Abstract
The naked mole rat (NMR) is a rodent that has gained importance as a biomedical research model for various conditions like hypoxic brain injury, cancer and nociception. This study was designed to investigate possible involvement of the noadrenergic receptor system in antinoception in the NMR, using the alpha-2 adrenergic receptor specific ligands clonidine (agonist) and yohimbine (antagonist) in the formalin test. Formalin test followed 30 min after intraperitoneal administration of ligands or control. A total of 96 naked mole rats were used. A significant reduction in nociceptive behaviours was demonstrated after administration of clonidine in the doses 1,3,10 and 30 μg/kg (n = 8 per group). Doses of clonidine above 30 μg/kg caused loss of motor and proprietion skills exhibited by prostration and failure to turn over when placed on their backs. The antinociception by 3 μg/kg clonidine was reversed by administration of 30 μg/kg of yohimbine. The present study demonstrates that the noradrenergic receptor system is present and involved in formalin test-related antinociceptive mechanisms in the NMR, similar to other mammals. Given the increasing importance of the NMR as a model for pain and nociception, the species may prove useful as an animal model for noradrenergic mechanisms in pain modulation.
Keywords: Formalin test, Clonidine, Naked mole rats, Noradrenergic, Nociception, Yohimbine, Statistics, Animal science, Neuroscience, Cell biology, Pharmaceutical science, Animal behavior
Formalin test; Clonidine; Naked mole rats; Noradrenergic; Nociception; Yohimbine; Statistics; Animal science; Neuroscience; Cell biology; Pharmaceutical science; Animal behavior.
1. Introduction
Naked mole rats (NMRs) are rodents that live a longer life compared to other rodents, sometimes living over 31 years and without showing signs of aging (Grimes et al., 2014; Kim et al., 2011). NMRs have evolved to survive underground in colonies of 100–300 animals. They are resistant to experimentally induced tumors and can survive under low oxygen levels owing to their hemoglobin's high affinity for oxygen. They also have few c-fibers and which lack the neurotransmitter substance P in the skin. Substance P is involved in sending pain signals to central nervous system, its lack in the NMR makes them behaviorally insensitive to itch induced by capsaicin, ammonia fumes and histamine. They are also insensitive to pain induced by acid and acidic fumes (Abiyselassie, 2018; Clarke and Faulkes 1998; Schuhmacher et al., 2015; Grimes et al., 2014; Kim et al., 2011).
Due to these unique characteristics, NMRs are used as research animal models for studying longevity, cancer, hypoxic brain injury and nociception (induced by heat, mechanical force, or chemicals). These studies in return assist in understanding mechanisms involved in various biological diseases affecting humans Abiyselassie (2018); Schuhmacher et al. (2015); Rochelle (2005).
Previous research show the interest to understand mechanisms related to pain modulation in the NMR. Kanui & Hole (1990) reported that morphine induces aggression and not analgesia, and therefore opioid systems in NMR may not be involved in nociception but motor and agonistic behavior regulation. Karim et al. (1993) and Kanui et al. (1993) reported that some opioid drugs significantly inhibit formalin induced pain behaviors. Kanui & Towett (1993) demonstrated that in the hot plate test, pethidine causes aggression, sensorimotor impairment and hyperalgesia while specific doses of acetylsalicylic acid and indomethacin cause significant increase in response latency.
Kanui et al. (2006) and Towett et al. (2009) demonstrated the role of opioid receptors mu, delta and kappa in NMR pain regulation. Dulu et al. (2014) reported the presence of cholinergic and opioid systems in NMR pain control. Jørgensen et al. (2016) reported the presence and involvement of acetylcholine muscarinic receptor subtypes M1 and M4 in NMR antinociception. These studies have thus established the involvement of both opioid and cholinergic receptor systems in NMR pain mechanisms.
The noradrenergic receptor system has been shown to play an important role in nociception and antinociception in several species. This receptor system is the target for alpha-2 adrenergic drugs that include agonists like clonidine, dexmedetomidine and xylazine and antagonists like yohimbine (Yasaei & Saadabadi, 2019; Briskas et al., 1987). These agonists are common in veterinary medicine where they are used for their fast onset, reversibility and analgesia. For example, in ruminants they are used as sedatives and analgesics for restraint, clinical work and minor surgeries though they are reported to cause several side effects, but these side effects are easily managed due to availability of specific antagonists (Alfa et al., 2014).
In African marsh terrapins (Pelomedusa subrufa), administration of clonidine causes a significant reduction in formalin induced pain-related behavior that is reversible with administration of its specific antagonist yohimbine (Makau et al., 2014). In Speke's hinged tortoises (Kiniskys spekii), administration of clonidine also causes a significant reduction in the duration of formalin induced pain-related behavior (Makau et al., 2016).
In rats, administration of alpha-2 adrenergic receptor ligands affects the release of intraspinal acetylcholine and thereby affecting nociceptive mechanisms (Abelson and Höglund, 2004). In mice, intrathecal administration of clonidine (0.46 and 0.92 μg), prazosin (3.75, 7.5 and 15 μg) and yohimbine (7.5 μg) injected intrathecally reduce pain behavior in both phases of formalin test, indicating that increased noradrenergic activity in the spinal cord inhibits nociception (Kanui et al., 1993).
An increased understanding of the noradrenergic receptor system – and its role in pain related mechanisms – is important for an increased understanding of transmission and processing of nociceptive information. This may in turn prove invaluable for future development of novel treatments against pain. Given the properties of the NMR presented above, this species could be an important animal model for a further understanding of noradrenergic antinociceptive mechanisms. To our knowledge, the involvement of the noradrenergic system in nociceptive and antinociceptive functions has never been investigated in the naked mole rat.
The aim of the present study was to study the role of the noradrenergic receptor system in the NMRs using the receptor agonist clonidine and antagonist yohimbine in the formalin test, in order to establish whether NMR can be used as animal model for noradrenergic antinociception. It was hypothesized that clonidine would reduce the formalin induced nocifensive behavior in a dose-related manner, and that this effect could be reversed by yohimbine.
2. Materials and methods
Ethical statement: The experiments were conducted after research approval licensing (Ref; KWS/BRM/5001) and obtaining a naked mole rat capture permit (Ref; KWS/904) from Kenya Wildlife Service (KWS). KWS licenses all research on wildlife in Kenya. The study also adhered to prevention of cruelty to animals act, chapter 360, laws of Kenya, and where applicable to Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes.
Animals: NMRs of both sexes were captured by opening underground burrows at their natural habitat in Makueni County, Kenya, while they were digging fresh mole hills. The weights of NMRs used during the experiment ranged from 26-35 g. Their ages were unknown since they were captured from the wild. They were acclimatized to the laboratory conditions for one month before start of the experiments. Health status monitoring was done daily and animals were handled carefully by skilled personnel to avoid incidences that could evoke fear, anxiety and stress.
Housing and husbandry: The NMRs were housed in cages partitioned into four equal chambers each measuring 35 × 25 × 20 cm in length, width and height respectively and joined by four tunnels, each measuring 30 × 5.5 × 5.5 cm in length, width and height respectively. Tunneling was to mimic underground burrows in their natural habitat. Ventilation of the cages was achieved by covering them with plywood that was not closely fitting. Each captured colony was kept in its own cage. The cage bedding consisted of wood shavings of fine texture made from local trees; was not pre-treated with any chemicals and was changed weekly. The cage bedding was about 2.5 cm deep. No additional materials were provided in the cage.
The housing conditions were almost similar to those in the wild i.e. temperatures of 28–31 °C and 24/0 h dark/light cycle. Relative humidity was maintained at 50–70% to prevent drying and scaling of the mole rat skin. Both temperature and humidity in the room were measured by a thermo hygrometer (Brannan-England). Room temperature was maintained by the use of two 250 W infrared lamps (Euro-matt) and a fan heater (1500 W, Intertronic, UK). By blowing hot air, the fan heater assisted evaporation of water from plastic basins placed in the animal room, thus raising humidity levels.
Food composed of fresh carrots, irish and sweet potatoes and was chopped into pieces of about 1 cm, which made it necessary for the animals to spend time chewing the food. Enough food was provided to allow ad libitum consumption. The NMRs obtained their water requirements from these succulent foods just like in the wild. Animals were monitored on a daily basis.
Study design: The experiment was carried out at South Eastern Kenya University. A total of 96 NMRs were used. The sample size (n) was eight NMRs per group of mixed sex per drug or control dose with each animal acting as an experimental unit. Females and males were equally distributed in groups to avoid any sex related bias. Each group of eight NMRs was injected with either clonidine (1,3,10 or 30 μg/kg), yohimbine (30 μg/kg), a combination of clonidine (3 μg/kg) and yohimbine (30 μg/kg), saline (0.9% NaCl) or 10% formalin. The sample size (n) was based on previous studies by Jørgensen et al. (2016), from which data was obtained for a group size determination through a power analysis: The control group was expected to have a maximal latency of approximately 100 s; a 50% decrease in latency in treated animals was considered biologically relevant; the standard deviation was estimated to 35 s; the alpha was set to 0.05 and the power to 80%. This gives a group size of 8 animals per group. Each NMR was randomly selected and used only once. The experimenter was blinded and thus not aware of the drugs or vehicle injected until after data analysis.
Procedures: The formalin test was performed based on previous studies by Towett et al. (2009), Dulu et al. (2014) and Jørgensen et al. (2016). Each NMR was injected subcutaneously with 20 μl of 10 % formalin dorsally on the right hind paw and immediately placed in a glass observation chamber measuring 15 × 14.5 × 14.5 cm for one hour, and observed for pain related behaviors in blocks of 5 min. The concentration of 10% formalin is higher than what normally is used in the formalin test. The reason for choosing the higher concentration for the naked mole rat is that this is considered necessary to trigger a sufficient response, and has been established as the standard concentration used for this species (Jørgensen et al. (2016); Dulu et al. (2014); Towett et al. (2009); Kanui et al. (1993a, b). The behaviors observed included the time in seconds spent licking, biting or flinching the injected paw. During investigation of the effects of clonidine and yohimbine, the ligands (or saline control) were injected intraperitoneally 30 min before formalin administration. All substances were administered using U-50 insulin syringe with a 30 Gauge needle. The experiments were carried out during daytime between 8.30 a.m. to 17.00 p. m at a room temperature of between 27.2-28.9 °C. Prior to the formalin test, each animal was acclimatized for 30 min to the observation chamber during the acclimation period and the day of experiment.
In connection to the formalin tests, the animals sensorimotor and proprioception skills were checked. Sensorimotor skills were checked by placing the dorsal surface of each injected hind paw on the table to see if the animal would sense this and turn the paw back to a normal position. For proprioception skills, the animals were placed on their back to see if they were capable of turning back on their feet.
Each animal was euthanized by cervical dislocation after the experiment to avoid any unnecessary pain following the procedure. Each animal was thus used only once.
Drugs: Clonidine hydrochloride (alpha-2 adrenergic agonist) and yohimbine hydrochloride (alpha-2 adrenergic antagonist) both from Sigma-Aldrich (Taufkirchen, Germany), 0.9 % NaCl (saline) and formalin were used. Saline was used to dissolve clonidine, diluting formalin to 10%, and injected as a control treatment. Yohimbine was dissolved in water as per manufacturer instructions. Drugs (formalin and ligands) and their controls were injected using U-50 insulin syringe with a 30-gauge needle.
Statistical analysis: The data were analyzed using two-way ANOVA with Bonferroni's post hoc test in Graph Pad Prism version 5.0. Data are presented as mean time in seconds (±SEM) in the graphs. P < 0.05 was considered a statistically significant difference.
3. Results
In a pilot study preceding the present study, it was found that doses of clonidine above 30 μg/kg caused loss of motor and proprioception skills exhibited by prostration and failure to turn over when placed on their backs. Sweating by the immobilised animals was also noted. At a clonidine dose of 300 μg/kg, all four NMRs used were completely immobilized for more than 30 min. At a clonidine dose of 100 μg/kg, one NMR was completely immobilized, two went under ventral reccumbency from 13 min onwards and one had only deppressed activity. Therefore doses of clonidine of 30 μg/kg and below were chosen for the present study. Co-administration of clonidine and yohimbine at high doses resulted in loss of pain related behaviour in the chronic phase of the formalin test. Yohimbine doses above 30 μg/kg did not cause antagonism to 3 μg/kg clonidine while even higher doses caused diarrhea. Doses of yohimbine below 30ug/kg were not evaluated in this study. Co-administration of clonidine 3 μg/kg with a yohimbine dose of 30 μg/kg was therefore chosen.
Subcutaneous injection with 20 μl of 10% formalin dorsally in the right hind paw resulted in a significant increase in pain related behavior compared to saline control in the first 5 min interval (p < 0.05) and later intervals between 40-60 min (P < 0.001) as shown in Figure 1.
Figure 1.
The effects of subcutaneous injection of 20μl 10% formalin (unfilled circle) or saline (unfilled triangle pointed down) dorsally on right hind paw on pain behaviours in the naked mole rat. Significance difference in pain behavior were observed in the time intervals 0–5 min (p < 0.05) and 40–60 min (P < 0.001). Shown as a∗∗ = P < 0.05 and a∗∗∗ = P < 0.001. Data are shown as mean ± SEM. Number of animals (n) = 8 for both saline and formalin. Data were analyzed using two way ANOVA with Bonferroni's post hoc test.
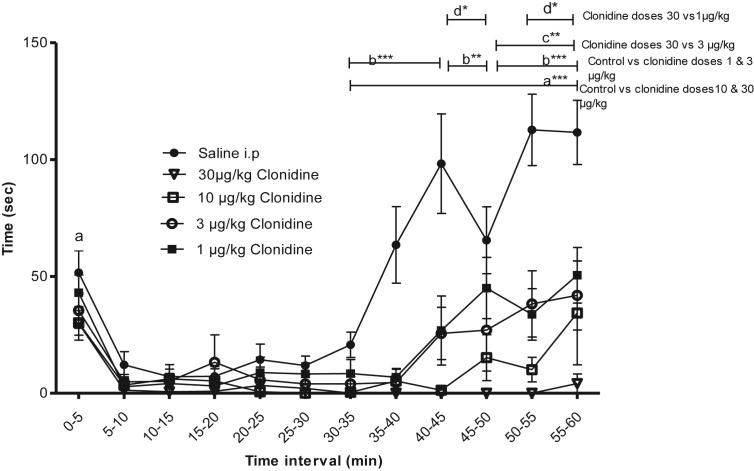
Intraperitoneal injection with 50 μl clonidine in the doses 1,3,10 or 30 μg/kg 30 min prior to formalin injection showed significant effect on the pain-related behavior compared to control. Significant differences were also observed between clonidine 30 μg/kg and doses 1 and 3 μg/kg respectively (Figure 2).
Figure 2.
The effect of intra-peritoneal (i. p) injection of clonidine in concentrations of 1.0, 3.0, or 10, 30 μg/kg compared to a control group of i. p saline injection followed by a formalin test after 30 min of treatment (clonidine) or control (saline) administration. Significant differences in pain behavior were found in the periods; p < 0.001 (a∗∗∗) during 35–60 min between control and clonidine dosages 30 and 10 μg/kg; p < 0.001 (b∗∗∗) during 35–45 and 50–60 min and p < 0.05 (b∗∗) during 45–50 min between control and clonidine dosages 3 and 1 μg/kg; p < 0.05 (c∗∗) during 50–60 min between clonidine dosages 30 and 3 μg/kg; p < 0.01 (d∗) during 45–50 and 55–60 min between clonidine dosages 30 and 1 μg/kg; Data are shown as mean time (±SEM). Number of animals (n) = 8 for control and clonidine dosages 1, 3, 10 and 30 μg/kg respectively. Data were analyzed using two way ANOVA with Bonferroni's post hoc test.
A dose of 30 μg/kg yohimbine was chosen for antagonizing clonidine. This dose was found not to significantly affect pain-related behaviours compared to its dissolvent (Figure 3). Administration of 30 μg/kg of yohimbine significantly reversed the effects of 3 μg/kg of clonidine in the formalin test (Figure 4).
Figure 3.
The effect of intra-peritoneal (i. p) injection of 20 μl water for injection (solvent for yohimbine hydrochloride powder) and 30 μg/kg yohimbine followed by a formalin test after 30 min of treatment (yohimbine) or control (water for injection) administration. Data are shown as mean ± SEM. Sample size (n) = 8 for both control and yohimbine. No significant difference in pain behavior was found between the two treatments.
Figure 4.
The effect of intra-peritoneal (i. p) injection of control, clonidine 3 μg/kg and co administration of yohimbine 30 μg/kg with 3 μg/kg clonidine. Significant differences in pain behavior were found in the periods; p < 0.05 (a∗∗) during 45–50 min between control and clonidine 3 μg/kg; p < 0.05 (b∗∗) during 45–55 min between clonidine 3 μg/kg and co-administered yohimbine 30 μg/kg & clonidine 3 ug/kg. Data are shown as mean ± SEM. Number of animals (n) = 8 for clonidine and control while n = 7 for yohimbine co-administered with clonidine. Data were analyzed using two way ANOVA with Bonferroni's post hoc test.
4. Discussion
This is to our sincere knowledge the first study to report antinociception in naked mole rats mediated by the noradrenergic receptor system.
The noradrenergic receptor system refers to the endogenous ligand noradrenaline and its receptors (Sofuoglu and Sewell, 2009; Smythies, 2005). While it is true that noradrenaline also binds to beta-adrenergic receptors, the affinity is much lower to these than to alpha-adrenergic receptors (Atzori et al., 2016), and therefore the term noradrenergic normally refers to alpha-, while adrenergic refers to beta-adrenergic receptors. Based on this, the term noradrenergic receptor system was chosen for this study. As clonidine primarily is an alpha 2 adrenergic agonist, the term alpha 2 adrenergic receptor system could have been chosen instead. However, this would not reflect the truth either. Although clonidine is a prototypical alpha 2 adrenergic receptor agonist, it also shows some affinity for alpha 1 adrenergic receptors (Giovannitti et al., 2015).
The current study in NMR established a biphasic pain response using the formalin test with a first phase between 0-5 min and a second phase between 40-60 min. A biphasic response has been reported in NMR at similar time periods to our study (Jørgensen et al., 2016). A biphasic response in NMR where first phase occurred at similar time periods to our study but different time periods for the second phase is also reported (Dulu et al., 2014; Towett et al., 2009).
This biphasic response is also commonly reported in other animal species (Reddy and Yaksh, 1980) although time periods for first and second phase differ among different species. Biphasic responses have been reported in monkeys (Sharma et al., 1993), guinea pigs (Leite-Panissi et al., 2001), mice and rats (Kanui et al., 1993; Kariuki et al., 2012), African toad (Ajao et al., 2007), and zebra fish (Francisco et al., 2017). However some animals like rabbits (Esmaeal, 2010), Speke's hinged tortoise (Wambugu et al., 2010; Makau et al., 2016), marsh terrapins (Makau et al., 2014), and crocodile (Kanui et al., 1990) have been reported to show a monophasic response.
Formalin test was first described in rats and cats by Dubuisson and Dennis in 1977 (Dubuisson and Dennis, 1977), and involves injecting small volumes of formalin subcutaneously or intradermally into the animal's paw. The responses observed include licking, biting or flinching the injected paw and occur in two phases; the early phase (acute phase) caused by C-fibers activation occurring in the first 5–10 min due to peripheral stimulus and the late phase (chronic phase) occurring 15–60 min later due to inflammatory reaction (Abelson and Roughan, 2011; Tjolsen et al., 1992). The acute phase of formalin test is used to model acute pain while chronic phase models chronic inflammatory pain (Sawynok and Liu, 2003), in other animals species and humans.
In the current experiment, intraperitoneal administration of clonidine at the doses 1,3,10 and 30 μg/kg significantly reduced formalin induced pain behavior compared to control (saline) in both the early and late phases in a dose dependent manner. This shows the antinociceptive effects of clonidine, the presence and involvement of noradrenergic receptor system of NMRs in pain modulation.
Intraperitoneal administration of clonidine under formalin test responds in a similar dose dependent manner in mice and rats (Yoon et al., 2015; Fukuda et al., 2006). Clonidine administration intrathecally also shows similar dose dependent responses under formalin test in the African marsh terrapin (Pelomedusa subrufa) and Speke's hinged tortoise (Makau et al., 2014, 2016); mice (Kanui et al., 1993), and Sprague-Dawley rats (Yoon et al., 2009).
Clonidine has a high affinity for alpha-2 adrenoceptors at both CNS and PNS. It is reported to have a predilection of 200: 1 for alpha-2 compared to alpha-1 adrenoceptors (Giovannitti et al., 2015). It acts by inhibiting nociceptive transmission through mimicking the action of norepinephrine. In the spinal cord, it inhibits action on alpha-2 adrenoceptors pre-synaptically and post-synaptically (Pertovaara, 2006). In addition, it acts by affecting release of acetylcholine in the dorsal horn of the spinal cord and therefore affecting nociception (Abelson and Höglund, 2004).
Clonidine and other alpha-2 adrenergic receptor agonists such as dexmedetomidine and xylazine are commonly used as sedatives and analgesics. In humans, clonidine is used in the management of hypertension, pain, treatment of attention deficit hyperactivity disorder (ADHD) in children among other off label uses (Yasaei & Saadabadi, 2019). The drug can cross the blood brain barrier and bind specific receptors in the central nervous system and other organs (Huwyler et al., 1997).
Clonidine at doses above 30 μg/kg resulted in altered motor and proprietion skills during pilot studies. Clonidine has been reported to cause motor blockade and hypnosis (Qingli et al., 2016), and hypothermia (Livingston et al., 1984) in experimental animals, which could explain the observed effect. Smaller clonidine doses, like those used in current study did not cause any side effects. This concurs with previous findings where small doses of clonidine had no effect on the sensorimotor performance in the mouse (Kanui et al., 1993).
Yohimbine is indicated as a prototypical alpha-2 adrenergic receptor antagonist due to its high selectivity (Zaretsky et al., 2015). In the current study, administration of 30 μg/kg yohimbine significantly reversed the effects of 3 μg/kg clonidine during the late phase of formalin test. Yohimbine is reported to show similar antagonistic effects to clonidine even in humans (Charney et al., 1983), rats (Normansell and Panksepp, 1985), marsh terrapins (Makau et al., 2014) and tortoises (Makau et al., 2016). Yohimbine acts by presynaptically inhibiting alpha-2 adrenergic receptors (Mekkaw and Al-Badr, 1987). Yohimbine alone is used in humans to manage drug induced hypotension and dry mouth, and as antidote for clonidine (Meyler, 2016).
Results from this study showed some high variation between each animal. This was probable since use of wild type strains causes phenotypic variation due to genetic diversity (Brekke et al., 2018). In addition, the ages of NMR used in this experiment were unknown since they were captured from their natural burrows. Furthermore, both sexes were included, which could have added to the variation, but both sexes were equally represented in all groups to avoid bias. However, despite the high variation, the conclusion drawn from the results should still be valid, since our power analysis show that our group size most likely is sufficient for finding any significant differences.
In conclusion, the present study indicates the presence and involvement of noradrenergic receptor system of naked mole rats in pain modulation. The study also supports that formalin test is a good test for studying nociception and antinociception in NMR. This makes the NMR a relevant animal model for studying noradrenergic receptor system using the formalin test.
Declarations
Author contribution statement
R. Mwobobia: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
T. Kanui: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
K. Abelson: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This study was conducted using funds provided by the Department of Experimental Medicine, University of Copenhagen, Denmark.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to express gratitude to Gilbert Mwanthi for taking care of naked mole rats in the laboratory.
References
- Abelson K.S.P., Höglund A.U. The effects of the α2-adrenergic receptor agonists clonidine and rilmenidine, and antagonists yohimbine and efaroxan, on the spinal cholinergic receptor system in the rat. Basic Clin. Pharmacol. Toxicol. 2004;94:153–160. doi: 10.1111/j.1742-7843.2004.pto940401.x. [DOI] [PubMed] [Google Scholar]
- Abelson K.S.P., Roughan J.V. Animal models in pain research. In: Hau J., Schapiro S.J., editors. Handbook of Laboratory Animal Science, Volume II, Third Edition: Animal Models. C R C Press LLC; Boca Raton: 2011. pp. 123–147. Chapter 7. [Google Scholar]
- Abiyselassie G.A. Overview of African naked mole-rat (Heterocephalus glaber) for bioprospecting and access and benefit sharing in Ethiopia. GSC Biol. Pharm. Sci. 2018;4(1):25–37. [Google Scholar]
- Ajao F., Afolabi A.O., Udoh U., Azeez O. The formalin test in African toad (Bufo regularis) - a novel pain model in Amphibians. Afr. J. Biomed. Res. 2007;8:123–125. [Google Scholar]
- Alfa K., Antagonistlerinin K., Ruminantlarda D., Ãœzerine Z., Shah D., Mingxing M., Hu-Li A review on the current use of alpha-2 agonists in small ruminants. Kafkas Univ. Vet. Fakult. Derg. 2014;20 [Google Scholar]
- Atzori M., Cuevas-Olguin R., Esquivel-Rendon E., Garcia-Oscos F., Salgado-Delgado R.C., Saderi N., Miranda-Morales M., Treviño M., Pineda J.C., Salgado H. Locus ceruleus NorepinephrineRelease: a central regulator of CNSSpatio-temporal activation? Front. Synaptic Neurosci. 2016;8:25. doi: 10.3389/fnsyn.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brekke T.D., Steele K.A., Mulley J.F. Inbred or outbred? Genet. Div. Lab. Rodent Col. 2018;8(2):679–686. doi: 10.1534/g3.117.300495. G3 (Bethesda, Md.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskas P., Tsiamitas C., Ayiannidis A. Xylazine-induced hyperglycaemia and α-adrenergic receptors in sheep. Transbound. Emer. Dis. 1987;34(1-10):58–60. doi: 10.1111/j.1439-0442.1987.tb00255.x. [DOI] [PubMed] [Google Scholar]
- Charney D.S., Heninger G.R., Redmond D.E. Yohimbine induced anxiety and increased noradrenergic function in humans: effects of diazepam and clonidine. Life Sci. 1983;33(1):19–29. doi: 10.1016/0024-3205(83)90707-5. [DOI] [PubMed] [Google Scholar]
- Clarke F.M., Faulkes C.G. Hormonal and behavioural correlates of male dominance and reproductive status in captive colonies of the naked mole-rat, Heterocephalus glaber. Proc. Biol. Sci. 1998;265(1404):1391–1399. doi: 10.1098/rspb.1998.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Directive 2010/63/EU of the european parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes (Accessed on Accessed on 4th August 2020). https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063.
- Dubuisson D., Dennis S.G. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Dulu T.D., Kanui T.I., Towett P.K., Maloiy G.M., Abelson K.S.P. Vol. 28. 2014. The effects of oxotremorine, epibatidine, atropine, mecamylamine and naloxone in the tail-flick, hot-plate, and formalin tests in the naked mole-rat (Heterocephalus glaber) in vivo; pp. 39–48. [PubMed] [Google Scholar]
- Esmaeal T. Central effect of exogenous histamine on pain induced by sub-plantar injection of formalin in rabbits. Vet. Res. Forum. 2010;1(1):1–6. [Google Scholar]
- Francisco E.A.M., Caio Á.P.B., Sacha A.A.R., Renata B.M., Francisco L.A.B., Ângela O.A., Messias V., Luiz F.W.G., Ramon da S.R., Adriana R.C. Vol. 14. 2017. Zebra Fish; pp. 422–429. 5. [Google Scholar]
- Fukuda T., Furukawa H., Hisano S. Systemic clonidine activates neurons of the dorsal horn, but not the locus ceruleus (A6) or the A7 area, after a formalin test: the importance of the dorsal horn in the antinociceptive effects of clonidine. J. Anesth. 2006;20:279–283. doi: 10.1007/s00540-006-0426-5. [DOI] [PubMed] [Google Scholar]
- Giovannitti J.A., Jr., Thoms S.M., Crawford J.J. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth. Prog. 2015;62(1):31–39. doi: 10.2344/0003-3006-62.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes K., Anilkumar K.R., Merry L.L., Rochelle B. And the beat goes on: maintained cardiovascular function during aging in the longest-lived rodent, the naked mole-rat. Am. J. Physiol. Heart Circ. Physiol. 2014 doi: 10.1152/ajpheart.00305.2014. Articles in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwyler J.R., Fricker Gert, Michael T.O., Schneider Markus, Drewe Rgen. Transport of Clonidine across cultured rain micro vessel endothelial cells. J. Pharmacol. Exp. Therapeut. 1997;282:81–85. [PubMed] [Google Scholar]
- Jørgensen K.B., Krogh- Jensen K., Pickering D.S., Kanui T.I., Abelson K.S.P. Investigation of the presence and antinociceptive function of muscarinic acetylcholine receptors in the African naked mole-rat (Heterocephalus glaber) J. Comp. Physiol. 2016;202:7–15. doi: 10.1007/s00359-015-1048-x. (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanui, Hole Morphine induces aggression but not analgesia in the naked mole-rat (Heterocephalus glaber) Comp. Biochem. Physiol. C Comp. Pharmacol. 1990;96(Issue 1):131. doi: 10.1016/0742-8413(90)90057-g. 1990. 13. [DOI] [PubMed] [Google Scholar]
- Kanui T.I., Towett P.K., Juma F.D. Stimulation of mu and delta opioid receptors induces hyperalgesia while stimulation of kappa receptors induces antinociception in the hot plate test in the naked mole-rat (Heterocephalus glaber) Brain Res. Bull. 2006;71(Issues 1–3):60–68. doi: 10.1016/j.brainresbull.2006.08.001. Elsevier. [DOI] [PubMed] [Google Scholar]
- Kanui T.I., Karim F., Towett P.K. The formalin test in the naked mole-rat (Heterocephalus glaber): analgesic effects of morphine, nefopam and paracetamol. Brain Res. 1993;600(1):123–126. doi: 10.1016/0006-8993(93)90409-g. [DOI] [PubMed] [Google Scholar]
- Kanui T.I., Tjolsen A., Lund A., Mjellem-Joly N., Hole K. Antinociceptive effects of intrathecal administration of alpha-adrenoceptor antagonists and clonidine in the formalin test in the mouse. J. Neuropharmacol. 1993;32:367–371. doi: 10.1016/0028-3908(93)90158-y. [DOI] [PubMed] [Google Scholar]
- Kanui T.I., Hole K., Miaron J. Nociception in crocodiles: capsaicin instillation, formalin and hot plate test. Zool. Sci. 1990;7:537–540. [Google Scholar]
- Kanui T.I., Towett P.K. Effects of pethidine, acetylsalicylic acid, and indomethacin on pain and behavior in the mole-rat. Pharmacol. Biochem. Behav. 1993;45(Issue 1):153–159. doi: 10.1016/0091-3057(93)90099-f. May 1993. [DOI] [PubMed] [Google Scholar]
- Karim F., Kanui T.I., Mbugua S. Effects of codeine, naproxen and dexamethasone on formalin-induced pain in the naked mole-rat. Neuroreport. 1993;4(1):25–28. doi: 10.1097/00001756-199301000-00006. [DOI] [PubMed] [Google Scholar]
- Kariuki H.N., Kanui T.I., Kioy P.G. Antinociceptive potentiation of pethidine (demerol) by clomipramine in the late phase of formalin test in mice. Pan Afr. Med. J. 2012;12:28. www.panafrican-med-journal.com/content/article/12/28/full/ [PMC free article] [PubMed] [Google Scholar]
- Kim E.B. Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature. 2011;479:223. doi: 10.1038/nature10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite-Panissi C.R.A., Rodrigues C.L., Brentegani M.R., Menescal-de-Oliveira L. Endogenous opiate analgesia induced by tonic immobility in Guinea pigs. Braz. J. Med. Biol. Res. 2001;34(2):245–250. doi: 10.1590/s0100-879x2001000200013. [DOI] [PubMed] [Google Scholar]
- Livingston A., Low J., Morris B. Effects of clonidine and xylazine on body temperature in the rat. Br. J. Pharmacol. 1984;81:189–193. doi: 10.1111/j.1476-5381.1984.tb10760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makau C.M., Towett P.K., Abelson K.S.P., Kanui T.I. Modulation of formalin-induced pain-related behavior by clonidine and yohimbine in the Speke’s hinged tortoise (Kiniskys spekii) J. Vet. Pharmacol. Therapeut. 2016 doi: 10.1111/jvp.12374. [DOI] [PubMed] [Google Scholar]
- Makau C.M., Towett P.K., Abelson K.S.P., Kanui T.I. Intrathecal administration of clonidine or yohimbine decreases the nociceptive behavior caused by formalin injection in the marsh terrapin (Pelomedusa subrufa) Brain Behav. 2014 doi: 10.1002/brb3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekkaw A.G., Al-Badr A.A. Vol. 16. Elsevier; 1987. pp. 731–768. (Analytical Profiles of Drug Substances). [Google Scholar]
- Meyler . The International Encyclopedia of Adverse Drug Reactions and Interactions. 2016. ELSEVIER; 2016. Side effects of drugs (sixteenth edition) pp. 541–543. [Google Scholar]
- Normansell L., Panksepp J. Effects of clonidine and yohimbine on the social play of juvenile rats. Pharmacol. Biochem. Behav. 1985;22(5):881–883. doi: 10.1016/0091-3057(85)90540-4. [DOI] [PubMed] [Google Scholar]
- Pertovaara A. Noradrenergic pain modulation. Prog. Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Prevention of Cruelty to Animals Act, Chapter 360, Laws of Kenya. Revised edition. 2012. http://extwprlegs1.fao.org/docs/pdf/ken63702.pdf [1983] [Google Scholar]
- Qingli D., Yuanyuan W., Yanan G., Hong Z. Effects of clonidine and intrathecal dexmedetomidine under ropivacaine spinal anesthesia. Int. J. Clin. Exp. Med. 2016;9(7):14929–14941. www.ijcem.com/ISSN: 1940-5901/IJCEM0021994 2016. [Google Scholar]
- Reddy S.V.R., Yaksh T.L. Spinal noradrenergic terminal system mediates antinociception. Brain Res. 1980;189:391–400. doi: 10.1016/0006-8993(80)90099-2. [DOI] [PubMed] [Google Scholar]
- Rochelle B. The naked mole-rat: a new long-living model for human aging. Res. J. Gerontol.: Biol. Sci. 2005;60A(11):1369–1377. doi: 10.1093/gerona/60.11.1369. Copyright 2005 by The Gerontological Society of America. [DOI] [PubMed] [Google Scholar]
- Sawynok J., Liu X. The formalin test: characteristics and usefulness of the model. Rev. Analg. 2003;7:145–163. [Google Scholar]
- Schuhmacher L., Husson Z., Smith E.S. The naked mole-rat as an animal model in biomedical research: current perspectives. Open Access Anim. Physiol. 2015;7:137–148. 2015. [Google Scholar]
- Sharma R., Mathur R., Nayar U. Gaba-B Mediated analgesia in tonic pain in Monkeys. Indian J. Physiol. 1993;37(3):189–193. [PubMed] [Google Scholar]
- Sofuoglu Mehmet, Sewell R. REVIEW: norepinephrine and stimulant addiction. Addiction Biol. 2009;14:119–129. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythies J. Section III. The norepinephrine system. Int. Rev. Neurobiol. 2005;64:173–211. doi: 10.1016/S0074-7742(05)64003-2. [DOI] [PubMed] [Google Scholar]
- Tjølsen A., Berge O., Hunskaar S., Rosland J.H., Hole K. The formalin test: an evaluation of the method. Pain. 1992;51(Issue 1):5–17. doi: 10.1016/0304-3959(92)90003-T. October 1992. [DOI] [PubMed] [Google Scholar]
- Towett P.K., Kanui T.I., Ole Maloiy G.M., Juma F., Ole Miaron J.O. Activation of mu, delta or kappa opioid receptors by DAMGO, DPDPE, U-50488 or U-69593 respectively causes antinociception in the formalin test in the naked mole-rat (Heterocephalus glaber) Pharmacol. Biochem. Behav. 2009;91(4):566–572. doi: 10.1016/j.pbb.2008.09.011. Elsevier. [DOI] [PubMed] [Google Scholar]
- Wambugu S., Towett P., Kiama S., Abelson K., Kanui T. Effects of opioids in the formalin test in the Speke’s hinged tortoise (Kinixy’s spekii) J. Vet. Pharmacol. Therapeut. 2010;33:347–351. doi: 10.1111/j.1365-2885.2009.01148.x. [DOI] [PubMed] [Google Scholar]
- Yasaei R., Saadabadi A. Stat Pearls [Internet]. Treasure Island (FL) Stat Pearls Publishing; 2019. Clonidine.https://www.ncbi.nlm.nih.gov/books/NBK459124/ 2020 Jan-. [Online] Available from: [Google Scholar]
- Yoon M.H., Kim C.M., Lee H.G. Synergistic antinociception of intrathecal sildenafil with clonidine in the rat formalin test. Pharmacol. Biochem. Behav. 2009;92(4):583–588. doi: 10.1016/j.pbb.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Yoon S.Y., Kang S.Y., Kim H.W., Kim H.C., Roh D.H. Clonidine reduces nociceptive responses in mouse orofacial formalin model: potentiation by sigma-1 receptor antagonist bd1047 without impaired motor coordination. Biol. Pharm. Bull. 2015;38(9):1320–1327. doi: 10.1248/bpb.b15-00183. [DOI] [PubMed] [Google Scholar]
- Zaretsky D.V., Zaretskaia M.V., DiMicco J.A., Rusyniak D.E. Yohimbine is a 5-HT1A agonist in rats in doses exceeding 1 mg/kg. Neurosci. Lett. 2015;606:215–219. doi: 10.1016/j.neulet.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]