Pregnant women and their infants are among the most vulnerable populations requiring special attention during the current Coronavirus disease (COVID-19) pandemic [1]. The infection is caused by a new enveloped RNA β-coronavirus, the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which can primarily infect type II alveolar epithelial cells of the lung through the binding to a specific receptor, angiotensin converting enzyme-2 (ACE-2) [2]. Since ACE-2 is widely expressed in different tissues, several extrapulmonary sites, such as heart, blood vessels, kidney, intestine, brain and placenta can be affected by the virus [3].
It is well known that physiological and immunological changes during pregnancy can mask or even increase the susceptibility to respiratory infections. Changes including increased heart rate, altered pulmonary volumes, as well as the natural physiological shift to a T-helper 2 response (Th2) which attenuates the cell-mediated immunity by Th1 system, leaving the mother vulnerable to viral infections [1]. In fact, adverse outcomes showing increased risk of maternal morbidity and mortality when compared to non-pregnant women have been demonstrated during the outbreaks caused by influenza virus and also other two coronavirus strains, the severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS) [4].
Regarding SARS-CoV-2 infection, the prevailing symptoms in pregnant women are cough, dyspnea, fever, chest pain and lymphopenia [4]. Similar to non-pregnant patients, severe cases evolve to pneumonia and vascular-related complications such as thrombocytopenia, elevated D-dimer levels and microthrombus formation [5,6]. Recently, two systematic reviews, including mainly retrospective and case reports studies, revealed an association of COVID-19 cases with expressive rates of miscarriage, preterm birth, premature prelabor rupture of membranes, fetal growth restriction, perinatal death and preeclampsia [1,4]. Reviewing published studies between January to September 2020 we found 14 studies reporting specifically cases of hypertensive disorders and/or preeclampsia associated with COVID-19 infection or even cases of patients that developed these pathological conditions during the course of infection (for details see Table S1). Accordingly, a series of 116 cases identified 4.3% of patients with hypertensive disorders, including 3.4% with preeclampsia [7]. Prabhu et al. [8] reported an incidence of preeclampsia in 15.7% in SARS-CoV-2 positive patients versus 9.3% in negative patients (Table S1). Evidence of fetal vascular malperfusion was noted among 48.3% positive placentas, while 11.3% placentas presented this condition among women without COVID-19. These placentas were noted to have thrombi in the fetal vessels. Moreover, Shanes et al. [9] analyzing 16 placentas from patients infected with SARS-CoV-2, found an increased prevalence of decidual arteriopathy and maternal vascular malperfusion, a pattern of placental injury which can be directly associated to hypertensive disorders, preeclampsia and abnormalities in oxygenation within the intervillous space.
Regarding SARS-CoV-2 infection, the prevailing symptoms in pregnant women are cough, dyspnea, fever, chest pain and lymphopenia [4]. Similar to non-pregnant patients, severe cases evolve to pneumonia and vascular-related complications such as thrombocytopenia, elevated D-dimer levels and microthrombus formation [5,6]. Recently, two systematic reviews, including mainly retrospective and case reports studies, revealed an association of COVID-19 cases with expressive rates of miscarriage, preterm birth, premature prelabor rupture of membranes, fetal growth restriction, perinatal death and preeclampsia [1,4]. Reviewing published studies between January to September 2020 we found 14 studies reporting specifically cases of hypertensive disorders and/or preeclampsia associated with COVID-19 infection or even cases of patients that developed these pathological conditions during the course of infection (for details see Table S1). Accordingly, a series of 116 cases identified 4.3% of patients with hypertensive disorders, including 3.4% with preeclampsia [7]. Prabhu et al. [8] reported an incidence of preeclampsia in 15.7% in SARS-CoV-2 positive patients versus 9.3% in negative patients (Table S1). Evidence of fetal vascular malperfusion was noted among 48.3% positive placentas, while 11.3% placentas presented this condition among women without COVID-19. These placentas were noted to have thrombi in the fetal vessels. Moreover, Shanes et al. [9] analyzing 16 placentas from patients infected with SARS-CoV-2, found an increased prevalence of decidual arteriopathy and maternal vascular malperfusion, a pattern of placental injury which can be directly associated to hypertensive disorders, preeclampsia and abnormalities in oxygenation within the intervillous space.
Since clinical evidence pointed to a correlation between COVID-19 and preeclampsia disease we performed the search for molecular alterations promoted by SARS-CoV-2 infection that might be linked to this clinical outcome. For this purpose, host Differentially Expressed Genes (DEGs) from clinical and experimental datasets of SARS-CoV-2 infection were obtained from published data [[10], [11], [12]]. Further, those host DEGs were checked to potential assignment to genes associated with preeclampsia disease found in the MalaCards Human Disease Database (https://www.malacards.org) [13]. The assignment checking was made manually for each DEG against all genes associated with preeclampsia in MalaCards Database. Afterwards, a Gene Set Enrichment Analysis (GSEA) of the DEGs linked to preeclampsia was also performed with the Reactome bioinformatics resource (https://reactome.org/), to identify classes of genes that are over-represented in a set of pathways [14], and may have an association with preeclampsia. A gene set was considered significantly enriched with FDR < 0.25 for the normalized enrichment score (NES). The network presented in Fig. 1 was constructed, visualized and analyzed by using the Cytoscape 3.8.2 software (Cytoscape Apps) 10 [15].
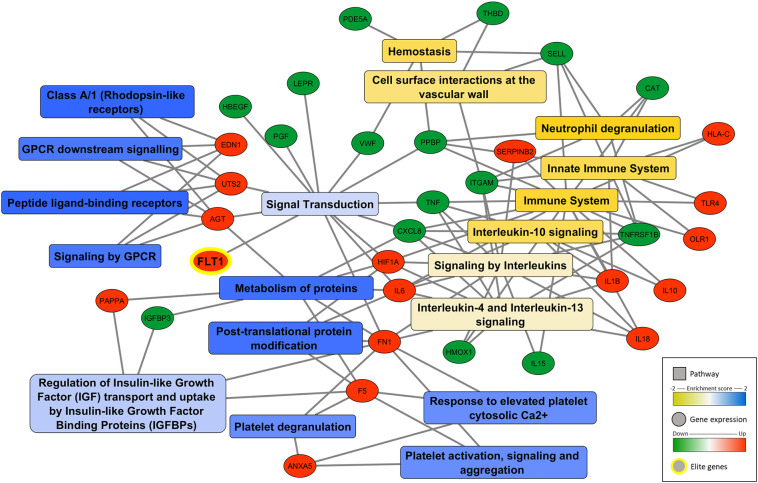
Fig. 1.
Network of DEG associated to preeclampsia during SARS-CoV-2 infection. Significant GSEA cluster/pathways are presented in rectangle (blue to yellow in color scale) and genes upregulated (red) or downregulated (green) are represented in circles. A gene set was considered significantly enriched with FDR < 0.25 for the normalized enrichment score (NES). The network was constructed using Cytoscape 3.8.2 software [15].
Interestingly, our data indicated that SARS-CoV-2 infection altered the expression of several biomarkers involved in preeclampsia (Fig. 1). Also, it was found classes of genes/proteins related to the four main molecular pathways involved in this disease, such as (Table S2): defective angiogenesis/vascular response (FLT1, PGF, ENG, FN1, ITGAM, SELL, HLA-C, IGFBP3, HBEGF), ischemia/hypoxia-related signaling (HIF-1A, CAT, HMOX1, OLR1), inflammatory signaling (TNF, TLR4, IL6, IL8, IL10, IL18, IL15, IL1B, LEPR, CXCL8, TNFRSF1B) and dysregulation of hemostasis/vasoactive peptides (EDN1, UTS2, F5, AGT, ANXA5, VWF, SERPINB2, THBD, PDE5A, PPBP). The release of antiangiogenic factors by the placenta is a key event in preeclampsia contributing to abnormal maternal spiral artery remodeling, reducing oxygen availability, leading to placental hypoxia and triggering inflammation and hemostasis. Our analysis indicated that SARS-CoV-2 infection up-regulates FLT1 and ENG, the main antiangiogenic factors involved in preeclampsia. Soluble FLT1 antagonizes both VEGF and PGF by binding them in the circulation and preventing interaction with their endogenous receptors (Table S2). Similarly, endoglins (ENG) also has potent anti-VEGF activity acting synergistically contributing to hypertension, proteinuria, and endothelial cell dysfunction associated with preeclampsia [16]. Corroborating our results, Mendoza et al. [17] showed that all cases of infected pregnant women developing preeclampsia features had increased serum levels of FLT-1/PGF (Table S1). The worst case had FLT-1/PGF ≥85/110, does not resolve spontaneously and delivery was the only definitive cure. Their overall conclusion was that COVID-19 can induce a preeclampsia-like syndrome [17].
Interestingly, our data indicated that SARS-CoV-2 infection altered the expression of several biomarkers involved in preeclampsia (Fig. 1). Also, it was found classes of genes/proteins related to the four main molecular pathways involved in this disease, such as (Table S2): defective angiogenesis/vascular response (FLT1, PGF, ENG, FN1, ITGAM, SELL, HLA-C, IGFBP3, HBEGF), ischemia/hypoxia-related signaling (HIF-1A, CAT, HMOX1, OLR1), inflammatory signaling (TNF, TLR4, IL6, IL8, IL10, IL18, IL15, IL1B, LEPR, CXCL8, TNFRSF1B) and dysregulation of hemostasis/vasoactive peptides (EDN1, UTS2, F5, AGT, ANXA5, VWF, SERPINB2, THBD, PDE5A, PPBP). The release of antiangiogenic factors by the placenta is a key event in preeclampsia contributing to abnormal maternal spiral artery remodeling, reducing oxygen availability, leading to placental hypoxia and triggering inflammation and hemostasis. Our analysis indicated that SARS-CoV-2 infection up-regulates FLT1 and ENG, the main antiangiogenic factors involved in preeclampsia. Soluble FLT1 antagonizes both VEGF and PGF by binding them in the circulation and preventing interaction with their endogenous receptors (Table S2). Similarly, endoglins (ENG) also has potent anti-VEGF activity acting synergistically contributing to hypertension, proteinuria, and endothelial cell dysfunction associated with preeclampsia [16]. Corroborating our results, Mendoza et al. [17] showed that all cases of infected pregnant women developing preeclampsia features had increased serum levels of FLT-1/PGF (Table S1). The worst case had FLT-1/PGF ≥85/110, does not resolve spontaneously and delivery was the only definitive cure. Their overall conclusion was that COVID-19 can induce a preeclampsia-like syndrome [17].
According to Shanes et al. [9] there were increased rates of maternal vascular malperfusion features, inflammation and intervillous thrombi deposition in placentas from SARS-CoV-2 infected women. Their data are in agreement with our findings indicating an up-regulation of vasoconstrictive peptides (EDN1, UTS2, AGT), nitric oxide modulators (PDE5A) and prothrombotic-related molecules (F5, ANXA5, VWF, THBD, PAI-1, ITGA1, PPBP) (Fig. 1, Table S2). Even proinflammatory cytokines such as IL-6 can have a central role inducing tissue factor expression, stimulating angiotensin system and then contributing to hypercoagulation and reduced placental blood perfusion [18]. Altogether these events lead to a decrease in oxygen supply, triggering ischemia/hypoxia-related signaling and changes in oxidative stress markers (HIF-1A, OLR1, CAT, HMOX1). In fact, markers of cellular oxygen deprivation such as hypoxia-inducible factors (HIF-1A and 2A) are expressed in proliferative trophoblasts and in the placentas of women with preeclampsia [19]. Preclinical data already detected that HIF-1A overexpression in pregnant mice is associated with hypertension, proteinuria, and fetal growth restriction [16].
According to Shanes et al. [9] there were increased rates of maternal vascular malperfusion features, inflammation and intervillous thrombi deposition in placentas from SARS-CoV-2 infected women. Their data are in agreement with our findings indicating an up-regulation of vasoconstrictive peptides (EDN1, UTS2, AGT), nitric oxide modulators (PDE5A) and prothrombotic-related molecules (F5, ANXA5, VWF, THBD, PAI-1, ITGA1, PPBP) (Fig. 1, Table S2). Even proinflammatory cytokines such as IL-6 can have a central role inducing tissue factor expression, stimulating angiotensin system and then contributing to hypercoagulation and reduced placental blood perfusion [18]. Altogether these events lead to a decrease in oxygen supply, triggering ischemia/hypoxia-related signaling and changes in oxidative stress markers (HIF-1A, OLR1, CAT, HMOX1). In fact, markers of cellular oxygen deprivation such as hypoxia-inducible factors (HIF-1A and 2A) are expressed in proliferative trophoblasts and in the placentas of women with preeclampsia [19]. Preclinical data already detected that HIF-1A overexpression in pregnant mice is associated with hypertension, proteinuria, and fetal growth restriction [16].
In summary, COVID-19 pandemic is the most important health challenge in the world nowadays. Pregnant women are susceptible individuals that require a differential care during an outbreak, mainly because of their altered immunological and physiological response which increases their susceptibility to infections and other clinical conditions. Although COVID-19 pathogenesis is far to be understood, the ongoing clinical data suggest an association between SARS-CoV-2 infection and the increase in potential life-threatening conditions to pregnant women and their infants, such as preeclampsia. Our data analysis supports these clinical evidences indicating that SARS-CoV-2 can affect different molecular pathways related to preeclampsia disease such as angiogenesis, hypoxia, inflammatory signaling, hypercoagulation and imbalance of vasoactive peptides. Thus, pregnant women are a higher risk population and antenatal surveillance for women with COVID-19 should be a priority.
The following are the supplementary data related to this article.
Main findings of studies reporting hypertensive disorders/preeclampsia associated with SARS-CoV-2 infection in pregnant women.
Differentially Expressed Genes (DEGs) from clinical and experimental datasets of SARS-CoV-2 infection and its potential role in preeclampsia.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbadis.2020.165999.
CRediT authorship contribution statement
MB/WOBS/EPP/JAG: Conceptualization; RFR/LS/EFT/WOBS: Methodology, Figure design, Data curation; PBT/MB: Clinical data search, Systematic review; MB/WOBS: Writing, Editing, Supervision; MB/WOBS/EPP/JAG/RFR/LS/EFT/PBT: Reviewing, Approval.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Dashraath P, Lin J, Wong J, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. 2020 [cited 2020 May 24];Available from: doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed]
- 2.Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. 2020 [cited 2020 Apr 14];Available from: doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed]
- 3.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Mascio D., Khalil A., Saccone G., et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM. 2020;2(2) doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu D., Li L., Wu X., et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR. 2020;215 doi: 10.2214/AJR.20.23072. www.ajronline.org Available from: (Internet, cited 2020 Aug 1) [DOI] [PubMed] [Google Scholar]
- 6.Bikdeli B, Madhavan M V., Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol [Internet] 2020 [cited 2020 Apr 21];Available from: https://linkinghub.elsevier.com/retrieve/pii/S0735109720350087. [DOI] [PMC free article] [PubMed]
- 7.Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol 2020;223(1):111.e1–111.e14. [DOI] [PMC free article] [PubMed]
- 8.Prabhu M, Cagino K, Matthews KC, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG An Int J Obstet Gynaecol [Internet] 2020 [cited 2020 Oct 4];Available from: /pmc/articles/PMC7361728/?report=abstract. [DOI] [PMC free article] [PubMed]
- 9.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am. J. Clin. Pathol. 2020;154(1):23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong Y., Liu Y., Cao L., et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. https://pubmed.ncbi.nlm.nih.gov/32228226/ Available from: (Internet, cited 2020 Aug 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell [Internet] 2020 [cited 2020 Aug 2];181(5). Available from: https://pubmed.ncbi.nlm.nih.gov/32416070/. [DOI] [PMC free article] [PubMed]
- 12.Bojkova D, Klann K, Koch B, et al. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature [Internet] 2020 [cited 2020 Aug 2];583(7816). Available from: https://pubmed.ncbi.nlm.nih.gov/32408336/. [DOI] [PMC free article] [PubMed]
- 13.Rappaport N., Twik M., Plaschkes I., et al. MalaCards: an amalgamated human disease compendium with diverse clinical and genetic annotation and structured search. Nucleic Acids Res. 2017;45(D1):D877–D887. doi: 10.1093/nar/gkw1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jassal B., Matthews L., Viteri G., et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020;48(D1):D498–D503. doi: 10.1093/nar/gkz1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shannon P., Markiel A., Ozier O., et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rana S., Lemoine E., Granger J., Karumanchi S.A. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094–1112. doi: 10.1161/CIRCRESAHA.118.313276. https://pubmed.ncbi.nlm.nih.gov/30920918/ Available from: (Internet, cited 2020 Aug 2) [DOI] [PubMed] [Google Scholar]
- 17.Mendoza M., Garcia-Ruiz I., Maiz N., et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG Int. J. Obstet. Gynaecol. 2020;127(11):1374–1380. doi: 10.1111/1471-0528.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaMarca B.D., Ryan M.J., Gilbert J.S., Murphy S.R., Granger J.P. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr. Hypertens. Rep. 2007;9(6):480–485. doi: 10.1007/s11906-007-0088-1. https://pubmed.ncbi.nlm.nih.gov/18367011/ Available from: (Internet, cited 2020 Aug 2) [DOI] [PubMed] [Google Scholar]
- 19.Rajakumar A., Brandon H.M., Daftary A., Ness R., Conrad K.P. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta. 2004;25(10):763–769. doi: 10.1016/j.placenta.2004.02.011. https://pubmed.ncbi.nlm.nih.gov/15451190/ Available from: (Internet, cited 2020 Jun 29) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Main findings of studies reporting hypertensive disorders/preeclampsia associated with SARS-CoV-2 infection in pregnant women.
Differentially Expressed Genes (DEGs) from clinical and experimental datasets of SARS-CoV-2 infection and its potential role in preeclampsia.