importance of studying protein distribution in human samples to increase translational confidence of animal data. Indeed, recent studies highlight the utility of immunostaining cadaveric temporal bones to validate inner ear expression patterns of proteins suggested by results from single‐cell RNA‐seq experiments in mice. 46 Furthermore, in addition to being useful for such validation studies, the reduced processing time and preserved immunogenicity in the approaches outlined here lends them to antibody screening endeavors. While there are already existing repositories of human temporal bones that have associated medical records, such as those in the NIDCD National Temporal Bone, Hearing and Balance Pathology Resource Registry, the temporal bone samples in these resources are still somewhat rare and are often already celloidin embedded or otherwise preserved for long periods of time. With the approaches outlined here, antibodies can first be screened for those with the greatest likelihood of working in older or more heavily fixed human inner ear samples. In the future we plan to employ the techniques outlined here for exactly these types of purposes: to validate murine single‐cell transcriptomic data, to screen commercial antibodies for efficacy in human inner ear tissues, and to investigate the expression of inner ear proteins in human cadavers, particularly those proteins suggested by animal data as being involved in hearing loss or other inner ear pathologies. Critically, the potential also exists to add samples and data to currently available resources such as the NIDCD National Temporal Bone, Hearing and Balance Pathology Resource Registry such that the number of human temporal bone samples available for research can be more readily increased to levels that allow for adequately powered statistical approaches. With an expanded pool of samples that covers a broader range of medical histories and other relevant characteristics such as age or career type, correlational studies can be undertaken to generate hypotheses about the effects of such variables on auditory function.
Despite the success of the current study, there are potential caveats. All the antibodies used for this study are well characterized and the proteins they are raised against are abundantly expressed in cochlear tissues. Also, the cadavers used in this study were embalmed within 6 hours from the time‐of‐death. Thus, it is not well understood whether demineralization with 0.5 M EDTA or RDO will yield similar results with proteins of low abundance, nor what effect increasing the time between death and embalming might have. Furthermore, in the case of RDO, it has been shown to interfere with the integrity of chromatin, 21 and the data presented here confirm this while also suggesting that RDO may not be ideal for immunostaining of nuclear proteins.
5. CONCLUSIONS
The data clearly indicate that both EDTA and RDO can be used to rapidly decalcify human temporal bones for immunofluorescence studies, though EDTA may be better than RDO for staining nuclear proteins. Furthermore, incubation with ISE significantly improved the immunofluorescent signal‐to‐noise ratio in cadaveric samples.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGMENTS
The authors would like to thank Drs Marianne Conway and Al Sinning for their assistance as directors of the UMMC body donation program, and of course, a tremendous thanks to the Mississippians who generously donated themselves or their loved ones to the UMMC body donation program. Their generosity has helped to advance both medical education and scientific research progress as well. This study was presented as a poster presentation during 42nd Annual Midwinter Meeting of the Association for Research in Otolaryngology (ARO), February 9‐13, 2019, Baltimore, Maryland. This work was supported through funding by the NIH (R01DC016365, R01DC016365 S1), the Office of Naval Research (N00014‐18‐1‐2716), the American Otological Society, and the University of Mississippi Medical Center (UMMC).
Ghosh S, Lewis MB, Walters BJ. Comparison of ethylenediaminetetraacetic acid and rapid decalcificier solution for studying human temporal bones by immunofluorescence. Laryngoscope Investigative Otolaryngology. 2020;5:919–927. 10.1002/lio2.449
Funding information American Otological Society; Marine Corps Warfighting Laboratory, Grant/Award Number: N00014‐18‐1‐2716; National Institute on Deafness and Other Communication Disorders, Grant/Award Numbers: R01DC016365, R01DC016365‐S1; The Joe W. and Dorothy Dorsett Brown Foundation
REFERENCES
- 1. Ren Y, Landegger LD, Stankovic KM. Gene therapy for human sensorineural hearing loss. Front Cell Neurosci. 2019;13:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xia JH, Liu CY, Tang BS, et al. Mutations in the gene encoding gap junction protein associated with autosomal dominant hearing impairment. Nat Genet. 1999. Feb;21(2):2413. [DOI] [PubMed] [Google Scholar]
- 3. Liu X‐Z. Mutations in connexin31 underlie recessive as well as dominant non‐syndromic hearing loss. Hum Mol Genet. 2000;9(1):63‐67. [DOI] [PubMed] [Google Scholar]
- 4. Plum A, Winterhager E, Pesch J, et al. Connexin31‐deficiency in mice causes transient placental dysmorphogenesis but does not impair hearing and skin differentiation. Dev Biol. 2001;231(2):334‐347. [DOI] [PubMed] [Google Scholar]
- 5. Van Laer L, Pfister M, Thys S, et al. Mice lacking Dfna5 show a diverging number of cochlear fourth row outer hair cells. Neurobiol Dis. 2005;19(3):386‐399. [DOI] [PubMed] [Google Scholar]
- 6. Hosoya M, Fujioka M, Ogawa K, Okano H. Distinct expression patterns of causative genes responsible for hereditary progressive hearing loss in non‐human primate cochlea. Sci Rep. 2016;6:22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hosoya M, Fujioka M, Okano H, Ogawa K. Distinct expression pattern of a deafness gene, KIAA1199, in a primate cochlea. Biomed Res Int. 2016;2016:1781894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matsuzaki S, Hosoya M, Okano H, Fujioka M, Ogawa K. Expression pattern of EYA4 in the common marmoset (Callithrix jacchus) cochlea. Neurosci Lett. 2018;662:185‐188. [DOI] [PubMed] [Google Scholar]
- 9. Suzuki N, Hosoya M, Oishi N, Okano H, Fujioka M, Ogawa K. Expression pattern of wolframin, the WFS1 (Wolfram syndrome‐1 gene) product, in common marmoset (Callithrix jacchus) cochlea. Neuroreport. 2016;27(11):833‐836. [DOI] [PubMed] [Google Scholar]
- 10. Hackam DG, Redelmeier DA. Translation of research evidence from animals to humans [9]. JAMA. 2006;296(14):1731‐1732. [DOI] [PubMed] [Google Scholar]
- 11. Roberts I, Kwan I, Evans P, Haig S. Does animal experimentation inform human healthcare? Observations from a systematic review of international animal experiments on fluid resuscitation. BMJ. 2002;324(7335):474‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shanks N, Greek R, Greek J. Are animal models predictive for humans? Philos Ethics Human Med. 2009;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McGonigle P, Ruggeri B. Animal models of human disease: challenges in enabling translation. Biochem Pharmacol. 2014;87(1):162‐171. [DOI] [PubMed] [Google Scholar]
- 14. Viana LM, O'Malley JT, Burgess BJ, et al. Cochlear neuropathy in human presbycusis: confocal analysis of hidden hearing loss in post‐mortem tissue. Hear Res. 2015;327:78‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ajileye AB, Esan EO, Adeyemi OA. Human embalming techniques: a review. Am J Biomed Sci. 2018;10(2):82‐95. [Google Scholar]
- 16. Brenner E. Human body preservation—old and new techniques. J Anat. 2014;224(3):316‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tian Q, Linthicum FH, Keithley EM. Application of labeling techniques to archival temporal bone sections. Ann Otol Rhinol Laryngol. 1999;108(1):47‐53. [DOI] [PubMed] [Google Scholar]
- 18. Shi SR, Cote C, Kalra KL, Taylor CR, Tandon AK. A technique for retrieving antigens in formalin‐fixed, routinely acid‐ decalcified, celloidin‐embedded human temporal bone sections for immunohistochemistry. J Histochem Cytochem. 1992;40(6):787‐792. [DOI] [PubMed] [Google Scholar]
- 19. Lam YM, Chen X, Pearson OM. Intertaxonomic variability in patterns of bone density and the differential representation of Bovid, Cervid, and Equid elements in the archaeological record. Am Antiq. 1999;64(2):343‐362. [Google Scholar]
- 20. Lopez IA, Ishiyama G, Hosokawa S, et al. Immunohistochemical techniques for the human inner ear. Histochem Cell Biol. 2016;146(4):367‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Choi SE, Hong SW, Yoon SO. Proposal of an appropriate decalcification method of bone marrow biopsy specimens in the era of expanding genetic molecular study. J Pathol Transl Med. 2015;49(3):236‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Montgomery SC, Cox BC. Whole mount dissection and immunofluorescence of the adult mouse cochlea. J Vis Exp. 2016;1 (107):53561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coleman B, Rickard NA, de Silva MG, Shepherd RK. A protocol for cryoembedding the adult Guinea pig cochlea for fluorescence immunohistology. J Neurosci Methods. 2009;176(2):144‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ghosh S, Sheth S, Sheehan K, et al. The endocannabinoid/cannabinoid receptor 2 system protects against cisplatin‐induced hearing loss. Front Cell Neurosci. 2018;12:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Du X, Choi C‐H, Chen K, Cheng W, Floyd RA, Kopke RD. Reduced formation of oxidative stress biomarkers and migration of mononuclear phagocytes in the cochleae of chinchilla after antioxidant treatment in acute acoustic trauma. Int J Otolaryngol. 2011;2011:612690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dallos P, Wu X, Cheatham MA, et al. Prestin‐based outer hair cell motility is necessary for mammalian Cochlear amplification. Neuron 2008. 2008;58(3):333‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zheng J, Shen W, He DZZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405(6783):149‐155. [DOI] [PubMed] [Google Scholar]
- 28. Simmons DD, Tong B, Schrader AD, Hornak AJ. Oncomodulin identifies different hair cell types in the mammalian inner ear. J Comp Neurol. 2010;518(18):3785‐3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weil D, Lévy G, Sahly I, et al. Human myosin VIIA responsible for the usher 1B syndrome: a predicted membrane‐associated motor protein expressed in developing sensory epithelia. Proc Natl Acad Sci USA. 1996;93(8):3232‐3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gibson F, Walsh J, Mburu P, et al. A type VII myosin encoded by the mouse deafness gene shaker‐1. Nature. 1995;374(6517):62‐64. [DOI] [PubMed] [Google Scholar]
- 31. Berglund AM, Ryugo DK. A monoclonal antibody labels type II neurons of the spiral ganglion. Brain Res. 1986;383(1–2):327‐332. [DOI] [PubMed] [Google Scholar]
- 32. Berglund AM, Ryugo DK. Neurofilament antibodies and spiral ganglion neurons of the mammalian cochlea. J Comp Neurol. 1991;306(3):393‐408. [DOI] [PubMed] [Google Scholar]
- 33. Hasson T, Gillespie PG, Garcia JA, et al. Unconventional myosins in inner‐ear sensory epithelia. J Cell Biol. 1997;137(6):1287‐1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nayagam BA, Muniak MA, Ryugo DK. The spiral ganglion: connecting the peripheral and central auditory systems. Hear Res. 2011;278(1–2):2‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bardhan T, Jeng JY, Waldmann M, et al. Gata3 is required for the functional maturation of inner hair cells and their innervation in the mouse cochlea. J Physiol. 2019;597(13):3389‐3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Walters BJ, Coak E, Dearman J, et al. In vivo interplay between p27Kip1, GATA3, ATOH1, and POU4F3 converts non‐sensory cells to hair cells in adult mice. Cell Rep. 2017;19(2):307‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu S, Wang Y, Lu Y, et al. The key transcription factor expression in the developing vestibular and auditory sensory organs: a comprehensive comparison of spatial and temporal patterns. Neural Plast. 2018;2018:7513258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dabdoub A, Puligilla C, Jones JM, et al. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci USA. 2008;105(47):18396‐18401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hume CR, Bratt DL, Oesterle EC. Expression of LHX3 and SOX2 during mouse inner ear development. Gene Expr Patterns. 2007;7(7):798‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lu N, Chen Y, Wang Z, et al. Sonic hedgehog initiates cochlear hair cell regeneration through downregulation of retinoblastoma protein. Biochem Biophys Res Commun. 2013;430(2):700‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. O'Malley JT, Merchant SN, Burgess BJ, Jones DD, Adams JC. Effects of fixative and embedding medium on morphology and immunostaining of the cochlea. Audiol Neurotol. 2009;14(2):78‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keithley EM, Truong T, Chandronait B, Billings PB. Immunohistochemistry and microwave decalcification of human temporal bones. Hear Res. 2000;148(1–2):192‐196. [DOI] [PubMed] [Google Scholar]
- 43. Cunningham CD, Schulte BA, Bianchi LM, et al. Microwave decalcification of human temporal bones. Laryngoscope. 2001;111(2):278‐282. [DOI] [PubMed] [Google Scholar]
- 44. Rask‐Andersen H, Liu W, Boström M, et al. Immunolocalization of prestin in the human cochlea. Audiol Med. 2010;8(2):56‐62. [Google Scholar]
- 45. Wu PZ, Liberman LD, Bennett K, de Gruttola V, O'Malley JT, Liberman MC. Primary neural degeneration in the human cochlea: evidence for hidden hearing loss in the aging ear. Neuroscience. 2019;407:8‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hoa M, Olszewski R, Li X, et al. Characterizing adult cochlear supporting cell transcriptional diversity using single‐cell RNA‐seq: validation in the adult mouse and translational implications for the adult human cochlea. Front Mol Neurosci. 2020;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]


