Abstract
Cholesterol is being recognized as a molecule involved in regulating the entry of the SARS-CoV-2 virus into the host cell. However, the data about the possible role of cholesterol carrying lipoproteins and their receptors in relation to infection are scarce and the connection of lipid-associated pathologies with COVID-19 disease is in its infancy. Herein we provide an overview of lipids and lipid metabolism in relation to COVID-19, with special attention on different forms of cholesterol. Cholesterol enriched lipid rafts represent a platform for viruses to enter the host cell by endocytosis. Generally, higher membrane cholesterol coincides with higher efficiency of COVID-19 entry. Inversely, patients with COVID-19 show lowered levels of blood cholesterol, high-density lipoproteins (HDL) and low-density lipoproteins. The modulated efficiency of viral entry can be explained by availability of SR-B1 receptor. HDL seems to have a variety of roles, from being itself a scavenger for viruses, an immune modulator and mediator of viral entry. Due to inverse roles of membrane cholesterol and lipoprotein cholesterol in COVID-19 infected patients, treatment of these patients with cholesterol lowering statins needs more attention. In conclusion, cholesterol and lipoproteins are potential markers for monitoring the viral infection status, while the lipid metabolic pathways and the composition of membranes could be targeted to selectively inhibit the life cycle of the virus as a basis for antiviral therapy.
Abbreviations: CoV, coronavirus; SARS-CoV, Severe Acute Respiratory Syndrome Coronavirus; MERS-CoV, Middle East Respiratory Syndrome Coronavirus; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; COVID-19, The Corona Virus Disease 2019; CoVs, coronaviruses; ACE2, angiotensin-converting enzyme 2; HDL-cholesterol, high-density lipoprotein cholesterol; LDL-cholesterol, low-density lipoprotein cholesterol; TC, total cholesterol; ApoA1, apolipoprotein A1; SR-B1, scavenger receptor class B type 1; PON1, paraoxonase 1; HEK, human embryonic kidney; FH, familial hypercholesterolemia; NPC, Niemann-Pick type C disease; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; NASH, steatohepatitis
Keywords: SARS-CoV-2, COVID-19, Cholesterol, Low density lipoprotein, High density lipoprotein, Statins
Highlights
-
•
Increased membrane cholesterol facilitates COVID-19 entry.
-
•
SARS-CoV-2 spike protein interacts with cholesterol as well as with HDL particles.
-
•
COVID-19 patients present with dyslipidemia.
-
•
Lipid associated chronic pathologies affect the SARS-CoV-2 infection.
-
•
Due to cholesterol lowering effect statins may interact with COVID-19 progression.
1. Introduction
After two outbreaks of Coronavirus (CoV) in the past two decades (Severe Acute Respiratory Syndrome Coronavirus, SARS-CoV; Middle East Respiratory Syndrome, MERS-CoV), the world is facing a newly emerging CoV, known as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the etiologic agent of severe respiratory illness called The Corona Virus Disease 2019 (COVID-19) [1,2]. The prevalence of SARS-CoV-2 is high, affecting a large number of people in a short period of time. However, the fatality rate of SARS-CoV-2 appears to be lower than that of SARS-CoV and MERS-CoV [3]. The characteristics of the three viruses are described in Table 1 . Most patients with SARS-CoV-2 infection have mild symptoms, with the main clinical manifestations being the common respiratory disorders, but in a handful of people the infection exceeds the severity, with possible fatal outcomes due to multi-organ complications [4]. Recent data show that no age group is excluded from the possibility of SARS-CoV-2 infection. However, it is more likely to affect elderly with comorbidities, such as cardiovascular and pulmonary diseases, diabetes, and hypertension, which could result in progressiveness of COVID-19 [[5], [6], [7], [8], [9], [10]].
Table 1.
Characteristics comparison between SARS-CoV, MERS-CoV and SARS-CoV-2 at the time of writing this article (7 October 2020) [1,3,5,[11], [12], [13]].a
| Outbreak | Number of countries/regions | Total number of confirmed cases | Total number of deaths | Case-fatality rate | |
|---|---|---|---|---|---|
| SARS-CoV | Guangdong Province, southern China (Nov. 2002–Aug. 2003) | 32 | 8422 | 919 | ~11% |
| MERS-CoV | Saudi Arabia, Middle East countries (2012) | 27 | 2494 | 858 | ~35% |
| SARS-CoV-2 | Wuhan, Hubei province, China; worldwide (Dec. 2019–ongoing) | 188 | >35.8 million | >1 million | ~3% |
SARS-CoV, Severe Acute Respiratory Syndrome Coronavirus; MERS-CoV, Middle East Respiratory Syndrome Coronavirus; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2.
2. SARS-CoV-2 structural components
Coronaviruses (CoVs) are widely distributed among humans and animals (Table 2 ). In addition to the respiratory system, they can also infect the enteric, hepatic and central nervous systems of humans and other species [7,14]. As RNA viruses, CoVs have a higher mutation rate and frequently undergo recombination. This leads to a greater genetic diversity, which hinders the development of vaccines [15]. In order to complete their replication cycle, they require a set of four major structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N) that are indispensable for the assembly of the virion and the infection of the host cell [14].
Table 2.
Comparison of protein composition, functional receptor and infected mammalian cells of SARS-CoV, MERS-CoV and SARS-CoV-2 [1,[15], [16], [17], [18], [19], [20], [21], [22]].
| Protein content | Functional receptor | Infected mammalian cells | |
|---|---|---|---|
| SARS-CoV | Replicase polyproteins: pp1a, pp1ab Structural proteins: spike (S) glycoprotein, membrane (M), envelope (E), nucleocapsid (N) 8 putative proteins |
ACE2 | airway epithelium, type I and type II pneumocytes, alveolar macrophages |
| MERS-CoV | Structural proteins: spike (S) glycoprotein, membrane (M), envelope (E), nucleocapsid (N) | hDPP4 | airway epithelium, type I and type II pneumocytes |
| SARS-CoV-2 | Non-structural proteins forming replicase-transcriptase complex: Nsp1-Nsp16 Structural proteins: spike (S) glycoprotein, membrane (M), envelope (E), nucleocapsid (N) 9 accessory factors |
ACE2 | types I and II pneumocytes, alveolar macrophages |
SARS-CoV, Severe Acute Respiratory Syndrome Coronavirus; MERS-CoV, Middle East Respiratory Syndrome Coronavirus; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; ACE2, Angiotensin-Converting Enzyme 2; hDPP4, human dipeptidyl peptidase 4.
SARS-CoV-2 is a positive-sense, single-stranded RNA virus, surrounded by lipid envelope and is, with its ~30 kb, one of the largest known viral RNAs [6]. Like SARS-CoV, it is of zoonotic origin and belongs to the family Coronaviridae, genus β-coronaviruses lineage B (β-B-CoVs), while MERS-CoV belongs to another lineage of the same genus (β-C-CoVs) [1,5,14,15,23]. The genome of the viral SARS-CoV-2 has been sequenced [16], revealing 96.2% identity with a bat coronavirus (BatCoV RaTG13). In addition, SARS-CoV-2 shares 79.6% homology with SARS-CoV [16], also derived from bats, and palm civet [1,3], and 51.8% identity with MERS-CoV [7,24]. Although the data are consistent with bats representing the reservoir of the newly CoV, the natural secondary host through which the virus reached humans has not yet been identified [24]. SARS-CoV, MERS-CoV and SARS-CoV-2 share many common characteristics, from epidemiology, clinical features, molecular mechanism and underlying infection process. However, unlike other β-B-CoVs, the spike protein of SARS-CoV-2 harbours furin-detectable site between the S1 and S2 subunits [1], indicating a potentially unique infectious property that could significantly increase the capacity of the viral spike protein [23].
3. Increased membrane cholesterol facilitates SARS-CoV-2 entry
Cholesterol is a fundamental lipid component of vertebrate cell membranes, mainly through lipid rafts, formations of ordered membrane microdomains that are enriched in cholesterol and sphingolipids [25,26]. Cholesterol enables proper functioning of the cell membrane where it is required to maintain membrane integrity and plays a pivotal role in modulating membrane fluidity and segregation, thereby affecting membrane heterogeneity [27,28]. Within lipid rafts, cholesterol also affects membrane permeability, signalling and transport [29]. In addition, it serves as an essential precursor for the synthesis of oxysterols, steroid hormones and bile acids [30], while cholesterol in the form of lipoproteins serves as a carrier of antioxidants, fat-soluble proteins, drugs and toxins [31]. Cholesterol is also a signalling molecule, regulates its own synthesis, the cell cycle and can modify proteins [30]. Due to its versatile roles and involvement in numerous physiological processes, the organism must maintain the cholesterol homeostasis, unless its excess could be potentially toxic. This is achieved by sophisticated regulation of de novo synthesis of cholesterol, its deposition in membranes, integration into lipoproteins or its storage in the form of cholesteryl esters and lipid droplets. The receptor-mediated uptake of cholesterol from the circulatory system and its metabolism into aforementioned downstream metabolites is also important for homeostasis [32]. The biosynthesis of cholesterol is an indispensable metabolic process in almost all mammalian cells, where the major site of its production is the liver. Congenital errors in cholesterol synthesis lead to deaths before birth, but when they are compatible with life, they result in congenital defects and severe malformations [33,34]. Cholesterol synthesis, its diurnal cycle and novel roles are discussed in more detail elsewhere [30,[32], [33], [34], [35], [36], [37], [38], [39], [40], [41]].
The successful entry of the virus to the host is a prerequisite of cross-species transmission. Therefore, understanding the exact mechanism of viral interaction with target cell provides a valuable information on viral pathogenesis and helps in vaccine and drug target design. The infectivity of certain viruses can be regulated by naturally-derived substances, thereby reducing their infectivity by interference with membrane lipid composition and consequently altering the viral lipid-dependent attachment [42]. Enveloped viruses, which also include CoVs, primarily engage plasma membrane fusion or endocytosis for entering the host cell [43]. Lipid raft microdomains with the unique protein composition are involved in the endocytosis-mediated process and serve as a platform and docking site for viruses to enter the host cell and release their genome [42]. By increasing the local concentration of entry receptors, lipid rafts mediate the entry process and influence other steps of the life viral cycle, such as assembly and budding [29].
Membrane composition plays a crucial role in the behaviour of fusion proteins and also influences membrane fusion by modulating the organization and dynamics of both included membranes [44]. However, the data are still contradictory and much more research in this area is needed. Rising cholesterol levels in human plasma membranes increased the infection rate of CoVs, by promoting membrane fusion [40]. Furthermore, Meher et al. [44] reported the effect of membrane cholesterol on the structure and oligomeric status of the fusion peptide of SARS-CoV whose binding affinity increased proportionally with increasing levels of membrane cholesterol. In contrast, cholesterol depletion physically disrupts the virion membrane [45]. Through the interference with lipid-dependent attachment to human host cells, naturally derived sterols and cyclodextrins can reduce the infectivity of CoVs (Fig. 1c) [42]. In vitro depletion of membrane-bound cholesterol from Angiotensin-Converting Enzyme 2 (ACE2)-expressing cells led to a reduced infectivity of CoVs, since the binding of the spike protein was reduced by half [46]. Also, interaction of phytosterols with lipid raft molecules can lead to a reduction of membrane cholesterol content or destabilization of its structure, thereby affecting viral infectivity (Fig. 1c) [42]. In addition, the viral infectivity is modulated by homeostatic control of cholesterol content and fatty acid metabolism [47].
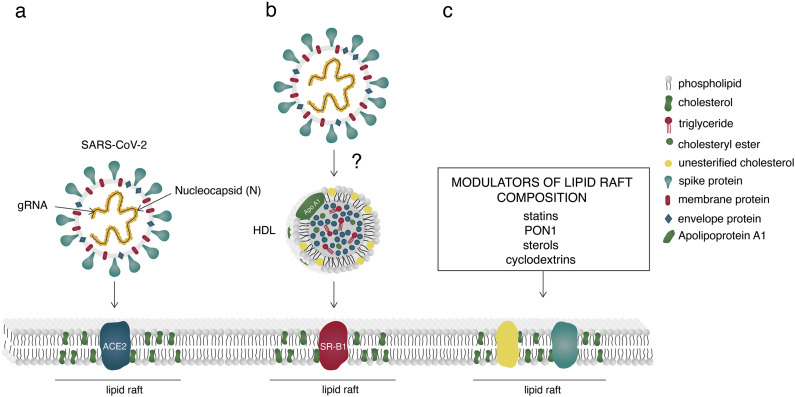
Fig. 1.
Entry of SARS-CoV-2 into the host cell (a) by engaging Angiotensin-Converting Enzyme 2 (ACE2), residing mainly within lipid rafts or (b) hypothetical entry through high-density lipoprotein (HDL) receptor Scavenger Receptor class B type 1 (SR-B1). (c) Modulators of lipid raft composition. SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; ACE2, Angiotensin-Converting Enzyme 2; gRNA, genomic ribonucleic acid; HDL, high density lipoprotein; SR-B1, Scavenger Receptor class B type 1; PON1, Paraoxonase 1.
Increased membrane cholesterol/fatty acid ratio enhances viral-mediated fusion with the host plasma membrane [48] while 25-hydroxycholesterol inhibits virus entry by blocking its fusion with the host membrane [49]. Cholesterol plays an essential role also in viral replication machinery and immune activation [50,51]. Interestingly, SARS-CoV-2 spike protein interacts with cholesterol (EC50 = 187.6 ± 120.5 nM). Moreover, both the spike as well as its S1 subunit interact with HDL particles, with spike exhibiting (5-fold) higher binding affinity [43].
Furthermore, 27-hydroxycholesterol, which inhibits the replication of a large diversity of both enveloped and non-enveloped human pathogens of viral origin, was shown to also inhibit SARS-CoV-2. Its physiological serum level significantly decreased in SARS-CoV-2 infected patients, reaching 50% reduction in severe cases of COVID-19. Additionally, serum concentration of lanosterol, lathosterol and desmosterol, all precursors of cholesterol, were also significantly reduced in moderate and severe COVID-19 patients, compared to healthy individuals [52]. Accumulated evidence indicate an important role of hydroxycholesterols as regulators of immune function, where their role in alteration of plasma membrane cholesterol content may possess antiviral, anti-inflammatory as well as proinflammatory effects [53].
Recently, it has been reported that individuals with AA genotype of SLC10A1 (encoding Na/taurocholate cotransporter NTCP, the entry receptor of Hepatitis B Virus) exhibit a decreased level of cholesterol as a result of impaired bile acid uptake which may enable escape from the Hepatitis B virus (HBV) infection [54]. In another cohort, the infectivity of the human parainfluenza virus type 3 (HPIV3) was markedly reduced due to an abnormal internalization capacity in the absence of viral envelope cholesterol, as shown in internalization assay in Human Embryonic Kidney 293 T cell line (HEK293T) [29].
4. The role of angiotensin-converting enzyme 2 and membrane proteinases
SARS-CoV-2, similarly to SARS-CoV, acquires human ACE2 as a functional receptor for host cell invasion (Fig. 1a) [1,15,16]. ACE2 resides mainly within lipid rafts [51]. It is widely expressed in organs that regulate blood pressure, in the heart, vessels, kidneys, the small intestine of gastrointestinal tract [2,13,55], and is abundantly distributed in alveolar type II epithelial cells [56,57], suggesting that these organs should be considered as potentially at high risk of infection. However, the expression level of ACE2 in the lungs which is the major site of SARS-CoV-2 infection is rather low [13,55], indicating that other viral entry mechanisms might be involved. ACE2 is also expressed in the mucosa of oral cavity and is highly enriched in epithelial cells of the tongue, suggesting that the oral cavity is also a potentially high-risk route of SARS-CoV-2 infection [56].
The spike glycoprotein of SARS-CoV-2 envelope has two subunits, S1 and S2, by which it attaches to the plasma membrane and after fusion mediates the viral entry [15,58]. After binding to the receptor, and prior to internalization, the spike protein is functionally cleaved by host Transmembrane Protease Serine 2 (TMPRSS2) [58]. The cleavage site is at the R685/S686, releasing the fusion peptide of spike and facilitating internalization of the virus [15,23]. Availability of the host proteases largely determines whether CoVs can enter the target cell through plasma membrane or endocytosis [1]. A lack of the host protease or incompatibility between the latter and the viral spike protein can inhibit virus entry [15]. After successful internalization, the virus uses the molecular machinery of the host in order to replicate, modifying the host metabolism and leading to major changes in the cellular lipid profile of the host [51,59].
5. Lipoproteins in viral infection
The role of lipoproteins as a first line of defence against microbes is well established [59], with most of them being able to bind and neutralize Gram-negative and Gram-positive bacterial membrane components, such as lipopolysaccharides and lipoteichoic acid, respectively [60,61]. Lipoprotein levels are altered during viral infections [60]. Hypolipidemia has been reported in critically ill patients, especially in septic conditions [60,[62], [63], [64]]. According to recent meta-analysis, the severity of a Dengue infection, a mosquito-borne tropical disease caused by the Dengue virus, inversely correlates with total cholesterol (TC) and LDL-cholesterol [60]. However, majority of data on lipoprotein interactions with viral infections involve HDL.
HDL consists mainly of free cholesterol, glycerophospholipids, sphingolipids and apolipoproteins (A1, A2) at the particle surface with cholesteryl esters and small amounts of triglycerides as components of the core [51,65]. HDL particles are constantly modified (both structurally and functionally) in response to physiological, pathological and acute inflammatory conditions (e.g. cytokine storm), which is reflected in their lipid and protein content and may lead to dysfunctional HDL particles [60,65,66]. Therapeutic agents, such as cholesteryl ester transfer protein (CETP) inhibitors (e.g. dalcetrapib, anacetrapib), nicotinic acid (niacin), fibric acid derivates (fibrates), and statins, which increase the level of HDL particles are well established. Some of them also induce favourable changes in their structure, composition and metabolism. However, it remains to be determined whether they also influence their qualitative properties [65].
Gangliosides in reconstituted HDL particles protect the cells from the polymeric Cholera toxin, suggesting the possibility that HDL containing lipids exhibit anti-infectious activity [60]. Apolipoprotein A1 (ApoA1), a major protein component of the HDL particles, binds the Dengue virus and enlarges its infectivity by facilitating its access to the cell via the Scavenger Receptor class B type 1 (SR-B1), the functional HDL receptor enriched in lipid rafts [27,60]. SR-B1 mediates the selective uptake of lipoprotein-derived cholesteryl esters into the cells [27]. It is also involved in reverse transport of cholesterol, the selective uptake of other HDL-bound lipid components, facilitates cholesterol secretion through bile acids and the outflow of cellular cholesterol to HDL particles [27]. SR-B1 can bind and internalize different types of HDL, including reconstituted HDL particles, HDL mimetic nanoparticles, although with a different affinity [27]. However, it was not yet shown that the uptake is affected by dysfunctional changes of HDL, since it depends on the presence of ApoA in the HDL. Pre-treatment of HEK293T cells with a potent SR-B1 antagonist ITX5061 strongly inhibited the entry of SARS-CoV-2-S pseudovirus to the host cells, where the treatment had no cytotoxic effect on cell survival [43]. The remaining question is whether SARS-CoV-2 could, in addition to ACE2, engage another route of entering the host cell, possibly via lipoprotein receptors (Fig. 1b).
HDL particles can have an antiviral effect on RNA and DNA viruses by neutralizing them, regardless of whether they are enveloped or not. However, the correlation between the latter and viral infections is not as clear as for bacteria [60]. The antiviral activity of HDL particles could be a consequence of ApoA1 interference with viral entry or with the target cell membrane during fusion, but HDL particles themselves could induce direct virus inactivation. Paraoxonase 1 (PON1) which is mainly transported by HDL displays antibacterial and antiviral properties [60]. By participating in cholesterol outflow from the cell membrane to HDL particles, PON1 contributes to lowering the cholesterol levels within lipid rafts, thus modulating viral infection (Fig. 1c). Pleiotropic nature of HDL particles that play an important role in cholesterol transport (reverse or not), act anti-inflammatory, and have antiviral and antioxidant properties, makes them a likely pathogen scavenger that could potentially be involved in the removal of infectious material [60,66]. The detailed elucidation of inflammatory pathways and determination of the triggers of inflammation could eventually lead to discovery of new therapeutic targets.
6. COVID-19 patients present with dyslipidemia
Viral infection triggers a specific lipid profile of the host which could serve as a potential biomarker to aid diagnostics. COVID-19 patients develop abnormalities, such as lymphocytopenia, progressive increase in pro-inflammatory cytokine levels (i.e. cytokine storm) and C-reactive protein (CRP), as well as a decrease in total protein, albumin, ApoA1, HDL-cholesterol, and TC, along with lowered CD3+ T, CD8+ T, and CD16+ T cell counts [10,51,67,68]. HDL-cholesterol and ApoA1 play protective roles in the maintenance of health and have beneficial effects on the lungs and various other disease states [68]. Described characteristics are useful as early warning indicators of the severity of COVID-19 disease (mild to severe) [68].
Hu et al. [51] recently described the lipid profile and other clinical features of COVID-19 patients from Wenzhou, China. Levels of serum TC, HDL- and LDL-cholesterol were significantly lower in the COVID-19 patients, where the level of HDL-cholesterol was more significantly altered in primarily infected patients who recently visited the city of Wuhan, compared to the patients, who were more likely infected by human-to-human transmission. Serum lipid composition was gender dependent, with male patients showing significantly lower HDL-cholesterol and a higher number of monocytes, a higher monocyte/HDL-cholesterol ratio and a higher lactate dehydrogenase compared to female patients. Serum levels of TC, HDL- and LDL-cholesterol decreased continuously until the 9th day of infection and then began to recover [51]. Similar changes in the lipid profile were also reported by other groups [43,67]. A significant decrease in the level of HDL-cholesterol was observed only in critical cases of COVID-19, while significant decrease of TC and LDL-cholesterol was observed in all patient groups (mild, severe, critical). Accordingly, it seems that hypolipidemia occurs in patients with mild symptoms and escalates with the progression and severity of the disease [67]. Taking into account increased serum levels of alanine aminotransferase (ALT), alkaline phosphatase (ALP) and aspartate aminotransferase (AST), the decrease of LDL-cholesterol may be explained by liver damage as a consequence of the SARS-CoV-2 infection. On the other hand, LDL-cholesterol levels may also decrease due to increase in Interleukin-6 (IL-6) [67]. In contrast, another study observed significantly increased level of serum LDL-cholesterol compared to the reference population where levels of HDL-cholesterol and TC were inversely correlated with the severity of COVID-19 [43].
7. Lipid associated chronic pathologies and relation to COVID-19 infection
Meta-analysis showed an inverse association between serum cholesterol and non-cardiovascular mortality in respiratory and digestive diseases, some cancers, and other residual causes of infectious origin [31]. A weak but statistically significant inverse association was found between level of TC and the incidence of some infectious diseases diagnosed in hospital setting [69].
Patients with familial hypercholesterolemia (FH) have a lifelong increase in plasma LDL-cholesterol [8,70]. They are at high risk of cardiovascular disease, and therefore have an increased risk of suffering a severe course of COVID-19 [8,70]. Statins are the first-choice treatment in heterozygous FH patients, while for most homozygous patients additional therapies are included, such as ezetimibe, bile acids sequestrants and LDL-apheresis [71]. Statins have a protective role against endothelial dysfunction. In the case of acute coronary event, which can be also a consequence of the viral infection, the treatment should not be withheld. While statins are undoubtedly beneficial due to their pleiotropic action, the question remains whether statin therapy in patients with acute coronary events enables additional protection from SARS-CoV-2 infection also on the cellular cholesterol level.
An autosomal recessive lysosomal storage disorder Niemann-Pick Type C disease (NPC) caused by a defect in the NPC1 or NPC2 proteins is characterized with disrupted intracellular cholesterol and sphingomyelin trafficking which leads to accumulation of sphingolipids and cholesterol within lysosomes [72]. Similarly, the distribution of certain proteins to the lipid rafts and maintenance of their function are impaired. Therefore, the internalization of raft-associated ACE2 receptor may be impaired in NPC disease [72,73]. It is tempting to speculate that changes in the composition of lipid rafts could significantly reduce the infectivity of SARS-CoV-2 and make NPC patients an unfavourable host with reduced susceptibility to infection. In addition, increased intracellular levels of 25-hydroxycholesterol that massively accumulates in NPC, reduces infectivity of several members of Coronaviridae, Hepatitis C Virus (HCV), Zika and others. 7-ketocholesterol, which also accumulates in NPC interferes with viral maturation, budding and release from host cells [72]. However, the exact mechanism of the antiviral activity of oxysterols is still unknown. It is proposed that NPC1 inhibitors could interfere with infectivity of SARS-CoV-2 via numerous lipid-dependent mechanisms [74].
Non-alcoholic Fatty Liver Disease (NAFLD), a hepatic manifestation of the metabolic syndrome [34,35,75], is the most common liver disease of developed world [76] as a result of obesity and diabetes epidemic [77]. NAFLD is a multifactorial condition that defines a spectrum of hepatic changes, ranging from simple steatosis, steatohepatitis (NASH), further progressing to fibrosis and cirrhosis, and eventually leading to hepatocellular carcinoma (HCC) [35,[75], [76], [77], [78]]. The disease is characterized by intrahepatic deposition of excess triglycerides, which continue to cause lipotoxicity, aggravate liver damage, and may lead to hepatocyte death [75,76,78]. Metabolomic analysis have shown increased cholesterol synthesis in NAFLD patients, while absorption of cholesterol was decreased. Plasma levels of LDL were also elevated, with abnormalities in lipoprotein incidence reflected in altered homeostasis of the major lipid components; cholesterol, lipoproteins, cholesterol esters and triglycerides [35]. Apart from limitations regarding the early prediction of NAFLD, there are no drugs for the direct treatment of the disease. However, patients are often treated with statins alone or in combination with antioxidants (e.g. vitamin E) [35,76]. Yet, the most effective treatment strategy for NAFLD is lifestyle intervention through a combination of diet, exercise and weight loss [76,79]. Therefore, it is intriguing to contemplate whether NAFLD patients without treatment are more susceptible for SARS-CoV-2 infection, or whether statin application may directly affect the entry of SARS-CoV-2 into the host cell by regulating cholesterol cell levels.
8. Do cholesterol lowering drugs statins influence the SARS-CoV-2 entry?
Statins have pleiotropic properties but are best known as cholesterol-lowering agents (Fig. 1c) that inhibit a rate-limiting enzyme of cholesterol synthesis, 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) [30,32,59,79,80]. Transcriptome analyses performed by our group revealed a modifying effect of rosuvastatin and atorvastatin on the expression of many metabolic and signalling pathways in the liver, including drug metabolism [81]. Statins are also ligands of Constitutive Androstane Receptor (CAR) and Pregnane X Receptor (PXR), both members of the nuclear receptor protein family [80]. Despite having adverse effects on the liver, statins are considered beneficial for the treatment of NASH [79].
Along with beneficial effects on cardiovascular and pulmonary function, statins can also strengthen the host defence. They have substantial anti-inflammatory, anti-thrombotic and immunomodulatory effects [82,83], which is why they may be used as a host-targeted treatment against pathogen infections. Furthermore, statin therapy also promotes stabilization of atherosclerotic plaques, which could be a subject of destabilization, caused by cytokine storm seen in COVID-19 [83]. Disruption of lipid rafts by lipid-lowering treatment has already been shown to affect infectivity of other CoVs [84]. As lipid lowering drugs, statins might thus significantly reduce the attachment and internalization of SARS-CoV-2 by lowering membrane cholesterol levels (Fig. 1c) [40,83]. It has been previously reported, that statin therapy increased viral clearance from the blood during chronic HCV infection, as well as reduced mortality and need for intubation due to influenza infection [83]. An in-silico study showed that fluvastatin, lovastatin, pitavastatin and rosuvastatin may efficiently inhibit the main protease of SARS-CoV-2, which plays a crucial role in proteolytic maturation [82]. Lowering cellular cholesterol might also trigger a greater uptake of cholesterol from the bloodstream, thereby lowering serum HDL- and LDL-cholesterol levels. Consequently, this would plausibly lead to an upregulation of the lipoprotein receptors, especially SR-B1, and to incorporation of cholesterol into the plasma membranes, resulting in higher SARS-CoV-2 infection rate.
9. Conclusion
The recent COVID-19 outbreak caused by SARS-CoV-2 poses a threat to the human population with an urgent need for rapid development of effective antiviral therapeutic agents. Understanding the exact molecular mechanism of viral pathogenesis is a fundamental step towards infection prevention. Cholesterol is involved in many cellular processes, one of which is regulating the entry of the virus into the host cell. Patients with lipid-associated pathologies may prove to be more or less prone to SARS-CoV-2 infection compared to healthy individuals. In most studies COVID-19 patients show lower levels of total, HDL- and LDL-cholesterol, which correlate with the disease severity and might be a potential prognostic blood biomarker. The lipid metabolic pathways and the composition of membranes could be targeted to selectively inhibit the life cycle of the virus as a basis for antiviral therapy. Additionally, emerging data indicate an important role of lipoproteins in SARS-CoV-2 infection. In particular HDL may facilitate a possible entry route of SARS-CoV-2 into the host cell via the SR-B1 receptor. Statins interact with SARS-CoV-2 infection and COVID-19 progression at many different levels. Limited data indicates an interaction also through cholesterol membrane composition. Further research on lipid components is necessary to provide valuable insights into the molecular mechanism underlying viral infection with SARS-CoV-2, and could therefore be used in the prevention and treatment of COVID-19.
Funding
The work was funded by Slovenian Research Agency (ARRS) programme grant P1-0390, project J1-9176 and the PhD grant for young researchers (EK).
Author's contribution
EK performed the literature search and wrote the manuscript, TR critically revised the lipid and lipoprotein part of the review; DR designed the manuscript and its contents. All authors actively contributed to writing and revising the text.
Ethics approval
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Touyz R.M., Li H., Delles C. ACE2 the Janus-faced protein - from cardiovascular protection to severe acute respiratory syndrome-coronavirus and COVID-19. Clin. Sci. (Lond). 2020;134:747–750. doi: 10.1042/CS20200363. [DOI] [PubMed] [Google Scholar]
- 3.Meo S.A., Alhowikan A.M., Khlaiwi T.A.L., Meo I.M., Halepoto D.M., Iqbal M., Usmani A.M., Hajjar W., Ahmed N. Novel coronavirus 2019-nCoV: Prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur. Rev. Med. Pharmacol. Sci. 2020;24:2012–2019. doi: 10.26355/eurrev_202002_20379. [DOI] [PubMed] [Google Scholar]
- 4.Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection——a review of immune changes in patients with viral pneumonia, Emerg. Microbes Infect. 2020;1751:1–14. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn D.-G., Shin H.-J., Kim M.-H., Lee S., Kim H.-S., Myoung J., Kim B.-T., Kim S.-J. Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19) J. Microbiol. Biotechnol. 2020;30:313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Q., Lin Q., Jin S., You L. Coronavirus 2019-nCoV: A brief perspective from the front line. J. Infect. 2020;80:373–377. doi: 10.1016/j.jinf.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K., Trilling M., Lu M., Dittmer U., Yang D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J. Med. Virol. 2020;92:491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vuorio A., Watts G.F., Kovanen P.T. Familial hypercholesterolemia and COVID-19: triggering of increased sustained cardiovascular risk. J. Intern. Med. 2020;287:746–747. doi: 10.1111/joim.13070. [DOI] [PubMed] [Google Scholar]
- 9.Sommerstein R., Kochen M.M., Messerli F.H., Gräni C. Coronavirus Disease 2019 (COVID-19): Do Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers Have a Biphasic Effect? J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhaskar S., Sinha A., Banach M., Mittoo S. Cytokine Storm in COVID-19 — Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong N.S., Zheng B.J., Li Y.M., Poon L.L.M., Xie Z.H., Chan K.H., Li P.H., Tan S.Y., Chang Q., Xie J.P., Liu X.Q., Xu J., Li D.X., Yuen K.Y., Peiris J.S.M. Y, Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nassar M.S., Bakhrebah M.A., Meo S.A., Alsuabeyl M.S., Zaher W.A. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: epidemiology, pathogenesis and clinical characteristics. Eur. Rev. Med. Pharmacol. Sci. 2018;22:4956–4961. doi: 10.26355/eurrev_201808_15635. [DOI] [PubMed] [Google Scholar]
- 13.Momtazi-Borojeni A.A., Banach M., Reiner Ž., Pirro M., Bianconi V., Al-Rasadi K., Sahebkar A. Interaction Between Coronavirus S-Protein and Human ACE2: Hints for Exploring Efficient Therapeutic Targets to treat COVID-19. Angiology. 2020 doi: 10.1177/0003319720952284. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y., Liu Q., Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keng C.T., Choi Y.W., Welkers M.R.A., Chan D.Z.L., Shen S., Gee Lim S., Hong W., Tan Y.J. The human severe acute respiratory syndrome coronavirus (SARS-CoV) 8b protein is distinct from its counterpart in animal SARS-CoV and down-regulates the expression of the envelope protein in infected cells. Virology. 2006;354:132–142. doi: 10.1016/j.virol.2006.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to SARS-CoV infection. J. Immunol. 2018;198:319–335. doi: 10.4049/jimmunol.1601896.Sex-based. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu H., Chan J.F.W., Wang Y., Yuen T.T.T., Chai Y., Hou Y., Shuai H., Yang D., Hu B., Huang X., Zhang X., Cai J.P., Zhou J., Yuan S., Kok K.H., K.K.W. To, Chan I.H.Y., Zhang A.J., Sit K.Y., Au W.K., Yuen K.Y. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020;71:1400–1409. doi: 10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian Z., Travanty E.A., Oko L., Edeen K., Berglund A., Wang J., Ito Y., Holmes K.V., Mason R.J. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am. J. Respir. Cell Mol. Biol. 2013;48:742–748. doi: 10.1165/rcmb.2012-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan R.W.Y., Chan M.C.W., Agnihothram S., Chan L.L.Y., Kuok D.I.T., Fong J.H.M., Guan Y., Poon L.L.M., Baric R.S., Nicholls J.M., Peiris J.S.M. Tropism of and Innate Immune Responses to the Novel Human Betacoronavirus Lineage C Virus in Human Ex Vivo Respiratory Organ Cultures. J. Virol. 2013;87:6604–6614. doi: 10.1128/jvi.00009-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Den Brand J.M.A., Smits S.L., Haagmans B.L. Pathogenesis of Middle East respiratory syndrome coronavirus. J. Pathol. 2015;235:175–184. doi: 10.1002/path.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., Qi F., Bao L., Du L., Liu S., Qin C., Sun F., Shi Z., Zhu Y., Jiang S., Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;80(327):46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 26.Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 27.Shen W.J., Asthana S., Kraemer F.B., Azhar S. Scavenger receptor B type 1: Expression, molecular regulation, and cholesterol transport function. J. Lipid Res. 2018;59:1114–1131. doi: 10.1194/jlr.R083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramstedt B., Slotte J.P. Sphingolipids and the formation of sterol-enriched ordered membrane domains. 2006;1758:1945–1956. doi: 10.1016/j.bbamem.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Tang Q., Liu P., Chen M., Qin Y. Virion-associated cholesterol regulates the infection of human parainfluenza virus type 3. Viruses. 2019;11 doi: 10.3390/v11050438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovač U., Skubic C., Bohinc L., Rozman D., Režen T. Oxysterols and gastrointestinal cancers around the clock. Front. Endocrinol. (Lausanne). 2019;10:1–19. doi: 10.3389/fendo.2019.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobs D.R., Blackburn H., Higgins M., Reed D., Iso H., McMillan G., Neaton J., Nelson J., Potter J., Rifkind B., Rossouw J., Shekelle R., Yusuf S. Report of the conference on low blood cholesterol: Mortality associations. Circulation. 1992;86:1046–1060. doi: 10.1161/01.CIR.86.3.1046. [DOI] [PubMed] [Google Scholar]
- 32.Rezen T., Rozman D., Pascussi J.M., Monostory K. Interplay between cholesterol and drug metabolism. Biochim. Biophys. Acta - Proteins Proteomics. 2011;1814:146–160. doi: 10.1016/j.bbapap.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Horvat S., McWhir J., Rozman D. Defects in cholesterol synthesis genes in mouse and in humans: Lessons for drug development and safer treatments. Drug Metab. Rev. 2011;43:69–90. doi: 10.3109/03602532.2010.540580. [DOI] [PubMed] [Google Scholar]
- 34.Lorbek G., Perše M., Jeruc J., Juvan P., Gutierrez-Mariscal F.M., Lewinska M., Gebhardt R., Keber R., Horvat S., Björkhem I., Rozman D. Lessons from Hepatocyte-Specific Cyp51 Knockout Mice: Impaired Cholesterol Synthesis Leads to Oval Cell-Driven Liver Injury. Sci. Rep. 2015;5:1–11. doi: 10.1038/srep08777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fon Tacer K., Rozman D. Nonalcoholic Fatty Liver Disease: Focus on Lipoprotein and Lipid Deregulation. J. Lipids. 2011;2011:1–14. doi: 10.1155/2011/783976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urlep Z., Rozman D. The interplay between circadian system, cholesterol synthesis, and steroidogenesis affects various aspects of female reproduction. Front. Endocrinol. (Lausanne). 2013;4:1–10. doi: 10.3389/fendo.2013.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marbach-Breitrück E., Matz-Soja M., Abraham U., Schmidt-Heck W., Sales S., Rennert C., Kern M., Aleithe S., Spormann L., Thiel C., Gerlini R., Arnold K., Klöting N., Guthke R., Rozman D., Teperino R., Shevchenko A., Kramer A., Gebhardt R. Tick-tock hedgehog-mutual crosstalk with liver circadian clock promotes liver steatosis. J. Hepatol. 2019;70:1192–1202. doi: 10.1016/j.jhep.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 38.Lewinska M., Juvan P., Perse M., Jeruc J., Kos S., Lorbek G., Urlep Z., Keber R., Horvat S., Rozman D. Hidden disease susceptibility and sexual dimorphism in the heterozygous knockout of Cyp51 from cholesterol synthesis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ačimovič J., Rozman D. Steroidal triterpenes of cholesterol synthesis. Molecules. 2013;18:4002–4017. doi: 10.3390/molecules18044002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abu-Farha M., Thanaraj T.A., Qaddoumi M.G., Hashem A., Abubaker J., Al-Mulla F. The Role of Lipid Metabolism in COVID-19 Virus Infection and as a Drug Target. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21103544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shahoei S.H., Nelson E.R. Nuclear receptors, cholesterol homeostasis and the immune system. J. Steroid Biochem. Mol. Biol. 2019;191 doi: 10.1016/j.jsbmb.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baglivo M., Baronio M., Natalini G., Beccari T., Chiurazzi P., Fulcheri E., Petralia P., Michelini S., Fiorentini G., Miggiano G.A., Morresi A., Tonini G., Bertelli M. Natural small molecules as inhibitors of coronavirus lipid-dependent attachment to host cells: A possible strategy for reducing SARS-COV-2 infectivity? Acta Biomed. 2020;91:161–164. doi: 10.23750/abm.v91i1.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei C., Wan L., Zhang Y., Fan C., Yan Q., Yang X., Gong J., Yang H., Li H., Zhang J., Zhang Z., Wang R., Wang X., Sun J., Zong Y., Yin F., Gao Q., Cao Y., Zhong H. 2020. Cholesterol Metabolism--Impact for SARS-CoV-2 Infection Prognosis, Entry, and Antiviral Therapies, MedRxiv. [DOI] [Google Scholar]
- 44.Meher G., Bhattacharjya S., Chakraborty H. Membrane Cholesterol Modulates Oligomeric Status and Peptide-Membrane Interaction of Severe Acute Respiratory Syndrome Coronavirus Fusion Peptide. J. Phys. Chem. B. 2019;123:10654–10662. doi: 10.1021/acs.jpcb.9b08455. [DOI] [PubMed] [Google Scholar]
- 45.Salimi H., Johnson J., Flores M.G., Zhang M.S., O’Malley Y., Houtman J.C., Schlievert P.M., Haim H. The lipid membrane of HIV-1 stabilizes the viral envelope glycoproteins and modulates their sensitivity to antibody neutralization. J. Biol. Chem. 2020;295:348–362. doi: 10.1074/jbc.RA119.009481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glende J., Schwegmann-Wessels C., Al-Falah M., Pfefferle S., Qu X., Deng H., Drosten C., Naim H.Y., Herrler G. Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2. Virology. 2008;381:215–221. doi: 10.1016/j.virol.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cervin M., Anderson R. Modulation of coronavirus‐mediated cell fusion by homeostatic control of cholesterol and fatty acid metabolism. J. Med. Virol. 1991;35:142–149. doi: 10.1002/jmv.1890350213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daya M., Cervin M., Anderson R. Cholesterol Enhances Mouse Hepatitis Virus-Mediated Cell Fusion. Virology. 1988;163:276–283. doi: 10.1016/0042-6822(88)90267-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu S., Aliyari R., Chikere K., Li G., Matthew D., Smith J.K., Pernet O., Guo H., Nusbaum R., a Zack A.N., Freiberg L. Su, Lee B., Cheng G. Viral Entry by Production of 25-Hydroxycholesterol. Immunity. 2013;38:92–105. doi: 10.1016/j.immuni.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adal M., Howe R., Kassa D., Aseffa A., Petros B. Associations of gender and serum total cholesterol with CD4+ T cell count and HIV RNA load in antiretroviral-naïve individuals in Addis Ababa. BMC Public Health. 2018;18:1–10. doi: 10.1186/s12889-018-5852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu X., Chen D., Wu L., He G., Ye W. Low Serum Cholesterol Level Among Patients with COVID-19 Infection in Wenzhou. China, Lancet. 2020 doi: 10.2139/ssrn.3544826. [DOI] [Google Scholar]
- 52.Marcello A., Civra A., Milan R., Nascimento L., Rajasekharan S., Giacobone C., Caccia C., Cavalli R., Adami M., Brambilla P., Lembo D., Poli G., Leoni V. The cholesterol metabolite 27-hydroxycholesterol inhibits SARS-CoV-2 and is markedly decreased in COVID-19 patients. Redox Biol. 2020;36 doi: 10.1016/j.redox.2020.101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee W., Ahn J.H., Park H.H., Kim H.N., Kim H., Yoo Y., Shin H., Hong K.S., Jang J.G., Park C.G., Choi E.Y., Bae J., Seo Y. COVID-19-activated SREBP2 disturbs cholesterol biosynthesis and leads to cytokine storm. Signal Transduct. Target. Ther. 2020;5 doi: 10.1038/s41392-020-00292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng X., Wang Y., Tian J., Zhou L., Chen X., Guo H., Zeng J., Shen N., Li J., Ke J., Zhu Y., Gong J., Chang J., Liu L., Zhong R. SLC10A1 S267F variant influences susceptibility to HBV infection and reduces cholesterol level by impairing bile acid uptake. J. Viral Hepat. 2019;26:1178–1185. doi: 10.1111/jvh.13157. [DOI] [PubMed] [Google Scholar]
- 55.Batlle D., Wysocki J., Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin. Sci. (Lond). 2020;134:543–545. doi: 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- 56.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12:1–5. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldstein M.R., Poland G.A., Graeber C.W. Are certain drugs associated with enhanced mortality in COVID-19? QJM An Int. J. Med. 2020;113:509–510. doi: 10.1093/qjmed/hcaa103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aronson J.K., Ferner R.E. Drugs and the renin-angiotensin system in covid-19. Bmj. 2020;369:m1313. doi: 10.1136/bmj.m1313. [DOI] [PubMed] [Google Scholar]
- 59.Minetti G. Mevalonate pathway, selenoproteins, redox balance, immune system, Covid-19: reasoning about connections. Med. Hypotheses. 2020;144:110128. doi: 10.31226/osf.io/73wy6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meilhac O., Tanaka S., Couret D. High-Density Lipoproteins Are Bug Scavengers. Biomolecules. 2020;10:1–18. doi: 10.3390/biom10040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kitchens R.L., Thompson P.A., Munford R.S., O’Keefe G.E. Acute inflammation and infection maintain circulating phospholipid levels and enhance lipopolysaccharide binding to plasma lipoproteins. J. Lipid Res. 2003;44:2339–2348. doi: 10.1194/jlr.M300228-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Barlage S., Fröhlich D., Böttcher A., Jauhiainen M., Müller H.P., Noetzel F., Rothe G., Schütt C., Linke R.P., Lackner K.J., Ehnholm C., Schmitz G. ApoE-containing high density lipoproteins and phospholipid transfer protein activity increase in patients with a systemic inflammatory response. J. Lipid Res. 2001;42:281–290. doi: 10.1016/S0022-2275(20)31690-4. [DOI] [PubMed] [Google Scholar]
- 63.Barlage S., Liebisch G., Glu T. Changes in HDL-associated apolipoproteins relate to mortality in human sepsis and correlate to monocyte and platelet activation. Intensive Care Med. 2009;35:1877–1885. doi: 10.1007/s00134-009-1609-y. [DOI] [PubMed] [Google Scholar]
- 64.Drobnik W., Liebisch G., Audebert F., Fröhlich D., Glück T., Vogel P., Rothe G., Schmitz G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J. Lipid Res. 2003;44:754–761. doi: 10.1194/jlr.M200401-JLR200. [DOI] [PubMed] [Google Scholar]
- 65.Otocka-Kmiecik A., Mikhailidis D.P., Nicholls S.J., Davidson M., Rysz J., Banach M. Dysfunctional HDL: A novel important diagnostic and therapeutic target in cardiovascular disease? Prog. Lipid Res. 2012;51:314–324. doi: 10.1016/j.plipres.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 66.Samadi S., Abolbashari S., Meshkat Z., Mohammadpour A.H., Kelesidis T., Gholoobi A., Mehramiz M., Tabadkani M., Sadabadi F., Dalirfardouei R., Ferns G.A., Ghayour-Mobarhan M., Avan A. Human T lymphotropic virus type 1 and risk of cardiovascular disease: High-density lipoprotein dysfunction versus serum HDL-C concentrations. BioFactors. 2019;45:374–380. doi: 10.1002/biof.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei X., Zeng W., Su J., Wan H., Yu X., Cao X., Tan W., Wang H. Hypolipidemia is associated with the severity of COVID-19. J. Clin. Lipidol. 2020;14:297–304. doi: 10.1016/j.jacl.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nie S., Zhao X., Zhao K., Zhang Z., Zhang Z., Zhang Z. Metabolic disturbances and inflammatory dysfunction predict severity of coronavirus disease 2019 (COVID-19): a retrospective study. MedRxiv. 2020 doi: 10.1101/2020.03.24.20042283. [DOI] [Google Scholar]
- 69.Iribarren C., Jacobs D.R., Sidney S., Claxton A.J., Feingold K.R. Cohort study of serum total cholesterol and in-hospital incidence of infectious diseases. Epidemiol. Infect. 1998;121:335–347. doi: 10.1017/S0950268898001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banach M., Penson P.E., Fras Z., Vrablik M., Pella D., Reiner Ž., Nabavi S.M., Sahebkar A., Kayikcioglu M., Daccord M. Brief recommendations on the management of adult patients with familial hypercholesterolemia during the COVID-19 pandemic. Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lambert C.T., Sandesara P., Isiadinso I., Gongora M.C., Eapen D., Bhatia N., Baer J.T., Sperling L.S. Current Treatment of Familial Hypercholesterolaemia. Coron. Artery Dis. Prev. 2014;9:76–81. doi: 10.15420/ecr.2014.9.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ballout R. The Lysosome: A Potential Therapeutic Juncture between the COVID-19 Pandemic and Niemann-Pick Type C Disease. FASEB J. 2020;34:7253–7264. doi: 10.20944/PREPRINTS202003.0340.V1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luo J., Yang H., Song B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020;21:225–245. doi: 10.1038/s41580-019-0190-7. [DOI] [PubMed] [Google Scholar]
- 74.Sturley S.L., Rajakumar T., Hammond N., Higaki K., Márka Z., Márka S., Munkacsi A.B. Potential COVID-19 therapeutics from a rare disease: Weaponizing lipid dysregulation to combat viral infectivity. J. Lipid Res. 2020;6:1–46. doi: 10.1016/j.surfcoat.2019.125084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naik A., Belič A., Zanger U.M., Rozman D. Molecular interactions between NAFLD and xenobiotic metabolism. Front. Genet. 2013;4:1–14. doi: 10.3389/fgene.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skubic C., Drakulić Ž., Rozman D. Personalized therapy when tackling nonalcoholic fatty liver disease: a focus on sex, genes, and drugs. Expert Opin. Drug Metab. Toxicol. 2018;14:831–841. doi: 10.1080/17425255.2018.1492552. [DOI] [PubMed] [Google Scholar]
- 77.Rozman D. From nonalcoholic fatty liver disease to hepatocellular carcinoma: A systems understanding. Dig. Dis. Sci. 2014;59:238–241. doi: 10.1007/s10620-013-2998-x. [DOI] [PubMed] [Google Scholar]
- 78.Kovac U., Rozman D. ELS. 2015. Genetics of Non-alcoholic Fatty Liver Disease. [DOI] [Google Scholar]
- 79.Lorbek G., Urlep Ž., Rozman D. Pharmacogenomic and personalized approaches to tackle nonalcoholic fatty liver disease. Pharmacogenomics. 2016;17:1273–1288. doi: 10.2217/pgs-2016-0047. [DOI] [PubMed] [Google Scholar]
- 80.Režen T., Hafner M., Kortagere S., Ekins S., Hodnik V., Rozman D. Rosuvastatin and atorvastatin are ligands of the human constitutive androstane receptor/retinoid X receptor α complex. Drug Metab. Dispos. 2017;45:974–976. doi: 10.1124/dmd.117.075523. [DOI] [PubMed] [Google Scholar]
- 81.Hafner M., Juvan P., Rezen T., Monostory K., Pascussi J.M., Rozman D. The human primary hepatocyte transcriptome reveals novel insights into atorvastatin and rosuvastatin action. Pharmacogenet. Genomics. 2011;21:741–750. doi: 10.1097/FPC.0b013e32834a5585. [DOI] [PubMed] [Google Scholar]
- 82.Reiner Ž., Hatamipour M., Banach M., Pirro M., Al-Rasadi K., Jamialahmadi T., Radenkovic D., Montecucco F., Sahebkar A. Statins and the COVID-19 main protease: in silico evidence on direct interaction. Arch. Med. Sci. 2020;16:490–496. doi: 10.5114/aoms.2020.94655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Radenkovic D., Chawla S., Pirro M., Sahebkar A., Banach M. Cholesterol in Relation to COVID-19: Should We Care about It? J. Clin. Med. 2020;9:1909. doi: 10.3390/jcm9061909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katsiki N., Banach M., Mikhailidis D. Lipid-lowering therapy and renin-angiotensin-aldosterone system inhibitors in the era of the COVID-19 pandemic. Arch. Med. Sci. 2020;16:485–489. doi: 10.5114/aoms.2020.94503. [DOI] [PMC free article] [PubMed] [Google Scholar]