Abstract
A general method for the synthesis of secondary homoallylic alcohols containing α-quaternary carbon stereogenic centers in high diastereo- and enantioselectivity (up to >20:1 dr and >99:1 er) is disclosed. Transformations employ readily accessible aldehydes, allylic diboronates, a chiral copper catalyst, and proceed by γ-addition of in situ generated enantioenriched boron-stabilized allylic copper nucleophiles. The catalytic protocol is general for a wide variety of aldehydes as well as a variety of 1,1-allylic diboronic esters. Hammett studies disclose that diastereoselectivity of the reaction is correlated to the electronic nature of the aldehyde, with dr increasing as aldehydes become more electron poor.
Graphical Abstract

Numerous biologically important compounds contain quaternary carbon stereogenic centers, and catalytic enantio- and diastereoselective reactions that deliver them are highly desirable.1 Homoallylic alcohols comprising a vicinal carbon stereocenter can be prepared by catalytic additions of substituted allyl fragments to aldehydes,2,3,4 however, related transformations that deliver quaternary carbon stereogenic centers enantio- and diastereoselectively, is difficult. While there are many examples for the construction of quaternary stereocenters via aldehyde allylation,5 few enantioselective methods exist. Prior disclosures include the enantiospecific intermolecular addition reactions of allylzincs derived from enantioenriched alkynylsulfoxides,6 as well as transformations with enantioenriched allylsilanes7 and allylboronic esters.8 In contrast, catalytic enantioselective versions are scarce.9 One study shows the catalytic enantioselective reactions of γ,γ-disubstituted allyl trichlorosilanes with aldehydes are efficiently promoted by chiral bisphosphoramide (Scheme 1A).10 Another disclosure includes a Cr-catalyzed allylation utilizing γ,γ-disubstituted allylchlorides (Scheme 1B); however, the use of four transition metals, two in stoichiometric amounts, detracts from the method.11 Elegant studies by Krische describe Ir-catalyzed reductive couplings of vinyl epoxides, dienes, and allenes with in situ generated aldehydes (Scheme 1C).12 A more recent approach involves a dual isomerization/enantioselective allylation sequence with allylic alcohols and homoallylic boronic esters, catalyzed by an Ir/chiral phosphoric acid protocol.13 In the case of quaternary stereogenic centers, however, a wide range of dr and er were observed.
Scheme 1.

Catalytic Enantioselective Additions to Aldehydes with γ,γ-Disubstituted Allyl Reagents
While significant advances in quaternary stereocenter synthesis by aldehyde allylation have been made, addition to alkyl aldehydes remains a significant challenge, and variation at the quaternary carbon center is largely absent. Furthermore, very few catalytic, enantioselective methods exist that afford products in high diastereo- and enantioselectivities. Our objective was the development of a general protocol for setting quaternary centers in homoallylic alcohols by employing γ,γ-disubstituted allylic 1,1-diboronate esters. Allylic 1,1-diboronic esters have been employed in the crotylation and prenylation of aldehydes,14,15 however, extension of these methods for enantio- and diastereoselective allyl addition beyond prenylation to set quaternary stereocenters has not been achieved.
Encouraged by our previous studies regarding the enantio- and diastereoselective reactions of γ,γ-disubstituted allylic 1,1-bis(boronates) with aldimines16 and ketones,17 we envisioned the stereoselective reaction in Scheme 1E. The enantio- and diastereoselective preparation of complex secondary homoallyl alcohols with vicinal quaternary carbon stereocenters bearing a functional E-alkenylboron can be achieved by catalytic reactions between an array of aldehydes (1) and readily accessible stereodefined 2.
We began our studies with the reaction of benzaldehyde (1a) and allyl diboronic ester 2a (Table 1). Initial control reaction between 1a and 2a in THF at −60 °C followed by an aqueous (entry 1) and NaBH4 (entry 2) workup, revealed significant background addition of unreacted 2a occurs upon workup (38% conv, >20:1 dr vs <2% conv). This outcome highlighted the need for a reductive quench during catalyst optimization. In the presence of 5 mol % CuOtBu, 10 mol % ligand, and 1.05 equiv MeOH in THF at −60 °C, several classes of chiral ligands were examined. Bidentate phosphines L1–3 proved ineffective; <2–8% conversion to 3a (entries 3–5). Switching to phosphoramidite L4 delivered 3a in 89% yield (>98:2 γ-allylation) in 2.7:1 dr and >99:1 er (entry 6). Notably, <2% conversion to 3a is observed in the absence of methanol (entry 7). Investigation of phosphoramidite 3,3’-aryl substitution (entries 8–10) identified 3,5-i-Pr-4-OMe substitution (L7) as optimal (>99:1 er, 4:1 dr) (entry 10). In an effort to further improve the dr, the reaction with L7 was run at −78 °C, however, this resulted in no improvement in selectivity. To test if a reductive workup was necessary given the high conversion with L7 in 16 h, the equivalent reaction with aqueous workup was found to afford a near identical result (98% NMR yield, 4:1 dr, 98.5:1.5 er). Consequently, provided catalytic reactions proceed to >98% consumption of aldehyde, a reductive work up is not required.18
Table 1.
Reaction Optimizationa
 | |||||
|---|---|---|---|---|---|
| entry | ligand | alcohol | NMR yield (%)b | drb | erc |
| 1d | - | - | 38 | >20:1 | - |
| 2 | - | - | <2 | - | - |
| 3 | L1 | MeOH | <2 | - | - |
| 4 | L2 | MeOH | <2 | - | - |
| 5 | L3 | MeOH | 8 | nd | nd |
| 6 | L4 | MeOH | 89 | 2.7:1 | >99:1 |
| 7 | L4 | <2 | - | - | |
| 8 | L5 | MeOH | 70 | 1.5:1 | >99:1 |
| 9 | L6 | MeOH | >98 | 1:1 | 99:1 |
| 10 | L7 | MeOH | 98 | 4:1 | >99:1 |
| 11e | L7 | MeOH | 94 | 4:1 | >99:1 |
| 12f | L7 | MeOH | 98 | 4:1 | 98.5:1.5 |
 | |||||
Reactions performed under N2 atmosphere.
NMR yield and diastereomeric ratios (dr) determined by analysis of 1H NMR spectra of crude reactions with hexamethyldisiloxane as internal standard.
Enantiomeric ratios (er) determined by SFC analysis.
No CuOtBu, ligand, or NaBH4 quench.
Reaction at −78 °C.
No NaBH4 quench. See the SI for details.
The robustness of the reaction conditions was surveyed, and it was found to be broad. As shown in Scheme 2A, a wide variety of aromatic substrates are tolerated, including those containing electron-donating groups (3b), halogens (3c–d), as well as electron-withdrawing ester (3e), nitrile (3f), nitro groups (3g–h) and trifluoromethyl (3i), to deliver products in excellent yields and er. Notably, meta and ortho substituted arenes undergo efficient and selective reaction (3h-k). Furthermore, a range of heteroarene products including pyridine (3k), furan (3l), and thiophene (3m) moieties are accessible in excellent yield and er albeit in 3:1–4:1 dr. In reaction with aldehydes containing extended π-systems, such as indole (3n), benzofuran (3o), and benzothiophene (3p), products are generated in higher diastereoselectivity in excellent yields and enantioselectivities. Moreover, synthesis of indole 3n on a 1.0 mmol scale demonstrated robustness of the protocol.
Scheme 2.

Aldehyde Scopea
aReactions performed under N2 atmosphere. Yields of purified products after SiO2 Chromatography. Experiments were run in duplicate. Diastereomeric ratios (dr) determined by analysis of 1H NMR spectra of purified products. Enantiomeric ratios (er) determined by HPLC or SFC analysis. See the SI for details. b1.0 mmol scale.
The catalytic protocol also extends to alkenyl, alkynyl, and alkyl aldehydes (Scheme 2B). Unsaturated cinnamyl, tiglic, and cyclohexenyl aldehyde substrates are converted to homoallylic alcohol products 5a–c in excellent yield and ≥99:1 er, and 4:1–6:1 dr, respectively. In addition, reaction with enantioenriched (–)-myrtenal delivers 5d in 82% yield and 9:1 dr. Reactions of alkynyl aldehydes also react efficiently, however, a decrease in diastereoselectivity results; for example, propargylic alcohol 5e is formed in 84% yield, 2:1 dr, and 98.5:1.5 er (major) and >99:1 er (minor). High yields, and selectivity are similarly observed with more challenging, less electrophilic aliphatic aldehydes. For example, a variety of alkyl-substituted aldehydes bearing t-Bu (5f), cyclic (5g–i), and β-branching (5j) were found to react smoothly to deliver desired products in 68–99% yield, ≥99:1 er, and >20:1–4:1 dr. Additionally, no adverse effects arising from a pendant alkene moiety in reactions with 4-pentenal and (–)-citronellal to afford 5k and 5l, were observed.
Finally, scope of the quaternary carbon stereocenter was investigated by varying the substituents introduced on theallyldiboron reagent. Notably, diastereomers 6b and 6c could be synthesized stereospecifically by subjecting either E- or Z-allyldiboronates to the reaction conditions, with both obtained in high dr and er. The increased sterics associated with α-branched cyclohexyl and cyclopropyl reagents are tolerated to produce 6d and 6e in excellent yield, and selectivity. Moreover, after a single recrystallization, 6e could be enriched to 20:1 dr, and the X-ray structure obtained to confirm the relative and absolute stereochemistry. Lastly, transformations of allyl diboronate reagents containing homobenzyl and silyl ether moieties generate competent nucleophiles; for example, alkenyl and aliphatic aldehyde derived products 6g–h delivered in 4:1–6:1 dr and ≥96.5:3.5 er, are illustrative.
To assess the importance of the allyl diboronate moiety, a comparison to monoboryl reagent 7 was evaluated (Scheme 4A). Under standard conditions with a NaBH4 quench, <5% conversion to homoallylic alcohol product 8 is observed; control reaction in the absence of Cu/L7 affords 8 in 66% NMR yield and 9:1 dr. Support for an enantio-determining transmetalation step in the reaction mechanism was provided by Swern oxidation of alcohol 3a to ketone 9 in 53% yield, and 97.5:2.5 er (Scheme 4B). The high er of the product strongly suggests an enantioselective transmetalation to form a stereodefined allylic nucleophile, and diastereoselectivity is the result of facial selectivity with the aldehyde. Further evidence for this is found in the high er of the minor diastereomer of 5e (see SI for details). To gain insight into interesting diastereoselectivity trends observed with aryl aldehydes, a Hammett plot was constructed (Scheme 4C). Satisfactory correlations between dr and the electronic effects of the substituents was observed. Notably, it was also found that substituent constant σp– values provided the best correlation for the electron-poor aldehydes (p-CO2Me, p-CN, p-NO2).19 The plot in Scheme 4C shows that electron-withdrawing substituents result in improved diastereoselectivity in the reaction (ρ = 0.96, R2= 0.95), indicating the electrophilicity of the aldehyde (vs C=O Lewis basicity) impacts the diastereoselectivity of the reaction.20 This data is consistent with C–C bond formation being diastereo-determining.21 At larger sigma values a change in slope of the Hammett plot of opposite sign is observed (ρ = −0.26, R2= 0.95), indicating a lower sensitivity of the allyl addition reaction to electronic changes. The break in the plot is suggestive of a change in diastereo-determining step likely towards aldehyde coordination influencing stereoselectivity.
Scheme 4.

Mechanism Experimentsa
aSee the SI for details.
The effect of catalyst versus substrate control with chiral aldehydes was examined with α-stereogenic amino aldehydes (Scheme 4D). Treatment of D-alanine-derived (R)-10 with optimal reaction conditions with Me,Me-diboron 2f results in high conversion to 11 as a 3.2:1 (C1:C2) mixture of diastereomers. In addition, when (R)-10 is subjected to the same conditions with n-Bu,Me diboron 2a, amino alcohol 12 is obtained in very similar diastereoselectivity (3.8:1 dr, (C1:C2)), indicating the substituents on the allylcopper do not play a significant role in affecting facial selectivity of the aldehyde. In contrast, reaction with aldehyde (S)-10 clearly indicates a matched-mismatched situation as homoallylic alcohol 13 is obtained in diminished stereoselectivity (86% yield, 1:1 dr, C1:C2).22
Based on the above findings a catalytic cycle and stereochemical model is proposed in Scheme 5 to rationalize our observations. The reaction likely proceeds via SE2’ transmetalation of (L)Cu–OMe (A) with diboronate 2.23,24 Subsequent, rapid 1,3-suprafacial shift to the less sterically encumbered boron-stabilized allyl copper species C must occur faster than C–C bond rotation in B to prevent isomerization of alkene geometry.25 Coordination of aldehyde 1 results in cyclization through D, affording Cu-bound product E. As apparent in the crystal structure of 6e, the large substituent occupies the pseudo-equatorial position in D, resulting in the observed diastereoselectivity. Finally, protonation of intermediate E with MeOH releases product 3 and regenerates catalyst A. The observations in Table 1, that show MeOH is required for product formation and to obtain high er, can be rationalized by slow reaction between E and 2, as well as the requirement of (L7)-Cu–OMe species to facilitate highly enantioselective transmetalation. The enantioselective formation of intermediate C is supported by the enantiomeric ratio of the minor diastereomers, which in most cases is high (see 5e, and SI for details).26
Scheme 5.

Proposed Catalytic Cycle and Stereochemical Model
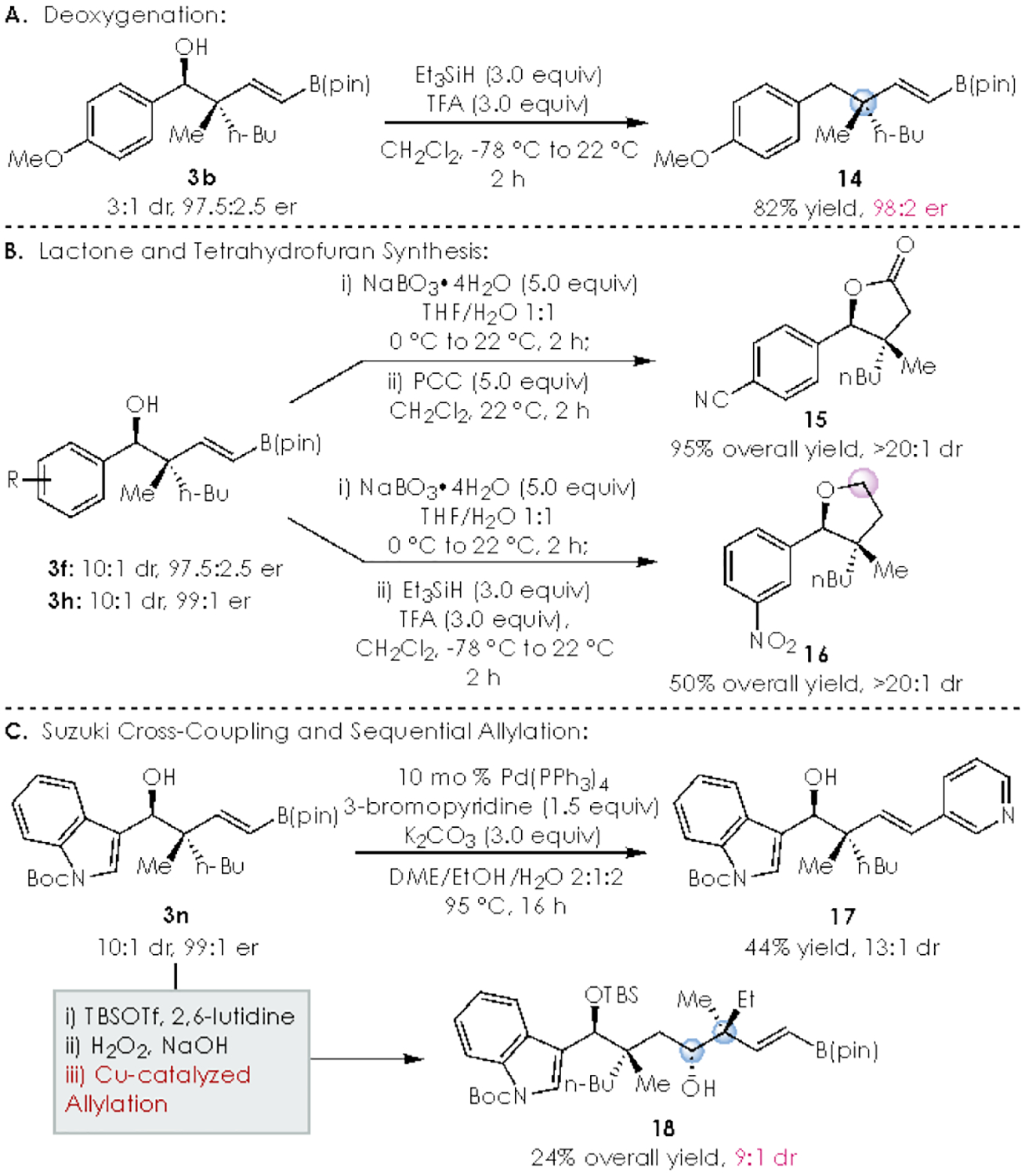
The utility of the method is showcased by the various chemical transformations depicted in Scheme 6. First, we investigated the reduction of enantio-enriched benzylic alcohol in 3b (3:1 dr) by treatment with CF3CO2H and HSiEt3 in CH2Cl2 to afford deoxygenated product 14 in 82% yield (Scheme 6A).27
Scheme 6.
Synthetic Utilitya
aSee the SI for details.
Oxidation of the alkenyl boronic esters 3f and 3h results in hemiacetal formation (Scheme 6B), which followed by either PCC oxidation or silane reduction delivers substituted lactone 15 and tetrahydrofuran 16 in 95% an 50% overall yield, respectively. The versatility of the alkenyl boronic esters moiety was also demonstrated to be effective in Suzuki cross couplings; for example, Pd-catalyzed reaction of 3n with 3-bromopyridine affords N-heterocycle 17 in 44% yield and 13:1 dr. Lastly, the ability of the method to efficiently construct multiple acyclic quaternary carbon stereocenters was demonstrated by a three-step telescoped sequence (Scheme 6C). Beginning with indole 3n, TBS protection of the alcohol followed by alkenyl boron oxidation results in the crude aldehyde, which was then subjected to Cu-catalyzed allyl addition with E-2b to afford alcohol 18 in 24% overall yield, and 9:1 dr with respect to the new stereogenic centers.
In conclusion, we have developed a versatile, robust Cu-catalyzed protocol for the enantio-, diastereo-, and anti-selective synthesis of vicinal homoallyl alcohol and quaternary carbon stereocenters. The method offers both broad aldehyde and quaternary stereocenter scopes utilizes a simple (phosphoramidite)-Cu catalyst. Mechanism studies provide support for the intermediacy of an enantioenriched allyl copper species, as well as reveal a pronounced electronic effect of substituents on diastereoselectivity. Studies are ongoing to understand the factors that control stereoselectivity, and to further develop stereoselective reactions with allyl diborons.
Supplementary Material
Scheme 3.

1,1-Allylic Diboron Scopea
ACKNOWLEDGMENTS
Financial support was provided by the United States National Institutes of Health, Institute of General Medical Sciences (R01GM116987). Core facilities in the Department of Chemistry at the University of North Carolina are supported by the National Science Foundation under grants: CHE1726291 (Mass spectroscopy); CHE0922858 and CHE1828183 (NMR spectroscopy). We thank Tia Cervarich of UNC for X-ray structure elucidation of 6e.
Footnotes
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.0c03495.
Experimental procedures and spectral and analytical data for all products (PDF)
Crystallographic data 6e (CIF)
Authors declare no competing financial interests.
REFERENCES
- (1).For recent reviews on the enantioselective synthesis of quaternary carbon stereogenic centers, see:; (a) Das JP; Marek I Enantioselective Synthesis of All-Carbon Quaternary Stereogenic Centers in Acyclic Systems. Chem. Commun 2011, 47, 4593–4623. [DOI] [PubMed] [Google Scholar]; (b) Minko Y; Marek I Stereodefined Acyclic Trisubstituted Metal Enolates towards the Asymmetric Formation of Quaternary Carbon Stereocentres. Chem Commun 2014, 50, 12597–12611. [DOI] [PubMed] [Google Scholar]; (c) Marek I; Minko Y; Pasco M; Mejuch T; Gilboa N; Chechik H; Das JP All-Carbon Quaternary Stereogenic Centers in Acyclic Systems through the Creation of Several C–C Bonds per Chemical Step. J. Am. Chem. Soc 2014, 136, 2682–2694. [DOI] [PubMed] [Google Scholar]; (d) Quasdorf KW; Overman LE Catalytic Enantioselective Synthesis of Quaternary Carbon Stereocenters. Nature 2014, 516, 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Liu Y; Han S-J; Liu W-B; Stoltz BM Catalytic Enantioselective Construction of Quaternary Stereocenters: Assembly of Key Building Blocks for the Synthesis of Biologically Active Molecules. Acc. Chem. Res 2015, 48, 740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zeng X-P; Cao Z-Y; Wang Y-H; Zhou F; Zhou J Catalytic Enantioselective Desymmetrization Reactions to All-Carbon Quaternary Stereocenters. Chem. Rev 2016, 116, 7330–7396. [DOI] [PubMed] [Google Scholar]; (g) Feng J; Holmes M; Krische MJ Acyclic Quaternary Carbon Stereocenters via Enantioselective Transition Metal Catalysis. Chem. Rev 2017, 117, 12564–12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).For a review on enantioselective allylation of carbonyl compounds, including crotylation of aldehydes, see:; Yus M; González-Gómez JC; Foubelo F Catalytic Enantioselective Allylation of Carbonyl Compounds and Imines. Chem. Rev 2011, 111, 7774–7854. [DOI] [PubMed] [Google Scholar]
- (3).For reviews of reductive couplings with aldehydes, including crotylation, see:; (a) Kim SW; Zhang W; Krishe MJ Catalytic Enantioselective Carbonyl Allylation and Propargylation via Alcohol-Mediated Hydrogen Transfer: Merging the Chemistry of Grignard and Sabatier. Acc. Chem. Res [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Holmes M; Schwartz LA; Krische MJ Intermolecular Metal-Catalyzed Reductive Coupling of Dienes, Allenes, and Enynes with Carbonyl Compounds and Imines. Chem. Rev 2018, 118, 6026–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).For representative examples of catalytic enantioselective crotylation of aldehydes, see:; (a) Traverse JF; Zhao Y; Hoveyda AH; Snapper ML Proline-Based N-Oxides as Readily Available and Modular Chiral Catalysts. Enantioselective Reactions of Allyltrichlorosilane with Aldehydes. Org. Lett 2005, 7, 3151–3154. [DOI] [PubMed] [Google Scholar]; (b) Rauniyar V; Zhai H; Hall DG Catalytic Enantioselective Allyl- and Crotylboration of Aldehydes Using Chiral Diol•SnCl4 Complexes. Optimization, Substrate Scope, and Mechanistic Investigations. J. Am. Chem. Soc 2008, 130, 8481–8490. [DOI] [PubMed] [Google Scholar]; (c) Zbieg JR; Yamaguchi E; McInturff EL; Krische MJ Enantioselective C-H Crotylation of Primary Alcohols via Hydrohydroxyalkylation of Butadiene. Science. 2012, 336, 324–327. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Meng F; Jang H; Jung B; Hoveyda AH Cu-Catalyzed Chemoselective Preparation of 2-(Pinacolato)boron-Substituted Allylcopper Complexes and their In Situ Site-, Diastereo-, and Enantioselective Additions to Aldehydes and Ketones. Angew. Chem. Int. Ed 2013, 52, 5046–5051. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Miura T; Nishida Y; Morimoto M; Murakami M Enantioselective Synthesis of Anti Homoallylic Alcohols from Terminal Alkynes and Aldehydes Based on Concomitant Use of a Cationic Iridium Complex and a Chiral Phosphoric Acid. J. Am. Chem. Soc 2013, 135, 11497–11500. [DOI] [PubMed] [Google Scholar]; (f) Miura T; Nishida Y; Murakami M Construction of Homoallylic Alcohols from Terminal Alkynes and Aldehydes with Installment of syn-Stereochemistry. J. Am. Chem. Soc 2014, 136, 6223–6226. [DOI] [PubMed] [Google Scholar]; (g) Li C; Shin K; Liu RY; Buchwald SL Engaging Aldehydes in CuH-Catalyzed Reductive Coupling Reactions: Stereoselective Allylation with Unactivated 1,3-Diene Pronucleophiles. Angew. Chem. Int. Ed 2019, 58, 17074–17080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).(a) Brown HC; Bhat KS; Randad RS Charge Reversal of Electrophilic π-Allylpalladium Intermediates: Carbonyl Allylation by Allylic Acetates with Pd(PPh3)4-Zn. J. Org. Chem 1987, 52, 3702–3704. [Google Scholar]; (b) Sato M; Yamamoto Y; Hara S; Suzuki A A Stereoselective Synthesis of 3,3’-Disubstituted Allylborane Derivatives Using Haloboration Reaction and their Application for the Diastereospecific Synthesis of Homoallylic Alcohols Having Quaternary Carbon. Tetrahedron Lett. 1993, 34, 7071–7074. [Google Scholar]; (c) Kobayashi S; Nishio K Facile and Highly Stereoselective Synthesis of Homoallylic Alcohols Using Organosilicon Intermediates. J. Org. Chem 1994, 59, 6620–6628. [Google Scholar]; (d) Nishigaichi Y; Fujimoto M; Takuwa A γ-Selective Pentadienylation of Aldehydes and Ketones with Pentadienyltins by the Use of ZnCl2. Synlett. 1994, 731–732. [Google Scholar]; (e) Nowotny S; Tucker CE; Jubert C; Knochel P Chromium(II)-Mediated Stereodivergent Additions of Allylic Phosphates and Halides to Aldehydes. J. Org. Chem 1995, 60, 2762–2772. [Google Scholar]; (f) Nishigaichi Y; Takuwa A Stereospecificity in the Lewis Acid Promoted Allylation Reaction of 3,3-Disubstituted Allyltins toward Aldehydes. Tetrahedron Lett. 1999, 40, 109–112. [Google Scholar]; (g) Hirashita T; Kambe S; Tsuji H; Omori H; Araki S Direct Preparation of Allylic Indium(III) Reagents from Allylic Alchols via a Reductive Transmetalation of π-Allylnickel(II) with Indium(I) Iodide. J. Org. Chem 2004, 69, 5054–5059. [DOI] [PubMed] [Google Scholar]; (h) Yanagisawa A; Aoki T; Arai T Dibutyltin Oxide Catalyzed Allyl-Transfer Reaction from Tertiary Homoallylic Alcohols to Aldehydes. Synlett. 2006, 2071–2074. [Google Scholar]; (i) Ngai M-Y; Skucas E; Krische MJ Ruthenium Catalyzed C–C Bond Formation via Transfer Hydrogenation: Branch-Selective Reductive Coupling of Allenes to Paraformaldehyde and Higher Aldehydes. Org. Lett 2008, 10, 2705–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Ely RJ; Morken JP Regio- and Stereoselective Ni-Catalyzed 1,4-Hydroboration of 1,3-Dienes: Access to Stereodefined (Z)-Allylboron Reagents and Derived Allylic Alcohols. J. Am. Chem. Soc 2010, 132, 2534–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Biggs RA; Lambadaris M; Ogilvie WW Highly Diastereoselective Generation of Various 3,3-Disubstituted Allyl Boronates for the Stereospecific Construction of Quaternary Centers. Tetrahedron Lett. 2014, 55, 6085–6087. [Google Scholar]; (l) Nakano T; Endo K; Ukaji Y Silver-Catalyzed Allylation of Ketones and Intramolecular Cyclization through Carbene Intermediates from Cyclopropenes Under Ambient Conditions. Chem. Asian J 2016, 11, 713–721. [DOI] [PubMed] [Google Scholar]; (m) Weber F; Ballmann M; Kohlmeyer C; Hilt G Nickel-Catalyzed Double Bond Transposition of Alkenyl Boronates for in Situ syn-Selective Allylboration Reactions. Org. Lett 2016, 18, 548–551. [DOI] [PubMed] [Google Scholar]
- (6).Sklute G; Marek I New Multicomponent Approach for the Creation of Chiral Quaternary Centers in the Carbonyl Allylation Reactions. J. Am. Chem. Soc 2006, 128, 4642–4649. [DOI] [PubMed] [Google Scholar]
- (7).Kacprzynski MA; Kazane SA; May TL; Hoveyda AH Cu-Catalyzed Asymmetric Conjugate Additions of Dialkyl- and Diarylzinc Reagents to Acyclic β-Silyl-α,β-Unsaturated Ketones. Synthesis of Allylsilanes in High Diastereo- and Enantiomeric Purity. Org. Lett 2007, 9, 3187–3190. [DOI] [PubMed] [Google Scholar]
- (8).(a) Yamamoto Y; Hara S; Suzuki A Enantioselective Synthesis of Quaternary Carbon in Homoallylic Alcohols by the Reaction of Tartrate Ester Derivatives of 3,3-Disubstituted Allylborane with Aldehydes. Synlett, 1996, 9, 883–884. [Google Scholar]; (b) Morgan JB; Morken JP Platinum-Catalyzed Tandem Diboration/Asymmetric Allylboration: Access to Nonracemic Functionalized 1,3-Diols. Org. Lett 2003, 5, 2573–2575. [DOI] [PubMed] [Google Scholar]; (c) Kliman LT; Mlynarski SN; Ferris GE; Morken JP Catalytic Enantioselective 1,2-Diboration of 1,3-Dienes: Versatile Reagents for Stereoselective Allylation. Angew. Chem. Int. Ed 2012, 51, 521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Potter B; Szymaniak AA; Edelstein EK; Morken JP Nonracemic Allylic Boronates through Enantiotopic-Group-Selective Cross-Coupling of Geminal Bis(boronates) and Vinyl Halides. J. Am. Chem. Soc 2014, 136, 17918–17921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).For examples of catalytic enantioselective allyl additions to ketones that generate quaternary carbon stereogenic centers, see:; (a) Alam R; Vollgraff T; Eriksson L; Szabó KJ Synthesis of Adjacent Quaternary Stereocenters by Catalytic Asymmetric Allylboration. J. Am. Chem. Soc 2015, 137, 11262–11265. [DOI] [PubMed] [Google Scholar]; (b) Feng J-J; Xu Y; Oestreich M Ligand-Controlled Diastereodivergent, Enantio- and Regioselective Copper-Catalyzed Hydroxyalkylboration of 1,3-Dienes with Ketones. Chem. Sci 2019, 10, 9679–9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) Denmark SE; Fu J Catalytic, Enantioselective Addition of Substituted Allylic Trichlorosilanes Using a Rationally-Designed 2,2’-Bispyrrolidine-Based Bisphosphoramide. J. Am. Chem. Soc 2001, 123, 9488–9489. [DOI] [PubMed] [Google Scholar]; (b) Denmark SE; Fu J Asymmetric Construction of Quaternary Centers by Enantioselective Allylation: Application to the Synthesis of the Serotonin Antagonist LY426965. Org. Lett 2002, 4, 1951–1953. [DOI] [PubMed] [Google Scholar]; (c) Denmark SE; Fu J; Lawler MJ Chiral Phosphoramide-Catalyzed Enantioselective Addition of Allylic Trichlorosilanes to Aldehydes. Preparative Studies with Bidentate Phosphorus-Based Amides. J. Org. Chem 2006, 71, 1523–1536. [DOI] [PubMed] [Google Scholar]
- (11).Xiong Y; Zhang G Enantioselective Synthesis of Quaternary Stereocenters via Chromium Catalysis. Org. Lett 2016, 18, 5094–5097. [DOI] [PubMed] [Google Scholar]
- (12).(a) Feng J; Garza VJ; Krische MJ Redox-Triggered C–C Coupling of Alcohols and Vinyl Epoxides: Diastereo- and Enantioselective Formation of All-Carbon Quaternary Centers via Tert -(Hydroxy)-Prenylation. J. Am. Chem. Soc 2014, 136, 8911–8914. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Nguyen KD; Herkommer D; Krische MJ Enantioselective Formation of All-Carbon Quaternary Centers via C–H Functionalization of Methanol: Iridium-Catalyzed Diene Hydrohydroxymethylation. J. Am. Chem. Soc 2016, 138, 14210–14213. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Holmes M; Nguyen KD; Schwartz LA; Luong T; Krische MJ Enantioselective Formation of CF3 -Bearing All-Carbon Quaternary Stereocenters via C–H Functionalization of Methanol: Iridium Catalyzed Allene Hydrohydroxymethylation. J. Am. Chem. Soc 2017, 139, 8114–8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Liu Y; Mazet C A Catalytic Dual Isomerization/Allylboration Sequence for the Stereoselective Construction of Congested Secondary Homoallylic Alcohols. J. Org. Chem 2020, 85, 5638–5650. [DOI] [PubMed] [Google Scholar]
- (14).(a) Miura T; Nakahashi J; Zhou W; Shiratori Y; Stewart SG; Murakami M Enantioselective Synthesis of anti-1,2-Oxaborinan-3-enes from Aldehydes and 1,1-Di(boryl)alk-3-enes Using Ruthenium and Chiral Phosphoric Acid Catallysts. J. Am. Chem. Soc 2017, 139, 10903–10908. [DOI] [PubMed] [Google Scholar]; (b) Miura T; Nakahashi J; Murakami M Enantioselective Synthesis of (E)-δ-Boryl-Substituted Anti-Homoallylic Alcohols Using Palladium and a Chiral Phosphoric Acid. Angew. Chem. Int. Ed 2017, 56, 6989–6993. [DOI] [PubMed] [Google Scholar]; (c) Park J; Choi S; Lee Y; Cho SH Chemo- and Stereoselective Crotylation of Aldehydes and Cyclic Aldimines with Allylic Gem-Diboronate Ester. Org. Lett 2017, 19, 4054–4057. [DOI] [PubMed] [Google Scholar]; (d) Gao S; Chen J; Chen M (Z)-α-Boryl-Crotylboron Reagents via Z -Selective Alkene Isomerization and Application to Stereoselective Syntheses of (E)-δ-Boryl-Syn-Homoallylic Alcohols. Chem. Sci 2019, 10, 3637–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wang M; Gao S; Chen M Stereoselective Syntheses of (E)-γ′,δ-Bisboryl-Substituted Syn-Homoallylic Alcohols via Chemoselective Aldehyde Allylboration. Org. Lett 2019, 21, 2151–2155. [DOI] [PubMed] [Google Scholar]; (f) Chen J; Chen M Enantioselective Syntheses of (Z)-6′-Boryl-Anti-1,2-Oxaborinan-3-Enes via a Dienylboronate Protoboration and Asymmetric Allylation Reaction Sequence. Org. Lett 2020, 22, 7321–7326. [DOI] [PubMed] [Google Scholar]; (g) Gao S; Duan M; Shao Q; Houk KN; Chen M Development of α,α-Disubstituted Crotylboronate Reagents and Stereoselective Crotylation via Brønsted or Lewis Acid Catalysis. J. Am. Chem. Soc 2020, 142 (43), 18355–18368. [DOI] [PubMed] [Google Scholar]
- (15).For an example aldehyde allyl addition via boron-stabilized allylic copper, see:; Gao S; Chen M Catalytic Carboboration of Dienylboronate for Stereoselective Synthesis of (E)-γ′,δ-Bisboryl-AntiHomoallylic Alcohols. Chem. Commun 2019, 55, 11199–11202. [DOI] [PubMed] [Google Scholar]
- (16).Green JC; Zanghi JM; Meek SJ Diastereo- and Enantioselective Synthesis of Homoallylic Amines Bearing Quaternary Carbon Centers. J. Am. Chem. Soc 2020, 142, 1704–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Zanghi JM; Meek SJ Cu-Catalyzed Diastereo- and Enantioselective Reactions of γ,γ-Disubstituted Allyldiboron Compounds with Ketones. Angew. Chem. Int. Ed 2020, 59, 8451–8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).If the catalytic reaction for 1a is stopped after 1 h by an aqueous work up 3a is formed in 94% NMR yield but 4.4:1 dr and diminished 86:14 er.
- (19).A plot utilizing only all the data points resulted in lower statistical significance (R2 = 0.62).
- (20).(a) Myers AG; Widdowson KL; Kukkola PJ Silicon-Directed Aldol Condensation. Evidence for a Pseudorotational Mechanism. J. Am. Chem. Soc 1992, 114, 2765–2767. [Google Scholar]; (b) Hosomi A; Kohra S; Ogata K; Yanagi T; Tominaga Y Studies in Organosilicon Chemistry. 100. Pentacoordinate Allylsiliconates in Organic Synthesis: Synthesis of Triethylammonium Bis(Catecholato)Allylsiliconates and Selective Allylation of Aldehydes. J. Org. Chem 1990, 55, 2415–2420. [Google Scholar]; (c) Denmark SE; Bui T Chiral Phosphoramide-Catalyzed, Enantioselective, Directed Cross-Aldol Reactions of Aldehydes. Proc. Natl. Acad. Sci. USA 2004, 101, 5439–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Malkov AV; Ramírez-López P; Biedermannová (née Bendová) L; Rulíšek L; Dufková L; Kotora M; Zhu F; Kočovský P On the Mechanism of Asymmetric Allylation of Aldehydes with Allyltrichlorosilanes Catalyzed by QUINOX, a Chiral Isoquinoline N-Oxide. J. Am. Chem. Soc 2008, 130, 5341–5348. [DOI] [PubMed] [Google Scholar]
- (21).Effectively quenching or in situ monitoring the allyl addition reactions proved difficult, as such C–C bond formation could not be confirmed as the rate-limiting step.
- (22).Control reaction between (R)-10 and 2f in the absence of (L4)-Cu (aqueous work up) results in 11% NMR yield and (1:10 dr, C1:C2).
- (23).Direct formation of α-borylcopper intermediate C is also possible.
- (24).For examples of site- and stereospecific M-OR SE2’ transmetalation with allylboron reagents, see:; (a) Yang Y; Buchwald SL Ligand-Controlled Palladium-Catalyzed Regiodivergent Suzuki-Miyaura Cross-Coupling of Allylboronates and Aryl Halides. J. Am. Chem. Soc 2013, 135, 10642–10645. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chausset-Boissarie L; Ghozati K; LaBine E; Chen JL-Y; Aggarwal VK; Crudden CM Enantiospecific, Regioselective Cross-Coupling Reactions of Secondary Allylic Boronic Esters. Chem. Eur. J 2013, 19, 17698–17701. [DOI] [PubMed] [Google Scholar]; (c) Potter B; Edelstein EK; Morken JP Modular, Catalytic Enantioselective Construction of Quaternary Carbon Stereocenters by Sequential Cross-Coupling Reactions. Org. Lett 2016, 18, 3286–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).For a crystal structure of an allyl α-boryl Cu species, see:; Sun Y; Zhou Y; Shi Y; del Pozo J; Torker S; Hoveyda AH Copper–Hydride-Catalyzed Enantioselective Processes with Allenyl Boronates. Mechanistic Nuances, Scope, and Utility in Target-Oriented Synthesis. J. Am. Chem. Soc 2019, 141, 12087–12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Due to the robustness and high reactivity of allyldiboronates, post-reaction non-catalytic allyl addition can lead to higher dr but lower er.
- (27).Kraus GA; Molina MT; Walling JA Reduction of Cyclic Hemiacetals. The Synthesis of Demethoxyeleutherin and nanaomycin A. J. Chem. Soc. Chem. Commun 1986, 1568–1569. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


