Abstract
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality among individuals with chronic kidney disease (CKD). Cardiac biomarkers of myocardial distention, injury, and inflammation may signal unique pathways underlying CVD in CKD. In this analysis, we studied the association of baseline levels and changes in 4 traditional and novel cardiac biomarkers with risk of all-cause, CV, and non-CV mortality in a large cohort of patients with CKD.
Methods
Among 3664 adults with CKD enrolled in the Chronic Renal Insufficiency Cohort Study, we conducted a cohort study to examine the associations of baseline levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP), cardiac high-sensitivity troponin T (hsTnT), growth differentiation factor−15 (GDF-15), and soluble ST-2 (sST-2) with risks of all-cause and cardiovascular (CV) mortality. Among a subcohort of 842 participants, we further examined the associations between change in biomarker levels over 2 years with risk of all-cause mortality. We used Cox proportional hazards regression models and adjusted for demographics, kidney function measures, cardiovascular risk factors, and medication use.
Results
After adjustment, elevated baseline levels of each cardiac biomarker were associated with increased risk of all-cause mortality: NT-proBNP (hazard ratio [HR] = 1.92, 95% confidence interval [CI] = 1.73−2.12); hsTnT (HR = 1.62, 95% CI = 1.48, 1.78]); GDF-15 (HR = 1.61, 95% CI = 1.46−1.78]); and sST-2 (HR = 1.26, CI = 1.16−1.37). Higher baseline levels of all 4 cardiac biomarkers were also associated with increased risk of CV. Declines in NT-proBNP (adjusted HR = 0.55, 95% CI = 0.36−0.86) and sST2 (HR = 0.55, 95% CI = 0.36−0.86]) over 2 years were associated with lower risk of all-cause mortality.
Conclusion
In a large cohort of CKD participants, elevations of NT-proBNP, hsTnT, GDF-15, and sST-2 were independently associated with greater risks of all-cause and CV mortality.
Keywords: cardiac biomarkers, cardiovascular, mortality
Graphical abstract
Cardiovascular disease (CVD) is the leading cause of mortality in patients with chronic kidney disease (CKD).1, 2, 3 The increased risk for CVD in CKD is only partially explained by the prevalence of traditional cardiovascular risk factors.4,5 Therefore, identifying novel pathways that uniquely contribute to CVD risk in CKD is an important step toward identifying underlying disease mechanisms and potentially developing effective therapies.
Cardiac biomarkers of myocardial distention, injury, and inflammation may signal unique pathways underlying CVD in CKD. N-terminal pro-B-type natriuretic peptide (NT-proBNP) is secreted from cardiac myocytes in response to ventricular wall stretch,6 and levels increase with increasing left ventricular mass.7, 8, 9 Concentrations of high-sensitivity cardiac troponin T (hsTnT) rise in response to myocardial injury.10,11 Growth differentiation factor-15 (GDF-15) is a member of the transforming growth factor−β cytokine family and plays a role in cardiomyocyte repair.12, 13, 14 Soluble ST2 (sST2) is a member of the interleukin-1 receptor family and its expression is up-regulated in the setting of myocardial injury.15 These 4 biomarkers have been extensively investigated in CVD patients and healthy individuals, with higher levels associated with greater risk of mortality.10,16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 Although a few studies have examined the association of these biomarkers with clinical outcomes in CKD, these studies are limited by small sample size and short duration of follow-up.34, 35, 36, 37, 38
We sought to determine the associations of baseline concentrations of NT-proBNP, hsTnT, GDF-15, and sST2 with subsequent all-cause and cardiovascular (CV) mortality in the large, multi-center cohort of CKD individuals from the Chronic Renal Insufficiency Cohort (CRIC) Study. We further examined the change in biomarkers over time in a subcohort of study participants.
Methods
Study Population
We performed an ancillary study of the Chronic Renal Insufficiency Cohort (CRIC) Study, a multi-center and prospective study of that enrolled 3939 adults with CKD between June 2003 and August 2008 at 7 clinical centers across the United States.39,40 The CRIC study enrolled participants with Modification of Diet in Renal Disease (MDRD) equation−based estimated glomerular filtration rate (eGFR) between 20 and 70 ml/min per 1.73 m2 for ages 21 to 44 years; 20 to 60 ml/min per 1.73 m2 for ages 45 to 64 years; and 20 to 50 ml/min per 1.73 m2 for ages 65 to 74 years.41 Inclusion and exclusion criteria have been previously described.39 Participants on maintenance dialysis or with a kidney transplant were not included at cohort entry. All study participants provided written informed consent, and the study protocol was approved by institutional review boards at each of the participating sites.
For our primary analysis, we excluded participants without baseline blood samples and those who were not able to have all 4 biomarkers measured concurrently, resulting in a final analytical cohort of 3664 participants. We further sampled and measured biomarkers in a random subcohort of 1002 participants at year 2 to assess longitudinal changes in biomarker levels. After excluding those with incomplete biomarkers measured at baseline and year 2, a total of 842 participants remained for this secondary analysis.
Cardiac Biomarkers
Both GDF-15 and sST2 were measured in batch from baseline plasma samples stored at −70°C and year 2 at the University of Pennsylvania Laboratory using an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). For GDF-15, the quantitative range was 23.4 to 1500 pg/ml, with a lower limit of detection of 2.0 pg/ml. At a concentration of 98.8 pg/ml, the intra-assay CV was 7.2%; at a concentration of 624 pg/ml, it was 4.5%. For sST2, the quantitative range was 0.63–40 ng/ml, with a limit of detection of 0.1 ng/ml. At a concentration of 2.6 ng/ml, the intra-assay CV was 11.2%; at 0.94 ng/ml, it was 8.5%.
Cardiac hs-TnT and NT-proBNP were measured at baseline in 2008 from ethylenediamine tetraacetic acid (EDTA) plasma stored at −70 °C, both by chemiluminescent microparticle immunoassay (Roche Diagnostics, Basel, Switzerland) on the Elecsys 2010. Cardiac hs-TnT was measured with a high-sensitivity assay with a range of values from 3 to 10,000 ng/l.42 The limit of blank was 3 ng/l, and limit of detection was <5 ng/l. For hsTnT, the intraassay CV was 3% at a concentration of 30 ng/l and 5.8% at 2213 ng/l. The value at the 99th percentile cutoff from a healthy reference population was 13.5 ng/l for hsTnT, with a 10% intraassay CV.42 The range of values for NT-proBNP was from 114 to 5900 ng/l, and the intraassay CV was 4.25% at a concentration of 132 ng/l and 5.3% at 4640 ng/l. For biomarkers with values that were below the limit of detection, we set the value to half of the lower limit of detection (n = 202 for NT-proBNP, n = 39 for hsTnT). Values that were below the limit of detection were set to half of the lower limit of detection. There were 202 of these for NT-proBNP and 39 of these for hsTnT (none for the other biomarkers).
In 2017, we added year 2 measures of NT-proBNP and hsTnT and remeasured a subset of baseline samples to calibrate the measures. The new measurements in 2017 were performed on the Roche E601. We remeasured NT-proBNP in 100 random samples from baseline and all the year 2 samples (n = 947). We developed and applied a Deming regression43 to calibrate the 2008 baseline NT-proBNP measures with the 2017 NT-proBNP measures.
Similarly, for hsTnT, we remeasured any baseline measure with a value <5 ng/l using the newer Roche E601 instrument, which had a limit of blank of 2.5 ng/l and limit of detection of <3 ng/l. At a concentration of 13.5 ng/l, the intraassay CV was 1.9%; at 4831 ng/l, the CV was 0.8% with the newer instrument. We also measured a random subset of 100 samples at baseline and all samples at the year 2 visit (n = 947). We developed and applied a Deming regression to calibrate the 2008 baseline hsTnT measures with the 2017 hsTnT measures.
Mortality
The primary outcome was all-cause mortality. Deaths in CRIC were ascertained by reports from next of kin, retrieval of death certificates, and state death files, if available. As secondary analyses, we assessed the association between biomarker concentrations with cardiovascular (CV) mortality. When a death occurred during a hospitalization that was adjudicated, concordant reviews by 2 physicians determined whether the death was CV related. For nonadjudicated deaths, a super learning algorithm that used adjudicated death events as the gold standard was used to predict the probability of CV-related events based on cause of death codes from the National Death Index data.44 Of the 918 deaths in our analytic population of 3664, 353 were classified as CV death, 411 classified as non-CV death, and 154 were unable to be classified as either CV or non-CV death.
Measurement of Covariates
At the baseline visit, participants provided information on their demographic characteristics, medical history, medication use, and social habits. Anthropometric measurements and blood pressure (BP) were assessed using standard protocols.45 Body mass index (BMI) was derived as weight in kilograms divided by height in meters squared. Serum creatinine was measured using an enzymatic method on an Ortho Vitros 950 (Ortho Clinical Diagnostics, Raritan, NJ) at the CRIC Central Laboratory and standardized to isotope dilution mass spectrometry−traceable values,46,47 and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to estimate the glomerular filtration rate (GFR).48 Additional assays measured included serum phosphorus, 24-hour urine total protein, glucose, low-density lipoprotein (LDL) cholesterol (mathematically derived), high-density lipoprotein (HDL) cholesterol, total parathyroid hormone level (PTH), and fibroblast growth factor (FGF) 23. All assays were performed at the central laboratory with the exception of plasma PTH (CV <5%; Scantibodies, Santee, CA).49
Statistical Analysis
We log-transformed biomarker concentrations due to skewed distributions. We modeled biomarker concentrations both continuously and in categories. To generate categories of roughly the same size, we examined hsTnT across quartiles and the other biomarkers across quintiles. We calculated mortality rates and created cumulative incidence curves for mortality across biomarker categories. We used Cox regression to estimate associations of baseline biomarker concentrations (exposure) with all-cause mortality and tested the proportional hazards assumption using Schoenfeld residuals.50 We also separately assessed associations with CV mortality. We constructed nested models to evaluate confounding characteristics. Model 1 adjusted for demographic factors including age, sex, race, clinical site, and traditional cardiovascular risk factors including diabetes, CVD, smoking, protein excretion, estimated glomerular filtration rate (GFR), systolic blood pressure, BMI, LDL, and HDL levels. Model 2 added additional adjustments for pertinent medication use and markers of mineral metabolism. These covariables for chosen a priori based on biological plausibility. We further adjusted for the other 3 biomarkers of interest under model 3 to assess independent associations of each biomarker. A small number (<5%) of participants were missing covariates used in modeling; these were multiply imputed using chained equations.51 The multiple analyses over the imputations were combined using the Rubin rules to account for the variability in the imputation procedure.52
We further assessed whether changes in biomarker levels were associated with all-cause mortality. We defined change by subtracting year 2 from baseline measurements. We then examined the distribution of absolute change of each biomarker and created 3 categories: the lowest quartile of absolute change; the middle 2 quartiles of absolute change; and the top quartile of absolute change. Participants were considered at risk from the date of the year 2 visit until death, or until they were censored because of dropout or loss of follow-up. For this analysis, adjustment models were the same as in the primary analysis.
In a secondary analysis, we examined the ability of each cardiac biomarker to predict all-cause and CV mortality, and evaluated the discriminatory ability via the 10-fold cross-validated Harrell C-index with accompanying 95% confidence intervals.53,54 We compared a baseline clinical model (age, sex, race/ethnicity, site, diabetes, CVD, BMI, smoking, systolic blood pressure (SBP), LDL, HDL, eGFR, log-transformed 24-hour urinary protein) to a baseline clinical model plus each cardiac biomarker.
A nominal P value of < 0.05 was taken as evidence of statistical significance in all analyses. All analyses were conducted using the R 3.6.0 computing environment (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Participant Characteristics
Among the 3664 participants, mean age was 58 ± 11 years, 46% were women, 42% were white, 41% were black, and 13% were Hispanic. Mean eGFR was 44.3 ± 14.8 ml/min per 1.73 m2 with a median protein excretion of 0.2 g/24 h. In all, 49% of the participants reported a history of diabetes, and 33% reported a history of CVD (Table 1). Compared to participants in the lowest quintile of NT-proBNP, those in the highest quintile tended to be older and to have more CV-related comorbidities and lower baseline eGFR (Table 1). Participants with higher levels of hsTnT and sST-2 were more likely to be male; otherwise, similar patterns were seen across levels of hsTnT, GDF-15, and sST-2 (Supplementary Tables S1−S3).
Table 1.
Baseline characteristics by quintile of baseline NT-proBNP level (N = 3664)
| Characteristic | Overall | ≤37.1 | 37.14–92.5 | 92.71–199.2 | 199.21–497 | >497 |
|---|---|---|---|---|---|---|
| Patients, n | 3664 | 733 | 733 | 732 | 733 | 733 |
| Age, yr | 57.8 (11.0) | 53.4 (11.6) | 56.6 (10.8) | 58.9 (10.7) | 59.6 (10.5) | 60.4 (9.9) |
| Women, n | 1673 (46) | 269 (37) | 350 (48) | 349 (48) | 390 (53) | 315 (43) |
| Race/ethnicity | ||||||
| Non-Hispanic white | 1544 (42) | 317 (43) | 311 (42) | 347 (47) | 318 (43) | 251 (34) |
| Non-Hispanic black | 1505 (41) | 332 (45) | 312 (43) | 268 (37) | 284 (39) | 309 (42) |
| Hispanic | 467 (13) | 50 (7) | 71 (10) | 92 (13) | 109 (15) | 145 (20) |
| Other | 148 (4) | 34 (5) | 39 (5) | 25 (3) | 22 (3) | 28 (4) |
| Estimated glomerular filtration rate, ml/min per 1.73 m2 | 44.3 (14.8) | 53.6 (14.2) | 48.0 (14.5) | 43.3 (13.8) | 40.0 (12.4) | 36.3 (12.3) |
| Urinary protein to creatinine ratio from 24-h urine test | 0.2 (0.1–0.8) | 0.1 (0.0–0.2) | 0.1 (0.0–0.5) | 0.1 (0.1–0.5) | 0.2 (0.1–1.1) | 0.6 (0.1–2.6) |
| Diabetes mellitus | 1785 (49) | 244 (33) | 312 (43) | 353 (48) | 402 (55) | 474 (65) |
| History of CVD | 1207 (33) | 100 (14) | 151 (21) | 210 (29) | 303 (41) | 443 (60) |
| History of atrial fibrillation | 609 (17) | 60 (8) | 82 (11) | 99 (14) | 147 (20) | 221 (30) |
| History of CHF | 348 (9) | 17 (2) | 22 (3) | 34 (5) | 90 (12) | 185 (25) |
| Current smoker | 464 (13) | 68 (9) | 74 (10) | 92 (13) | 113 (15) | 117 (16) |
| Alcohol use | 2312 (63) | 540 (74) | 510 (70) | 455 (62) | 419 (57) | 388 (53) |
| Body mass index, kg/m2 | 32.1 (7.9) | 31.9 (6.8) | 32.1 (7.7) | 32.4 (8.5) | 32.3 (8.3) | 31.9 (7.9) |
| Systolic blood pressure, mm Hg | 128.6 (22.1) | 119.3 (15.9) | 123.9 (18.6) | 126.0 (19.2) | 132.9 (22.4) | 141.1 (26.3) |
| Diastolic blood pressure, mm Hg | 71.5 (12.8) | 72.5 (11.2) | 71.9 (11.6) | 70.3 (12.1) | 71.0 (13.5) | 71.8 (15.3) |
| Hemoglobin, g/dl | 12.6 (1.8) | 13.5 (1.6) | 12.8 (1.6) | 12.6 (1.6) | 12.2 (1.7) | 11.8 (1.9) |
| LDL cholesterol, mg/dl | 103.0 (35.3) | 107.2 (33.5) | 104.7 (35.2) | 102.5 (35.0) | 101.5 (34.1) | 99.1 (38.2) |
| HDL cholesterol, mg/dl | 47.7 (15.5) | 47.3 (14.3) | 48.5 (15.7) | 48.0 (15.3) | 48.1 (16.4) | 46.5 (15.8) |
| ACEi/ARBs | 2509 (68) | 486 (66) | 509 (69) | 508 (69) | 514 (70) | 492 (67) |
| Diuretics | 2164 (59) | 327 (45) | 387 (53) | 441 (60) | 449 (61) | 560 (76) |
| β-Blockers | 1795 (49) | 181 (25) | 269 (37) | 360 (49) | 450 (61) | 535 (73) |
| Fibroblast growth factor−23 (RU/ml), median (IQR) | 145.0 (96.5–235.7) | 102.5 (75.7–149.7) | 124.3 (87.1–188.1) | 142.6 (97.4–218.1) | 169.8 (115.9–258.8) | 221.8 (142.1–361.6) |
| Serum phosphorus, mg/dl | 3.7 (0.7) | 3.5 (0.6) | 3.6 (0.6) | 3.7 (0.7) | 3.8 (0.7) | 3.9 (0.7) |
| Total parathyroid hormone, pg/ml, median (IQR) | 54.0 (35.0–89.0) | 41.2 (30.0–58.0) | 47.8 (32.6–78.0) | 53.7 (33.0–84.0) | 62.0 (39.0–101.2) | 80.0 (49.5–128.1) |
ACEi, angiotensin-converting enzyme inhibitor; ARBs, angiotensin receptor blockers; CHF, congestive heart failure; CVD, cardiovascular disease; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Entries are mean (SD) or n (%), except as noted. All plasma concentrations are in nanograms per liter (ng/l).
Associations of Baseline NT-proBNP, hsTNT, GDF-15, and sST-2 With All-Cause Mortality
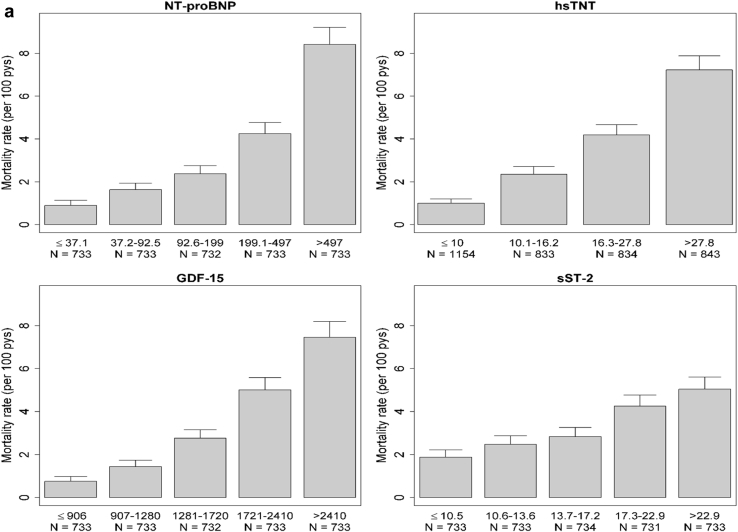
Over a median (IQR) follow-up period of 8.5 (6.8−9.6) years, there were 918 deaths (3.2 deaths per 100 person-years). Unadjusted rates of all-cause mortality increased in a graded fashion with higher levels of each biomarker (Figure 1a). After adjustment for potential confounders, cumulative incidence of all-cause mortality also varied across levels of each biomarker (Supplementary Figure S1A).
Figure 1.
(a) Rates of all-cause mortality, by biomarker category. (b) Rates of cardiovascular (CV) mortality, by biomarker category. GDF-15, growth differentiation factor−15; hsTNT, high-sensitivity troponin T; NT-proBNP, N-terminal pro-B-type natriuretic peptide; pys, person-years; sST-2, soluble ST-2.
All 4 biomarkers were significantly associated with all-cause mortality, with the strongest association observed for NT-proBNP (Table 2). After adjustments for demographics, comorbidities, clinical characteristics, and kidney function measures (model 1), every 1-SD higher log-transformed NT-proBNP level was associated with almost 2-fold higher risk of all-cause mortality, and this association remained robust after further adjustments for mineral metabolism markers and medication use (model 2). hsTnT and GDF-15 demonstrated comparable associations with all-cause mortality. The association of sST-2 with all-cause mortality was the weakest among the 4 biomarkers, although it remained robust after covariate adjustment. Additional adjustments for alternative biomarkers in our combined analysis (model 3) slightly attenuated the observed associations, but they remained strong and statistically significant for all biomarkers.
Table 2.
Association of cardiac biomarker with all-cause mortality
| Cardiac biomarker | Number at risk (n events) | Model 0 |
Model 1 |
Model 2 |
Model 3 (combined analysis) |
||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Continuous predictors | |||||||||
| Log(NT-proBNP) per 1 SD (1.71) increase | 2.48 (2.29–2.69) | <0.0001 | 1.95 (1.77–2.15) | <0.0001 | 1.92 (1.73–2.12) | <0.0001 | 1.76 (1.59–1.95) | <0.0001 | |
| Log(hsTnT) per 1 SD (0.82) increase | 1.96 (1.83–2.09) | <0.0001 | 1.66 (1.52–1.81) | <0.0001 | 1.62 (1.48–1.78) | <0.0001 | 1.49 (1.36–1.63) | <0.0001 | |
| Log(GDF-15) per 1 SD (0.59) increase | 2.24 (2.09–2.40) | <0.0001 | 1.69 (1.53–1.86) | <0.0001 | 1.61 (1.46–1.78) | <0.0001 | 1.49 (1.34–1.65) | <0.0001 | |
| Log(sST-2) per 1 SD (0.56) increase | 1.50 (1.39–1.62) | <0.0001 | 1.31 (1.20–1.42) | <0.0001 | 1.26 (1.16–1.37) | <0.0001 | 1.16 (1.07–1.25) | 0.0003 | |
| Categorical predictors | |||||||||
| NT-proBNP, pg/ml | |||||||||
| (Reference: ≤37.1) | 733 (56) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| 37.2–92.5 | 733 (98) | 1.83 (1.32–2.55) | <0.0001 | 1.48 (1.06–2.06) | <0.0001 | 1.47 (1.05–2.06) | <0.0001 | 1.49 (1.06–2.08) | <0.0001 |
| 92.6–199 | 732 (142) | 2.71 (1.99–3.69) | 1.76 (1.28–2.43) | 1.75 (1.26–2.42) | 1.69 (1.22–2.33) | ||||
| 199.1–497 | 733 (236) | 4.97 (3.71–6.65) | 2.80 (2.04–3.84) | 2.81 (2.04–3.87) | 2.54 (1.83–3.51) | ||||
| >497 | 733 (386) | 10.36 (7.80–13.76) | 4.71 (3.41–6.49) | 4.51 (3.24–6.27) | 3.88 (2.78–5.43) | ||||
| hsTnT, pg/ml | |||||||||
| (Reference: ≤10) | 1154 (99) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| 10.1–16.2 | 833 (159) | 2.39 (1.86–3.06) | <0.0001 | 1.61 (1.24–2.09) | <0.0001 | 1.58 (1.22–2.04) | <0.0001 | 1.56 (1.21–2.02) | <0.0001 |
| 16.3–27.8 | 834 (260) | 4.34 (3.44–5.46) | 2.41 (1.86–3.12) | 2.33 (1.80–3.02) | 2.21 (1.70–2.86) | ||||
| >27.8 | 843 (400) | 7.82 (6.27–9.75) | 3.67 (2.79–4.82) | 3.43 (2.60–4.52) | 2.90 (2.19–3.84) | ||||
| GDF-15 | |||||||||
| (Reference: ≤906) | 733 (48) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| 907–1280 | 733 (88) | 1.92 (1.35–2.73) | <0.0001 | 1.23 (0.85–1.77) | <0.0001 | 1.19 (0.83–1.71) | <0.0001 | 1.20 (0.83–1.73) | <0.0001 |
| 1281–1720 | 732 (163) | 3.76 (2.73–5.19) | 1.90 (1.35–2.67) | 1.80 (1.28–2.54) | 1.80 (1.28–2.54) | ||||
| 1721–2410 | 733 (267) | 7.03 (5.17–9.57) | 2.82 (1.99–3.99) | 2.64 (1.86–3.73) | 2.58 (1.81–3.67) | ||||
| >2410 | 733 (352) | 10.77 (7.95–14.6) | 3.86 (2.69–5.55) | 3.49 (2.42–5.01) | 3.11 (2.15–4.51) | ||||
| sST-2 | |||||||||
| (Reference: ≤10.5) | 733 (114) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| 10.6–13.6 | 733 (146) | 1.33 (1.04–1.69) | <0.0001 | 1.05 (0.82–1.35) | <0.0001 | 1.06 (0.83–1.35) | <0.0001 | 1.07 (0.84–1.37) | 0.02 |
| 13.7–17.2 | 734 (163) | 1.54 (1.21–1.96) | 1.16 (0.91–1.47) | 1.14 (0.90–1.45) | 1.12 (0.88–1.43) | ||||
| 17.3–22.9 | 731 (231) | 2.33 (1.86–2.91) | 1.51 (1.20–1.90) | 1.48 (1.18–1.87) | 1.38 (1.10–1.74) | ||||
| >22.9 | 733 (264) | 2.79 (2.24–3.47) | 1.67 (1.32–2.11) | 1.56 (1.23–1.97) | 1.32 (1.04–1.68) | ||||
CI, confidence interval; GDF-15, growth differentiation factor−15; HR, hazard ratio; hsTnT, high-sensitivity troponin T; NT-proBNP, N-terminal pro-B-type natriuretic peptide; sST-2, soluble ST-2.
Model 0: unadjusted. Model 1: age, sex, race/ethnicity, site, diabetes, cardiovascular disease, body mass index, smoking, systolic blood pressure, low-density lipoprotein, high-density lipoprotein, estimated glomerular filtration rate, log-transformed 24-hour urinary protein.. Model 2: Model 1 + fibroblast growth factor−23, phosphorus, β-blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, diuretics. Model 3: Model 2 + alternative cardiac biomarker.
Associations of Baseline NT-proBNP, hsTNT, GDF-15, and sST-2 With CV Mortality
Over a median (IQR) follow-up period of 8.5 (6.8−9.6) years, there were 353 deaths (1.2 deaths per 100 person-years) from CV mortality. Rates of CV mortality increased incrementally across levels of each biomarker (Figure 1b). Adjusted cumulative incidence of CV mortality rates varied across categories of the 4 biomarkers, although the observed differences were less for sST-2 (Supplementary Figure S1B).
All 4 biomarkers were significantly associated with death from CV mortality. Again, we observed the strongest association for NT-proBNP (Table 3 and Supplementary Table S4). Per SD higher log-transformed baseline concentration of NT-proBNP was associated with increased risk of CV mortality (adjusted hazard ratio [HR] = 2.04, 95% confidence interval [CI] = 1.74−2.39) (Table 3). Analogously, hsTnT and GDF-15 were also associated with CV mortality. Again, sST-2 demonstrated the weakest, albeit statistically robust, associations with CV mortality. Adjustment for alternative biomarkers in our combined analysis (model 3) attenuated the observed associations with CV mortality for all four biomarkers.
Table 3.
Association of cardiac biomarkers with CV mortality
| Cardiac biomarker | Model 0 |
Model 1 |
Model 2 |
Model 3 (combined analysis) |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| CV mortality | ||||||||
| Log(NT-proBNP) per 1 SD (1.71) increase | 2.66 (2.35, 3.00) | <0.0001 | 2.10 (1.80, 2.45) | <0.0001 | 2.04 (1.74, 2.39) | <0.0001 | 1.89 (1.60, 2.22) | <0.0001 |
| Log(hsTnT) per 1 SD (0.82) increase | 2.12 (1.94, 2.31) | <0.0001 | 1.87 (1.65, 2.11) | <0.0001 | 1.82 (1.60, 2.06) | <0.0001 | 1.71 (1.50, 1.95) | <0.0001 |
| Log(GDF-15) per 1 SD (0.59) increase | 2.22 (2.01, 2.45) | <0.0001 | 1.63 (1.41, 1.89) | <0.0001 | 1.55 (1.33, 1.81) | <0.0001 | 1.44 (1.23, 1.68) | <0.0001 |
| Log(sST-2) per 1 SD (0.56) increase | 1.50 (1.34, 1.67) | <0.0001 | 1.27 (1.11, 1.44) | 0.0004 | 1.22 (1.08, 1.39) | 0.002 | 1.13 (1.00, 1.27) | 0.04 |
CI, confidence interval; CV, cardiovascular; GDF-15, growth differentiation factor−15; HR, hazard ratio; hsTnT, high-sensitivity troponin T; NT-proBNP, N-terminal pro-B-type natriuretic peptide; sST-2, soluble ST-2.
Model 0: unadjusted. Model 1: age, sex, race/ethnicity, site, diabetes, cardiovascular disease, body mass index, smoking, systolic blood pressure, low-density lipoprotein, high-density lipoprotein, estimated glomerular filtration rate, log-transformed 24-hour urinary protein.. Model 2: Model 1 + fibroblast growth factor−23, phosphorus, β-blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, diuretics. Model 3: Model 2 + alternative cardiac biomarker.
Associations of 2-Year Change in Cardiac Biomarkers With All-Cause Mortality
There were 842 participants in the subcohort who had repeated measures of each cardiac biomarker 2 years apart. Characteristics of the 842 participants from the random subcohort who had a repeat cardiac biomarker measurement at year 2 were comparable to those in the overall study population (Supplementary Table S5). Compared with participants in the middle 2 quartiles of absolute change (referent group), declines in NT-proBNP and sST2 were associated with lower risk of all-cause mortality (adjusted HR = 0.55, 95% CI = 0.36−0.86, and HR = 0.60, 95% CI = 0.40−0.92], respectively. Declines in hsTnT and GDF-15 did not have significant associations with mortality risk (Table 4). For all 4 biomarkers, the quartile with the greatest increase over 2 years was not significantly associated with mortality risk compared with participants in the middle 2 quartiles.
Table 4.
Among subcohort only, association of change in cardiac biomarkers with all-cause mortality (n = 842)
| Cardiac biomarker | N at risk | N events | Unadjusted |
Model 1 |
Model 2 |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Absolute change | |||||
| NT-proBNP (pg/ml) | |||||
| ≤ −14.3 | 211 | 56 | 0.33 (0.22, 0.49) | 0.55 (0.36, 0.86) | 0.55 (0.36, 0.86) |
| −14.2 to 285 | 420 | 42 | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| > 285 | 211 | 64 | 1.17 (0.82, 1.68) | 1.31 (0.89, 1.94) | 1.24 (0.84, 1.84) |
| hsTnT (ng/ml) | |||||
| ≤ −0.247 | 211 | 37 | 0.83 (0.55, 1.24) | 0.95 (0.63, 1.45) | 0.99 (0.65, 1.50) |
| −0.246 to 11.1 | 420 | 65 | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| >11.1 | 211 | 60 | 1.67 (1.11, 2.52) | 1.31 (0.85, 2.02) | 1.33 (0.86, 2.06) |
| GDF-15 (pg/ml) | |||||
| ≤ −122 | 211 | 39 | 0.76 (0.51, 1.13) | 1.25 (0.79, 1.97) | 1.28 (0.81, 2.03) |
| −121 to 585 | 421 | 63 | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| >585 | 210 | 60 | 1.64 (1.09, 2.45) | 1.50 (0.93, 2.42) | 1.54 (0.95, 2.48) |
| sST-2 (ng/ml) | |||||
| ≤ −3.91 | 211 | 56 | 0.49 (0.34, 0.71) | 0.67 (0.44, 1.01) | 0.60 (0.40, 0.92) |
| −3.9 to 3.46 | 420 | 60 | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| >3.46 | 211 | 46 | 0.78 (0.53, 1.15) | 0.88 (0.57, 1.38) | 0.88 (0.56, 1.37) |
CI, confidence interval; GDF-15, growth differentiation factor−15; HR, hazard ratio; hsTnT, high-sensitivity troponin T; NT-proBNP, N-terminal pro-B-type natriuretic peptide; sST-2, soluble ST-2.
Model 1: age, sex, race/ethnicity, site, diabetes, cardiovascular disease, body mass index, smoking, systolic blood pressure, low-density lipoprotein, high-density lipoprotein, estimated glomerular filtration rate, log-transformed urine protein, and baseline level of biomarker. Model 2: Model 1 + fibroblast growth factor−23, phosphorus, beta blockers, ACEi/ARBs, diuretics.
Discriminatory Ability of Cardiac Biomarkers to Predict All-Cause and CV Mortality
The C-statistic for a baseline clinical model for all-cause mortality was 0.74 (95% CI = 0.72−0.76) and for CV mortality was 0.77 (95% CI = 0.75−0.80). Addition of NT-proBNP, hsTnT, GDF-15, and sST2, but not galectin-3, improved the C-statistic for both all-cause and CV mortality (with the greatest improvement seen with NT-proBNP; Table 5).
Table 5.
Discriminatory ability of cardiac biomarker to predict all-cause and CV mortality in the CRIC study
| Cardiac biomarker | Model 0 |
Model 1 |
|
|---|---|---|---|
| 10-Fold CV C-index (95% bootstrap CI) | 10-Fold CV C-index (95% bootstrap CI) | Difference in C-index, compared to model 0 (95% bootstrap CI) | |
| Overall mortality | |||
| NT-proBNP | 0.74 (0.72, 0.76) | 0.77 (0.75, 0.78) | 0.03 (0.02, 0.04) |
| hsTNT | 0.74 (0.72, 0.76) | 0.76 (0.74, 0.78) | 0.02 (0.01, 0.03) |
| GDF-15 | 0.74 (0.72, 0.76) | 0.76 (0.74, 0.77) | 0.02 (0.01, 0.02) |
| Galectin-3 | 0.74 (0.72, 0.76) | 0.75 (0.73, 0.76) | 0.00 (0.00, 0.01) |
| sST-2 | 0.74 (0.72, 0.76) | 0.75 (0.73, 0.76) | 0.01 (0.00, 0.01) |
| CV mortality | |||
| NT-proBNP | 0.77 (0.75, 0.80) | 0.80 (0.78, 0.82) | 0.03 (0.02, 0.04) |
| hsTNT | 0.77 (0.75, 0.80) | 0.79 (0.77, 0.81) | 0.02 (0.01, 0.03) |
| GDF-15 | 0.77 (0.75, 0.80) | 0.79 (0.76, 0.81) | 0.01 (0.00, 0.02) |
| Galectin-3 | 0.77 (0.75, 0.80) | 0.77 (0.75, 0.80) | 0.00 (0.00, 0.01) |
| sST-2 | 0.77 (0.75, 0.80) | 0.78 (0.75, 0.80) | 0.01 (0.00, 0.01) |
CRIC, Chronic Renal Insufficiency Cohort; CV, cardiovascular; GDF-15, growth differentiation factor−15; hsTnT, high-sensitivity troponin T; NT-proBNP, N-terminal pro-B-type natriuretic peptide; sST-2, soluble ST-2.
Model 0: age, sex, race/ethnicity, site, diabetes, cardiovascular disease, body mass index, smoking, systolic blood pressure, low-density lipoprotein, high-density lipoprotein, estimated glomerular filtration rate, log-transformed 24-hour urinary protein. Model 1: M0 + log-transformed biomarker.
Discussion
In a well-characterized cohort of more than 3000 adults with CKD, we observed robust associations of higher baseline concentrations of NT-proBNP, hsTnT, GDF-15, and sST-2 with increased risks of all-cause and CV mortality. Among individuals of a randomly sampled subcohort, declines in NT-proBNP and sST-2 over 2 years were associated with lower risk of all-cause mortality. These results suggest that circulating cardiac biomarkers of myocardial injury and inflammation are associated with adverse clinical outcomes in CKD individuals independent of traditional CVD risk factors.
Numerous large-scale, community-based studies have demonstrated associations between elevations of NT-proBNP and hsTnT with increased risk of CV and all-cause deaths among CVD patients and healthy individuals independent of CVD risk factors.10,16, 17, 18, 19, 20, 21 Cross-sectional studies of CKD patients have shown that elevations of NT-proBNP and hsTnT are associated with increased incidence of left ventricular hypertrophy and left ventricular dysfunction.9,11,55 However, longitudinal data in CKD are more limited. Among 994 participants with moderate CKD in the African-American Study of Kidney Disease and Hypertension Study, each 2-fold higher concentration of NT-proBNP was associated with an 80% increased risk of CV-related death.34 Of the 940 CKD individuals from the Atherosclerosis Risk in Communities Study, each 1-SD higher NT-proBNP was associated with 50% increased risk of developing coronary artery disease, stroke, or heart failure.35
We also observed a robust and independent association between GDF-15 with mortality. GDF-15 exerts anti-inflammatory properties and plays a vital counterregulatory role in the context of CV injury. Higher GDF-15 level predicts mortality among CVD and heart failure patients.24,27, 28, 29, 30 Large-scale studies of healthy individuals without CVD have also demonstrated elevated GDF-15 levels to be associated with higher risk of CV and all-cause mortality.56,57
Similarly, we observed an association between sST-2 with higher risk of all-cause mortality. As a membrane of the interleukin-1 receptor family, ST2 exists in both membrane-bound and soluble forms. Soluble ST2 (sST2) functions as a “decoy” receptor and prevents the binding of interleukin-33, a fibroblast protein with known antihypertrophic properties, to the membrane-bound version of ST2. Among patients with heart failure, elevated sST2 is associated with depressed systolic function and increased risk of CV mortality.31, 32, 33 In a prior study of 883 individuals with moderate CKD, we showed that every 1-SD higher concentration of sST2 was associated with 36% higher risk of all-cause mortality.38
Among the subcohort of participants who had repeated biomarkers measurements, declines in both NT-proBNP and sST-2 over 2 years were associated with lower risk of all-cause mortality. We did not observe associations between increases in biomarker levels with mortality risk; the reason for this is unclear. Our data both support as well as differ from existing studies in non-CKD populations. Among community-dwelling elderly individuals, increase in NT-proBNP levels over 3 years were associated with higher risk of incident heart failure and CV death, whereas declines were associated with improved clinical outcomes.58 A few clinical trials in heart failure have used BNP thresholds to guide therapies in heart failure patients. Compared with routine clinical care or symptom-based therapy, titrating heart failure therapy to BNP thresholds has demonstrated clinical benefit in a few studies,59, 60, 61 suggesting that using serial measurements of biomarkers levels to guide medical therapy may improve clinical outcomes. However, a larger clinical trial of patients with more advanced heart failure did not show any clinical benefit.62 Our findings may suggest that biomarker-guided medical therapy for heart failure may be extended to individuals with CKD.
Our findings have several important clinical implications. Elevations in these biomarkers may provide new insight into key pathways involved in the pathogenesis of CVD in CKD patients and may be used to guide development of new therapies. Assessment of markers of cardiac stress and inflammation may be used in the clinical setting to help risk-stratify CKD patients who are at heighted risk for poor clinical outcomes, even among those without overt CVD. Targeting biomarker levels may also serve as therapeutic targets for treatment of CVD-related complications in CKD patients. Future large, prospective studies and clinical trials of CKD patients are needed to test these hypotheses.
Our study has several strengths. We used data from a large, racially diverse, well-characterized CKD cohort. We also adjusted for a broad range of potential confounders in our analyses. We recognize several limitations as well. First, the prediction of nonadjudicated CV-related deaths may introduce bias in our results. We were not able to determine the cause of death (CV or non-CV) for 16.8% of the deaths in our analytic population. Finally, we observed relatively fewer events among participants of the subcohort with repeated measurements, therefore limiting our power to detect meaningful associations between longitudinal changes in biomarker levels with clinical outcomes.
In summary, elevated baseline levels of NT-proBNP, hs-TnT, GDF-15, and sST-2 were independently associated with increased risk of all-cause and CV mortality among patients with CKD. Declines in NT-proBNP and sST-2 over 2 years were also associated with lower risk of all-cause mortality. Further studies are needed to elucidate the underlying biological mechanisms and to determine whether targeting these biomarkers with therapy can mitigate CVD burden in patients with CKD.
Disclosure
All the authors declare no competing interests.
Acknowledgments
This study was supported by R01 DK103612 (Bansal). Roche Diagnostics provided partial funding for the NT-proBNP and hsTnT assays. Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131, and an unrestricted fund from the Northwest Kidney Centers.
Footnotes
Table S1. Baseline characteristics by category of baseline hsTnT level (N = 3664).
Table S2. Baseline characteristics by category of baseline GDF-15 level (N = 3664).
Table S3. Baseline characteristics by category of baseline sST-2 level (N = 3664).
Table S4. Association of cardiac biomarkers with CV mortality (categorical predictor).
Table S5. Characteristics of subcohort with longitudinal measurements (N = 842).
Figure S1. (A) Kaplan−Meier curves (adjusted) of each biomarker with all-cause mortality. (B) Kaplan−Meier curves (adjusted) of each biomarker with CV mortality.
STROBE Statement for Cohort Studies.
Contributor Information
Nisha Bansal, Email: nbansal@uw.edu.
CRIC Study Investigators:
Lawrence J. Appel, Harold I. Feldman, Alan S. Go, Jiang He, James P. Lash, Panduranga S. Rao, Mahboob Rahman, and Raymond R. Townsend
Supplementary Material
References
- 1.Saran R., Robinson B., Abbott K.C. US Renal Data System 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69:7–8. doi: 10.1053/j.ajkd.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter A.C., Lash J.P., Xie D. Predictors and outcomes of health-related quality of life in adults with CKD. Clin J Am Soc Nephrol. 2016;11:1154–1162. doi: 10.2215/CJN.09990915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go A.S., Chertow G.M., Fan D. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Muntner P., He J., Chen J. Prevalence of non-traditional cardiovascular disease risk factors among persons with impaired fasting glucose, impaired glucose tolerance, diabetes, and the metabolic syndrome: analysis of the Third National Health and Nutrition Examination Survey (NHANES III) Ann Epidemiol. 2004;14:686–695. doi: 10.1016/j.annepidem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Wattanakit K., Coresh J., Muntner P. Cardiovascular risk among adults with chronic kidney disease, with or without prior myocardial infarction. J Am Coll Cardiol. 2006;48:1183–1189. doi: 10.1016/j.jacc.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 6.Yasue H., Yoshimura M., Sumida H. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. doi: 10.1161/01.cir.90.1.195. [DOI] [PubMed] [Google Scholar]
- 7.Vickery S., Price C.P., John R.I. B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis. 2005;46:610–620. doi: 10.1053/j.ajkd.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Mishra R.K., Li Y., Ricardo A.C. Association of N-terminal pro-B-type natriuretic peptide with left ventricular structure and function in chronic kidney disease (from the Chronic Renal Insufficiency Cohort [CRIC]) Am J Cardiol. 2013;111:432–438. doi: 10.1016/j.amjcard.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeFilippi C.R., Fink J.C., Nass C.M. N-terminal pro-B-type natriuretic peptide for predicting coronary disease and left ventricular hypertrophy in asymptomatic CKD not requiring dialysis. Am. J Kidney Dis. 2005;46:35–44. doi: 10.1053/j.ajkd.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 10.de Lemos J.A., Drazner M.H., Omland T. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra R.K., Li Y., Defilippi C. Association of cardiac troponin T with left ventricular structure and function in CKD. Am J Kidney Dis. 2013;61:701–709. doi: 10.1053/j.ajkd.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlittenhardt D., Schober A., Strelau J. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res. 2004;318:325–333. doi: 10.1007/s00441-004-0986-3. [DOI] [PubMed] [Google Scholar]
- 13.de Jager S.C., Bermudez B., Bot I. Growth differentiation factor 15 deficiency protects against atherosclerosis by attenuating CCR2-mediated macrophage chemotaxis. J Exp Med. 2011;208:217–225. doi: 10.1084/jem.20100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kempf T., Eden M., Strelau J. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98:351–360. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- 15.Seki K., Sanada S., Kudinova A.Y. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail. 2009;2:684–691. doi: 10.1161/CIRCHEARTFAILURE.109.873240. [DOI] [PubMed] [Google Scholar]
- 16.Kragelund C., Gronning B., Kober L. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666–675. doi: 10.1056/NEJMoa042330. [DOI] [PubMed] [Google Scholar]
- 17.Geng Z., Huang L., Song M. N-terminal pro-brain natriuretic peptide and cardiovascular or all-cause mortality in the general population: a meta-analysis. Sci Rep. 2017;7:41504. doi: 10.1038/srep41504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kistorp C., Raymond I., Pedersen F. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293:1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 19.Scheven L., de Jong P.E., Hillege H.L. High-sensitive troponin T and N-terminal pro-B type natriuretic peptide are associated with cardiovascular events despite the cross-sectional association with albuminuria and glomerular filtration rate. Eur Heart J. 2012;33:2272–2281. doi: 10.1093/eurheartj/ehs163. [DOI] [PubMed] [Google Scholar]
- 20.deFilippi C.R., de Lemos J.A., Christenson R.H. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saunders J.T., Nambi V., de Lemos J.A. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eggers K.M., Kempf T., Lagerqvist B. Growth-differentiation factor-15 for long-term risk prediction in patients stabilized after an episode of non-ST-segment-elevation acute coronary syndrome. Circ Cardiovasc Genet. 2010;3:88–96. doi: 10.1161/CIRCGENETICS.109.877456. [DOI] [PubMed] [Google Scholar]
- 23.Wollert K.C., Kempf T., Peter T. Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation. 2007;115:962–971. doi: 10.1161/CIRCULATIONAHA.106.650846. [DOI] [PubMed] [Google Scholar]
- 24.Anand I.S., Kempf T., Rector T.S. Serial measurement of growth-differentiation factor-15 in heart failure: relation to disease severity and prognosis in the Valsartan Heart Failure Trial. Circulation. 2010;122:1387–1395. doi: 10.1161/CIRCULATIONAHA.109.928846. [DOI] [PubMed] [Google Scholar]
- 25.Khan S.Q., Ng K., Dhillon O. Growth differentiation factor-15 as a prognostic marker in patients with acute myocardial infarction. Eur Heart J. 2009;30:1057–1065. doi: 10.1093/eurheartj/ehn600. [DOI] [PubMed] [Google Scholar]
- 26.Hagstrom E., James S.K., Bertilsson M. Growth differentiation factor-15 level predicts major bleeding and cardiovascular events in patients with acute coronary syndromes: results from the PLATO study. Eur Heart J. 2016;37:1325–1333. doi: 10.1093/eurheartj/ehv491. [DOI] [PubMed] [Google Scholar]
- 27.Kempf T., von Haehling S., Peter T. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1054–1060. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 28.Chan M.M., Santhanakrishnan R., Chong J.P. Growth differentiation factor 15 in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail. 2016;18:81–88. doi: 10.1002/ejhf.431. [DOI] [PubMed] [Google Scholar]
- 29.Dallmeier D., Brenner H., Mons U. Growth differentiation factor 15, its 12-month relative change, and risk of cardiovascular events and total mortality in patients with stable coronary heart disease: 10-year follow-up of the KAROLA study. Clin Chem. 2016;62:982–992. doi: 10.1373/clinchem.2016.254755. [DOI] [PubMed] [Google Scholar]
- 30.Hagstrom E., Held C., Stewart R.A. Growth differentiation factor 15 predicts all-cause morbidity and mortality in stable coronary heart disease. Clin Chem. 2017;63:325–333. doi: 10.1373/clinchem.2016.260570. [DOI] [PubMed] [Google Scholar]
- 31.Rehman S.U., Mueller T., Januzzi J.L., Jr. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52:1458–1465. doi: 10.1016/j.jacc.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 32.Januzzi J.L., Jr., Peacock W.F., Maisel A.S. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol. 2007;50:607–613. doi: 10.1016/j.jacc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Bayes-Genis A., de Antonio M., Vila J. Head-to-head comparison of 2 myocardial fibrosis biomarkers for long-term heart failure risk stratification: ST2 versus galectin-3. J Am Coll Cardiol. 2014;63:158–166. doi: 10.1016/j.jacc.2013.07.087. [DOI] [PubMed] [Google Scholar]
- 34.Astor B.C., Yi S., Hiremath L. N-terminal prohormone brain natriuretic peptide as a predictor of cardiovascular disease and mortality in blacks with hypertensive kidney disease: the African American Study of Kidney Disease and Hypertension (AASK) Circulation. 2008;117:1685–1692. doi: 10.1161/CIRCULATIONAHA.107.724187. [DOI] [PubMed] [Google Scholar]
- 35.Matsushita K., Sang Y., Ballew S.H. Cardiac and kidney markers for cardiovascular prediction in individuals with chronic kidney disease:the Atherosclerosis Risk in Communities study. Arterioscler Thromb Vasc Biol. 2014;34:1770–1777. doi: 10.1161/ATVBAHA.114.303465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bansal N., Hyre Anderson A., Yang W. High-sensitivity troponin T and N-terminal pro-B-type natriuretic peptide (NT-proBNP) and risk of incident heart failure in patients with CKD: the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol. 2015;26:946–956. doi: 10.1681/ASN.2014010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nair V., Robinson-Cohen C., Smith M.R. Growth differentiation factor-15 and risk of CKD progression. J Am Soc Nephrol. 2017;28:2233–2240. doi: 10.1681/ASN.2016080919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuegel C., Katz R., Alam M. GDF-15, galectin 3, soluble ST2, and risk of mortality and cardiovascular events in CKD. Am J Kidney Dis. 2018;72:519–528. doi: 10.1053/j.ajkd.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldman H.I., Appel L.J., Chertow G.M. The Chronic Renal Insufficiency Sohort (CRIC) Study: design and methods. J Am Soc Nephrol. 2003;14:S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 40.Lash J.P., Go A.S., Appel L.J. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levey A.S., Bosch J.P., Lewis J.B. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 42.Giannitsis E., Kurz K., Hallermayer K. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 43.Linnet K. Necessary sample size for method comparison studies based on regression analysis. Clin Chem. 1999;45:882–894. [PubMed] [Google Scholar]
- 44.van der Laan M.J., Polley E.C., Hubbard A.E. Super learner. Stat Appl Genet Mol Biol. 2007;6:25. doi: 10.2202/1544-6115.1309. [DOI] [PubMed] [Google Scholar]
- 45.National Center for Health Statistics (NCHS) Centers for Disease Control and Prevention; 2000. National Health and Nutrition Examination Survey Anthropometry Procedures Manual. Available at: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf. Accessed October 14, 2020. [Google Scholar]
- 46.Joffe M., Hsu C.Y., Feldman H.I. Variability of creatinine measurements in clinical laboratories: results from the CRIC Study. Am J Nephrol. 2010;31:426–434. doi: 10.1159/000296250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levey A.S., Coresh J., Greene T. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 48.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Batacchi Z., Robinson-Cohen C., Hoofnagle A.N. Effects of vitamin D2 supplementation on vitamin D3 metabolism in health and CKD. Clin J Am Soc Nephrol. 2017;12:1498–1506. doi: 10.2215/CJN.00530117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grambsch P., Therneau T. Proportional hazards tests and diagnostics based on weighted residuals Biometrika. 1994;81:515–526. [Google Scholar]
- 51.Royston P. Multiple imputation of missing values. Stata J. 2004;4:227–241. [Google Scholar]
- 52.Rubin D.B. Wiley; New York, NY: 1987. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 53.Harrell F.E., Jr., Lee K.L., Mark D.B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 54.Uno H.T.L., Cai T., Kohane I.S., Wei L.J. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med. 2013;32:2430–2442. doi: 10.1002/sim.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.deFilippi C., Seliger S.L., Kelley W. Interpreting cardiac troponin results from high-sensitivity assays in chronic kidney disease without acute coronary syndrome. Clin Chem. 2012;58:1342–1351. doi: 10.1373/clinchem.2012.185322. [DOI] [PubMed] [Google Scholar]
- 56.Brown D.A., Breit S.N., Buring J. Concentration in plasma of macrophage inhibitory cytokine-1 and risk of cardiovascular events in women: a nested case-control study. Lancet. 2002;359:2159–2163. doi: 10.1016/S0140-6736(02)09093-1. [DOI] [PubMed] [Google Scholar]
- 57.Daniels L.B., Clopton P., Laughlin G.A. Growth-differentiation factor-15 is a robust, independent predictor of 11-year mortality risk in community-dwelling older adults: the Rancho Bernardo Study. Circulation. 2011;123:2101–2110. doi: 10.1161/CIRCULATIONAHA.110.979740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.deFilippi C.R., Christenson R.H., Gottdiener J.S. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N-terminal pro-B-type natriuretic peptide testing. J Am Coll Cardiol. 2010;55:441–450. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfisterer M., Buser P., Rickli H. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. JAMA. 2009;301:383–392. doi: 10.1001/jama.2009.2. [DOI] [PubMed] [Google Scholar]
- 60.Jourdain P., Jondeau G., Funck F. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol. 2007;49:1733–1739. doi: 10.1016/j.jacc.2006.10.081. [DOI] [PubMed] [Google Scholar]
- 61.Felker G.M., Hasselblad V., Hernandez A.F. Biomarker-guided therapy in chronic heart failure: a meta-analysis of randomized controlled trials. Am Heart J. 2009;158:422–430. doi: 10.1016/j.ahj.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 62.Felker G.M., Anstrom K.J., Adams K.F. Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2017;318:713–720. doi: 10.1001/jama.2017.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.