Abstract
Purpose
Specific language impairment (SLI) is characterized by a delay in language acquisition despite a lack of other developmental delays or hearing loss. Genetics of SLI is poorly understood. The purpose of this study is to identify SLI genetic loci through family-based linkage mapping.
Method
We performed genome-wide parametric linkage analysis in six families segregating with SLI. An age-appropriate standardized omnibus language measure was used to categorically define the SLI phenotype.
Results
A suggestive linkage region replicated a previous region of interest with the highest logarithm of odds (LOD) score of 2.40 at 14q11.2-q13.3 in Family 489. A paternal parent-of-origin effect associated with SLI and language phenotypes on a nonsynonymous single nucleotide polymorphism (SNP) in NOP9 (14q12) was reported previously. Linkage analysis identified a new SLI locus at 15q24.3-25.3 with the highest parametric LOD score of 3.06 in Family 315 under a recessive mode of inheritance. Suggestive evidence of linkage was also revealed at 4q31.23-q35.2 in Family 300, with the highest LOD score of 2.41. Genetic linkage was not identified in the other three families included in parametric linkage analysis.
Conclusions
These results are the first to report genome-wide suggestive linkage with a total language standard score on an age-appropriate omnibus language measure across a wide age range. Our findings confirm previous reports of a language-associated locus on chromosome 14q, report new SLI loci, and validate the pedigree-based parametric linkage analysis approach to mapping genes for SLI.
Supplemental Material
Most children acquire language without any formal instruction. Contrary to common expectations, language does not come easily to all children. Specific language impairment (SLI) is a language disorder that delays the acquisition of language skills in children who have no hearing loss or other developmental delays (National Institute on Deafness and Other Communication Disorders, 2019). The estimated prevalence of SLI (excluding children with low/borderline normal nonverbal IQ) is 7% in the United States (Tomblin et al., 1997), an estimate that was recently replicated for children in England (Norbury et al., 2016). When children with borderline normal nonverbal IQ are included in these affected samples, they add approximately 3% to the percentage (Norbury et al., 2016). Language ability is associated with multiple aspects of quality of life including stronger social relationships and academic success (Conti-Ramsden et al., 2018; Johnson et al., 2010; Rice, 2017; Tomblin, 2014). The causes of language delay in children with SLI are not known.
Family aggregation and twin studies provide evidence that the language acquisition difficulties children with SLI experience are inherited (Bishop et al., 2006, 1995; Bishop & Hayiou-Thomas, 2008; Rice et al., 1998, 2020, 2018; Tallal et al., 2001). Twin studies of individuals with and without SLI have revealed strong heritability estimates for multiple speech and language phenotypes in dizygotic and monozygotic twins (Bishop & Hayiou-Thomas, 2008; Rice et al., 2020, 2018). Heritability estimates vary, ranging from .20 to .94, depending on the phenotype, age of children, and dimensions of language assessed. Of interest here, standardized omnibus language measures (that assess multiple domains of language) are often in the higher heritability range (Bishop & Hayiou-Thomas, 2008; Rice et al., 2020, 2018).
Although SLI is known to aggregate in families (Rice et al., 1998; Tallal et al., 2001), family-based genetic studies in SLI pose challenges. SLI is a complex disorder, with age-variable language outcomes among individuals with SLI (although with similar overall developmental outcomes), that does not seem to align with a simple Mendelian inheritance pattern (Strachan et al., 2014; van Heyningen & Yeyati, 2004). Mendelian disorders follow Mendelian laws of inheritance characterized by the involvement of factors in a single gene, which can be heritable (changes in germ cells) or not heritable (de novo/new changes). Classification of Mendelian traits is dichotomous, while complex traits are those that affect individuals on more of a continuous spectrum of phenotypes (Strachan et al., 2014). Under simple Mendelian inheritance, contributing gene mutations show a complete genotype–phenotype correlation or complete disease penetrance (Jordan, 2014). Non-Mendelian traits are more likely the result of mutations in a single gene or multiple genes transmitted from parents to offspring under an undefined inheritance pattern with variable disease penetrance (van Heyningen & Yeyati, 2004). However, the expression of such disorders can be caused by a combination of genetic and environmental factors, further complicating the discovery of contributing factors in complex disorders, especially those that are phenotyped behaviorally, like SLI (Jordan, 2014; van Heyningen & Yeyati, 2004).
Linkage analysis is often the first step in family-based genetic investigation to narrow regions to identify causative genes and variations. The purpose of parametric linkage analysis is to identify coinheritance of chromosomal loci with a phenotype (Read & Strachan, 2011). Linkage is estimated by logarithm of odds (LOD) scores. In parametric linkage, factors such as mode of inheritance, disease frequency, and disease penetrance must be set to complete the analysis. The mode of inheritance can be set to dominant or recessive with variable disease penetrance. Although the use of multiple models during analysis is not ideal, past studies have shown it produces powerful results for analysis of complex phenotypes (Abreu et al., 1999; Bartlett et al., 2002; Greenberg et al., 1998). Traditional LOD score-based gene mapping methods are conventionally successful for simple Mendelian disorders, but similar approaches have also been successful for complex genetic disorders in which the mode of inheritance is not determined (Abreu et al., 1999). Linkage analysis methods utilizing dominant and recessive inheritance models were tested against a nonparametric linkage model and shown to be more powerful for heterogenous phenotypes (Abreu et al., 1999). Pedigree-based analysis of complex disorders with a well-defined phenotype serves to reduce trait variance across related individuals (Neale & Cardon, 2013). At the same time, the power of linkage in complex disorders is influenced by variable disease penetrance, phenocopy rate, and unknown segregation patterns (Clerget-Darpoux & Elston, 2007; Ott et al., 2011).
Multiple genetic studies of SLI are reported, with mixed outcomes for linkage and association; consistently high heritability estimates and genetic loci are reported (Reader et al., 2014; Rice et al., 2020; Smith, 2007). SLI is heterogeneous genetically and phenotypically (Bishop & Hayiou-Thomas, 2008). The SLI Consortium (SLIC) completed the first linkage analysis in individuals with SLI, using small nuclear families and analyses relying on sib-pair information (SLIC, 2002). Genetic analyses in nuclear SLI families were completed for three phenotype measures: (a) a test of nonword repetition (NWR) measuring phonological short-term memory (Gathercole et al., 1994), and omnibus standard scores on the (b) Receptive and (c) Expressive Language subtests of the Clinical Evaluation of Language Fundamentals–Revised (CELF-R; Semel et al., 1987). The resulting significant loci were reported on chromosome 16q24, linked with the NWR test, and on chromosome 19q13, linked with the CELF-R Expressive Language subtest (SLIC, 2002). However, in the follow-up targeted genetic study including new families, both linkage regions (chromosome 16q and 19q) were linked to the NWR test, whereas no linkage was observed with the CELF-R Receptive or Expressive subtests, counter to the previous findings (SLIC, 2004).
Similarly, an investigation of Canadian families phenotyped as reading impaired (RI), language impaired, or clinically impaired showed linkage on chromosome 13q; subsequent follow-up investigation of American families replicated the linkage on 13q (Bartlett et al., 2004, 2002). Participants in this study were grouped based on their performance on age-appropriate standardized measures of language and non-verbal intelligence, a battery of other tests, and a family history interview (Bartlett et al., 2004, 2002). The most significant linkage was revealed at 13q21 with an LOD score of 3.92 for individuals identified with RI (Bartlett et al., 2002). It was hypothesized that the RI group showed a reading deficit as a result of underlying language impairment (Bartlett et al., 2002). When an additional 22 American families were included in the analysis, an LOD score > 4.0 was noted at two locations on chromosome 13q (Bartlett et al., 2004). More recently, significant linkage overlapping with the identified region on 13q was mapped in an extended family with a history of verbal trait disorder (Truong et al., 2016). These studies provide support for a pedigree focused approach in the study of SLI, especially with appropriate phenotype measures.
Founder populations and consanguineous families provide a unique opportunity for family-based analysis. The founder-based population of Robinson Crusoe Island, near Chile, has a high incidence (35%) of SLI (Villanueva et al., 2011, 2008). Research participants, who lived on Robinson Crusoe Island, were comprehensively assessed with a nonverbal IQ measure, multiple language measures (including omnibus standard scores) in Spanish, and screened for hearing impairments, motor difficulties, and comorbid diagnoses (Villanueva et al., 2010, 2011). Family-wide parametric linkage analysis under autosomal dominant and recessive models of inheritance did not show significant linkage (Villanueva et al., 2011). However, nonparametric linkage analysis resulted in five significant linkage loci, including chromosome 6q, 7q, 12, 13, and 17, suggesting a polygenic pattern of SLI transmission (Villanueva et al., 2011). Similarly, linkage mapping and homozygosity mapping of extended consanguineous families, with language deficits, ascertained from Pakistan and phenotyped with a vocabulary measure, resulted in multiple suggestive loci: chromosome 2q, 5p, 8q, 14q, 17q, and 22q, suggesting locus heterogeneity (Andres et al., 2019).
Another approach considered regions previously linked to the phenotypically related impairment of reading disorder (RD). Previous behavioral investigations support a hypothesized relationship between SLI and RD, such that early language impairments are predicative of later reading impairments (Adlof & Hogan, 2019; Catts et al., 2006, 2005; Snowling et al., 2000). An earlier genetics study of SLI using a cohort of individuals and family members with SLI (N = 322), compiled at the University of Kansas (KU) Cohort, targeted the chromosomal loci 1p36, 3p12-q13, 6p22, and 15q21 (Rice et al., 2009). Sib-pair and family-based analyses utilized the full sample at targeted loci (Rice et al., 2009). Linkage analysis was performed using quantitative and categorical phenotypes. Nonparametric linkage analysis was performed in MERLIN for the categorical phenotypes. LOD scores above 0.6 were found for the standardized omnibus language categorical and quantitative phenotypes within the chromosome 1p36, 6p22, 7q31, and 15q21 loci (Rice et al., 2009). This targeted approach could be applied to novel populations, especially when the phenotype measures overlap. This indicates the possibility of an overlap in reading and language phenotypes, both genetically and behaviorally. Also, of note, several individuals included in the study of targeted RD loci are members of the families included in the current genome-wide linkage mapping study.
The power of family-based methods of genetic studies of SLI is supported by early linkage studies and more recent studies utilizing unique populations and exome sequencing (Andres et al., 2019; Rakhlin et al., 2013; Truong et al., 2016; Villanueva et al., 2011, 2015; Wiszniewski et al., 2013). Such studies have provided strong evidence for candidate genes including TM4SF20, NFXL1, and SETBP1 (Rakhlin et al., 2013; Villanueva et al., 2015; Wiszniewski et al., 2013). One study of note is the family-based Robinson Crusoe Island investigation of a founder population, which underscores the need for further family-based study (Mountford et al., 2019; Villanueva et al., 2011, 2015). Genetic studies in the founder population identified a protein coding change in NFXL1 with a higher frequency in individuals from the population with language impairment (Villanueva et al., 2015). Upon sequencing of NFXL1 in SLIC probands, three more protein coding variations were identified in four additional SLI cases (Villanueva et al., 2015). NFXL1 only explains a fraction of SLI cases, although with large effect (Villanueva et al., 2015). The combination of initial behavioral investigation (indicating a high rate of language impairment in the population), family-based linkage, and homozygosity mapping ultimately contributed to the utility of next-generation sequencing (NGS) and convincing exome sequencing results (Villanueva et al., 2011).
Across the reported linkage regions, many candidate genes have been identified and these studies indicate that more genes are involved in SLI than previously expected (Chen et al., 2017; Mountford & Newbury, 2018; Mountford et al., 2019). The current list of language impairment candidate genes is extensive, although across studies and reviews about 30 candidates are more consistently and recently discussed (Chen et al., 2017; Mountford & Newbury, 2018; Mountford et al., 2019). These reports are crucial for further investigation to acknowledge in analyses and reporting.
Our study uses the logic of the previous investigations to perform familywise genetic analyses of our sample. The goal of our study is to map loci, which can be used to identify candidate genes and rare variations of large effect associated with SLI in our sample and across previous and future studies in the field. The current study analyzed probands with SLI and their family members, assessed longitudinally (e.g., Rice & Hoffman, 2015). The rate of SLI in the family members (of the KU cohort) is significantly higher than the rate of SLI and related phenotypes in members of control probands (Rice et al., 1998). The elevated rate of SLI in family members is reflected in the six families investigated in the current study. We used traditional family-based LOD score methods to map genetic loci to performance on age-appropriate standardized omnibus language assessments. The rationale for this approach is to reduce trait variance within complex phenotypes at the behavioral level and capture the genetic transmission.
Method
Participants and Ethics Approval
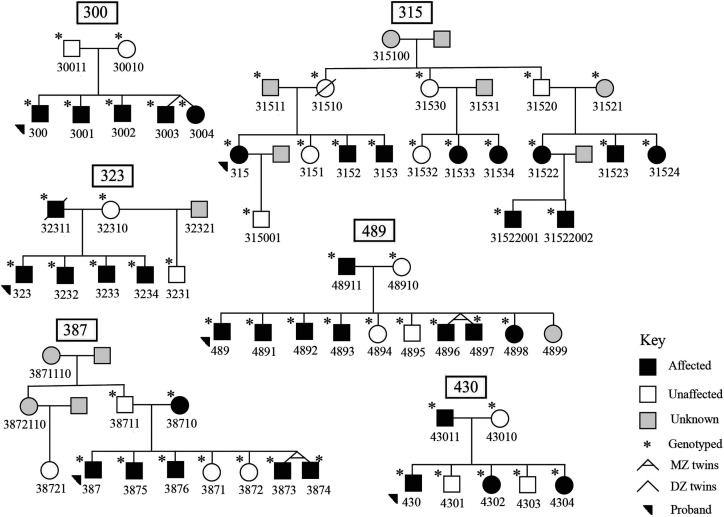
The families included in this study are a part of a larger ongoing longitudinal study in Dr. Mabel Rice's Language Acquisition Studies Lab at the KU. Most of the families were recruited from school speech pathology caseloads, except Family 315, who self-referred to the study. The institutional review board at the KU approved the study (Institutional Review Board No. 8223) and we acquired appropriate informed consent from all participants. Parents provided consent for all participants under 18 years of age. In total, 60 participants make up the six families, including six probands, 12 parents, 28 siblings, and 14 other relatives; 38 individuals are categorized as affected (see Figure 1). Affection status was determined based on standard scores from age-appropriate standardized omnibus language measures (described in detail below). As noted in the opening review of precedents in the literature, omnibus standard scores provide the same psychometric index across a wide age range, which is needed in family studies, and these measures have shown sensitivity to genetic effects in previous studies. The families in the current analysis were chosen based on their size, the high proportion of affected individuals, and availability of extended family members. Twin pairs are present in three families, one dizygotic (Family 300) and two monozygotic twin pairs (Families 387 and 489; see Figure 1). All six families are Caucasian.
Figure 1.
Six pedigrees used for linkage mapping. Family 315 Branch 1 (proband branch) includes descendants of 31510 and 31511, Family 315 Branch 2 includes descendants of 31520 and 31521, and Family 315 Branch 3 includes descendants of 31530 and 31531.
Family 315 is the largest in the study; 18 individuals were available for single nucleotide polymorphism (SNP) genotyping and behavioral data was collected from 16 individuals. Genetic and language data were collected in Family 315 from three nuclear families (branches) descending from 315100. Branch 1 refers to the proband branch (descendants of 31510 and 31511), Branch 2 refers to the descendants of 31520 and 31521, and Branch 3 refers to the descendants of 31530 and 31531. Importantly, since the completion of genotyping in this study, additional behavioral information was collected in family members of Branch 2, including that one individual has a diagnosis of a rare syndromic condition, which presents with multiple severe phenotypes (not shown in Figure 1). This individual was not included in the present analysis, as their genetic and behavioral data were not available at the time of the genotyping. We note the existence of the rare condition in a young member of the family as of possible import for interpretation of possible outcomes, which we return to in the Discussion section.
Phenotype Measures
A full list of the phenotype measures collected as part of the ongoing longitudinal study of families in Rice's lab is described by Rice et al. in an earlier publication (Rice et al., 2009). Probands entered the study as children affected with SLI. The exclusionary criteria were met by four entrance screening criteria: no cognitive impairment (average or above average nonverbal IQ), no hearing loss, no other diagnoses of developmental delay, neurological disorder or autism at the initial time of assessment (based on parent report), and intelligible speech. There were no probands with childhood apraxia of speech (CAS; https://www.asha.org/public/speech/disorders/childhood-apraxia-of-speech/), an uncommon motor speech disorder that makes it hard to speak, nor are there any in the full sample of participants. In addition, probands were monolingual native speakers of English and screened for nonstandard dialects. Family members (parents and siblings) of probands were also monolingual native speakers of English, without diagnosis of neurological conditions related to language, without hearing loss, and ≤ 55 years of age. Family members were not screened/exempt from participation for cognitive impairments or other developmental delays or autism at the initial time of assessment (based on parent report). Intelligible speech was required for validity of some of the phenotypes. Persons with hearing loss were not assessed/included in the study because the phenotype measures are not valid estimates of their language abilities.
Speech articulation was assessed with the Goldman-Fristoe Test of Articulation–Second Edition (Goldman & Fristoe, 2015). All participants were also assessed with an age-appropriate standardized nonverbal IQ assessment: Columbia Mental Maturity Scale for children between age 3;6 and 6;11 (years;months), Wechsler Intelligence Test for Children–Third Edition for ages 7 years through 16 years, and the Wechsler Intelligence Test for Adults–Third Edition for ages 17 years and older (Burgemeister et al., 1972; Wechsler, 1991, 1997). Participants recruited as children (i.e., probands, siblings, and young extended family members) were followed longitudinally, with annual administration of standardized measures. Parents, aunts, uncles, and other older extended family members were tested 1 time.
Omnibus Language Measure
The omnibus language scores were obtained from a standardized language measure appropriate for the participant's age. Standardized omnibus language measures are designed to assess multiple dimensions of language, and age-standardized versions have been developed across a wide age range, following psychometric standards for reliability and validity. Although they do not measure all possible aspects of language acquisition, they are generally robust for broadly defined semantic and syntactic dimensions of language, across expressive versus receptive task demands. The omnibus language measures were chosen based on their high levels of reliability and validity, which is verified by monitoring of test–retest reliability across times of measurement in this longitudinal study. We note any potential psychometric weaknesses work against our hypotheses of genetic risk in the families.
The current study began following participants longitudinally in 1993 using an accelerated longitudinal design in which multiple age cohorts are sampled, such that children vary continuously in age at first assessment. The measures and their versions have remained the same throughout the course of the study in order to avoid confounds related to different items sets and different norming samples. It is essential to minimize this type of possible measurement error when comparing performance over time both within and across individuals. Training in the administration of the tasks includes reading the manuals, practice administration with adult trainers in the lab, observed administration with practice children in the lab, and followed by observed administration in the field with actual participants before being authorized to administer assessments independently. Routine intermittent live observations of actual data collection are conducted for monitoring reliability and validity of task administration, supplemented by viewing of video recordings of each session. Scoring of outcomes is also monitored and checked at various levels of processing, involving the examiners and other team members, on an ongoing basis. The measures were versions of two tests available at the outset of the study: for ages 4–6;11, the TOLD-2–Primary Spoken Language Standard Score (Newcomer & Hammill, 1988), and for 7 years of age through adulthood, the CELF-3 (Semel et al., 1995). Age norms on the CELF-3 stop at age 21;11. Our age range for this measure is 6.03–43.43 years. Thus, standard language scores for older adults in this study were based on norms for age 21;11, based on the assumption that language skills do not vary much per person, relative to the outcomes at 22 years, for the tasks included in the CELF-3.
We explored this expectation in our database, which includes participants with longitudinal assessments from childhood into adulthood. The sample was annually assessed beyond the ceiling age level. They entered as SLI (N = 18) or Controls (N = 10). The oldest time of sequential annual assessments was for one participant in the SLI group, a total of 13 assessments with the final at 33.69 years; for the Control group, the oldest was 30.11, a total of 12 assessments. For a comparison between “early adulthood” and “adulthood,” we calculated average omnibus standard scores per person in the age range 18–21 (“early adulthood”) and 22+ years (“adulthood”). For the SLI group, the difference between these averages ranges from 2 to 6 standard score points (within standard error of measurement) for all but one participant who had a traumatic experience that coincided with two lower scores that bounced back into the usual level in subsequent assessments. For the Control group, the range in differences for the “early” average versus the “later” average is 2–7, again within standard error of measurement, with one exceptional case of a period of three consecutive lower-than-average scores that later resolved to the previous levels. Overall, in our data, the general pattern of outcomes met our assumption that standard scores on the CELF-3 were unlikely to increase with age. Furthermore, we note that if language skills do improve in adulthood, then the use of younger age norms would inflate the adult standard scores, resulting in a lower likelihood of affection status, which would work against the study hypotheses. If such an inflation did occur, it is very small or nonexistent, as the average omnibus language standard score among proband parents in this study is 88.55 (SD = 15.16). Finally, recent analysis in this sample shows high test–retest reliability, with correlations between occasions of measurement ranging from r = .715 (p < .0001) to r = .975 (p < .0001), offering further support for stability of measurement over time.
Individuals with a standard score ≤ 85 on an age-appropriate standardized omnibus language measure were categorized as affected. Following precedents in previous longitudinal studies for assigning affectedness status, individuals were classified as affected based on their lowest standardized omnibus language score across all occasions of measurement. This method is consistent with those used in the SLIC studies where past or current language performance was used to identify probands (Falcaro et al., 2008). Importantly, the lowest performance estimate also captures the affectedness status of siblings. Individuals who never scored in the affected range across times of measurement (i.e., standard score > 85) were classified as unaffected, and those who did not complete a standardized omnibus language measure were assigned an unknown status. The age range for participants was 4.01–43.43 years; standard score range was 50–118 (lowest is a proband, and highest is a parent). Overall, on the phenotype level, the individuals seemed to share the common description of SLI. Note that the probands were recruited with exclusionary criteria to rule out possible confounding conditions. Siblings and parents could be affected as SLI, or with developmental disorders including autism. One sibling with autism is included among the participants in this study. The incidence of other conditions, such as autism or other developmental disorders, in our full database seems to align with population estimates. In this study, the family members, including the probands, were generally undetectable in a general population. Recent studies document that children with SLI are unlikely to be detected by their classroom teachers (Chrisopulos & Kean, 2020). Parents often comment that, if they scored low on testing (results are provided upon request, but few make the request), they were just like their child when they (a parent) were little (indicating one of the children who scored as affected, although that information was known only when parents asked). Almost all the parents were employed; some had advanced degrees, and others had high school degrees; employment included high-level engineering design, management positions, private businesses, and service positions.
Genetic Analyses
DNA Collection and Preparation
Saliva was collected from participants at the time of initial or follow-up behavioral assessment. Saliva samples/buccal swabs were collected using the Oragene-Discover OGR-500 or OGR-575 Kits (DNA Genotek, Oragene). DNA was purified from the saliva samples using a standardized protocol with the Oragene prepIT⸰L2P kit. Extracted DNA was resuspended and stored in 1x Tris-EDTA (1X TE) buffer at −80°C for long-term storage.
Linkage Simulation
To obtain estimates of linkage, we performed familywise 2-point parametric linkage simulations using the FastSLink package in easyLINKAGE-Plus (Hoffmann & Lindner, 2005). Linkage simulation was tested in each family under dominant and recessive modes of inheritance with complete penetrance, consistent with our planned linkage analyses. Simulation used 1,000 replicates of one marker at a time, across the autosomal genome, with four equally frequent alleles and a 0% phenocopy rate with a 0.05 increment of recombination fraction. The primary goal of the linkage simulation was to determine the familywise maximum estimated LOD (ELOD) scores across the whole genome.
Suggestive and significant LOD score thresholds for complex traits have been proposed based on previous linkage simulations (Lander & Kruglyak, 1995). Proposed suggestive LOD score thresholds range between 1.9 and 2.4, while significant LOD score thresholds range between 3.3 and 3.8, depending on the family relationships tested (Lander & Kruglyak, 1995). In the current study, suggestive regions were defined around SNPs with calculated LOD scores greater than 1.9, consistent with suggestive linkage thresholds. Additional regions were defined around SNPs with calculated LOD scores greater than 1.2, in the case that they overlapped with regions previously linked to SLI or related phenotypes (see Supplemental Table S1). Related phenotypes included in the search were performance on NWR tests, autism spectrum disorders (ASDs), CAS, and RD (see Supplemental Table S1). The boundaries for all reported regions were determined based on the segment in which the majority of the markers surrounding the highest LOD score showed a positive LOD score.
SNP Genotyping
SNP genotyping was performed on the Illumina Infinium QC Array-24 that has 15,949 SNP markers equally spaced throughout the genome (https://www.illumina.com/products/by-type/microarray-kits/infinium-qc.html). All DNA samples were diluted with 1X TE buffer to 50-ng/ul concentration, and 200 ng of DNA was used for SNP genotyping. Genome-wide SNP genotyping was outsourced to the Genetic Resources Core Facility at Johns Hopkins University School of Medicine. Genotype data were consistent with reported family relationships in all individuals (call rate above 99.8%). The array has 12,032 SNPs distributed across the autosomal chromosomes, though 38 were located within pseudo-autosomal regions. Additionally, 3,917 SNPs were located on the sex chromosomes (X chromosome = 1,840 markers; Y chromosome = 1,401 markers; XY chromosome = 535 markers) and mitochondrial DNA (141 markers).
Parametric Linkage Analysis
Whole genome parametric linkage analysis was used to identify candidate chromosomal loci in the six families. Linkage analysis was performed using Superlink-Online SNP 1.1, a publicly available genetic linkage analysis program at http://cbl-hapw.cs.technion.ac.il/superlink-snp/ (Silberstein et al., 2012). Superlink-Online SNP 1.1 has a graphical interphase, allowing it to handle large pedigrees and thousands of SNP markers for single-point linkage analysis.
Linkage analysis was performed in each family individually using autosomal dominant and recessive inheritance models with variable disease penetrance. Previous studies recommended using reduced disease penetrance levels when working with complex phenotypes (Hodge et al., 1997). For each inheritance model, we performed analysis at two disease penetrance levels, complete (99%) and reduced (70%; see Table 1). A rare disease allele frequency of 0.001 with a 0% phenocopy rate was used in all analyses.
Table 1.
Penetrance levels used for analyses.
| Inheritance pattern | Disease penetrance | WT/WT homozygous | WT/MUT heterozygous | MUT/MUT homozygous |
|---|---|---|---|---|
| Dominant | Complete | 0.00 | 0.99 | 0.99 |
| Reduced | 0.00 | 0.70 | 0.70 | |
| Recessive | Complete | 0.00 | 0.00 | 0.99 |
| Reduced | 0.00 | 0.00 | 0.70 |
Note. WT = wild type; MUT = mutant.
Out of 15,949 SNPs, a total of 13,834 SNPs (autosomal and X chromosome) were used in the Superlink-Online SNP 1.1 analysis. Superlink-Online SNP 1.1 has a cleaning tool, which removes markers that are uninformative or erroneous for various reasons (Silberstein et al., 2012). In total, the cleaning tool removed 3,983 markers (includes 1,773 X chromosome markers) across the six families, leaving 9,851 informative markers for linkage analyses.
The linkage program MERLIN was used to draw haplotypes in Family 315 (Abecasis et al., 2002). Given the family history of rare syndromic conditions in one part of Family 315 (Branch 2), additional analyses were performed, including analysis of the individual branches and analysis removing one branch at a time. Loss of heterozygosity analysis was performed in Family 315 using Homozygosity Mapper (http://www.homozygositymapper.org; Seelow et al., 2009).
Results
Six probands with SLI and their family members were included in genome-wide parametric linkage analysis, and the highest LOD scores were compared with the maximum ELODs (see Table 2). In alignment with the simulated results, we report suggestive evidence of linkage to three independent loci in three families (300, 315, 489; see Table 3), while evidence of suggestive or significant linkage was not identified in the other three families (323, 387, and 430; see Figure 1 and Supplemental Figure S1). In Family 387, under the recessive model of inheritance, two markers spanning chrX:89712929-90955058 showed single-point LOD scores equal to the maximum ELOD (1.81; see Table 2). This agrees with visual inspection of the mode of inheritance in Family 387, which shows the possibility of sex-linked recessive inheritance (see Figure 1).
Table 2.
Maximum estimated LOD scores in all families.
| Family | Inheritance pattern |
|
|---|---|---|
| Dominant | Recessive | |
| 300 | NA | 2.41 |
| 315 | 4.09 | 4.63 |
| 323 | 0.87 | 0.90 |
| 387 | 1.81 | 1.81 |
| 430 | 1.20 | 1.20 |
| 489 | 2.41 | 2.41 |
Note. We did not perform simulation under the dominant model of inheritance in Family 300 because both parents are unaffected for the omnibus phenotype while all children are affected. NA = not available.
Table 3.
Suggestive specific language impairment loci (LOD score > 2.2).
| Family | Locus | hg19 position (Mbp) | rsIDs | Single-point LOD scores |
No. of genes | |
|---|---|---|---|---|---|---|
| Dominant inheritance | Recessive inheritance | |||||
| 300 | 4q31.3–q34.3 | 154.88–179.98 | rs1878449–rs12499500 | 1.20 | 2.41 a | 106 |
| 315 | 15q23–q25.2 | 68.68–83.22 | rs1075991–rs1267657 | 1.46 | 3.06 | 196 |
| 489 | 14q11.2–q13.3 | 20.83–37.06 | rs2700–rs1955739 | 1.50 | 2.40 a | 216 |
Note. Mbp = megabases.
LOD scores that reached the maximum estimated LOD.
The highest calculated single-point LOD score was greater than 1.9 in the reported suggestive loci and matched the maximum ELOD in Family 300 and 489 (see Table 3). The highest single-point and multipoint LOD score of 2.4 was obtained in Family 300 to 4q (154.9-180.0 Mbp) and in Family 489 to 14q (20.82–37.06 Mbp), under the recessive mode of inheritance (see Table 3). However, in Family 315, the highest single-point LOD score of 3.06 to rs11072823 at 15q (79.29 Mbp) was less than the maximum ELOD score (see Table 3). Genes within the suggestive regions are listed in Supplemental Table S2 (4q), Supplemental Table S3 (15q), and Supplemental Table S4 (14q). To provide additional context for the suggestive regions, the background linkage and the families with no significant linkage, the genome-wide linkage results for all six families are presented in Supplemental Figure S1. There were 10 additional markers in Family 315 with LOD scores greater than 2.0 (see Supplemental Figure S1 and Supplemental Table S5). However, the markers, in the vicinity of the highest scoring markers, showed negative LOD scores (see Supplemental Figure S1).
The reduced single-point LOD score (compared to the maximum ELOD) in Family 315 led us to perform branch-wise linkage analysis, especially considering the presence of a rare syndromic condition (details of diagnosis not available) in Branch 2. We performed linkage analysis separately in Branch 1, Branch 2, and Branch 3 and all possible combinations of these branches (see Figure 1 and Supplemental Figure S2). The highest single-point LOD scores of 1.3 and 0.55 were obtained in Branches 1 and 3, respectively, to chromosome 15q24.3-25 linkage region (see Table 4). The highest single-point LOD score of 2.26 revealed to rs11072823 when linkage analysis was performed in Branches 1 and 3 (keeping the ancestral relationship intact) and the highest multipoint LOD score increased to 2.65 at rs11072823 (see Table 5). Although the highest LOD scores are suggestive, the branch-wise analysis provides further support for linkage at 15q to SLI (see Supplemental Figure S2).
Table 4.
Single-point LOD scores (15q region) in Family 315, branches, and possible combinations.
| rsID | hg19 position (Mbp) | Family 315 | Branch 1 | Branch 2 | Branch 3 | Branches 1 and 2 | Branches 1 and 3 | Branches 2 and 3 |
|---|---|---|---|---|---|---|---|---|
| rs1075991 | 68.68 | −5.15 | −0.67 | −2.27 | 0.13 | −3.08 | −0.47 | −2.32 |
| rs896999 | 69.10 | 0.36 | 0.00 | 0.12 | 0.03 | 0.00 | 0.15 | 0.28 |
| rs748681 | 69.20 | −2.97 | 1.33 | −4.40 | 0.04 | 0.59 | 1.74 | −4.45 |
| rs2415047 | 69.89 | −1.32 | 0.72 | −2.27 | 0.03 | −0.95 | 0.88 | −2.12 |
| rs8035183 | 70.33 | −3.90 | 0.43 | −4.40 | 0.13 | 0.28 | 0.63 | −4.36 |
| rs2001597 | 70.42 | −3.31 | 1.33 | −5.10 | 0.04 | −0.76 | 1.74 | −4.94 |
| rs652937 | 71.47 | 0.63 | 0.00 | 0.00 | 0.42 | 0.18 | 0.55 | 0.55 |
| rs11632479 | 72.01 | 0.36 | 0.00 | 0.12 | 0.03 | 0.00 | 0.15 | 0.28 |
| rs12594531 | 72.07 | −3.44 | 0.72 | −4.40 | 0.03 | 0.88 | 0.88 | −4.24 |
| rs2957740 | 72.31 | −1.74 | 0.72 | −2.70 | 0.03 | 0.89 | 0.88 | −2.55 |
| rs1374092 | 72.81 | 0.36 | 0.00 | 0.12 | 0.03 | 0.00 | 0.15 | 0.28 |
| rs11632630 | 73.25 | −1.39 | 0.72 | −2.27 | 0.04 | −1.81 | 0.84 | −2.12 |
| rs12903941 | 73.55 | 0.36 | 0.00 | 0.12 | 0.03 | 0.00 | 0.15 | 0.28 |
| rs17773784 | 74.08 | −2.17 | 0.00 | −2.40 | 0.03 | −1.23 | 0.15 | −2.25 |
| rs896588 | 74.11 | −6.06 | 0.43 | −7.10 | 0.55 | 0.28 | 1.36 | −6.64 |
| rs1823718 | 74.15 | −5.67 | 1.33 | −7.10 | 0.04 | 0.59 | 1.74 | −7.15 |
| rs1484214 | 74.58 | −1.60 | 0.43 | −2.27 | 0.13 | −1.69 | 0.63 | −2.32 |
| rs741761 | 74.70 | −3.77 | 0.00 | −4.40 | 0.42 | −0.01 | 0.55 | −3.85 |
| rs2075590 | 74.71 | −2.17 | 0.00 | −2.40 | 0.03 | −1.23 | 0.15 | −2.25 |
| rs2075589 | 74.71 | −2.17 | 0.00 | −2.40 | 0.03 | −1.23 | 0.15 | −2.25 |
| rs11857558 | 74.71 | 0.36 | 0.00 | 0.12 | 0.03 | 0.00 | 0.15 | 0.28 |
| rs11852760 | 74.72 | −1.65 | 0.00 | −2.27 | 0.42 | −1.40 | 0.55 | −1.73 |
| rs1992145 | 74.72 | −1.65 | 0.00 | −2.27 | 0.42 | −1.40 | 0.55 | −1.73 |
| rs12898794 | 74.88 | −4.87 | 0.00 | −5.10 | 0.03 | −1.40 | 0.15 | −4.95 |
| rs41279188 | 75.01 | −4.04 | 0.00 | −4.27 | 0.03 | 0.59 | 0.15 | −4.12 |
| rs762551 | 75.04 | 0.63 | 0.00 | 0.00 | 0.42 | 0.18 | 0.55 | 0.55 |
| rs2470890 | 75.05 | 0.63 | 0.00 | 0.00 | 0.42 | 0.18 | 0.55 | 0.55 |
| rs12050778 | 76.13 | −4.21 | 0.43 | −5.10 | 0.04 | −1.89 | 0.84 | −4.94 |
| rs2955736 | 76.38 | −1.77 | 0.00 | −2.40 | 0.42 | −1.23 | 0.55 | −1.85 |
| rs744336 | 76.67 | −1.77 | 0.00 | −2.40 | 0.42 | −2.36 | 0.55 | −1.85 |
| rs11636648 | 77.34 | −3.32 | 0.72 | −4.27 | 0.03 | 1.48 | 0.88 | −4.12 |
| rs11639314 | 77.54 | −4.04 | 0.00 | −4.27 | 0.03 | 0.59 | 0.15 | −4.12 |
| rs74025333 | 77.93 | −2.17 | 0.00 | −2.40 | 0.03 | −1.23 | 0.15 | −2.25 |
| rs4243047 | 77.98 | 0.63 | 0.00 | 0.00 | 0.42 | −0.01 | 0.55 | 0.55 |
| rs1519819 | 78.54 | 0.93 | 0.00 | 0.60 | 0.42 | 0.17 | 0.55 | 0.85 |
| rs965604 | 78.79 | 0.36 | 0.00 | 0.12 | 0.03 | 0.00 | 0.15 | 0.28 |
| rs11072823 | 79.29 | 3.06 | 1.33 | 0.73 | 0.55 | 0.92 | 2.26 | 1.35 |
| rs1402760 | 79.44 | −6.14 | 0.72 | −7.10 | 0.03 | 0.88 | 0.88 | −6.94 |
| rs4778752 | 80.40 | −2.47 | 0.00 | −2.70 | 0.03 | −2.36 | 0.15 | −2.55 |
| rs2034247 | 80.61 | 0.63 | 0.00 | 0.00 | 0.42 | 0.46 | 0.55 | 0.55 |
| rs11072930 | 80.91 | −1.81 | 0.43 | −2.70 | 0.04 | −0.01 | 0.84 | −2.54 |
| rs1553650 | 81.11 | 1.57 | 1.33 | 0.00 | 0.42 | 0.59 | 1.53 | −0.05 |
| rs4075641 | 81.78 | 1.08 | 0.72 | 0.12 | 0.03 | 0.89 | 0.88 | 0.28 |
| rs1567897 | 82.02 | −6.87 | 0.00 | −7.10 | 0.03 | −2.40 | 0.15 | −6.94 |
| rs12916134 | 82.19 | −3.05 | 0.72 | −4.40 | 0.42 | −1.33 | 1.27 | −3.85 |
| rs1267657 | 83.22 | −5.35 | 0.43 | 0.12 | −4.70 | −0.01 | −4.51 | −4.58 |
Note. The parameters were set for recessive inheritance, omnibus language affected, full penetrance, and a disease frequency of 0.001. The SNP in bold has the highest single-point LOD score when linkage analysis included all members of Family 315. Mbp = megabases.
Table 5.
Highest multipoint LOD scores within chromosome 15q24.3-q25 in Family 315 branch analysis.
| Analysis | Branches included | Multipoint LOD score |
|---|---|---|
| Individual branches | Branch 1 | 1.33 |
| Branch 2 | −7.5 | |
| Branch 3 | 0.73 | |
| One branch removed; other relationships intact | Branch 1 and 2 | 0.29 |
| Branches 1 and 3 | 2.65 | |
| Branches 2 and 3 | −7.12 |
Note. The parameters were set for recessive inheritance, omnibus language affected, full penetrance, and a disease frequency of 0.001.
Discussion
In this study, we performed a parametric genome-wide scan in SLI families with multiple omnibus affected individuals. Our study suggested new linkage loci and confirmed previously reported loci in SLI, suggesting locus heterogeneity. We reported three independent linkage loci in three families that reach the likelihood of suggestive linkage (see Table 3). Though these results are suggestive, all three regions are uniquely important in their contribution to explaining transmission of SLI. In Family 300, a large suggestive region was identified under recessive inheritance and the family's inheritance pattern of performance on the omnibus measure shows a clear recessive pattern. In Family 489, the region identified includes a previously reported gene showing a paternal parent-of-origin effect, and the gene is involved in mechanisms implicated in other neurological disorders. Finally, the largest family (315) provides further support for family-based analysis and reveals a novel region of interest. In the other three families, suggestive linkage was not estimated, possibly due to family size or structure (see Figure 1).
Our findings are consistent with the conclusion that genome-wide parametric linkage analysis is an authentic and powerful method to map disease genes in disorders like SLI, dyslexia, and stuttering, given the phenotype is well characterized and mapped to multiple individuals in the family (Nopola-Hemmi et al., 2001; Raza et al., 2012 , 2013; Truong et al., 2016). The outcomes here are consistent with the use of family-based linkage mapping to provide sufficient information to identify DNA variations in genes responsible for stuttering, a speech phenotype (Raza et al., 2012 , 2013). With easy access to next NGS and its reduced cost over time, researchers adopt NGS to identify disease associated genes (Koboldt et al., 2013). We recognize that this is mostly useful and cost-effective for rare/Mendelian disorders but is less successful for non-Mendelian disorders. For complex phenotypes, like SLI, initial gene mapping information is likely to provide genomic targets to prioritize DNA variations in NGS data (Bailey-Wilson & Wilson, 2011; Hastings et al., 2016; Raza et al., 2013, 2015). The linkage data presented in this article, although suggestive, will be useful to narrow the focus for gene identification efforts through targeted/whole genome/exome sequencing.
Our study follows the precedents in the literature for phenotyping SLI using standard scores. However, previous methods differ from those used in this study in ways that may matter in sensitivity to detection of genetic effects. As noted earlier, the CELF-R (omnibus language measure) was used by the SLIC and linkage loci were reported (SLIC, 2002). Their analysis divided the CELF language phenotype into the two component subtests: Receptive and Expressive Language scores. Although the initial analyses revealed linkage with the CELF-R expressive language score on chromosome 19q, the follow-up analyses did not replicate these results (SLIC, 2002, 2004). In additional follow-up analyses by the SLIC, multivariate linkage analysis was used to simultaneously analyze multiple CELF-R subtests (Monaco & SLI Consortium, 2007). The multivariate analysis indicated the 16q locus may be involved in language, reading, and spelling phenotypes, as well as NWR performance, while the 19q locus was not strongly linked to reading and spelling (Monaco & SLI Consortium, 2007). These results indicate support for initial genetic analysis utilizing omnibus phenotypes; in that language, reading, spelling, and memory may share common causal pathways, especially given the prevalence of individuals with both language and reading difficulties. Other studies have used omnibus language measures in conjunction with additional language measures to assign affection status (Villanueva et al., 2010, 2011, 2008) or focused on performance on the NWR task, following up on the early results from the SLIC (Newbury et al., 2009; Whitehouse et al., 2011). A recent report showed high heritability of performance on omnibus language measures, in 6-year-old twins, using the TOLD-P:3, a more recent version of the TOLD measure used in this study (Newcomer & Hammill, 1997; Rice et al., 2020). In the context of previous studies, the study reported here contributes new information about omnibus language phenotypes, for the purpose of grouping participants as SLI. Further studies are needed to establish replicability of omnibus phenotypes. Replicability could be influenced due to the use of omnibus measures to establish affectedness status, as more recent research suggests. Perhaps more importantly and more likely, replicability could also be influenced by the level of standards in place for training examiners and possible differences across studies in the editions of tests carrying the same name.
Omnibus measures are also of interest as a phenotype because of the possible insight they can provide into the overlap with other related disorders that may share common causal pathways. For example, recall that early language impairments predict later reading impairments. Specifically, evidence from a longitudinal study of children with SLI shows that those with the poorest reading comprehension skills had significantly lower scores on both the Peabody Picture Vocabulary Test–Fourth Edition and the CELF in eighth grade, as compared to those who had the poorest reading decoding skills (Catts et al., 2006). These results show that while reading involves both decoding and language knowledge, underlying language deficits play a more crucial role in overall comprehension of text (Catts et al., 2006). These behavioral and genetic results combined leave an open question in terms of the potential of genetic study of related phenotypes. Three regions previously reported in RD, chromosome 1p36, 3p12, and 18p11, overlapped with regions where we observed LOD scores above 1.0 in Families 315, 387, and 430 (see Supplemental Table S1; Fisher & Francks, 2006; Fisher et al., 2002; Grigorenko et al., 2001; Hannula-Jouppi et al., 2005; Nopola-Hemmi et al., 2001; Rabin et al., 1993; Tzenova et al., 2004). This overlap also supports the fact that the participants in the current study were included in the previous targeted investigation of reported RD loci, including 1p36, 3p12-q13.
In the context of the many candidate SLI regions and genes already reported, only one candidate gene NOP9 overlapped with our three reported suggestive regions, though other regions with LOD scores greater than 1.2 in four families (315, 387, 430, and 489) are also noted if they coincided with previously reported regions or candidate genes associated with SLI, RD, NWR performance, ASD, and CAS (see Supplemental Table S1; Chen et al., 2017; Mountford & Newbury, 2018; Mountford et al., 2019). A few candidate genes of note overlapped with the regions showing markers with LOD scores above 1.2 including ROBO1, ROBO2, and FOXP1 (see Supplemental Table S1; Chen et al., 2017; Mountford & Newbury, 2018; Mountford et al., 2019). The lack of overlap of the reported region and some of the most promising candidate genes, like TM4SF20, NFXL1, and SETBP1, is not surprising given that these were identified in unique populations or appear to be linked to language in a specific race, in the case of TM4SF20 (Rakhlin et al., 2013; Villanueva et al., 2011; Wiszniewski et al., 2013). The previous results emphasized focused investigations of well-characterized samples with similar genetic backgrounds in the identification of causative genes. Our sample and procedures are consistent with these criteria, supporting the utility of the current foundational study.
Other challenges for gene discovery are at the level of variations in the size of gene regions of interest. The linkage region (~25 MB) in Family 300 is large, which could be due to a small family size. However, this could be a new SLI locus to be considered in the future with a larger data set. This locus may be a useful target for genome sequencing in this family showing a recessive inheritance patterns for language phenotypes. Additionally, some SNPs at 4q with the LOD score above 1.2 were observed in Families 387 and 430, indicating replication, although with less significance (see Supplemental Table S1). This overlap could help to narrow the region of interest in follow-up investigation of exome variations.
Another feature of interest with Family 300 is that previous reports overlapping with the 4q region include a case study of ASD in which a deletion was identified on 4q (Ramanathan et al., 2004). The one child with ASD in our sample is in Family 300. Therefore, we conducted follow-up analysis excluding the child with ASD, revealing that the LOD score on chromosome 4q decreased to 1.8, while LOD scores in two other regions increased to 1.8 (chr7p14.3-p22.2 and chr22q11.21-q12.3). Notably, the chromosome 7p and 22q regions did not contain any markers with negative LOD scores. The chromosome 22q region overlaps with two previous reports (Gialluisi et al., 2014; Landi et al., 2013). One end of the 22q region overlaps with an SNP in RBFOX2 gene (RNA binding Fox-1 homolog 2) associated with language and reading phenotypes (Gialluisi et al., 2014). The other end of the boundary overlaps with the gene COMT, in which a variation has been associated with better readers (Landi et al., 2013). Although these regions are consistent with the previous reports, the linkage findings in Family 300 are not definitive, warranting further investigation.
The overlapping report of the suggestive region we report on chromosome 14 in Family 489 (14q11.2-q13.3) is especially noteworthy. A paternal parent-of-origin effect was identified previously at chromosome 14q12, specifically a nonsynonymous variant in the NOP9 gene (nucleolar protein 9; Nudel et al., 2014; Pettigrew et al., 2016). Note that, in Family 489, the father is the affected parent (see Figure 1). However, we did not see an indication of paternal parent-of-origin effect based on our analysis of the pattern of inheritance in this region. The SNP closest to the NOP9 gene available on our array is rs1957528, and its LOD score is equal to the highest observed LOD score of 2.4 in Family 489 (see Table 2). The NOP9 protein is linked to RNA-binding proteins, which have been associated with multiple neurological disorders (Kapeli & Yeo, 2012; Nudel et al., 2014). NOP9 encodes a nucleolar protein that is involved in processing ribosomal RNA. Cells depleted with NOP9 were unable to process the mature ribosomal RNA, which is an important component of ribosome assembly (Thomson et al., 2007). Efficient ribosomal assembly is crucial for protein synthesis and is involved in chromatin organization. There is growing evidence that dysregulated ribosomal biogenesis results in neurodevelopmental disorders like autism, intellectual disability, and progressive neurodegenerative disorders (Hetman & Slomnicki, 2019). It is also worth mentioning that the 14q region overlaps with our previously reported homozygosity region in consanguineous families with language deficits, from 14q12-q32.2 (Andres et al., 2019). Other genes within the 14q region may also be considered strong candidates because this region reported in multiple studies previously.
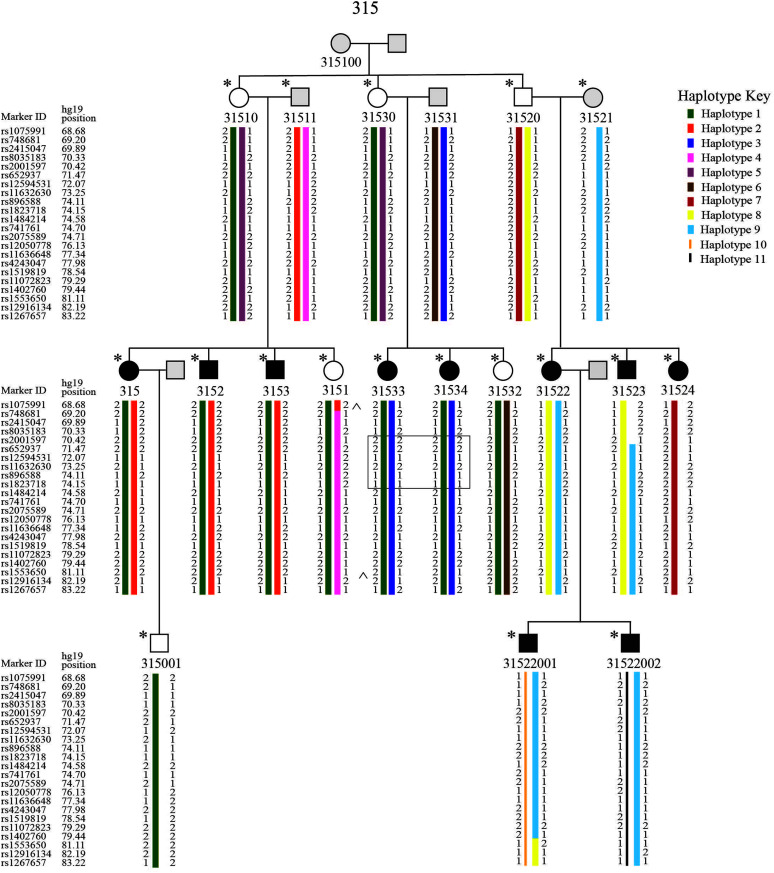
We noted in the introduction that one of the advantages of pedigree analysis is that it can reduce trait variance of complex phenotypes at the behavioral level in order to better capture the genetic factors involved in the transmission of the trait (Neale & Cardon, 2013). Yet, additional factors must be considered, at more detailed levels of analysis. For example, inclusion of additional extended family members may introduce phenotypic and genotypic variance that must be parsed out. In the case of Family 315, the largest family in the study, additional branch-wise and branch combination analysis further supported and narrowed the region of interest. The branch-wise analysis revealed that Branch 2 contributed to the reduced calculated LOD score in Family 315 compared to the ELOD. This could mean the language phenotype within Branch 2 varies more meaningfully in comparison to the members of the other branches. To understand how the alleles are inherited to the 15q locus in Family 315, we computed haplotypes, using MERLIN and Haplopainter (see Figure 2; Abecasis et al., 2002; Thiele & Nürnberg, 2004). Two cross-over events delimit the 14.54-Mb linkage region in Branches 1 and 3 (see ^ on Figure 2). Two haplotypes (Haplotypes 1 and 2) cosegregate in Branch 1 and a similar pattern observed in Branch 3 but with Haplotypes 1 and 3 (see Figure 2). We observed that an affected individual, 31524, in Branch 2, inherited Haplotype 1, but this haplotype and Haplotype 2 or 3 were not identified in other affected individuals of Branch 2, indicating that there might be another locus segregating in this branch of the family. Haplotype 1 is the ancestral haplotype shared among all affected in Branches 1 and 3. Haplotypes 2 and 3 are inherited from the married-in individuals and co-occur with Haplotype 1 in the affected members of Branches 1 and 3, indicating that more than one haplotype segregates with the trait in Branches 1 and 3. We also noticed that Haplotype 2 and Haplotype 3 shared a small section (74.15-77.34 Mb; on Figure 2), which indicates the possibility that 31511 and 31531 are distantly related or an unknown ancestral recombination occurred. Also, affected members of Branch 1 share a ~3-Mb region of homozygosity (77.98–81.11 Mb; on Figure 2) containing the highest scoring marker. A founder effect is suggested when multiple haplotypes or copies of the same haplotype are segregated in the pedigree by an unknown distant founder (Drayna, 2005). Despite the high scoring markers under the recessive model of inheritance, Family 315 did not show loss of heterozygosity regions greater than 4 Mb, the size that is reportedly common in outbred populations (McQuillan et al., 2008). Additionally, inspection of the region shared by affected members of Branch 1 (77.98–81.11 Mb) showed one heterozygous marker and homozygosity analysis of Branches 1 and 3 (relationships intact, Branch 2 removed) did not reveal any homozygosity regions. However, homozygosity analysis of Branches 1 and 3 individually showed homozygous regions between 3.5 and 4 Mb on chromosome 15q in affected members (chr15:47686263-51204364 in Branch 1 and chr15:70421861-74147244 in Branch 3), though only the homozygous region in Branch 3 (solid outline on Figure 2) overlapped with the reported suggestive linkage region. Interestingly, the homozygous region in the affected members of Branch 3 indicates Haplotypes 1 and 3 could be part of the same ancestral chromosome as it appears in individual 31531, though the genotypes are inferred. Additionally, one homozygous region was shared by affected individuals of Branch 1 on chromosome 14q (chr14:333909916-37059525), but it was still less than 4 Mb. The linkage results on chromosome 14q in Branch 1 shows positive LOD scores, but no markers showing LOD scores greater than the highest background LOD score in Branch 1 (see Supplemental Figure S2). Unlike with the region on chromosome 15q, no overlap was observed with other branches individually or in combination, indicating the homozygous region on 14q may not be of further interest in Family 315. Our haplotype analysis suggested a possibility of a distant common ancestor, but genome-wide homozygosity analysis negates it. The segregation of more than one haplotype in Branches 1 and 3 on chromosome 15q indicates that multiple alleles at this locus may be the cause of SLI in Family 315.
Figure 2.
Haplotype assignments in Family 315 at chromosome 15q23-q25.2 (68.68–83.22 Mb). ^ indicates the cross-over events in 3151 and 31533, * indicates the genotyped individuals, and the solid outline indicates a homozygous region in Individuals 31533 and 31534.
The SNP on chromosome 15, rs11072823 that reached an LOD score of 3.06, is also of interest because it is found on RasGRF1 (Ras protein specific guanine nucleotide releasing factor). RasGRF1 is a regulator of the extracellular signal-regulated kinase signaling pathway via binding with a N-methyl-D-aspartate receptor subunit, specifically NR2B (Krapivinsky et al., 2003). RasGRF1 is directly activated by calcium and is exclusively expressed in the nervous system (Krapivinsky et al., 2003). Previous studies have disrupted the function of the RasGRF1 protein in mice and found effects on the learning and memory mechanisms controlled by the amygdala and the hippocampus (Brambilla et al., 1997; Giese et al., 2001). This could be another candidate for future investigation in Family 315 and other cases of SLI, due to the role this protein plays in learning and memory pathways.
Conclusions
Overall, our family-based genetic study provides important genomic targets suggesting genotype–phenotype associations. We provided evidence of new loci and confirmed a previously reported locus. The replication of a previously reported SLI locus in the current study illustrates the strength of traditional family-based gene mapping. Our findings are consistent with the hypothesis that there are several loci involved in the transmission of SLI. Future family-based genetic studies and investigation of the reported loci are warranted. This is the first genome-wide linkage analysis of families coming from the longitudinal study of the KU cohort, providing an important foundational step for future identification of causal genetic markers involved in the regulation of gene pathways in individuals with SLI.
Although the results are encouraging, we recognize the limitations of a family-based study design. In particular, the design assumes that major gene effects are shared even though this is a complex disorder. It is also true that the replication across families brings more confidence in potential shared genetic effects across families. It remains for future studies to provide further clarification of the exact nature of gene effects.
Ultimately, replicated findings of specific genetic pathways may be helpful in clinical counseling with the families of children with SLI. Furthermore, it could increase the likelihood of identification of affected family members. Clinical speech/language pathology services could expand to a familial approach with support for the possible social and academic consequences of SLI, as well as the identification of clinical speech and language goals from phenotypes demonstrated to be associated with genetic influence, benchmarked to age expectations.
Author Contributions
Erin M. Andres: Formal analysis (Lead), Investigation (Equal), Methodology (Equal), Writing – original draft (Equal), Writing – review & editing (Equal). Kathleen Kelsey Earnest: Data curation (Equal), Supervision (Equal), Writing – review & editing (Equal). Shelley D. Smith: Data curation (Equal), Writing – review & editing (Equal). Mabel L. Rice: Conceptualization (Equal), Funding acquisition (Lead), Project administration (Equal), Resources (Equal), Supervision (Lead), Writing – review & editing (Equal). Muhammad Hashim Raza: Conceptualization (Equal), Investigation (Equal), Methodology (Lead), Project administration (Equal), Resources (Equal), Supervision (Lead), Visualization (Lead), Writing – original draft (Equal), Writing – review & editing (Equal).
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Deafness and Other Communication Disorders awarded to M. L. R. (T32DC000052, R01DC001803) and M. H. R. (R21 DC017830). This work was also supported in part by the start-up research funds and Research Excellence Initiative-Child Language Doctoral Program funds provided to M. H. R. by the KU, Lawrence. The funders had no role in study design, collection, analysis, and interpretation of data; writing of the report; or in the decision to submit the article for publication.
We appreciate all the children and families who participated in this study. We like to thank examiners and students in the Child Language Doctoral Program for assistance in collecting behavioral data and saliva samples. We would also like to thank Silvia Paracchini and Adnan Yousaf for their valuable opinions about selecting families and data management.
Funding Statement
This work was supported by the National Institute on Deafness and Other Communication Disorders awarded to M. L. R. (T32DC000052, R01DC001803) and M. H. R. (R21 DC017830). This work was also supported in part by the start-up research funds and Research Excellence Initiative-Child Language Doctoral Program funds provided to M. H. R. by the KU, Lawrence. The funders had no role in study design, collection, analysis, and interpretation of data; writing of the report; or in the decision to submit the article for publication.
References
- Abecasis, G. R. , Cherny, S. S. , Cookson, W. O. , & Cardon, L. R. (2002). Merlin-rapid analysis of dense genetic maps using sparse gene flow trees. Nature Genetics, 30(1), 97–101. https://doi.org/10.1038/ng786 [DOI] [PubMed] [Google Scholar]
- Abreu, P. C. , Greenberg, D. A. , & Hodge, S. E. (1999). Direct power comparisons between simple LOD scores and NPL scores for linkage analysis in complex diseases. The American Journal of Human Genetics, 65(3), 847–857. https://doi.org/10.1086/302536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlof, S. M. , & Hogan, T. P. (2019). If we don't look, we won't see: Measuring language development to inform literacy instruction. Policy Insights from the Behavioral and Brain Sciences, 6(2), 210–217. https://doi.org/10.1177/2372732219839075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres, E. M. , Hafeez, H. , Yousaf, A. , Riazuddin, S. , Rice, M. L. , Basra, M. A. R. , & Raza, M. H. (2019). A genome-wide analysis in consanguineous families reveals new chromosomal loci in specific language impairment (SLI). European Journal of Human Genetics, 27(8), 1274–1285. https://doi.org/10.1038/s41431-019-0398-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Wilson, J. E. , & Wilson, A. F. (2011). Linkage analysis in the next-generation sequencing era. Human Heredity, 72(4), 228–236. https://doi.org/10.1159/000334381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett, C. W. , Flax, J. F. , Logue, M. W. , Smith, B. J. , Vieland, V. J. , Tallal, P. , & Brzustowicz, L. M. (2004). Examination of potential overlap in autism and language loci on chromosomes 2, 7, and 13 in two independent samples ascertained for specific language impairment. Human Heredity, 57(1), 10–20. https://doi.org/10.1159/000077385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett, C. W. , Flax, J. F. , Logue, M. W. , Vieland, V. J. , Bassett, A. S. , Tallal, P. , & Brzustowicz, L. M. (2002). A major susceptibility locus for specific language impairment is located on 13q21. The American Journal of Human Genetics, 71(1), 45–55. https://doi.org/10.1086/341095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, D. V. M. , Adams, C. V. , & Norbury, C. F. (2006). Distinct genetic influences on grammar and phonological short-term memory deficits: Evidence from 6-year-old twins. Genes, Brain and Behavior, 5(2), 158–169. https://doi.org/10.1111/j.1601-183X.2005.00148.x [DOI] [PubMed] [Google Scholar]
- Bishop, D. V. M. , & Hayiou-Thomas, M. E. (2008). Heritability of specific language impairment depends on diagnostic criteria. Genes, Brain and Behavior, 7(3), 365–372. https://doi.org/10.1111/j.1601-183X.2007.00360.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, D. V. M. , North, T. , & Donlan, C. (1995). Genetic basis of specific language impairment: Evidence from a twin study. Developmental Medicine & Child Neurology, 37(1), 56–71. https://doi.org/10.1111/j.1469-8749.1995.tb11932.x [DOI] [PubMed] [Google Scholar]
- Brambilla, R. , Gnesutta, N. , Minichiello, L. , White, G. , Roylance, A. J. , Herron, C. E. , Ramsey, M. , Wolfer, D. P. , Cestari, V. , & Rossi-Arnaud, C. (1997). A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature, 390(6657), 281–286. https://doi.org/10.1038/36849 [DOI] [PubMed] [Google Scholar]
- Burgemeister, B. B. , Blum, L. H. , & Lorge, I. (1972). The Columbia Mental Maturity Scale. The Psychological Corporation. [Google Scholar]
- Catts, H. W. , Adlof, S. M. , & Ellis Weismer, S. (2006). Language deficits in poor comprehenders: A case for the simple view of reading. Journal of Speech, Language, and Hearing Research, 49(2), 278–293. https://doi.org/10.1044/1092-4388(2006/023) [DOI] [PubMed] [Google Scholar]
- Catts, H. W. , Adlof, S. M. , Hogan, T. P. , & Ellis Weismer, S. (2005). Are specific language impairment and dyslexia distinct disorders? Journal of Speech, Language, and Hearing Research, 48(6), 1378–1396. https://doi.org/10.1044/1092-4388(2005/096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. S. , Reader, R. H. , Hoischen, A. , Veltman, J. A. , Simpson, N. H. , Francks, C. , Newbury, D. F. , & Fisher, S. E. (2017). Next-generation DNA sequencing identifies novel gene variants and pathways involved in specific language impairment. Scientific Reports, 7, Article 46105. https://doi.org/10.1038/srep46105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisopulos, T. T. , & Kean, J. (2020). General education teachers' contribution to the identification of children with language disorders. Perspectives of the ASHA Special Interest Groups, 5(4), 770–777. https://doi.org/10.1044/2020_persp-19-00166 [Google Scholar]
- Clerget-Darpoux, F. , & Elston, R. C. (2007). Are linkage analysis and the collection of family data dead? Prospects for family studies in the age of genome-wide association. Human Heredity, 64(2), 91–96. https://doi.org/10.1159/000101960 [DOI] [PubMed] [Google Scholar]
- Conti-Ramsden, G. , Durkin, K. , Toseeb, U. , Botting, N. , & Pickles, A. (2018). Education and employment outcomes of young adults with a history of developmental language disorder. International Journal of Language & Communication Disorders, 53(2), 237–255. https://doi.org/10.1111/1460-6984.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayna, D. (2005). Founder mutations. Scientific American, 293(4), 78–85. https://doi.org/10.1038/scientificamerican1005-78 [DOI] [PubMed] [Google Scholar]
- Falcaro, M. , Pickles, A. , Newbury, D. F. , Addis, L. , Banfield, E. , Fisher, S. E. , Monaco, A. P. , Simkin, Z. , Conti-Ramsden, G. , & SLI Consortium (2008). Genetic and phenotypic effects of phonological short-term memory and grammatical morphology in specific language impairment. Genes, Brain and Behavior, 7(4), 393–402. https://doi.org/10.1111/j.1601-183X.2007.00364.x [DOI] [PubMed] [Google Scholar]
- Fisher, S. E. , & Francks, C. (2006). Genes, cognition and dyslexia: Learning to read the genome. Trends in Cognitive Sciences, 10(6), 250–257. https://doi.org/10.1016/j.tics.2006.04.003 [DOI] [PubMed] [Google Scholar]
- Fisher, S. E. , Francks, C. , Marlow, A. J. , MacPhie, I. L. , Newbury, D. F. , Cardon, L. R. , Ishikawa-Brush, Y. , Richardson, A. J. , Talcott, J. B. , Gayan, J. , Olson, R. K. , Pennington, B. F. , Smith, S. D. , DeFries, J. C. , Stein, J. F. , & Monaco, A. P. (2002). Independent genome-wide scans identify a chromosome 18 quantitative-trait locus influencing dyslexia. Nature Genetics, 30(1), 86–91. https://doi.org/10.1038/ng792 [DOI] [PubMed] [Google Scholar]
- Gathercole, S. E. , Willis, C. S. , Baddeley, A. D. , & Emslie, H. (1994). The children's test of nonword repetition: A test of phonological working memory. Memory, 2(2), 103–127. https://doi.org/10.1080/09658219408258940 [DOI] [PubMed] [Google Scholar]
- Gialluisi, A. , Newbury, D. F. , Wilcutt, E. G. , Olson, R. K. , DeFries, J. C. , Brandler, W. M. , Pennington, B. F. , Smith, S. D. , Scerri, T. S. , Simpson, N. H. , SLI Consortium, Luciano, M. , Evans, D. M. , Bates, T. C. , Stein, J. F. , Talcott, J. B. , Monaco, A. P. , Paracchini, S. , Francks, C. , & Fisher, S. E. (2014). Genome-wide screening for DNA variants associated with reading and language traits. Genes, Brain and Behavior, 13(7), 686–701. https://doi.org/10.1111/gbb.12158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese, K. P. , Friedman, E. , Telliez, J. B. , Fedorov, N. B. , Wines, M. , Feig, L. A. , & Silva, A. J. (2001). Hippocampus-dependent learning and memory is impaired in mice lacking the Ras-guanine-nucleotide releasing factor 1 (Ras-GRF1). Neuropharmacology, 41(6), 791–800. https://doi.org/10.1016/S0028-3908(01)00096-X [DOI] [PubMed] [Google Scholar]
- Goldman, R. , & Fristoe, M. (2015). Goldman-Fristoe Test of Articulation–Third Edition. AGS. [Google Scholar]
- Greenberg, D. A. , Abreu, P. C. , & Hodge, S. E. (1998). The power to detect linkage in complex disease by means of simple LOD-score analyses. The American Journal of Human Genetics, 63(3), 870–879. https://doi.org/10.1086/301997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorenko, E. L. , Wood, F. B. , Meyer, M. S. , Pauls, J. E. D. , Hart, L. A. , & Pauls, D. L. (2001). Linkage studies suggest a possible locus for developmental dyslexia on chromosome 1p. American Journal of Medical Genetics, 105(1), 120–129. https://doi.org/10.1002/1096-8628(20010108)105:1<120::AID-AJMG1075>3.0.CO;2-T [PubMed] [Google Scholar]
- Hannula-Jouppi, K. , Kaminen-Ahola, N. , Taipale, M. , Eklund, R. , Nopola-Hemmi, J. , Kääriäinen, H. , & Kere, J. (2005). The axon guidance receptor gene ROBO1 is a candidate gene for developmental dyslexia. PLoS Genetics, 1(4), Article e50. https://doi.org/10.1371/journal.pgen.0010050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings, R. , de Villiers, C. P. , Hooper, C. , Ormondroyd, L. , Pagnamenta, A. , Lise, S. , Salatino, S. , Knight, S. J. L. , Taylor, J. C. , Thomson, K. L. , Arnold, L. , Chatziefthimiou, S. D. , Konarev, P. V. , Wilmanns, M. , Ehler, E. , Ghisleni, A. , Gautel, M. , Blair, E. , Watkins, H. , & Gehmlich, K. (2016). Combination of whole genome sequencing, linkage, and functional studies implicates a missense mutation in titin as a cause of autosomal dominant cardiomyopathy with features of left ventricular noncompaction. Circulation: Cardiovascular Genetics, 9(5), 426–435. https://doi.org/10.1161/CIRCGENETICS.116.001431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetman, M. , & Slomnicki, L. P. (2019). Ribosomal biogenesis as an emerging target of neurodevelopmental pathologies. Journal of Neurochemistry, 148(3), 325–347. https://doi.org/10.1111/jnc.14576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge, S. E. , Abreu, P. C. , & Greenberg, D. A. (1997). Magnitude of type I error when single-locus linkage analysis is maximized over models: A simulation study. American Journal of Human Genetics, 60(1), 217–227. [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, K. , & Lindner, T. H. (2005). easyLINKAGE-Plus-automated linkage analyses using large-scale SNP data. Bioinformatics, 21(17), 3565–3567. https://doi.org/10.1093/bioinformatics/bti571 [DOI] [PubMed] [Google Scholar]
- Johnson, C. J. , Beitchman, J. H. , & Brownlie, E. B. (2010). Twenty-year follow-up of children with and without speech-language impairments: Family, educational, occupational, and quality of life outcomes. American Journal of Speech-Language Pathology, 19(1), 51–65. https://doi.org/10.1044/1058-0360(2009/08-0083) [DOI] [PubMed] [Google Scholar]
- Jordan, B. (2014). Genes and non-Mendelian diseases: Dealing with complexity. Perspectives in Biology and Medicine, 57(1), 118–131. https://doi.org/10.1353/pbm.2014.0002 [DOI] [PubMed] [Google Scholar]
- Kapeli, K. , & Yeo, G. (2012). Genome-wide approaches to dissect the roles of RNA binding proteins in translational control: Implications for neurological diseases. Frontiers in Neuroscience, 6, Article 144. https://doi.org/10.3389/fnins.2012.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koboldt, D. C. , Steinberg, K. M. , Larson, D. E. , Wilson, R. K. , & Mardis, E. R. (2013). The next-generation sequencing revolution and its impact on genomics. Cell, 155(1), 27–38. https://doi.org/10.1016/j.cell.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky, G. , Krapivinsky, L. , Manasian, Y. , Ivanov, A. , Tyzio, R. , Pellegrino, C. , Ben-Ari, Y. , Clapham, D. E. , & Medina, I. (2003). The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron, 40(4), 775–784. https://doi.org/10.1016/S0896-6273(03)00645-7 [DOI] [PubMed] [Google Scholar]
- Lander, E. , & Kruglyak, L. (1995). Genetic dissection of complex traits: Guidelines for interpreting and reporting linkage results. Nature Genetics, 11(3), 241–247. https://doi.org/10.1038/ng1195-241 [DOI] [PubMed] [Google Scholar]
- Landi, N. , Frost, S. J. , Mencl, W. E. , Preston, J. L. , Jacobsen, L. K. , Lee, M. , Yrigollen, C. , Pugh, K. R. , & Grigorenko, E. L. (2013). The COMT Val/Met polymorphism is associated with reading-related skills and consistent patterns of functional neural activation. Developmental Science, 16(1), 13–23. https://doi.org/10.1111/j.1467-7687.2012.01180.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillan, R. , Leutenegger, A.-L. , Abdel-Rahman, R. , Franklin, C. S. , Pericic, M. , Barac-Lauc, L. , Smolej-Narancic, N. , Janicijevic, B. , Polasek, O. , Tenesa, A. , Macleod, A. K. , Farrington, S. M. , Rudan, P. , Hayward, C. , Vitart, V. , Rudan, I. , Wild, S. H. , Dunlop, M. G. , Wright, A. F. , … Wilson, J. F. (2008). Runs of homozygosity in European populations. The American Journal of Human Genetics, 83(3), 359–372. https://doi.org/10.1016/j.ajhg.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco, A. P. , & SLI Consortium. (2007). Multivariate linkage analysis of specific language impairment (SLI). Annals of Human Genetics, 71(5), 660–673. https://doi.org/10.1111/j.1469-1809.2007.00361.x [DOI] [PubMed] [Google Scholar]
- Mountford, H. S. , & Newbury, D. F. (2018). The genomic landscape of language: Insights into evolution. Journal of Language Evolution, 3(1), 49–58. https://doi.org/10.1093/jole/lzx019 [Google Scholar]
- Mountford, H. S. , Villanueva, P. , Fernández, M. A. , De Barbieri, Z. , Cazier, J. B. , & Newbury, D. F. (2019). Candidate gene variant effects on language disorders in Robinson Crusoe Island. Annals of Human Biology, 46(2), 109–119. https://doi.org/10.1080/03014460.2019.1622776 [DOI] [PubMed] [Google Scholar]
- National Institute on Deafness and Other Communication Disorders. (2019, October 21). Specific language impairment. Retrieved November 10, 2019, from https://www.nidcd.nih.gov/health/specific-language-impairment
- Neale, M. C. , & Cardon, L. R. (2013). Methodology for genetic studies of twins and families. Springer Science & Business Media. [Google Scholar]
- Newbury, D. F. , Winchester, L. , Addis, L. , Paracchini, S. , Buckingham, L. L. , Clark, A. , Cohen, W. , Cowie, H. , Dworzynski, K. , Everitt, A. , Goodyer, I. M. , Hennessy, E. , Kindley, A. D. , Miller, L. L. , Nasir, J. , O'Hare, A. , Shaw, D. , Simkin, Z. , Simonoff, E. , … Monaco, A. P. (2009). CMIP and ATP2C2 modulate phonological short-term memory in language impairment. The American Journal of Human Genetics, 85(2), 264–272. https://doi.org/10.1016/j.ajhg.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer, P. L. , & Hammill, D. D. (1988). Test of Language Development–Primary, Second Edition (TOLD-P:2) (Vol. 2). Pro-Ed. [Google Scholar]
- Newcomer, P. L. , & Hammill, D. D. (1997). Test of Language Development–Primary (TOLD-P:3) (Vol. 3). Pro-Ed. [Google Scholar]
- Nopola-Hemmi, J. , Myllyluoma, B. , Haltia, T. , Taipale, M. , Ollikainen, V. , Ahonen, T. , Voutilainen, A. , Kere, J. , & Widén, E. (2001). A dominant gene for developmental dyslexia on chromosome 3. Journal of Medical Genetics, 38(10), 658–664. https://doi.org/10.1136/jmg.38.10.658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury, C. F. , Gooch, D. , Wray, C. , Baird, G. , Charman, T. , Simonoff, E. , Vamvakas, G. , & Pickles, A. (2016). The impact of nonverbal ability on prevalence and clinical presentation of language disorder: Evidence from a population study. The Journal of Child Psychology and Psychiatry, 57(11), 1247–1257. https://doi.org/10.1111/jcpp.12573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudel, R. , Simpson, N. H. , Baird, G. , O'Hare, A. , Conti-Ramsden, G. , Bolton, P. F. , Hennessy, E. R. , Ring, S. M. , Davey Smith, G. , Francks, C. , Paracchini, S. , Monaco, A. P. , Fisher, S. E. , & Newbury, D. F. (2014). Genome-wide association analyses of child genotype effects and parent-of-origin effects in specific language impairment. Genes, Brain and Behavior, 13(4), 418–429. https://doi.org/10.1111/gbb.12127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott, J. , Kamatani, Y. , & Lathrop, M. (2011). Family-based designs for genome-wide association studies. Nature Reviews Genetics, 12(7), 465–474. https://doi.org/10.1038/nrg2989 [DOI] [PubMed] [Google Scholar]
- Pettigrew, K. A. , Frinton, E. , Nudel, R. , Chan, M. T. M. , Thompson, P. , Hayiou-Thomas, M. E. , Talcott, J. B. , Stein, J. , Monaco, A. P. , Hulme, C. , Snowling, M. J. , Newbury, D. F. , & Paracchini, S. (2016). Further evidence for a parent-of-origin effect at the NOP9 locus on language-related phenotypes. Journal of Neurodevelopmental Disorders, 8(1), Article 24. https://doi.org/10.1186/s11689-016-9157-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin, M. , Wen, X. L. , Hepburn, M. , Lubs, H. A. , Feldman, E. , & Duara, R. (1993). Suggestive linkage of developmental dyslexia to chromosome 1p34-p36. The Lancet, 342(8864), 178. https://doi.org/10.1016/0140-6736(93)91384-X [DOI] [PubMed] [Google Scholar]
- Rakhlin, N. , Kornilov, S. A. , Palejev, D. , Koposov, R. A. , Chang, J. , & Grigorenko, E. L. (2013). The language phenotype of a small geographically isolated Russian-speaking population: Implications for genetic and clinical studies of developmental language disorder. Applied Psycholinguistics, 34(5), 971–1003. https://doi.org/10.1017/S0142716412000094 [Google Scholar]
- Ramanathan, S. , Woodroffe, A. , Flodman, P. L. , Mays, L. Z. , Hanouni, M. , Modahl, C. B. , Steinberg-Epstein, R. , Bocian, M. E. , Spence, M. A. , & Smith, M. (2004). A case of autism with an interstitial deletion on 4q leading to hemizygosity for genes encoding for glutamine and glycine neurotransmitter receptor sub-units (AMPA 2, GLRA3, GLRB) and neuropeptide receptors NPY1R, NPY5R. BMC Medical Genetics, 5(1), Article 10. https://doi.org/10.1186/1471-2350-5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza, M. H. , Amjad, R. , Riazuddin, S. , & Drayna, D. (2012). Studies in a consanguineous family reveal a novel locus for stuttering on chromosome 16q. Human Genetics, 131(2), 311–313. https://doi.org/10.1007/s00439-011-1134-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza, M. H. , Gertz, E. M. , Mundorff, J. , Lukong, J. , Kuster, J. , Schaffer, A. A. , & Drayna, D. (2013). Linkage analysis of a large African family segregating stuttering suggests polygenic inheritance. Human Genetics, 132(4), 385–396. https://doi.org/10.1007/s00439-012-1252-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza, M. H. , Mattera, R. , Morell, R. , Sainz, E. , Rahn, R. , Gutierrez, J. , Paris, E. , Root, J. , Solomon, B. , Brewer, C. , Basra, M. A. R. , Khan, S. , Riazuddin, S. , Braun, A. , Bonifacino, J. S. , & Drayna, D. (2015). Association between rare variants in AP4E1, a component of intracellular trafficking, and persistent stuttering. The American Journal of Human Genetics, 97(5), 715–725. https://doi.org/10.1016/j.ajhg.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read, A. P. , & Strachan, T. (2011). Human molecular genetics. Taylor & Francis Group. [Google Scholar]
- Reader, R. H. , Covill, L. E. , Nudel, R. , & Newbury, D. F. (2014). Genome-wide studies of specific language impairment. Current Behavioral Neuroscience Reports, 1(4), 242–250. https://doi.org/10.1007/s40473-014-0024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, M. L. (2017). Overlooked by public health: Specific language impairment. Open Access Government. [Google Scholar]
- Rice, M. L. , Haney, K. R. , & Wexler, K. (1998). Family histories of children with SLI who show extended optional infinitives. Journal of Speech, Language, and Hearing Research, 41(2), 419–432. https://doi.org/10.1044/jslhr.4102.419 [DOI] [PubMed] [Google Scholar]
- Rice, M. L. , & Hoffman, L. (2015). Predicting vocabulary growth in children with and without specific language impairment: A longitudinal study from 2; 6 to 21 years of age. Journal of Speech, Language, and Hearing Research, 58(2), 345–359. https://doi.org/10.1044/2015_JSLHR-L-14-0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, M. L. , Smith, S. D. , & Gayán, J. (2009). Convergent genetic linkage and associations to language, speech and reading measures in families of probands with specific language impairment. Journal of Neurodevelopmental Disorders, 1(4), 264–282. https://doi.org/10.1007/s11689-009-9031-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, M. L. , Taylor, C. L. , Zubrick, S. R. , Hoffman, L. , & Earnest, K. K. (2020). Heritability of specific language impairment and nonspecific language impairment at ages 4 and 6 years across phenotypes of speech, language, and nonverbal cognition. Journal of Speech, Language, and Hearing Research, 63(3), 793–813. https://doi.org/10.1044/2019_JSLHR-19-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, M. L. , Zubrick, S. R. , Taylor, C. L. , Hoffman, L. , & Gayán, J. (2018). Longitudinal study of language and speech of twins at 4 and 6 years: Twinning effects decrease, zygosity effects disappear, and heritability increases. Journal of Speech, Language, and Hearing Research, 61(1), 79–93. https://doi.org/10.1044/2017_JSLHR-L-16-0366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelow, D. , Schuelke, M. , Hildebrandt, F. , & Nürnberg, P. (2009). HomozygosityMapper—An interactive approach to homozygosity mapping. Nucleic Acids Research, 37(Suppl. 2), W593–W599. https://doi.org/10.1093/nar/gkp369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel, E. , Wiig, E. H. , & Secord, W. (1987). Clinical Evaluation of Language Fundaments–Revised. The Psychological Corporation. [Google Scholar]
- Semel, E. , Wiig, E. H. , & Secord, W. A. (1995). Clinical Evaluation of Language Fundamentals–Third Edition. The Psychological Corporation. [Google Scholar]
- Silberstein, M. , Weissbrod, O. , Otten, L. , Tzemach, A. , Anisenia, A. , Shtark, O. , Tuberg, D. , Galfrin, E. , Gannon, I. , Shalata, A. , Borochowitz, Z. U. , Dechter, R. , Thompson, E. , & Geiger, D. (2012). A system for exact and approximate genetic linkage analysis of SNP data in large pedigrees. Bioinformatics, 29(2), 197–205. https://doi.org/10.1093/bioinformatics/bts658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLI Consortium. (2002). A genomewide scan identifies two novel loci involved in specific language impairment. The American Journal of Human Genetics, 70(2), 384–398. https://doi.org/10.1086/338649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLI Consortium. (2004). Highly significant linkage to the SLI1 locus in an expanded sample of individuals affected by specific language impairment. The American Journal of Human Genetics, 74(6), 1225–1238. https://doi.org/10.1086/421529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. D. (2007). Genes, language development, and language disorders. Mental Retardation and Developmental Disabilities Research Reviews, 13(1), 96–105. https://doi.org/10.1002/mrdd.20135 [DOI] [PubMed] [Google Scholar]
- Snowling, M. , Bishop, D. V. M. , & Stothard, S. E. (2000). Is preschool language impairment a risk factor for dyslexia in adolescence? The Journal of Child Psychology and Psychiatry, 41(5), 587–600. https://doi.org/10.1111/1469-7610.00651 [DOI] [PubMed] [Google Scholar]
- Strachan, T. , Goodship, J. , & Chinnery, P. (2014). Genetics and genomics in medicine. Taylor & Francis. https://doi.org/10.1201/9781317594307 [Google Scholar]
- Tallal, P. , Hirsch, L. S. , Realpe-Bonilla, T. , Miller, S. , Brzustowicz, L. M. , Bartlett, C. W. , & Flax, J. F. (2001). Familial aggregation in specific language impairment. Journal of Speech, Language, and Hearing Research, 44(5), 1172–1182. https://doi.org/10.1044/1092-4388(2001/091) [DOI] [PubMed] [Google Scholar]
- Thiele, H. , & Nürnberg, P. (2004). HaploPainter: A tool for drawing pedigrees with complex haplotypes. Bioinformatics, 21(8), 1730–1732. https://doi.org/10.1093/bioinformatics/bth488 [DOI] [PubMed] [Google Scholar]
- Thomson, E. , Rappsilber, J. , & Tollervey, D. (2007). Nop9 is an RNA binding protein present in pre-40S ribosomes and required for 18S rRNA synthesis in yeast. RNA, 13(12), 2165–2174. https://doi.org/10.1261/rna.747607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin, J. B. (2014). Education and psychosocial outcomes of language impairment in kindergarten. In Tomblin J. B. & Nippold M. A. (Eds.), Understanding individual differences in language development across the school years (pp. 166–203). Psychology Press. https://doi.org/10.4324/9781315796987 [Google Scholar]
- Tomblin, J. B. , Records, N. L. , Buckwalter, P. , Zhang, X. , Smith, E. , & O'Brien, M. (1997). Prevalence of specific language impairment in kindergarten children. Journal of Speech, Language, and Hearing Research, 40(6), 1245–1260. https://doi.org/10.1044/jslhr.4006.1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong, D. T. , Shriberg, L. D. , Smith, S. D. , Chapman, K. L. , Scheer-Cohen, A. R. , DeMille, M. M. C. , Adams, A. K. , Nato, A. Q. , Wijsman, E. M. , Eicher, J. D. , & Gruen, J. R. (2016). Multipoint genome-wide linkage scan for nonword repetition in a multigenerational family further supports chromosome 13q as a locus for verbal trait disorders. Human Genetics, 135(12), 1329–1341. https://doi.org/10.1007/s00439-016-1717-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzenova, J. , Kaplan, B. J. , Petryshen, T. L. , & Field, L. L. (2004). Confirmation of a dyslexia susceptibility locus on chromosome 1p34-p36 in a set of 100 Canadian families. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 127(1), 117–124. https://doi.org/10.1002/ajmg.b.20139 [DOI] [PubMed] [Google Scholar]
- van Heyningen, V. , & Yeyati, P. L. (2004). Mechanisms of non-Mendelian inheritance in genetic disease. Human Molecular Genetics, 13(Suppl. 2), R225–R233. https://doi.org/10.1093/hmg/ddh254 [DOI] [PubMed] [Google Scholar]
- Villanueva, P. , Jara, L. , & Palomino, H. (2010). Association of D16S515 microsatellite with specific language impairment on Robinson Crusoe Island, an isolated Chilean population: A possible key to understanding language development. Human Biology, 82(4), 395–408. https://doi.org/10.3378/027.082.0404 [DOI] [PubMed] [Google Scholar]
- Villanueva, P. , Newbury, D. F. , Jara, L. , De Barbieri, Z. , Mirza, G. , Palomino, H. M. , Fernández, M. A. , Cazier, J. B. , Monaco, A. P. , & Palomino, H. (2011). Genome-wide analysis of genetic susceptibility to language impairment in an isolated Chilean population. European Journal of Human Genetics, 19(6), 687–695. https://doi.org/10.1038/ejhg.2010.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva, P. , Nudel, R. , Hoischen, A. , Fernández, M. A. , Simpson, N. H. , Gilissen, C. , Reader, R. H. , Jara, L. , Echeverry, M. M. , Francks, C. , Baird, G. , Conti-Ramsden, G. , O'Hare, A. , Bolton, P. F. , Hennessy, E. R. , SLI Consortium, Palomino, H. , Carvajal-Carmona, L. , Veltman, J. A. , … Newbury, D. F. (2015). Exome sequencing in an admixed isolated population indicates NFXL1 variants confer a risk for specific language impairment. PLOS Genetics, 11(3), Article e1004925. https://doi.org/10.1371/journal.pgen.1004925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva, P. , Palomino, H. M. , & Palomino, H. (2008). High prevalence of specific language impairment in Robinson Crusoe Island. A possible founder effect. Revista Medica de Chile, 136(2), 186–192. https://doi.org/10.4067/S0034-98872008000200007 [PubMed] [Google Scholar]
- Wechsler, D. (1991). Wechsler Intelligence Scale for Children–Third Edition. The Psychological Corporation. [Google Scholar]
- Wechsler, D. (1997). Adult Intelligence Scale–Third Edition. The Psychological Corporation. https://doi.org/10.1037/t49755-000 [Google Scholar]
- Whitehouse, A. J. O. , Bishop, D. V. M. , Ang, Q. W. , Pennell, C. E. , & Fisher, S. E. (2011). CNTNAP2 variants affect early language development in the general population. Genes, Brain and Behavior, 10(4), 451–456. https://doi.org/10.1111/j.1601-183X.2011.00684.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiszniewski, W. , Hunter, J. V. , Hanchard, N. A. , Willer, J. R. , Shaw, C. , Tian, Q. , Illner, A. , Wang, X. , Cheung, S. W. , Patel, A. , Campbell, I. M. , Gelowani, V. , Hixson, P. , Ester, A. R. , Azamian, M. S. , Potocki, L. , Zapata, G. , Hernandez, P. P. , Ramocki, M. B. , … Lalani, S. R. (2013). TM4SF20 ancestral deletion and susceptibility to a pediatric disorder of early language delay and cerebral white matter hyperintensities. The American Journal of Human Genetics, 93(2), 197–210. https://doi.org/10.1016/j.ajhg.2013.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a