Abstract

A novel photodeactivation strategy for controlling gene expression has been developed based on light-induced activation of cAMP response element binding protein (CREB). Light-induced cleavage of the photoresponsive protecting group of an antagonist of CREB binding protein (CBP) results in photocleaved products with weak binding affinity for CBP. This photodissociation reaction enables protein–protein interactions between CBP and CREB that trigger the formation of a multiprotein transcription complex to turn gene expression “on”. This enables irradiation of antagonist-treated HEK293T cells to be used to trigger temporal recovery of CREB-dependent transcriptional activity and endogenous gene expression under photolytic control.
Short abstract
A photodeactivatable inhibitor has been developed for allowing protein−protein interactions and upregulating CREB-dependent gene expression in a temporal manner under photochemical control.
Introduction
A range of optochemical and optogenetic strategies have been developed that employ light as a phototrigger to control biological activity and function in cellular systems.1−3 These approaches include inactive caged probes whose bioactivities can be modulated through photolytic cleavage of their photoresponsive protecting groups (PPGs).4−9 These probes employ temporal light-triggered cleavage of photocleavable constructs to release agonists (Figure 1a) or antagonists (Figure 1b) that then act to regulate protein activity. Caged derivatives of nucleotides (e.g., ATP and cAMP),10,11 intracellular messenger ions (e.g., Ca2+),12−15 and neurotransmitters (e.g., acetylcholine)16,17 have been reported, whose photolysis has been used to modulate the activity of target proteins involved in different cellular pathways. For example, this type of photocleavage strategy has been used to trigger spatiotemporal photorelease of caged neurotransmitters in the brain as a method to investigate neuronal activity.18
Figure 1.
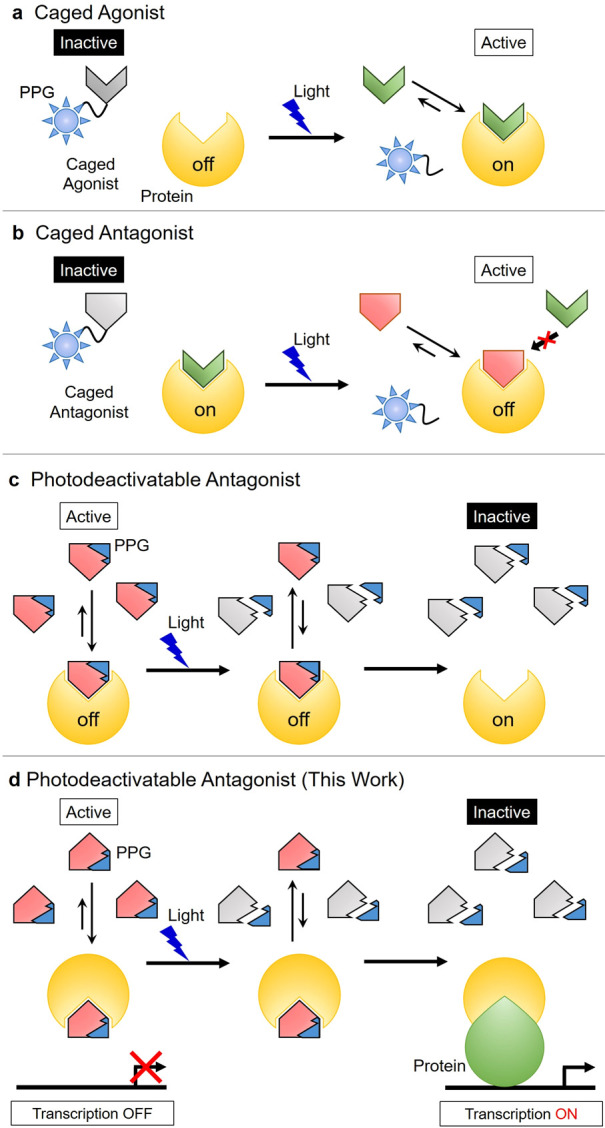
Four photolytic strategies for the optochemical spatiotemporal control of protein activity. (a) Photolytic release of a caged agonist to turn protein activity “on”. (b) Photolytic release of a caged antagonist to turn protein activity “off”. (c) Photolytic cleavage of a photodeactivatable antagonist to turn protein activity “on”. (d) Photolytic cleavage of a photodeactivatable antagonist results in protein–protein interactions affording a complex that turns “on” protein activity and gene expression.
An alternative photodegradation strategy has also been described based on the use of a photolabile enzyme antagonist that allows an enzyme’s activity to be turned “on” under photochemical control. In this approach, binding of a photodeactivatable antagonist to a target enzyme results in its activity being inhibited. Subsequent irradiation of the PPG group of the antagonist results in photofragmentation to afford cleavage products with much weaker binding affinity, which results in the enzyme’s active site becoming unblocked, and its activity being turned “on” (Figure 1c). For example, Li and co-workers described the use of a peptide kinase antagonist containing a photodegradable linker that was photolytically cleaved to turn “on” kinase activity.19 Alternatively, Porter et al. reported a covalent antagonist approach that is based on photolytic cleavage of a bound antagonist from the active site of a protease to turn proteolytic activity “on”.20 We reasoned that this type of photodeactivatable antagonist strategy might also be useful for turning “on” gene expression pathways in ex vivo cellular systems. We envisaged that this could be achieved using light to photodegrade the PPG of an inactive protein–antagonist complex to unblock a protein binding domain that would allow an active transcription factor protein to be formed that could upregulate gene transcription (Figure 1d).
cAMP-responsive element binding protein (CREB) was identified as a potential target to develop this photodeactivatable antagonist approach for the control of gene expression. CREB is an important mediator of gene transcription, which is activated through phosphorylation by protein kinase A (PKA) and Ca2+/calmodulin-dependent protein kinase IV (CaMK4).21,22 This results in phosphorylated CREB forming protein–protein interactions with CREB binding protein (CBP) and cAMP response elements (CREs) to produce an active multiprotein complex. This complex then combines with CREB-regulated transcription coactivator 1 (CRTC1) to upregulate gene expression pathways that are involved in neuronal growth pathways and synaptic plasticity.23 Gene knockdown and knockout approaches have previously been developed as tools to genetically suppress CREB-mediated transcriptional activity, with these modified cell lines used to explore the role of CREB in memory and learning in the brain.24 However, lack of a direct genetic activator of CREB means that genetic methods cannot be used for the spatiotemporal upregulation of CREB-mediated transcriptional activity. To circumvent this limitation, we previously described how irradiation of inactive caged CBP inhibitors25 could be used to generate active CBP antagonists to turn “off” CREB activity in cellular systems (Figure 2a).26−28 Once released, these CBP antagonists inhibit key protein–protein interactions between CBP and CREB to prevent formation of the active CREB multiprotein complex required for upregulation of gene expression.
Figure 2.
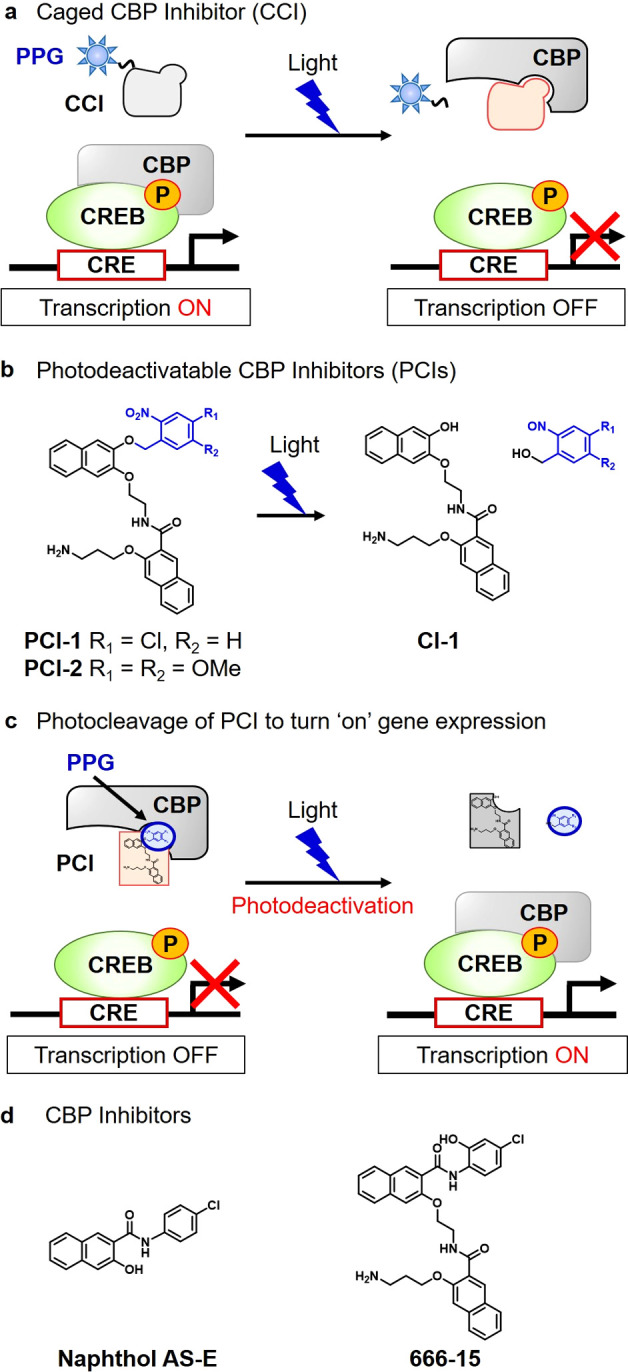
Design of photodeactivatable CBP inhibitors (PCIs). (a) Caged CBP inhibitor strategy for photochemical downregulation of CREB-mediated gene expression.25 (b) Photocleavage of the o-nitrobenzyl PPG of a PCI affords CI-1 that exhibits weak CBP binding affinity. (c) Photocleavage of a CBP bound antagonist (PCI) results in upregulation of CREB-mediated gene expression. (d) CBP inhibitors, naphthol AS-E and 666-15.27,28
In this report, we now describe the development of an alternative photodeactivatable CBP inhibitor (PCI) strategy that can be used to upregulate CREB-mediated gene expression in real time. This approach involves photocleavage of the PPG of a CBP antagonist (PCI) to produce photofragments that exhibit poor binding affinity for CBP (Figure 2b). This enables irradiation of PCI-treated cells to be used to photocleave the PPG of a CBP bound PCI to unblock its CREB binding site which enables free CBP to bind to CREB to initiate formation of the multiprotein complex required for gene transcription to occur (Figure 2c).
Results
Design and Photochemical Properties of PCIs
Our first goal was to identify a photodeactivatable antagonist with good affinity for the CREB-binding site of CBP. Previous studies had revealed that naphthol AS-E and its derivative 666-15 were effective inhibitors of the CREB binding site of CBP (Figure 2d). Structure–activity relationship studies revealed that the chloroarylamido fragments of these antagonists were important for strong CBP binding, with replacement of their chloroarylamido groups with other aryl groups generally well tolerated.27,28 Consequently, we reasoned that replacement of the chloroarylamido fragment of 666-15 with a well-established o-nitrobenzyl PPG would afford a photoaddressable PCI (e.g., PCI-1 or PCI-2) that would retain good binding affinity for the CREB binding site of CBP. PCI-1 (containing a 4-chloro-2-nitrobenzyl PPG) and PCI-2 (containing a 4,5-dimethoxy-2-nitrobenzyl PPG) were prepared using standard synthetic procedures (see Schemes S1 and S2 for details), and their photolytic and CREB inhibitory properties were investigated. HPLC analysis revealed that photolysis of PCI-1 and PCI-2 in 20 mM HEPES buffer at 365 nm resulted in rapid photocleavage to afford CI-1 as their major cleavage product (Figure 3a,b).
Figure 3.
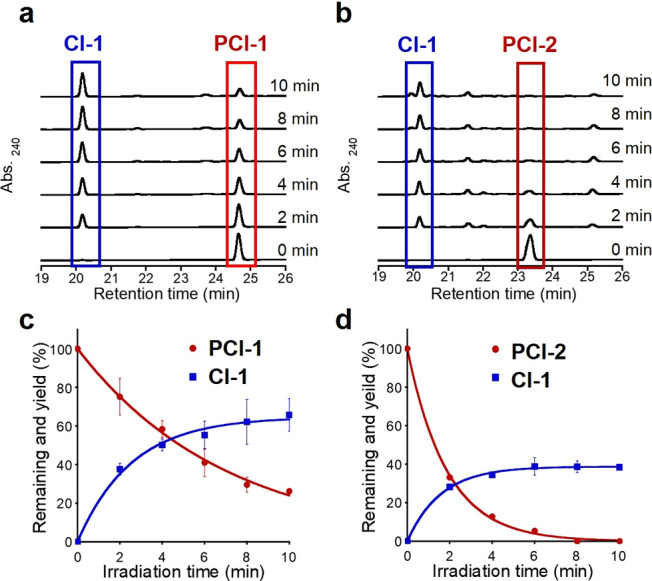
Photolysis of PCIs. HPLC analyses of the photolytic cleavage products of 50 μM samples of (a) PCI-1 and (b) PCI-2 in 20 mM HEPES buffer (pH 7.4). Time course of the photolysis reactions of (c) PCI-1 and (d) PCI-2 to produce CI-1. Light intensity: 4 mW cm–2, λ = 365 nm ±5 nm. Error bars denote ± SD (N = 3).
The wavelength dependency of these PCI photolysis reactions is consistent with their absorption spectra (Figures S1 and S2), with no PCI cleavage occurring in the absence of illumination. Quantitative analysis of the amount of CI-1 produced in these photolysis reactions was carried out by comparing its concentration with a benzoic acid internal standard (Figure 3c,d). These photolysis reactions revealed that PCI-1 levels were reduced to 20% of its original level after 10 min, forming CI-1 in around 60% theoretical yield, with PCI-1 totally consumed after 30 min (Figure S3). Alternatively, PCI-2 was completely photolyzed after 10 min of illumination at 365 nm to afford CI-1 in 39% theoretical yield. Therefore, although PCI-1 and PCI-2 exhibit similar quantum yields (Φ), the larger molar absorption coefficient of PCI-2 means that it is cleaved approximately 4-fold faster than PCI-1 when irradiated at 365 nm (Table 1 and Figure S2). The amount of CI-1 produced in these PCI illumination reactions is less than expected from the rate of PCI photocleavage, even though CI-1 (see Scheme S3 for synthesis) was found to be stable to prolonged illumination at 365 nm (Figure S4). This indicates that more than one PCI photocleavage pathway is likely to be operating in these reactions; however, we were unable to isolate/identify any other photocleavage products produced in these photocleavage reactions.
Table 1. Photochemical Properties of PCI-1 and PCI-2.
| PCI-1 | PCI-2 | |
|---|---|---|
| k (s–1) | 2.4 × 10–3 | 9.0 × 10–3 |
| ε365nm (M–1 cm–1) | 1.5 × 103 | 4.5 × 103 |
| Φ | 2.6 × 10–2 | 3.3 × 10–2 |
| ε365nmΦ (M–1 cm–1) | 39 | 147 |
CREB Inhibitory Activities of PCIs
The inhibitory activities of the photodeactivatable CBP inhibitors PCI-1 and PCI-2 were then evaluated in human embryo kidney 293T cells (HEK293T) expressing a photoluminescent dual luciferase reporter assay. This cell line expresses a firefly luciferase reporter gene containing a CREB-activity-sensing CRE promoter as well as a Renilla luciferase reporter gene that functions as an internal control. This reporting system enables the luminescence generated by firefly luciferase to be calibrated against the luminescent output of the Renilla luciferase reporter assay. This allows the inhibitory activities of the PCIs to be determined through measurement of cellular photoactivity levels, thus minimizing variability caused by differences in cellular conditions and/or transfection efficiencies. This reporter assay enabled the inhibitory activities of both PCIs to be determined by measuring cellular photoluminescent responses determined by cellular CREB-induced firefly luciferase expression levels. This photoluminescent reporter system revealed similarly good IC50 values (50% inhibition concentration of CREB transcriptional activity) for PCI-1 and PCI-2 against CBP of 3.4 ± 0.2 and 3.3 ± 0.3 μM, respectively (Figure 4). Although these IC50 values were significantly higher than those reported for 666-15 (IC50 = 81 nM),28 they are similar in value to the IC50 value of 2.26 μM previously reported for the effective CBP inhibitor naphthol AS-E27 and so were predicted to effectively inhibit CBP.
Figure 4.
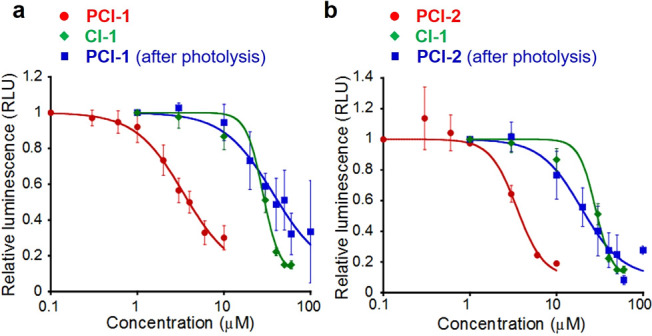
CREB inhibitory activities of PCIs before and after photolysis. Inhibition of CREB activity using (a) PCI-1 and (b) PCI-2, with both data sets presented in comparison to CI-1 and PCI photoproducts. Relative luminescence values are reported as a ratio of Firefly:Renilla luciferase luminescent intensities. Photolysis of PCIs (100 μM) carried out using UV illumination (10 mW cm–2, λ = 365 ± 5 nm) for 10 min in Hanks’s buffered saline solution (HBSS). Error bars denote ± SD (N = 3).
PCI-1 and PCI-2 were then preirradiated at 365 nm in HEPES buffer (pH 7.4) for 10 min, and the products of these photolysis reactions were analyzed by HPLC (Figure S5). The inhibitory activities of the photocleavage products of PCI-1 and PCI-2 were reduced markedly when compared to their parent PCIs (Figure 4), with preirradiated PCI-1 and PCI-2 not significantly inhibiting CREB transcriptional activity at concentration levels between 1 and 10 μM. The differences in inhibitory profiles for the PCI-1 and PCI-2 photocleavage products, when compared to pure CI-1, are likely to be due to the formation of other photolytic products with lower or higher binding affinities for CBP, respectively. Nevertheless, the inhibitory profiles of PCI-1 and PCI-2 for CBP meant that they should function as effective photodeactivatable inhibitors of CREB activity, with their weakly binding photoproducts only exhibiting a negligible effect on CREB activity levels.
Photochemical Recovery of CREB Activity in Cells Treated with PCIs
CREB-mediated gene expression levels were largely suppressed when HEK293T cells were treated with PCI-1 and PCI-2 at a concentration level of 6 μM (Figure 5). Illumination of these PCI-1-treated cells at 365 nm led to an approximate 1.2-fold upregulation of luciferase expression levels, corresponding to recovery of around 40% of CREB-mediated gene expression activity levels of untreated HEK293T cells; however, this increase was considered not to be statistically significant. More promisingly, illumination of cells containing 6 μM PCI-2 resulted in 5-fold upregulation of CREB-mediated luciferase activity, affording CREB activity levels that were essentially identical to those of untreated HEK293T cells. The differences in cellular photolytic efficiencies between PCI-1 and PCI-2 were investigated by carrying out HPLC analyses of irradiated cell extracts (Figure S6). Unreacted PCI-1 was detected in irradiated cell extracts (and their supernatants); however, no evidence of any intact PCI-2 could be found in irradiated cell extracts. Therefore, we propose that the increased gene expression levels observed for irradiated cells treated with PCI-2 are most likely to be due to its more efficient cellular photocleavage at 365 nm. Importantly, cytotoxicity assays revealed that the observed changes in luciferase expression levels in cells containing PCIs and irradiated PCIs (up to 10 μM) were not caused by nonspecific cellular damage caused by the photocleavage process (see Figures S7 and S8). We also confirmed that the PCI compounds did not inhibit luciferase activity at up to 10 μM PCI concentration (Figure S9).
Figure 5.
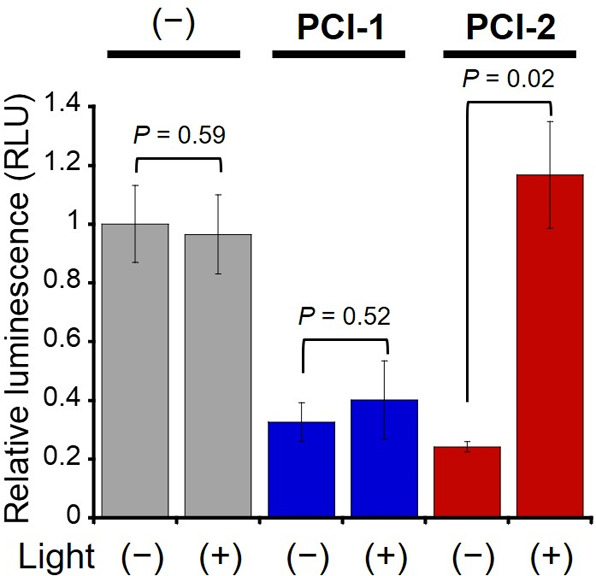
Light-mediated recovery of CREB activity in PCI-treated HEK293T cells. CREB-mediated gene expression levels after treatment of cells with 6 μM PCI-1 and PCI-2 before and after illumination (λex = 365 nm, 10 mW cm–2 for 5 min) in HBSS (1% DMSO). Error bars denote ± SD (N = 3). Significance evaluated using the Student t-test.
The transcriptional inhibitory effects of the PCIs were then confirmed by measuring mRNA expression levels of the endogenous CREB target gene NR4A2 (nuclear receptor subfamily 4 group A member 2)29 using quantitative reverse transcriptional polymerase chain reaction (qRT-PCR) experiments (Figure 6). Treatment of cells with PCI-1 and PCI-2 resulted in 15.0- and 4.7-fold suppression of NR4A2 mRNA expression levels, respectively. After cellular irradiation, NR4A2 gene expression levels in PCI-1-treated cells were increased by 2.3-fold, while expression levels in PCI-2-treated cells were increased by 6.9-fold. These transcription results confirmed the CREB activity results obtained from the luciferase expression assay (Figure 5), with irradiation of PCI-2-treated cells resulting in full recovery of endogenous CREB target gene expression levels. Therefore, these results clearly confirm that irradiation of PCI-2-treated cells results in photocleavage of PCI-2 to upregulate mRNA levels associated with CREB-signaling gene expression pathways.
Figure 6.
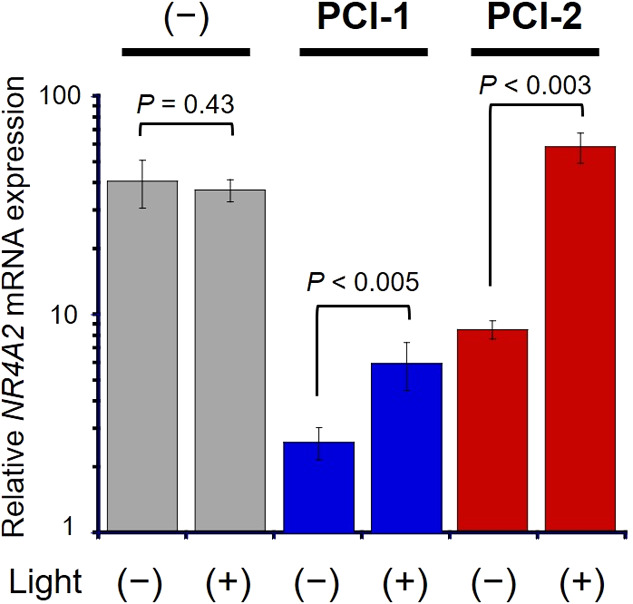
Results of qRT-PCR experiments used to determine NR4A2 gene expression levels in HEK293T cells. CREB-dependent NR4A2 mRNA expression levels of HEK293T cells treated with 6 μM PCI-1 and PCI-2, before and after illumination (λex = 365 nm, 10 mW cm–2 for 5 min) in DMEM (0.6% DMSO). Error bars denote ± SD (N = 3). Significance evaluated using the Student t-test.
Discussion
This study reports the development of a novel optochemical approach that enables a CREB-mediated gene expression pathway to be turned “on” in a temporal manner through photocleavage of the PPG of a photodeactivatable antagonist of a transcription coactivator protein (Figures 1d and 2c). The design of this type of photolytic antagonist is challenging, because it requires access to a PPG-containing antagonist of a gene activating protein whose PPG group can be efficiently photolyzed to afford cleavage products with low binding affinity. This approach has been realized using CBP antagonist PCI-2 which contains a photolabile o-nitrobenzyl PPG, whose photolytic cleavage products exhibit weak CBP binding activity (Figures 3 and 4). This enables ex vivo irradiation of PCI-2-treated HEK293T cells to be used to unblock the CREB binding site of CBP to trigger recovery of CREB-mediated transcription activity (Figures 5 and 6) and upregulation of protein expression in ex vivo cellular systems. The ability to photodegrade an inhibitor of a known protein–protein binding interaction to restore biological function is noteworthy, since these types of regulatory protein–protein interactions are known to be responsible for controlling numerous biological systems and pathways.
Prior to irradiation, the CBP bound PCI-2 antagonist will exist in dynamic equilibria with a large pool of unbound PCI antagonist within the cell, with the micromolar IC50 binding value of PCI-2 for CBP allowing for rapid exchange between its bound and unbound states. Upon illumination, photolytic cleavage of both bound and unbound PCI-2 will occur to afford cleavage products with weak CBP affinities, thus resulting in CREB activity being turned “on”. Faster photolytic cleavage of the unbound PCI-2 cellular reservoir may result in its faster depletion, which would perturb the CBP-PCI binding equilibria leading to dissociation of bound PCI-2 from the CBP binding site. This type of equilibrium controlled photodissociation mechanism would be potentially beneficial, because preferential photocleavage of unbound PCI would protect the CBP binding site from damage from reactive radical intermediates/products produced from photofragmentation of bound PCI.30
Safety Statement
No unexpected or unusually high safety hazards were encountered
Conclusions
A new photodeactivatable antagonist strategy has been developed that enables gene expression to be turned “on” in a temporal manner under photochemical control. Illumination of a photolytically labile antagonist (PCI-2) of CBP results in its PPG being cleaved to afford photoproducts that exhibit low CBP binding affinity. This light-induced degradation reaction results in irreversible photodissociation of the antagonist from the CBP binding site, allowing protein–protein interactions between CBP and CREB to initiate the formation of a multiprotein complex that triggers recovery of CREB-dependent transcriptional activity and gene expression under photochemical control. Spatiotemporal upregulation of CREB activity in real time provides a potentially powerful tool for probing the role of CREB-mediated pathways in neuronal cell proliferation and long-term memory processes. Furthermore, numerous antagonists of other key protein–protein interactions are known, whose aryl fragments could potentially be replaced with photocleavable o-nitrobenzyl groups (or other PPGs), while retaining binding affinity. This provides the opportunity to prepare new types of photodeactivatable inhibitors to modulate other protein–protein interactions and photochemically upregulate other gene expression pathways in real time.31−34
Acknowledgments
We would like to thank Dr. Fuminori Sugihara (Osaka Univ.) for the use of a real-time PCR instrument. This research was supported by the following grants: Grant-in-Aid for Scientific Research (Grant 16K01933 and 20K05747 to M.M.; 25220207, 18H039351, and 19K22255 to K.K.); Innovative Areas “Frontier Research on Chemical Communications” (17H06409 to K.K.); “Brain Information Dynamics” (17H06312 to H.B.) of Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan; AMED (18he0902005h0004, 17ae0101041h9902, and 18fm0208018h0002 to K.K.); Takeda Science Foundation (to K.K. and H.B.); JSPS A3 Foresight Program; JSPS CORE-to-CORE Program “Asian Chemical Biology Initiative”; Japan (JSPS)-UK (RSC) Research Cooperative Program (JPJSBP120195705 to K.K.) and Royal Society International Exchange (IEC\R3\183068 to S.D.B.).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.0c00736.
Experimental procedures and additional figures including syntheses, photolysis results, absorption spectra, time course results, photostability results, cytotoxicity, luciferase activity, and NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Yizhar O.; Fenno L. E.; Davidson T. J.; Mogri M.; Deisseroth K. Optogenetics in Neural Systems. Neuron 2011, 71, 9–34. 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Ellis-Davies G. C. R. Caged Compounds: Photorelease Technology for Control of Cellular Chemistry and Physiology. Nat. Methods 2007, 4, 619–628. 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrentz T.; Schonberger M.; Trauner D. Optochemical Genetics. Angew. Chem., Int. Ed. 2011, 50, 12156–12182. 10.1002/anie.201103236. [DOI] [PubMed] [Google Scholar]
- Klán P.; Šolomek T.; Bochet C. G.; Blanc A.; Givens R.; Rubina M.; Popik V.; Kostikov A.; Wirz J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013, 113, 119–191. 10.1021/cr300177k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip R. W.; Wen Y. X.; Gravel D.; Giasson R.; Sharma D. K Photochemistry of the ortho-Nitrobenzyl System in Solution - Identification of the Biradical Intermediate in the Intramolecular Rearrangement. J. Phys. Chem. 1991, 95, 6078–6081. 10.1021/j100169a009. [DOI] [Google Scholar]
- Takezaki M.; Hirota N.; Terazima M. Nonradiative Relaxation Processes and Electronically Excited States of Nitrobenzene Studied by Picosecond Time-Resolved Transient Grating Method. J. Phys. Chem. A 1997, 101, 3443–3448. 10.1021/jp963095t. [DOI] [Google Scholar]
- Das P. K.; Encinas M. V.; Small R. D.; Scaiano J. C. Photoenolization of ortho-Alkyl-substituted Carbonyl-Compounds - Use of Electron-Transfer Processes to Characterize Transient Intermediates. J. Am. Chem. Soc. 1979, 101, 6965–6970. 10.1021/ja00517a030. [DOI] [Google Scholar]
- Small R. D.; Scaiano J. C. Role of Biradical Intermediates in Photochemistry of ortho-Methylacetophenone. J. Am. Chem. Soc. 1977, 99, 7713–7714. 10.1021/ja00465a055. [DOI] [Google Scholar]
- Givens R. S.; Matuszewski B. Photochemistry of Phosphate-esters - an Efficient Method for the Generation of Electrophiles. J. Am. Chem. Soc. 1984, 106, 6860–6861. 10.1021/ja00334a075. [DOI] [Google Scholar]
- Walker J. W.; Reid G. P.; McCray J. A.; Trentham D. R. Photolabile 1-(2-nitrophenyl)ethyl Phosphate-Esters of Adenine-Nucleotide Analogs - Synthesis and Mechanism of Photolysis. J. Am. Chem. Soc. 1988, 110, 7170–7177. 10.1021/ja00229a036. [DOI] [Google Scholar]
- Kaplan J. H.; Forbush B.; Hoffman J. F. Rapid Photolytic Release of Adenosine 5′-triphosphate from a Protected Analog - Utilization by the Na-K Pump of Human Red Blood-Cell Ghosts. Biochemistry 1978, 17, 1929–1935. 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- Ellis-Davies G. C. R.; Kaplan J. H. A New Class of Photolabile Chelators for the Rapid Release of Divalent-Cations - Generation of Caged-Ca and Caged-Mg. J. Org. Chem. 1988, 53, 1966–1969. 10.1021/jo00244a022. [DOI] [Google Scholar]
- Ellis-Davies G. C. R.; Kaplan J. H. Nitrophenyl-EGTA, a Photolabile Chelator that Selectively Binds Ca2+ with High-Affinity and Releases it Rapidly upon Photolysis. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 187–191. 10.1073/pnas.91.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams S. R.; Kao J. P. Y.; Grynkiewicz G.; Minta A.; Tsien R. Y. Biologically Useful Chelators that Release Ca2+ upon Illumination. J. Am. Chem. Soc. 1988, 110, 3212–3220. 10.1021/ja00218a034. [DOI] [Google Scholar]
- Tertyshnikova S.; Fein A. Inhibition of Inositol 1,4,5-trisphosphate-induced Ca2+ Release by cAMP-dependent Protein Kinase in a Living Cell. Proc. Natl. Acad. Sci. U. S. A. 1998, 95, 1613–1617. 10.1073/pnas.95.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. W.; McCray J. A.; Hess G. P. Photolabile Protecting Groups for an Acetylcholine-Receptor Ligand - Synthesis and Photochemistry of a New Class of ortho-Nitrobenzyl Derivatives and their Effects on Receptor Function. Biochemistry 1986, 25, 1799–1805. 10.1021/bi00355a052. [DOI] [PubMed] [Google Scholar]
- Milburn T.; Matsubara N.; Billington A. P.; Udgaonkar J. B.; Walker J. W.; Carpenter B. K.; Webb W. W.; Marque J.; Denk W.; McCray J. A.; Hess G. P. Synthesis, Photochemistry, and Biological-Activity of a Caged Photolabile Acetylcholine-Receptor Ligand. Biochemistry 1989, 28, 49–55. 10.1021/bi00427a008. [DOI] [PubMed] [Google Scholar]
- Jerome J.; Heck D. H. The Age of Enlightenment: Evolving Opportunities in Brain Research through Optical Manipulation of Neuronal Activity. Front. Syst. Neurosci. 2011, 5, 1–11. 10.3389/fnsys.2011.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Hah J.-M.; Laurence D. S. Light-Mediated Liberation of Enzymatic Activity: “Small Molecule” Caged Protein Equivalents. J. Am. Chem. Soc. 2008, 130, 10474–10475. 10.1021/ja803395d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimer J.; Pávová M.; Anders M.; Pachl P.; Šácha P.; Cígler P.; Weber J.; Majer P.; Řezáčová P.; Kräusslich H.-G.; Müller B.; Konvalinka J. Triggering HIV Polyprotein Processing by Light Using Rapid Photodegradation of a Tight-Binding Protease Inhibitor. Nat. Commun. 2015, 6, 6461. 10.1038/ncomms7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze B. E.; Ginty D. D. Function and Regulation of CREB Family Transcription Factors in the Nervous System. Neuron 2002, 35, 605–623. 10.1016/S0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Bito H.; Takemoto-Kimura S. Ca2+/CREB/CBP-dependent gene regulation: a shared mechanism critical in long-term synaptic plasticity and neuronal survival. Cell Calcium 2003, 34, 425–430. 10.1016/S0143-4160(03)00140-4. [DOI] [PubMed] [Google Scholar]
- Nonaka M.; Kim R.; Fukushima H.; Sasaki K.; Suzuki K.; Okamura M.; Ishii Y.; Kawashima T.; Kamijo S.; Takemoto-Kimura S.; Okuno H.; Kida S.; Bito H. Region-Specific Activation of CRTC1-CREB Signaling Mediates Long-Term Fear Memory. Neuron 2014, 84, 92–106. 10.1016/j.neuron.2014.08.049. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R.; Frenguelli B.; Blendy J.; Cioffi D.; Schutz G.; Silva A. J. Deficient Long-Term-Memory in Mice with a Targeted Mutation of the Camp-Responsive Element-Binding Protein. Cell 1994, 79, 59–68. 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Imoto T.; Kawase A.; Minoshima M.; Yokoyama T.; Bito H.; Kikuchi K. Photolytic Release of a Caged Inhibitor of an Endogenous Transcription Factor Enables Optochemical Control of CREB-Mediated Gene Expression. Org. Lett. 2020, 22, 22–25. 10.1021/acs.orglett.9b03568. [DOI] [PubMed] [Google Scholar]
- Best J. L.; Amezcua C. A.; Mayr B.; Flechner L.; Murawsky C. M.; Emerson B.; Zor T.; Gardner K. H.; Montminy M. Identification of Small-Molecule Antagonists that Inhibit an Activator:Coactivator Interaction. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 17622–17627. 10.1073/pnas.0406374101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B. X.; Xie F. C.; Fan Q. H.; Barnhart K. M.; Moore C. E.; Rheingold A. L.; Xiao X. S. Novel Type of Prodrug Activation through a Long-Range O,N-Acyl Transfer: A Case of Water-Soluble CREB Inhibitor. ACS Med. Chem. Lett. 2014, 5, 1104–1109. 10.1021/ml500330n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F. C.; Li B. X. B.; Kassenbrock A.; Xue C. H.; Wang X. Y.; Qian D. Z.; Sears R. C.; Xiao X. S. Identification of a Potent Inhibitor of CREB-Mediated Gene Transcription with Efficacious in Vivo Anticancer Activity. J. Med. Chem. 2015, 58, 5075–5087. 10.1021/acs.jmedchem.5b00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk J. D.; Abel T. The Role of NR4A Transcription Factors in Memory Formation. Brain Res. Bull. 2011, 85, 21–29. 10.1016/j.brainresbull.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth A.; Corrie J. E.; Gradwell M. J.; Maeda Y.; Mäntele W.; Meier T.; Trentham D. R. Time-resolved Infrared Spectroscopy of Intermediates and Products from Photolysis of 1-(2-nitrophenyl) ethyl phosphates: Reaction of the 2-nitrosoacetophenone Byproduct with Thiols. J. Am. Chem. Soc. 1997, 119, 4149–4159. 10.1021/ja964430u. [DOI] [Google Scholar]
- For previous examples where photochemical tools have been used for the optical control of gene expression for investigation of dynamic gene regulatory mechanisms, see refs 32–34 and see:; Cambridge S. B.; Geissler D.; Calegari F.; Anastassiadis K.; Hasan M. T.; Stewart A. F.; Huttner W. B.; Hagen V.; Bonhoeffer T. Doxycycline-dependent Photoactivated Gene Expression in Eukaryotic Systems. Nat. Methods 2009, 6, 527–531. 10.1038/nmeth.1340. [DOI] [PubMed] [Google Scholar]
- Reis S. A.; Ghosh B.; Hendricks J. A.; Szantai-Kis D. M.; Törk L.; Ross K. N.; Lamb J.; Read-Button W.; Zheng B.; Wang H.; Salthouse C.; Haggarty S. J.; Mazitschek R. Light-controlled Modulation of Gene Expression by Chemical Optoepigenetic Probes. Nat. Chem. Biol. 2016, 12, 317–323. 10.1038/nchembio.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert L.; Xu J.; Wan R.; Srinivasan V.; Dou Y.; Vázquez O. Controlled Inhibition of Methyltransferases Using Photoswitchable Peptidomimetics: Towards an Epigenetic Regulation of Leukemia. Chem. Sci. 2017, 8, 4612–4618. 10.1039/C7SC00137A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumschlager A.; Rullan M.; Khammash M. Exploiting Natural Chemical Photosensitivity of Anhydrotetracycline and Tetracycline for Dynamic and Setpoint Chemo-optogenetic Control. Nat. Commun. 2020, 11, 3834. 10.1038/s41467-020-17677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


